Upcycling Orange-Based Waste into Functional CNCs for Greener L-Lactide Ring-Opening Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositional Analysis of Biomass
2.3. Alkaline/Peroxide Treatment of Orange Peel Waste
2.4. Isolation of Cellulosic Materials by Acid Hydrolysis
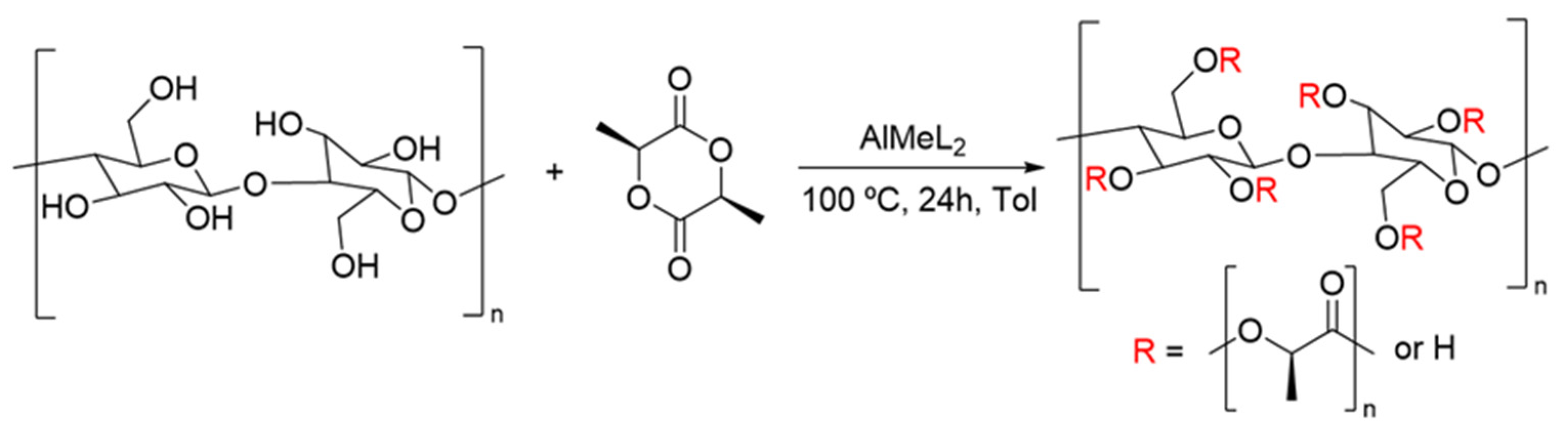
2.5. Surface-Initiated ROP of LLA
