Characterization of a Hydrogel Composite Containing Bioactive Moringa as a Novel Pulp-Capping Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extract Preparation
2.2. High-Performance Liquid Chromatography (HPLC)
2.3. Hydrogel Composite
2.3.1. Hydrogel Composite Preparation
2.3.2. Addition of Moringa to Hydrogel Composite
2.4. pH Value Assessment
2.5. Antibacterial Assay (Well Diffusion Method)
2.6. Antioxidant Assay (DPPH Test)
2.7. Cytotoxicity Test (MTT Assay)
2.8. Drying Time
2.9. Film Thickness
2.10. Fourier Transform Infrared Spectroscopy (FTIR)
2.11. X-Ray Diffraction (XRD)
2.12. Shear Viscosity
2.13. Scanning Electron Microscopy (SEM)
2.14. Folding Endurance
2.15. Degradation Rate
2.16. Swelling Index
2.17. In Vitro Drug Release
- First-order:
- Higuchi:
2.18. Statistical Analysis
3. Results
3.1. HPLC
3.2. pH
3.3. Antibacterial Assay (Well Diffusion Method)
3.4. Antioxidant Assay (DPPH Test)
3.5. Cytotoxicity Test
3.6. Drying Time
3.7. Film Thickness
3.8. FTIR
3.9. XRD
3.10. Shear Viscosity
3.11. SEM
3.12. Folding Endurance
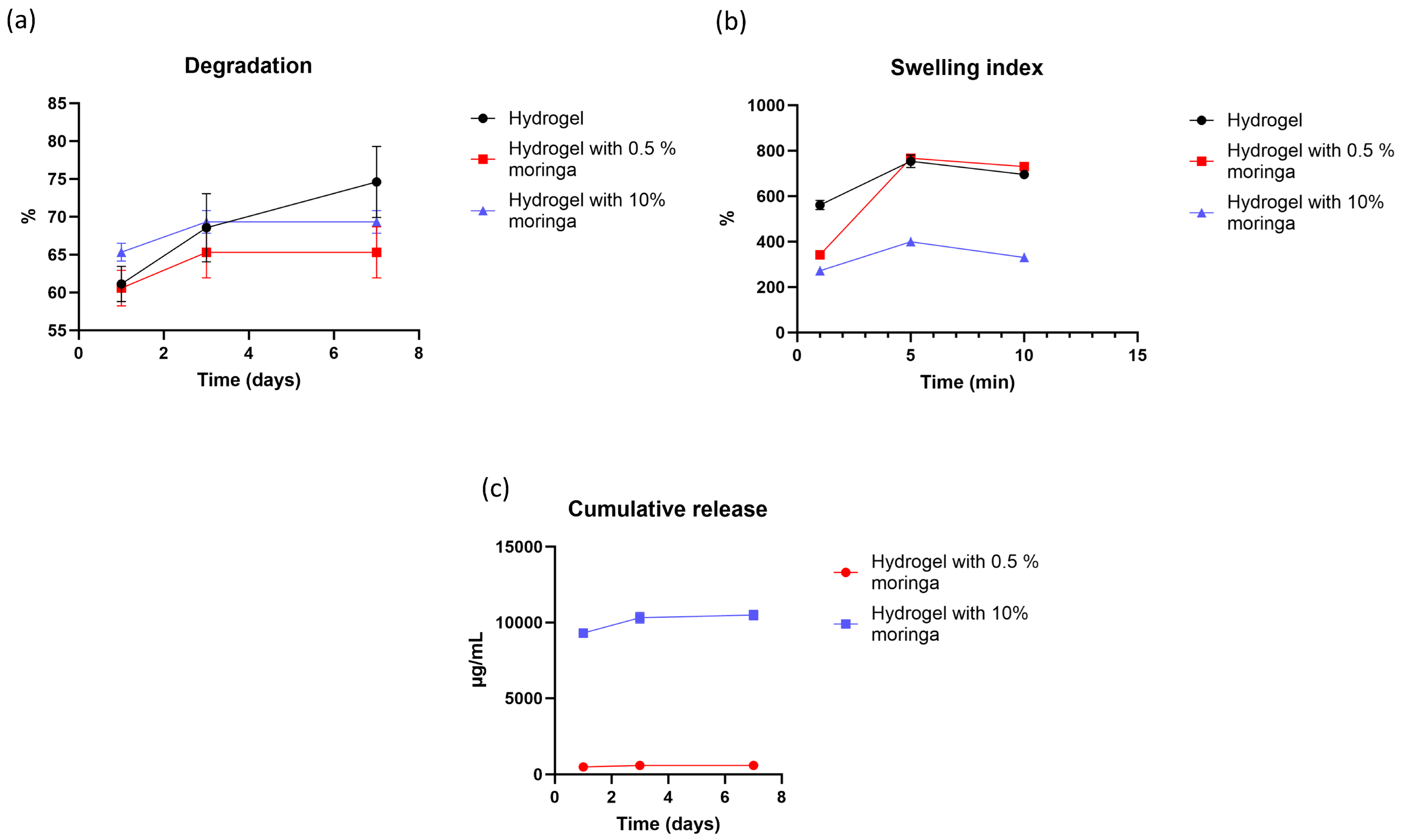
3.13. Degradation Rate
3.14. Swelling Index
3.15. In Vitro Drug Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High performance liquid chromatography |
| PVA | Polyvinyl alcohol |
| HA | Hyaluronic acid |
| SA | Sodium alginate |
| SEM | Scanning electron microscope |
| FTIR | Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| NHF | Normal human fibroblast |
| MEM | Minimum essential media |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate buffer saline |
References
- Hilton, T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef]
- Qureshi, A. Recent Advances in Pulp Capping Materials: An Overview. J. Clin. Diagn. Res. 2014, 8, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Alp, S.; Ulusoy, N. Current Approaches in Pulp Capping: A Review. Cyprus J. Med. Sci. 2024, 9, 154–160. [Google Scholar] [CrossRef]
- Al-Hyali, N.A. Comparison among Pulp Capping Materials in Calcium Ion Release, pH Change, Solubility and Water Sorption: An in Vitro Study. J. Baghdad Coll. Dent. 2017, 29, 9–16. [Google Scholar] [CrossRef][Green Version]
- Ali, A.; Mahdee, A.; Fadhil, N. Preferences of treatments and materials used in the management of exposed pulps: A web-based questionnaire study. J. Stomatol. 2022, 75, 99–106. [Google Scholar] [CrossRef]
- Mutar, M.T.; Mahdee, A.F. Different pulp capping agents and their effect on pulp inflammatory response: A narrative review. Saudi Dent. J. 2024, 36, 1295–1306. [Google Scholar] [CrossRef]
