Molecular Properties of Starch–Water Interactions in the Presence of Bioactive Compounds from Barley and Buckwheat—LF NMR Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Gels Preparation
2.3. Determination of Amylose Content
2.4. LF NMR Relaxometry
3. Results and Discussion
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BH | Buckwheat hull fiber |
| BPP | Bloembergen–Purcell–Pound equations |
| GB | Green burley |
| FID | Free induction decay |
| LF NMR | Low-Field Nuclear Magnetic Resonance |
| NPS | Non-waxy potato starch |
| NPS/BH | Non-waxy potato starch with buckwheat hull fiber |
| NPS/GB | Non-waxy potato starch with green barley |
| WPS | Waxy potato starch |
| WPS/BH | Waxy potato starch with buckwheat hull fiber |
| WPS/GB | Waxy potato starch with green barley |
References
- Rashwan, A.K.; Younis, H.A.; Abdelshafy, A.M.; Osman, A.I.; Eletmany, M.R.; Hafouda, M.A.; Chen, W. Plant Starch Extraction, Modification, and Green Applications: A Review. Environ. Chem. Lett. 2024, 22, 2483–2530. [Google Scholar] [CrossRef]
- Palma-Rodríguez, H.M.; Vargas-Torres, A.; Leyva-López, R. Porous Starch: Enzymatic Methods to Obtain It and Apply It as a Carrier Material in the Food Area. Biointerface Res. Appl. Chem. 2024, 14, 45. [Google Scholar] [CrossRef]
- Raj, N.; Dalal, N.; Bisht, V.; Dhakar, U. Potato Starch: Novel Ingredient for Food Industry. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1718–1724. [Google Scholar] [CrossRef]
- Choton, S.; Bandral, J.D.; Singh, J.; Bhat, A.; Sood, M.; Gupta, N.; Reshi, M.; Kaur, D. Enzymatic Modification of Starch: A Review. Saudi J. Med. Pharm. Sci. 2024, 10, 1–8. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Djali, M. The Effect of Starch-Hydrocolloid Interaction on Starch Digestibility, Pasting and Physicochemical Properties: A Review. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012084. [Google Scholar] [CrossRef]
- Mahfouzi, M.; Zhang, H.; Haoran, L.; McClements, D.J.; Hadidi, M. Starch-Based Particles as Stabilizers for Pickering Emulsions: Modification, Characteristics, Stabilization, and Applications. Crit. Rev. Food Sci. Nutr. 2025, 65, 1841–1856. [Google Scholar] [CrossRef]
- Naveen, R.; Loganathan, M. Role of Varieties of Starch in the Development of Edible Films—A Review. Starch–Stärke 2024, 76, 2300138. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Jeżowski, P.; Menzel, J.; Baranowska, H.M.; Kowalczewski, P.Ł. Microwaved-Assisted Synthesis of Starch-Based Biopolymer Membranes for Novel Green Electrochemical Energy Storage Devices. Materials 2023, 16, 7111. [Google Scholar] [CrossRef] [PubMed]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef]