2.6. Characterization Techniques
3. Results and Discussion
3.1. Compositional Analysis of Orange Peel Waste
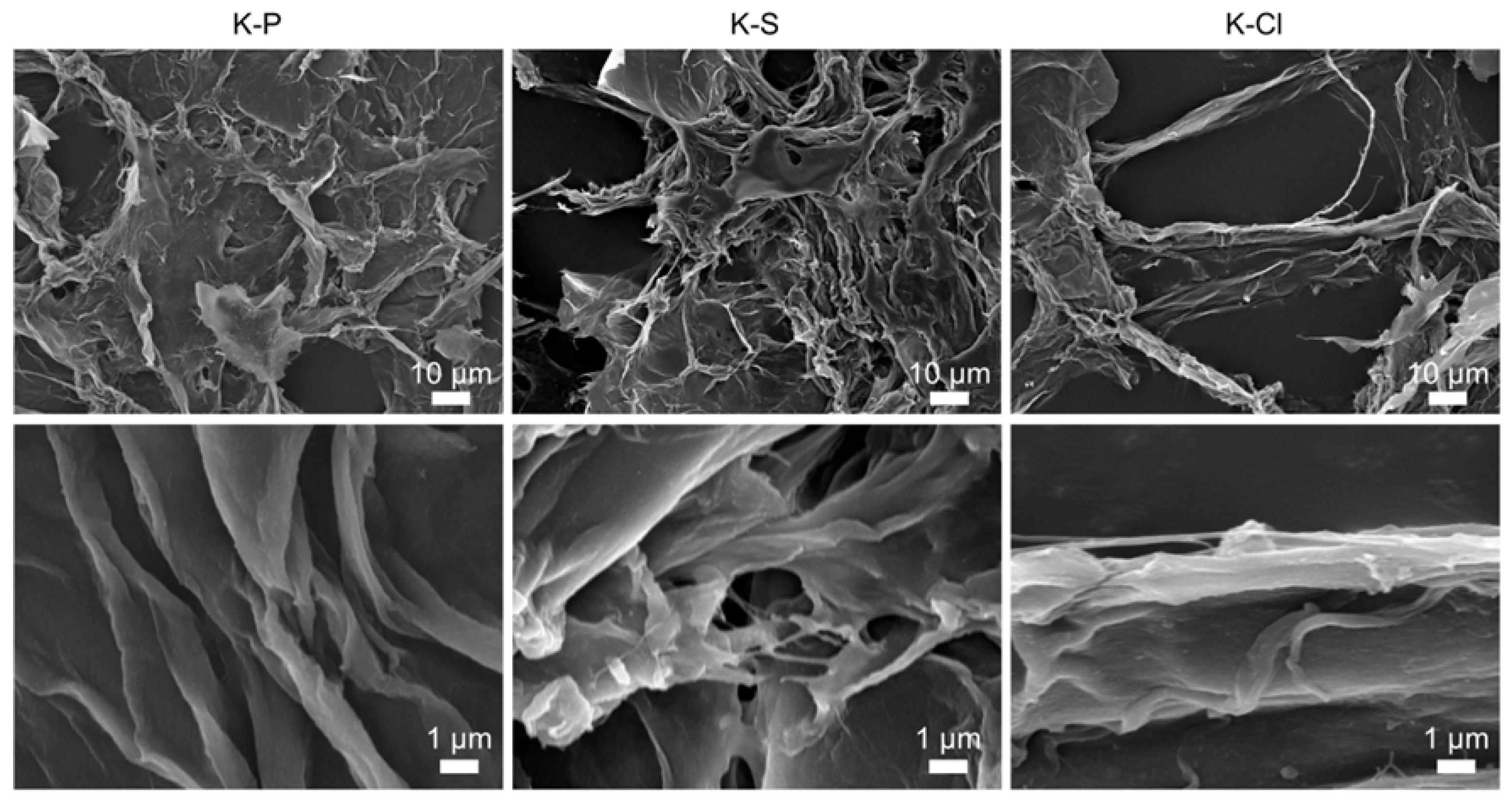
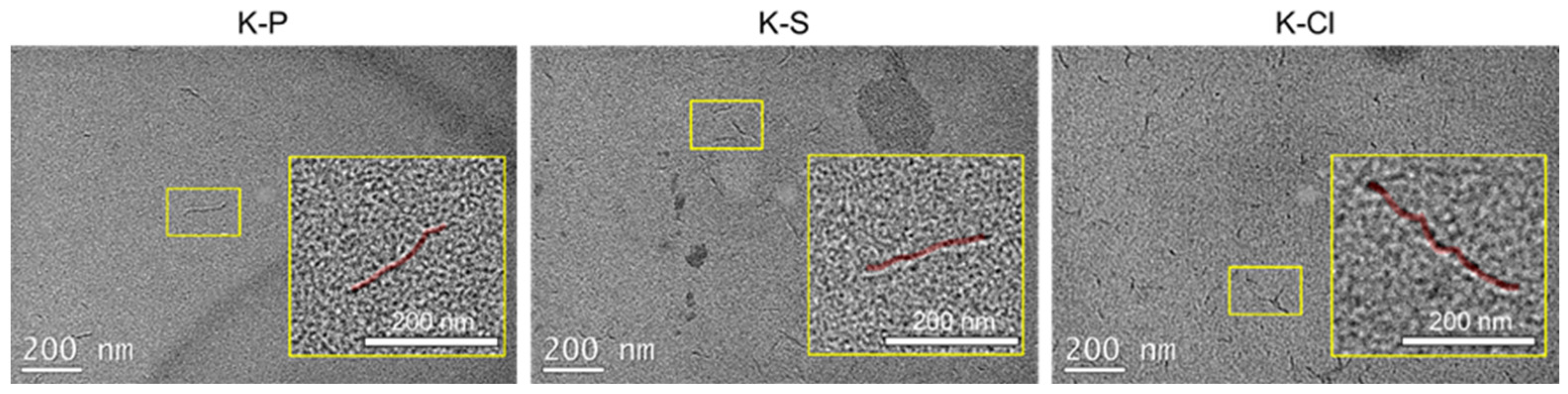
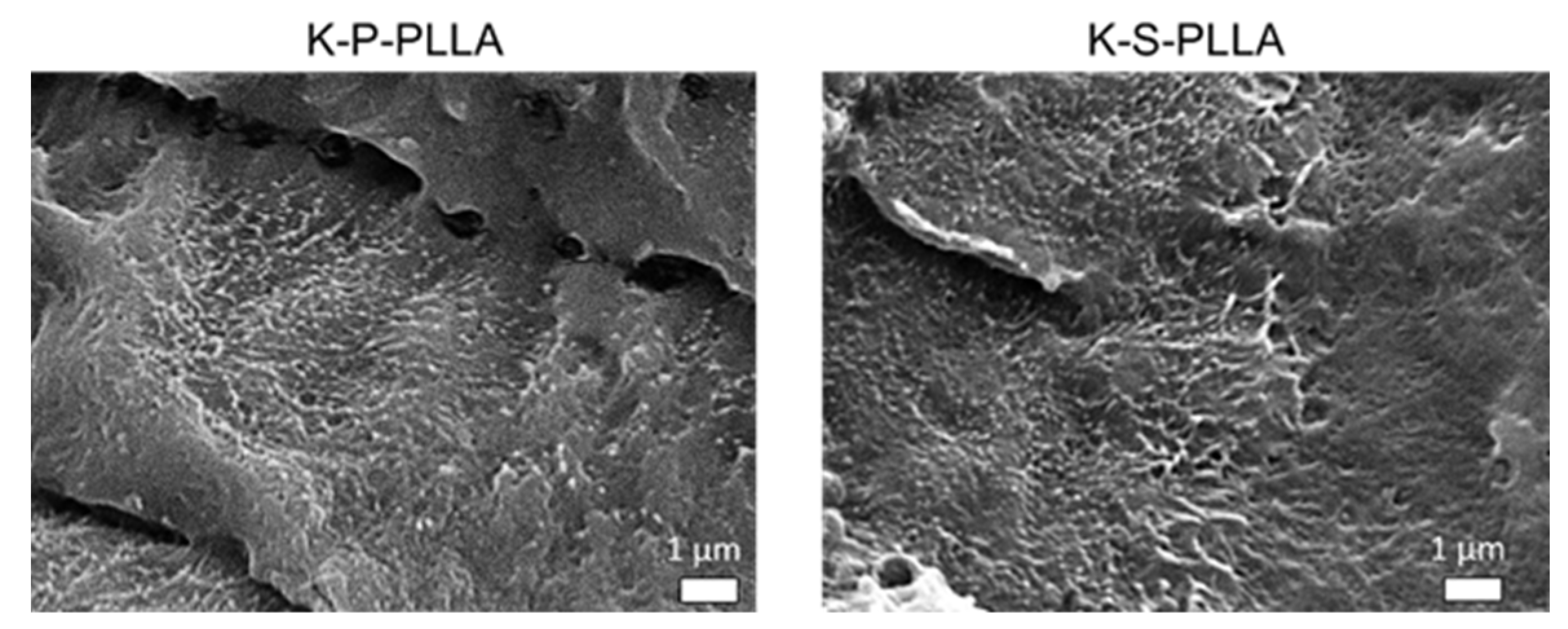
3.2. Morphological Characterization (SEM and TEM)
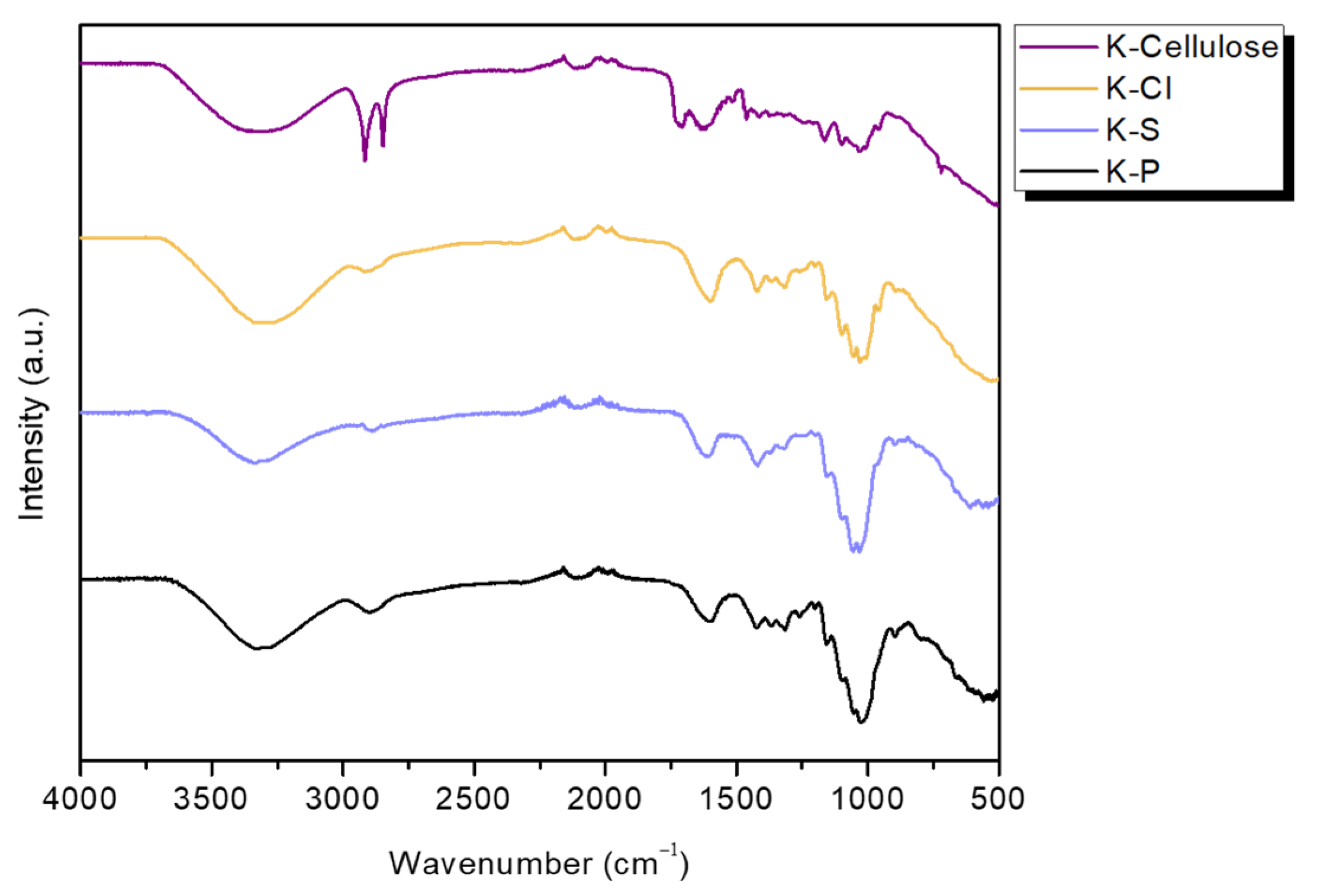
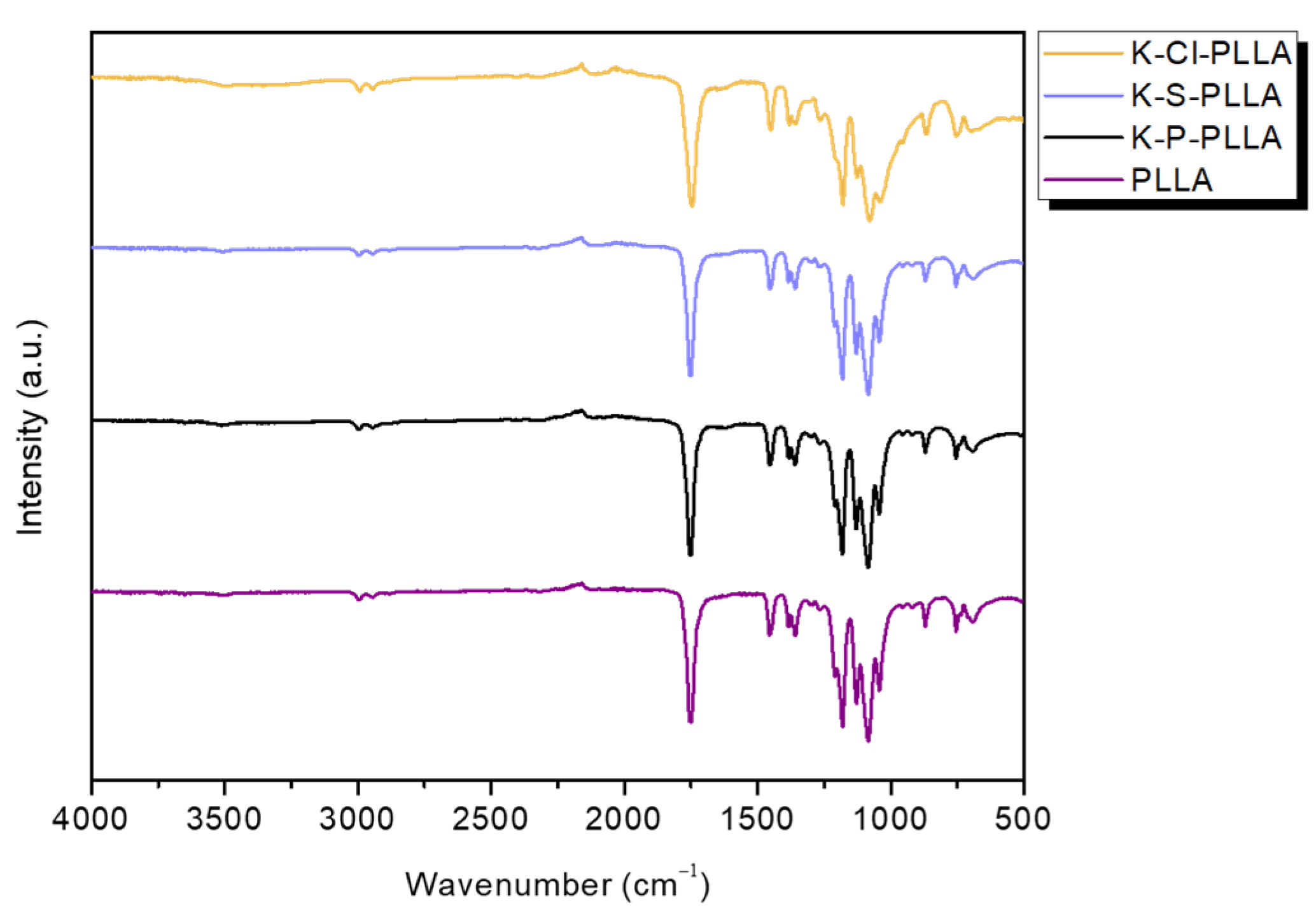