- Shafiq, N.E.; Mahdee, A.F. Moringa oleifera Use in Maintaining Oral Health and Its Potential Use in Regenerative Dentistry. Sci. World J. 2023, 2023, 8876189. [Google Scholar] [CrossRef]
- Shafiq, N.E.; Mahdee, A.F.; Hasan, Z.Y.M. Leaf Extracts of Moringa oleifera Cultivated in Baghdad: Characterization and Antimicrobial Potential against Endodontic Pathogens. Sci. World J. 2024, 2024, 58164. [Google Scholar] [CrossRef] [PubMed]
- Alsaraf, K.M.; Abd, S.T.; Husain, N.S. Husain, An Antimicrobial Activity of Moringa Oleifera Extract in Comparison to Chlorhexidene Gluconate: In Vitro Study. J. Baghdad Coll. Dent. 2016, 28, 183–187. [Google Scholar] [CrossRef][Green Version]
- Shafiq, N.A.; (University of Baghdad, Baghdad, Iraq); Mahdee, A.F.; (University of Baghdad, Baghdad, Iraq). Assessment of Moringa oleifera Leaf Extracts as a Novel Bioactive Material in Dental Pulp Regeneration (Comparative In Vitro Study). Unpublished work. 2024. [Google Scholar][Green Version]
- Parhizkar, A.; Asgary, S. Local Drug Delivery Systems for Vital Pulp Therapy: A New Hope. Int. J. Biomater. 2021, 2021, 5584268. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Q.; Yu, H.; Shao, L.; Cui, X.; Pang, Q.; Zhu, Y.; Hou, R. Construction methods and biomedical applications of PVA-based hydrogels. Front. Chem. 2024, 12, 1376799. [Google Scholar] [CrossRef]
- Dawood, R.M.; Mahdee, A.F. Fabrication and characterization of 3D-printed polymeric-based scaffold coated with bioceramic and naringin for a potential use in dental pulp regeneration (in vitro study). Int. Endod. J. 2025, 58, 627–642. [Google Scholar] [CrossRef]
- Dawood, R.M.; Mahdee, A.F. Inducing Osteogenesis in Human Pulp Stem Cells Cultured on Nano-Hydroxyapatite and Naringin-Coated 3D-Printed Poly Lactic Acid Scaffolds. Polymers 2025, 17, 596. [Google Scholar] [CrossRef]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery. J. Control. Release 2017, 265, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly(vinyl alcohol)-hyaluronic Acid Membranes for Wound Dressing Applications: Synthesis and in vitro Bio-Evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Kodavaty, J. Poly (vinyl alcohol) and hyaluronic acid hydrogels as potential biomaterial systems—A comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 71, 103298. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2012.