- Compart, J.; Singh, A.; Fettke, J.; Apriyanto, A. Customizing Starch Properties: A Review of Starch Modifications and Their Applications. Polymers 2023, 15, 3491. [Google Scholar] [CrossRef]
- Hermans, P.H.; Weidinger, A. X-ray Studies on the Crystallinity of Cellulose. J. Polym. Sci. 1949, 4, 135–144. [Google Scholar] [CrossRef]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a Phenomenological Definition of the Term “Gel”. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Wang, K. 17. Elastic and Viscoelastic Models of Crustal Deformation in Subduction Earthquake Cycles. In The Seismogenic Zone of Subduction Thrust Faults; Columbia University Press: New York, NY, USA, 2016; pp. 540–575. [Google Scholar] [CrossRef]
- Singh, R.; Davies, P.; Bajaj, A.K. Estimation of the Dynamical Properties of Polyurethane Foam through Use of Prony Series. J. Sound. Vib. 2003, 264, 1005–1043. [Google Scholar] [CrossRef]
- Sudheesh, C.; Varsha, L.; Sunooj, K.V.; Pillai, S. Influence of Crystalline Properties on Starch Functionalization from the Perspective of Various Physical Modifications: A Review. Int. J. Biol. Macromol. 2024, 280, 136059. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, W.; Qiao, S.; Song, W.; Tan, W. Advances in Processing Techniques and Determinants of Sweet Potato Starch Gelatinization. Foods 2025, 14, 545. [Google Scholar] [CrossRef]
- Fonseca, L.M.; El Halal, S.L.; Dias, A.R.; da Rosa Zavareze, E. Physical Modification of Starch by Heat-Moisture Treatment and Annealing and Their Applications: A Review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef]
- Nwaiwu, O.; Onyeaka, H. New Model High Temperature Pasting Analysis of Fermented Cassava Granules. Fermentation 2022, 8, 89. [Google Scholar] [CrossRef]
- Roulet, P.; Macinnes, W.M.; Gumy, D.; Würsch, P. Retrogradation Kinetics of Eight Starches. Starch–Stärke 1990, 42, 99–101. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Gilbert, R. The Effects of Starch Molecular Fine Structure on Thermal and Digestion Properties of Rice Starch. Foods 2022, 11, 4012. [Google Scholar] [CrossRef]
- Walkowiak, K.; Przybył, K.; Baranowska, H.M.; Koszela, K.; Masewicz, Ł.; Piątek, M. The Process of Pasting and Gelling Modified Potato Starch with LF-NMR. Polymers 2022, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Duda, A.; Poliszko, N.; Różańska, M.B.; Jeżowski, P.; Tomkowiak, A.; Mildner-Szkudlarz, S.; Baranowska, H.M. Wheat Bread Enriched with Raspberry and Strawberry Oilcakes: Effects on Proximate Composition, Texture and Water Properties. Eur. Food Res. Technol. 2019, 245, 2591–2600. [Google Scholar] [CrossRef]
- Tako, M.; Tamaki, Y.; Teruya, T.; Takeda, Y. The Principles of Starch Gelatinization and Retrogradation. Food Nutr. Sci. 2014, 05, 280–291. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A Comprehensive Review of the Factors Influencing the Formation of Retrograded Starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef]