3.3. FTIR Spectroscopy
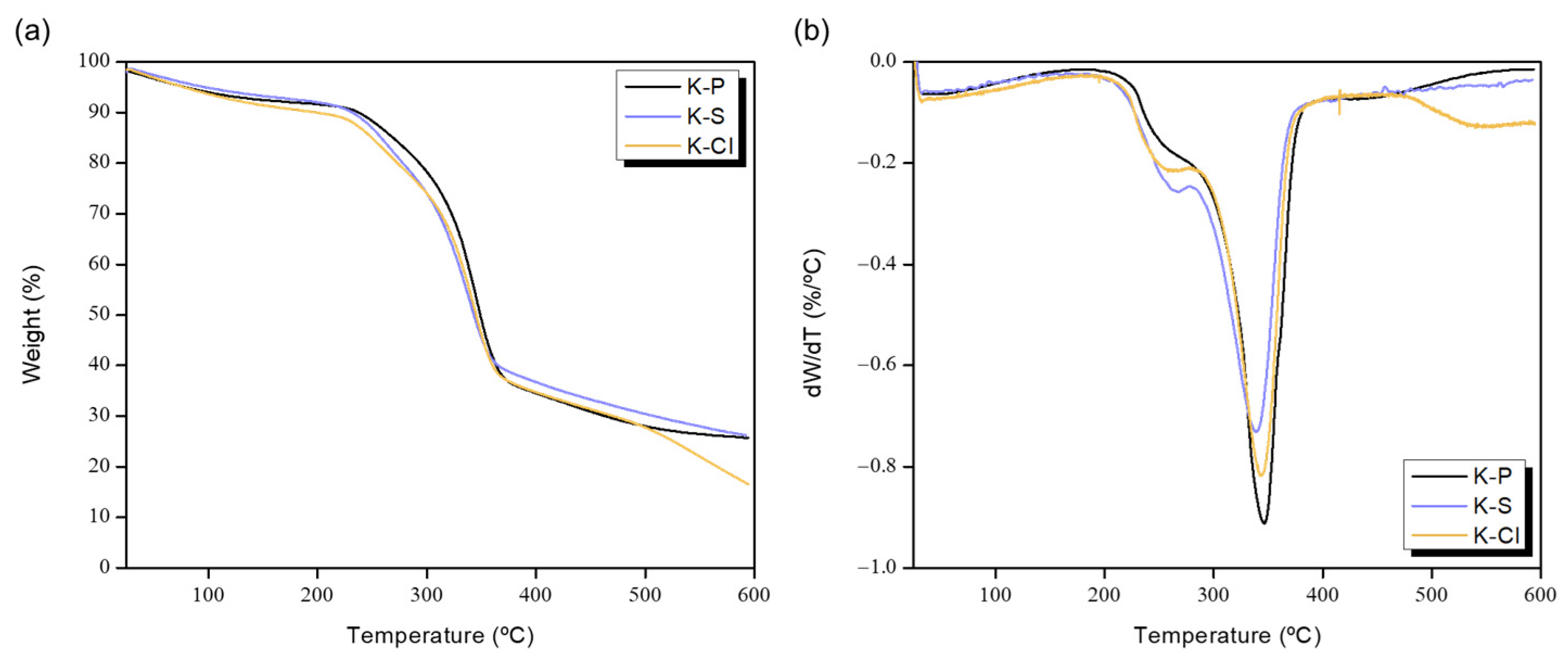
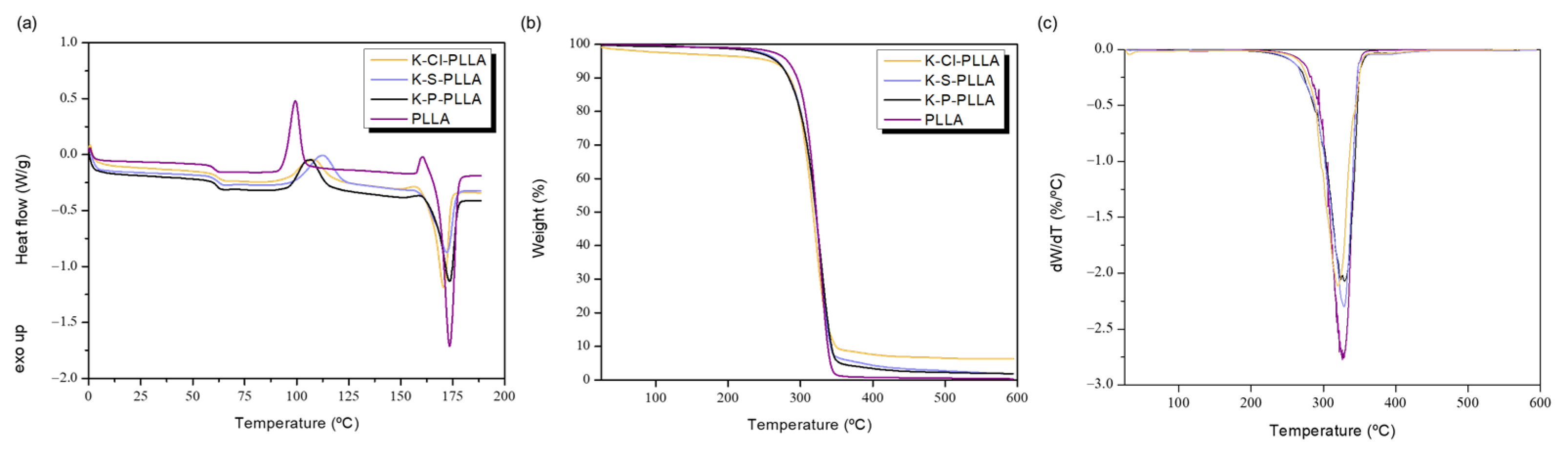
3.4. Thermal Degradation Behavior
3.5. Zeta Potential Analysis
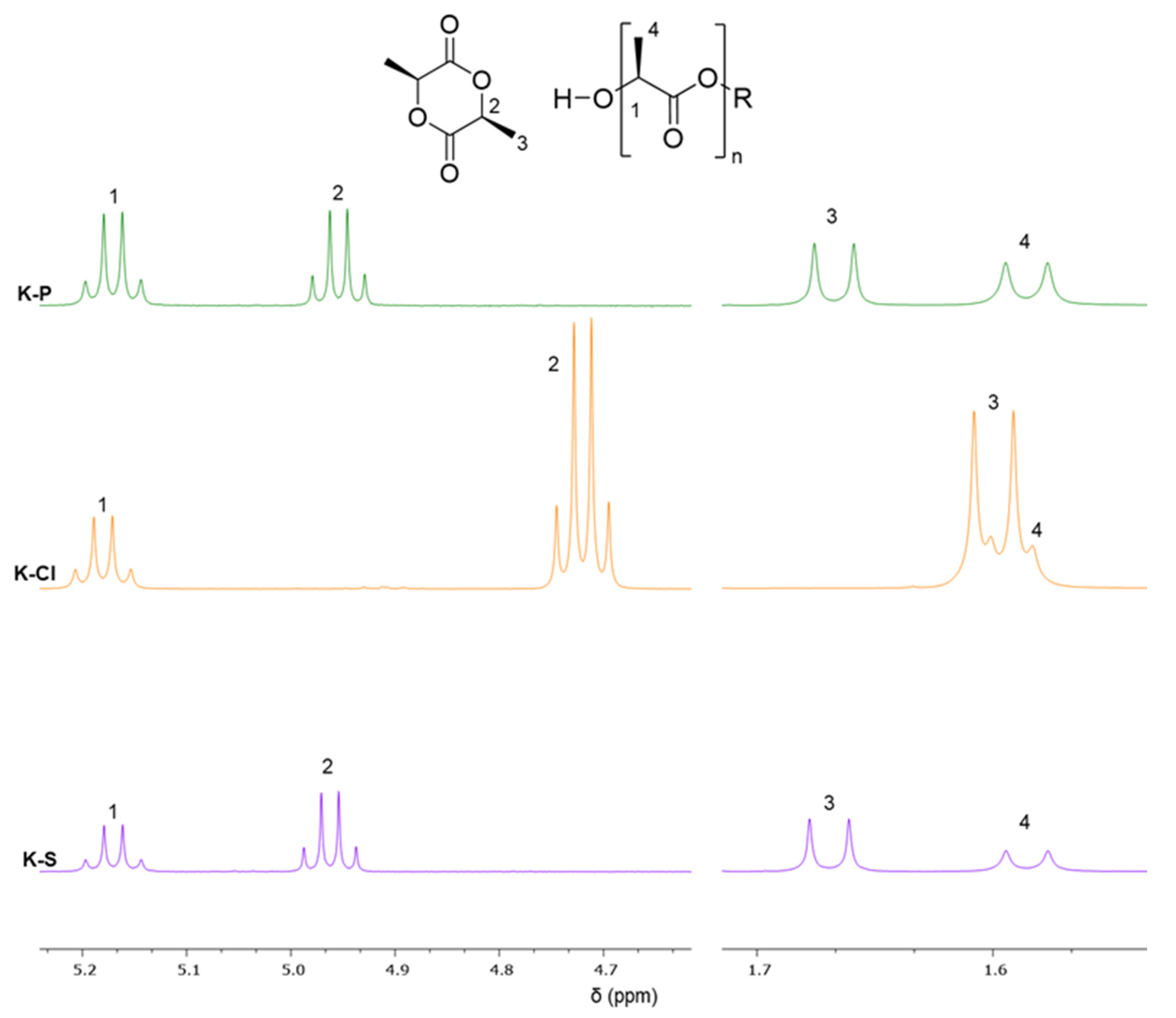
3.6. Surface-Initiated ROP of Lactide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedro, A.C.; Maciel, G.M.; Lima, N.P.; Lima, N.F.; Ribeiro, I.S.; Pinheiro, D.F.; Haminiuk, C.W.I. Valorization