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Guo, J.; Liu, L.; Fu, H.; Yao, J.; Krucińska, I.; Draczyński, Z. Highly Strong, Tough, and Stretchable Conductive Hydrogels Based on Silk Sericin-Mediated Multiple Physical Interactions for Flexible Sensors. ACS Appl. Polym. Mater. 2021, 4, 618–626. [Google Scholar] [CrossRef]
- Aruldass, S.; Mathivanan, V.; Mohamed, A.; Tye, C. Factors affecting hydrolysis of polyvinyl acetate to polyvinyl alcohol. J. Environ. Chem. Eng. 2019, 7, 103238. [Google Scholar] [CrossRef]
- Queen, D.; Orsted, H.; Sanada, H.; Sussman, G. A dressing history. Int. Wound J. 2004, 1, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Peng, G. TLR-mediated metabolic reprogramming in the tumor microenvironment: Potential novel strategies for cancer immunotherapy. Cell. Mol. Immunol. 2018, 15, 428–437. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar]
- Rizfa, M.S.; Yudiati, E.; Wijayanti, D.P. Improving The Antioxidant Activity of Sodium Alginate from Sargassum sp. by Thermal Heating and Chemical Methods. J. Kelaut. Trop. 2020, 23, 284–290. [Google Scholar] [CrossRef]
- Latifah, R.N.; Rahmania, S.; Rohmah, B.L. The effect of extraction time on the quality of brown seaweed Na-Alginate Sargassum polycisteum as the base material for SBK Edible Film. J. Phys. Conf. Ser. 2022, 2190, 012001. [Google Scholar] [CrossRef]
- Karakuş, N.R.; Türk, S.; Guney Eskiler, G.; Syzdykbayev, M.; Appazov, N.O.; Özacar, M. Investigation of Tannic Acid Crosslinked PVA/PEI-Based Hydrogels as Potential Wound Dressings with Self-Healing and High Antibacterial Properties. Gels 2024, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, T.; Soloman, A.M.; Annamalai, D.; Thiruppathi, V.; Srinivasan, P.; Masilamani, D.; Gopinath, A.; Madhan, B. Facile crosslinking of PVA scaffolds using quercetin for biomaterial applications. Mater. Lett. 2024, 364, 136307. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Gupta, A.S.; Mukherjee, K.; Giri, T.K. Borax cross-linked polysaccharide-based self-healing hydrogels for drug delivery and regenerative medicine. J. Drug Delivery Sci. Technol. 2025, 105, 106592. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.S.M. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Shivakumara, L.R.; Demappa, T. Synthesis and Swelling Behavior of Sodium Alginate/Poly(vinyl alcohol) Hydrogels. Turk. J. Pharm. Sci. 2019, 16, 252–260. [Google Scholar] [CrossRef]
- Hapipi, N.M.; Mazlan, S.A.; Ubaidillah, U.; Homma, K.; Aziz, S.A.A.; Nordin, N.A.; Bahiuddin, I.; Nazmi, N. The Rheological Studies on Poly(vinyl) Alcohol-Based Hydrogel Magnetorheological Plastomer. Polymers 2020, 12, 2332. [Google Scholar] [CrossRef]
- Gajra, B.; Pandya, S.S.; Singh, S.; Rabari, H.A. Mucoadhesive Hydrogel Films of Econazole Nitrate: Formulation and Optimization Using Factorial Design. J. Drug Deliv. 2014, 2014, 305863. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Johari, N.; Adabavazeh, Z.; Baino, F. PVA-based bioinks for 3D bioprinting: A comprehensive review of their applications in tissue engineering. Bioprinting 2025, 49, e00419. [Google Scholar] [CrossRef]