- Yan, W.; Yin, L.; Zhang, M.; Zhang, M.; Jia, X. Gelatinization, Retrogradation and Gel Properties of Wheat Starch–Wheat Bran Arabinoxylan Complexes. Gels 2021, 7, 200. [Google Scholar] [CrossRef]
- Zhu, F. NMR Spectroscopy of Starch Systems. Food Hydrocoll. 2017, 63, 611–624. [Google Scholar] [CrossRef]
- Choi, S.-G.; Kerr, W.L. 1H NMR Studies of Molecular Mobility in Wheat Starch. Food Res. Int. 2003, 36, 341–348. [Google Scholar] [CrossRef]
- Farrar, T.C.; Becker, E.D. Pulse and Fourier Transform NMR; Elsevier: Amsterdam, The Netherlands, 1971; ISBN 9780122496509. [Google Scholar]
- Brosio, E.; Gianferri, R.R. Low-Resolution NMR—An Analytical Tool in Foods Characterization and Traceability. In Basic NMR in Foods Characterization; Brosio, E., Ed.; Research Signpost: Kerala, India, 2009; pp. 9–37. ISBN 978-81-308-0303-6. [Google Scholar]
- Blümich, B. Introduction to Compact NMR: A Review of Methods. TrAC Trends Anal. Chem. 2016, 83, 2–11. [Google Scholar] [CrossRef]
- Stangierski, J.; Baranowska, H.M. The Influence of Heating and Cooling Process on the Water Binding in Transglutaminase-Modified Chicken Protein Preparation, Assessed Using Low-Field NMR. Food Bioproc Technol. 2015, 8, 2359–2367. [Google Scholar] [CrossRef]
- Conte, P.; Bubici, S.; Palazzolo, E.; Alonzo, G. Solid-State 1H-NMR Relaxation Properties of the Fruit of a Wild Relative of Eggplant at Different Proton Larmor Frequencies. Spectrosc. Lett. 2009, 42, 235–239. [Google Scholar] [CrossRef]
- Atambayeva, Z.; Nurgazezova, A.; Amirkhanov, K.; Assirzhanova, Z.; Khaimuldinova, A.; Charchoghlyan, H.; Kaygusuz, M. Unlocking the Potential of Buckwheat Hulls, Sprouts, and Extracts: Innovative Food Product Development, Bioactive Compounds, and Health Benefits—A Review. Pol. J. Food Nutr. Sci. 2024, 74, 293–312. [Google Scholar] [CrossRef]
- Koç, S.T.; Coşkun, F. Buckwheat: Nutritional Value, Health Effects and Applications in Foods. Turk. J. Agric. Food Sci. Technol. 2025, 13, 1665–1674. [Google Scholar] [CrossRef]
- Sonawane, S.; Shams, R.; Dash, K.K.; Patil, V.; Pandey, V.K.; Dar, A.H. Nutritional Profile, Bioactive Properties and Potential Health Benefits of Buckwheat: A Review. EFood 2024, 5, e171. [Google Scholar] [CrossRef]
- Ariyarathna, P.; Mizera, P.; Walkowiak, J.; Dziedzic, K. Physicochemical and Functional Properties of Soluble and Insoluble Dietary Fibers in Whole Grains and Their Health Benefits. Foods 2025, 14, 2447. [Google Scholar] [CrossRef]
- Dziedzic, K.; Ariyarathna, P.; Szwengiel, A.; Hęś, M.; Ratajczak, K.; Górecka, D.; Sulewska, H.; Walkowiak, J. Changes in the Content of Dietary Fiber, Flavonoids, and Phenolic Acids in the Morphological Parts of Fagopyrum tataricum (L.) Gaertn Under Drought Stress. Molecules 2025, 30, 270. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding Buckwheat for Nutritional Quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and Therapeutic Role of Functional Ingredients of Barley Grass for Chronic Diseases in Human Beings. Oxid. Med. Cell Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef]
- Raj, R.; Shams, R.; Pandey, V.K.; Dash, K.K.; Singh, P.; Bashir, O. Barley Phytochemicals and Health Promoting Benefits: A Comprehensive Review. J. Agric. Food Res. 2023, 14, 100677. [Google Scholar] [CrossRef]
- Sikora, M.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Walkowiak, K.; Masewicz, Ł.; Kowalczewski, P.Ł.; Baranowska, H.M. Molecular Analysis of Retrogradation of Corn Starches. Polymers 2019, 11, 1764. [Google Scholar] [CrossRef]
- Adamczyk, G.; Krystyjan, M.; Witczak, M. The Impact of Fiber from Buckwheat Hulls Waste on the Pasting, Rheological and Textural Properties of Normal and Waxy Potato Starch Gels. Polymers 2021, 13, 4148. [Google Scholar] [CrossRef]
- Eid, O.; Elkady, W.M.; Ezzat, S.; El Sayed, A.; Abd Elsattar, E. Comprehensive Overview: The Effect of Using Different Solvents for Barley Extraction with Its Anti-Inflammatory and Antioxidant Activity. Chem. Biodivers. 2023, 20, e202200935. [Google Scholar] [CrossRef]
- Morrison, W.R.; Laignelet, B. An Improved Colorimetric Procedure for Determining Apparent and Total Amylose in Cereal and Other Starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Węglarz, W.P.; Harańczyk, H. Two-Dimensional Analysis of the Nuclear Relaxation Function in the Time Domain: The Program CracSpin. J. Phys. D Appl. Phys. 2000, 33, 1909–1920. [Google Scholar] [CrossRef]
- Mostoufi, N.; Constantinides, A. Linear and Nonlinear Regression Analysis. In Applied Numerical Methods for Chemical Engineers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 403–476. [Google Scholar]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Li, C. Unraveling the Complexities of Starch Retrogradation: Insights from Kinetics, Molecular Interactions, and Influences of Food Ingredients. Food Rev. Int. 2024, 40, 3159–3182. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Mijović, J.; Zhang, H. Local Dynamics and Molecular Origin of Polymer Network−Water Interactions as Studied by Broadband Dielectric Relaxation Spectroscopy, FTIR, and Molecular Simulations. Macromolecules 2003, 36, 1279–1288. [Google Scholar] [CrossRef]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Tang, H.; Belton, P.S. Proton Relaxation in Plant Cell Walls and Model Systems. In Advances in Magnetic Resonance in Food Science; Elsevier: Amsterdam, The Netherlands, 1999; pp. 166–184. [Google Scholar]