- Kim, J.-T.; Lee, D.-Y.; Kim, Y.-H.; Lee, I.-K.; Song, Y.-S. Effect of pH on Swelling Property of Hyaluronic Acid Hydrogels for Smart Drug Delivery Systems. J. Sens. Sci. Technol. 2012, 21, 256–262. [Google Scholar] [CrossRef]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Lin, Y.-S. Effects of pH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2021, 120, 111667. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Taha, G.M. Investigation and kinetics of hydrogel scaffold with sustained release ciprofloxacin hydrochloride. Polym. Bull. 2024, 81, 17393–17411. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Mahmood, A.; Munir, A.; Qalawlus, A.H.M.; Jabeen, N.; Kousar, M.; Anwar, Z. Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair. Int. J. Pharm. 2023, 643, 123244. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, Y.; Fang, J.; Liu, W.; Zhao, Y.; Huang, T.; Cui, J.; Yang, Y.; Zhou, Z. Preparation and characterization of PVA/SA/HA composite hydrogels for wound dressing. Int. J. Polym. Anal. Charact. 2019, 24, 132–141. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. FTIR spectral band shifts explained by OM–cation interactions. J. Plant Nutr. Soil. Sci. 2021, 184, 388–397. [Google Scholar] [CrossRef]
- Kamel, S.; Dacrory, S.; Hesemann, P.; Bettache, N.; Ali, L.M.A.; Postel, L.; Akl, E.M.; El-Sakhawy, M. Wound Dressings Based on Sodium Alginate–Polyvinyl Alcohol–Moringa oleifera Extracts. Pharmaceutics 2023, 15, 1270. [Google Scholar] [CrossRef]
- Kovtun, G.; Casas, D.; Cuberes, T. Influence of Glycerol on the Surface Morphology and Crystallinity of Polyvinyl Alcohol Films. Polymers 2024, 16, 2421. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef] [PubMed]
- Ningrum, D.R.; Hanif, W.; Mardhian, D.F.; Asri, L.A.T.W. In Vitro Biocompatibility of Hydrogel Polyvinyl Alcohol/Moringa oleifera Leaf Extract/Graphene Oxide for Wound Dressing. Polymers 2023, 15, 468. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 9, 568. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2015, 53, 326–336. [Google Scholar] [CrossRef]
- Chousidis, N. Polyvinyl alcohol (PVA)-based films: Insights from crosslinking and plasticizer incorporation. Eng. Res. Express 2024, 6, 025010. [Google Scholar] [CrossRef]
- Nayak, S.; Khuntia, S.K. Development and study of properties of Moringa oleifera fruit fibers/ polyethylene terephthalate composites for packaging applications. Compos. Commun. 2019, 15, 113–119. [Google Scholar] [CrossRef]
- Kopka, B.; Kost, B.; Wrześniewska, J.; Rajkowska, K.; Kadłubowski, S.; Kunicka-Styczyńska, A.; Baryga, A.; Gonciarz, W.; Basko, M.; Brzeziński, M. Supramolecular poly(vinyl alcohol)-based hydrogels containing quercetin for bacterial and fungal elimination. Eur. Polym. J. 2023, 187, 111881. [Google Scholar] [CrossRef]
- Bar, A.; Ramon, O.; Cohen, Y.; Mizrahi, S. Shrinkage behaviour of hydrophobic hydrogel during dehydration. J. Food Eng. 2002, 55, 193–199. [Google Scholar] [CrossRef]
- Gheorghita, R.; Filip, R.; Lupaescu, A.-V.; Iavorschi, M.; Anchidin-Norocel, L.; Gutt, G. Innovative Materials with Possible Applications in the Wound Dressings Field: Alginate-Based Films with Moringa oleifera Extract. Gels 2023, 9, 560. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J.; Mali, K.K.; Pargaonkar, S.S.; Shinde, P.V.; Dhane, N.S. Citric acid crosslinked carboxymethylcellulose-poly(ethylene glycol) hydrogel films for delivery of poorly soluble drugs. Int. J. Biol. Macromol. 2018, 118, 783–791. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Pan, N.C.; Bersaneti, G.T.; Mali, S.; Celligoi, M.A.P.C. Films Based on Blends of Polyvinyl Alcohol and Microbial Hyaluronic Acid. Braz. Arch. Biol. Technol. 2020, 63, 1–14. [Google Scholar] [CrossRef]