- Steinrücken, E.; Kloth, S.; Vogel, M. Decoupling of Water and Ion Dynamics in Nanophase-Segrated Mixtures of an Ionic Liquid and Water Studied by NMR Experiments and MD Simulations. J. Phys. D Appl. Phys. 2025, 58, 295302. [Google Scholar] [CrossRef]
- Cao, P.; Wu, G.; Yao, Z.; Wang, Z.; Li, E.; Yu, S.; Liu, Q.; Gilbert, R.G.; Li, S. Effects of Amylose and Amylopectin Molecular Structures on Starch Electrospinning. Carbohydr. Polym. 2022, 296, 119959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, M.; Wei, W.; He, T.; Zhang, X.; Xu, H.; Sun, D. Multiscale Insights into the Role of Water Content in the Extrusion-Crosslinked Starch. Int. J. Biol. Macromol. 2025, 307, 142118. [Google Scholar] [CrossRef]
- Liu, W.; Xu, J.; Shuai, X.; Geng, Q.; Guo, X.; Chen, J.; Li, T.; Liu, C.; Dai, T. The Interaction and Physicochemical Properties of the Starch-Polyphenol Complex: Polymeric Proanthocyanidins and Maize Starch with Different Amylose/Amylopectin Ratios. Int. J. Biol. Macromol. 2023, 253, 126617. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Yoo, S.-H.; Lim, S.-T. Water Effect on the Interaction between Amylose and Amylopectin during Retrogradation. Carbohydr. Polym. 2011, 86, 1671–1674. [Google Scholar] [CrossRef]
- Adamczyk, G.; Hanus, P.; Bobel, I.; Krystyjan, M. Enrichment of Starch Desserts with the Addition of Apple Juice and Buckwheat Fiber. Polymers 2023, 15, 717. [Google Scholar] [CrossRef]
- Adamczyk, G.; Krystyjan, M.; Jaworska, G. The Effect of the Addition of Dietary Fibers from Apple and Oat on the Rheological and Textural Properties of Waxy Potato Starch. Polymers 2020, 12, 321. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Baranowska, H.M.; Le Thanh-Blicharz, J.; Makowska, A. Water Binding Capacity in Waxy and Normal Rice Starch Pastes. In Proceedings of the 1st International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 5–7 November 2015; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2015; pp. 69–72. [Google Scholar]
- Makowska, A.; Dwiecki, K.; Kubiak, P.; Baranowska, H.M.; Lewandowicz, G. Polymer-Solvent Interactions in Modified Starches Pastes–Electrokinetic, Dynamic Light Scattering, Rheological and Low Field Nuclear Magnetic Resonance Approach. Polymers 2022, 14, 2977. [Google Scholar] [CrossRef] [PubMed]
- Rachman, A.; Chen, L.; Brennan, M.; Brennan, C. Effects of Addition of Buckwheat Bran on Physicochemical, Pasting Properties and Starch Digestion of Buckwheat Gels. Eur. Food Res. Technol. 2020, 246, 2111–2117. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, L.; Wang, L.; Li, Z.; Qiu, J. Multi-Scale Structure, Rheological and Digestive Properties of Starch Isolated from Highland Barley Kernels Subjected to Different Thermal Treatments. Food Hydrocoll. 2022, 129, 107630. [Google Scholar] [CrossRef]
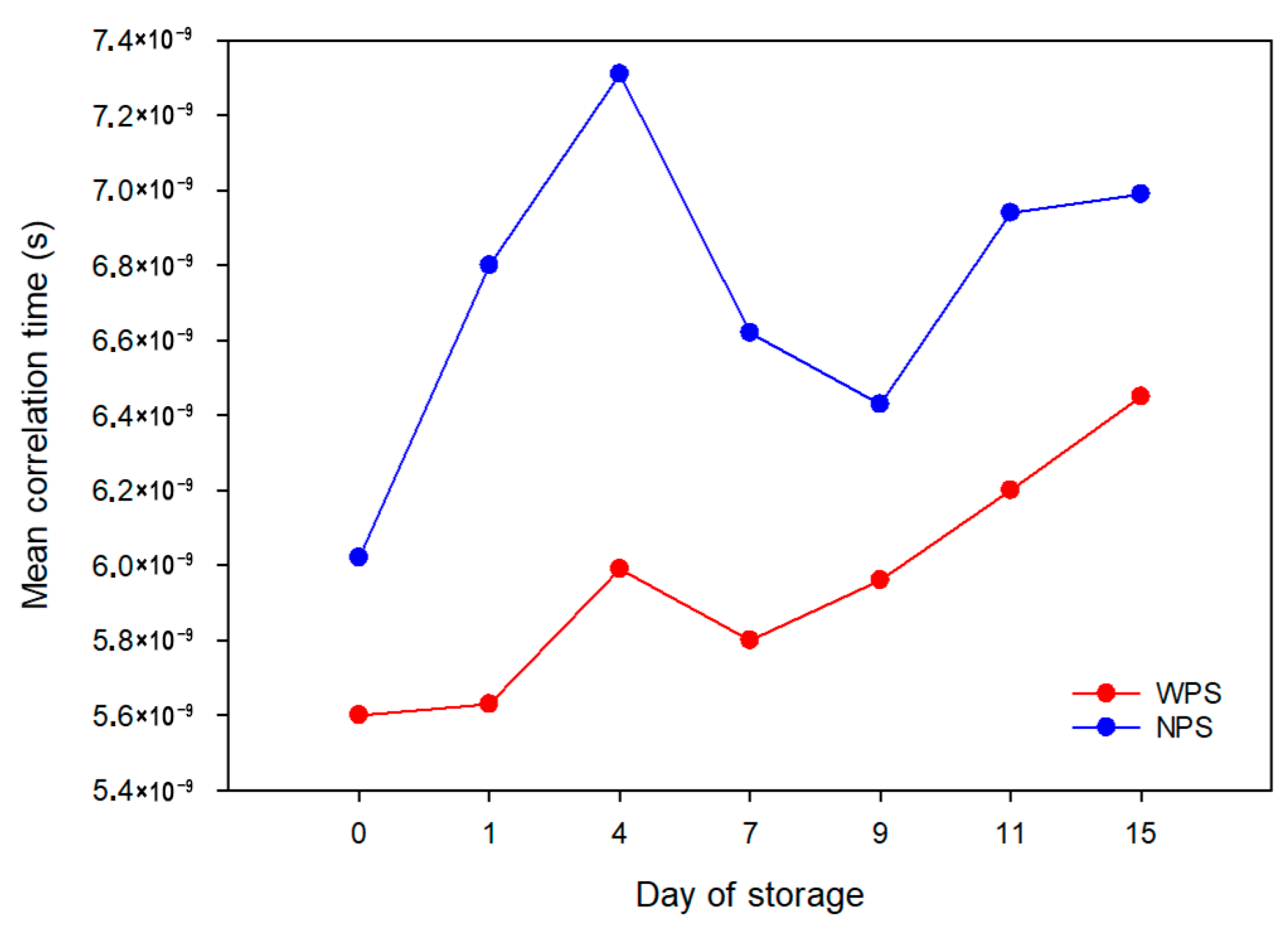
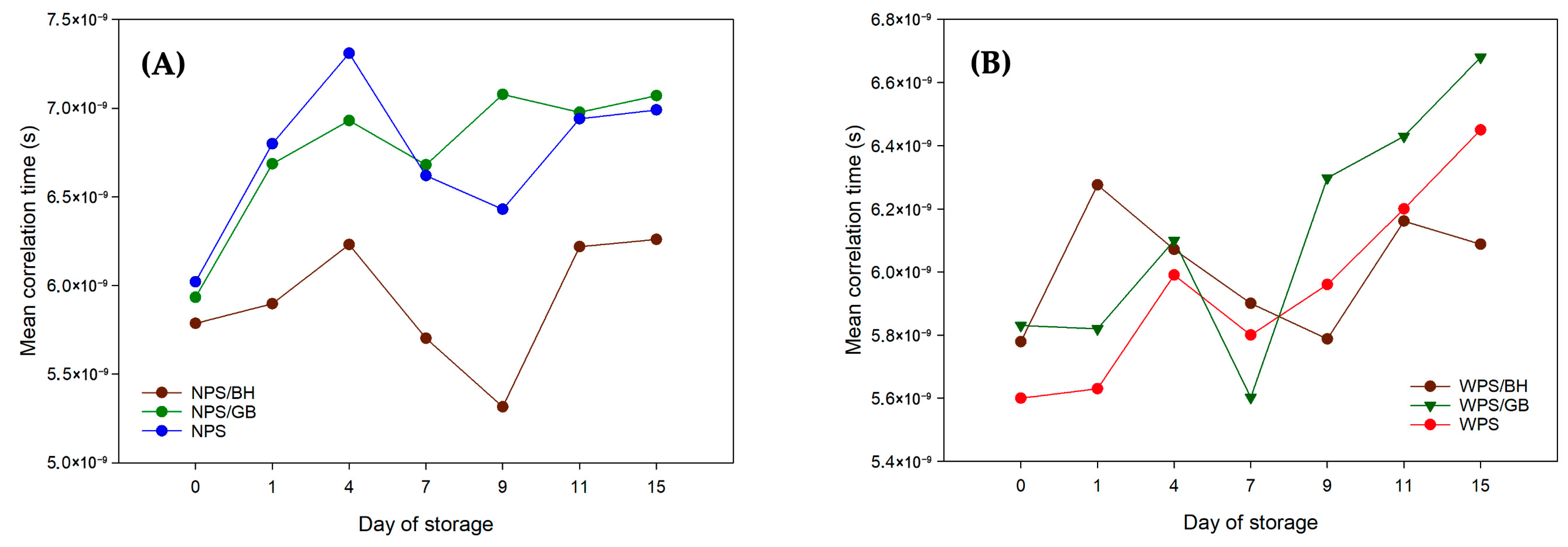
| Sample | Relaxation Time (ms) | Day of Storage | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 11 | 15 | ||
| WPS | T1 | 1478 ± 2 | 1476 ± 1 | 1433 ± 2 | 1440 ± 2 | 1498 ± 1 | 1509 ± 2 |
| T2 | 900 ± 4 | 910 ± 3 | 990 ± 4 | 991 ± 4 | 961 ± 4 | 867 ± 4 | |
| WPS/BH | T1 | 1723 ± 3 | 1767 ± 2 | 1770 ± 1 | 1722 ± 1 | 1713 ± 2 | 1733 ± 2 |
| T2 | 1014 ± 5 | 977 ± 4 | 1004 ± 3 | 998 ± 3 | 1007 ± 5 | 972 ± 5 | |
| WPS/GB | T1 | 1806 ± 2 | 1807 ± 2 | 2029 ± 2 | 1969 ± 2 | 2169 ± 2 | 2130 ± 1 |
| T2 | 1056 ± 5 | 1058 ± 4 | 1058 ± 4 | 1185 ± 4 | 1196 ± 3 | 1127 ± 3 | |
| NPS | T1 | 1567 ± 3 | 1539 ± 1 | 1612 ± 1 | 1655 ± 1 | 1699 ± 1 | 1720 ± 2 |
| T2 | 1142 ± 6 | 1160 ± 3 | 1122 ± 3 | 1082 ± 5 | 1012 ± 4 | 999 ± 4 | |
| NPS/BH | T1 | 1738 ± 1 | 1709 ± 1 | 1847 ± 1 | 1750 ± 2 | 1783 ± 2 | 1772 ± 6 |
| T2 | 1022 ± 3 | 991 ± 4 | 1027 ± 4 | 1040 ± 5 | 1113 ± 4 | 989 ± 4 | |
| NPS/GB | T1 | 1703 ± 2 | 1722 ± 1 | 1939 ± 2 | 1949 ± 1 | 1942 ± 2 | 1950 ± 1 |
| T2 | 983 ± 5 | 905 ± 4 | 989 ± 5 | 1025 ± 5 | 973 ± 4 | 989 ± 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, G.; Masewicz, Ł.; Przybył, K.; Zaryczniak, A.; Kowalczewski, P.Ł.; Beszterda-Buszczak, M.; Cichocki, W.; Baranowska, H.M. Molecular Properties of Starch–Water Interactions in the Presence of Bioactive Compounds from Barley and Buckwheat—LF NMR Preliminary Study. Polymers 2025, 17, 2606. https://doi.org/10.3390/polym17192606
Adamczyk G, Masewicz Ł, Przybył K, Zaryczniak A, Kowalczewski PŁ, Beszterda-Buszczak M, Cichocki W, Baranowska HM. Molecular Properties of Starch–Water Interactions in the Presence of Bioactive Compounds from Barley and Buckwheat—LF NMR Preliminary Study. Polymers. 2025; 17(19):2606. https://doi.org/10.3390/polym17192606
Chicago/Turabian StyleAdamczyk, Greta, Łukasz Masewicz, Krzysztof Przybył, Aleksandra Zaryczniak, Przemysław Łukasz Kowalczewski, Monika Beszterda-Buszczak, Wojciech Cichocki, and Hanna Maria Baranowska. 2025. "Molecular Properties of Starch–Water Interactions in the Presence of Bioactive Compounds from Barley and Buckwheat—LF NMR Preliminary Study" Polymers 17, no. 19: 2606. https://doi.org/10.3390/polym17192606
APA StyleAdamczyk, G., Masewicz, Ł., Przybył, K., Zaryczniak, A., Kowalczewski, P. Ł., Beszterda-Buszczak, M., Cichocki, W., & Baranowska, H. M. (2025). Molecular Properties of Starch–Water Interactions in the Presence of Bioactive Compounds from Barley and Buckwheat—LF NMR Preliminary Study. Polymers, 17(19), 2606. https://doi.org/10.3390/polym17192606