- Mahkam, M.; Doostie, L. The Relation Between Swelling Properties and Cross-Linking of Hydrogels Designed for Colon-Specific Drug Delivery. Drug Deliv. 2005, 12, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ganji, F.; Farahani, S.V.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Shafiei, F.; Ghavami-Lahiji, M.; Kashi, T.S.J.; Najafi, F. Drug release kinetics and biological properties of a novel local drug carrier system. Dent. Res. J. 2021, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yan, S.; Wang, H.; Zhao, L.; Zhu, H.; Wen, Z. Antimicrobial and antibiofilm effects of total flavonoids from Potentilla kleiniana Wight et Arn on Pseudomonas aeruginosa and its potential application to stainless steel surfaces. LWT 2022, 154, 112631. [Google Scholar] [CrossRef]
- Berg, J.v.D.; Kuipers, S. The antibacterial action of Moringa oleifera: A systematic review. S. Afr. J. Bot. 2022, 151, 224–233. [Google Scholar] [CrossRef]



| Phenolic Compound | Area (Standards) | Area (Sample) | Concentration (Sample) µg/mL |
|---|---|---|---|
| Gallic acid | 45,793 | 320,026 | 69.88535 |
| Vanillic acid | 175,796 | 179,648 | 10.21912 |
| Quercetin Anhydrous | 59,797 | 33,161 | 5.545596 |
| Apigenin | 20,523 | 16,078 | 7.834137 |
| Kaempferol | 94,304 | 73,597 | 7.804229 |
| Chlorogenic acid | 10,927 | - | - |
| Myricetin | 2385 | - | - |
| Rutin | 29,912 | 27,766 | 9.282562 |
| Isorhamnetin | 9484 | 8585 | 9.052088 |
| Name of Material | Concentration of moringa | pH | Antibacterial Assay, Inhibition Zones (mm) (±SD) | Antioxidant Assay, Inhibition Rate (%) (±SD) | Drying Time (s) (±SD) | Film Thickness (±SD) and [RR %] |
|---|---|---|---|---|---|---|
| PVA | - | 6.4 | - | - | - | - |
| PVA + moringa | 0.5% W/W | 5.8 | - | - | - | - |
| PVA + HA + SA | - | 7.3 | No inhibition | 23.83% A (0.036) | 218.33 A (7.63) | 0.054 A (0.006) [96.72%] |
| Hydrogel + moringa | 0.5% W/W | 7.2 | No inhibition | 37.7% B (0.264) | 146.33 B (5.5) | 0.054 A (0.004) [96.72%] |
| 5% W/W | 5.3 | No inhibition | 39.8% C (0.36) | - | - | |
| 7.5% W/W | 5.1 | No inhibition | 40.1% C,D,E (0.1) | - | - | |
| 10% W/W | 5 | 1.03 A (0.057) | 40.4% C,D,E (0.2) | 45 C (5) | 0.115 B (0.004) [93.06%] | |
| 20% W/W | 4.8 | 1.33 A (0.057) | 40.6% E (0.2) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutar, M.T.; Mahdee, A.F. Characterization of a Hydrogel Composite Containing Bioactive Moringa as a Novel Pulp-Capping Material. Polymers 2025, 17, 2626. https://doi.org/10.3390/polym17192626
Mutar MT, Mahdee AF. Characterization of a Hydrogel Composite Containing Bioactive Moringa as a Novel Pulp-Capping Material. Polymers. 2025; 17(19):2626. https://doi.org/10.3390/polym17192626
Chicago/Turabian StyleMutar, Mustafa Tariq, and Anas F. Mahdee. 2025. "Characterization of a Hydrogel Composite Containing Bioactive Moringa as a Novel Pulp-Capping Material" Polymers 17, no. 19: 2626. https://doi.org/10.3390/polym17192626
APA StyleMutar, M. T., & Mahdee, A. F. (2025). Characterization of a Hydrogel Composite Containing Bioactive Moringa as a Novel Pulp-Capping Material. Polymers, 17(19), 2626. https://doi.org/10.3390/polym17192626