of Bioactive Compounds from Juice Industry Waste: Applications, Challenges, and Future Prospects. Trends Food Sci. Technol. 2024, 152, 104693. [Google Scholar] [CrossRef]
- Senit, J.J.; Velasco, D.; Gomez Manrique, A.; Sanchez-Barba, M.; Toledo, J.M.; Santos, V.E.; Garcia-Ochoa, F.; Yustos, P.; Ladero, M. Orange Peel Waste Upstream Integrated Processing to Terpenes, Phenolics, Pectin and Monosaccharides: Optimization Approaches. Ind. Crops Prod. 2019, 134, 370–381. [Google Scholar] [CrossRef]
- Sikorska, W.; Musioł, M.; Zawidlak-Wȩgrzyńska, B.; Rydz, J. End-of-Life Options for (Bio)Degradable Polymers in the Circular Economy. Adv. Polym. Technol. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Drozd, N.N.; Paderin, N.M.; Tarabukin, D.V.; Udoratina, E.V. Hemocompatibility, Biodegradability and Acute Toxicity of Acetylated Cellulose Nanocrystals of Different Types in Comparison. Carbohydr. Polym. 2021, 269, 118307. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of Cellulose Nanocrystals for Advanced Applications. J. Colloid. Interface Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline Cellulose Isolation via Acid Hydrolysis from Non-Woody Biomass: Importance of Hydrolysis Parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef]
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic Modulus and Stress-Transfer Properties of Tunicate Cellulose Whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(Ethylene Glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Mantovan, J.; Alejandra Gil Giraldo, G.; Marjorie Marim, B.; Salomão Garcia, P.; Machado Baron, A.; Mali, S. Cellulose-Based Materials from Orange Bagasse Employing Environmentally Friendly Approaches. Biomass Convers. Biorefin. 2023, 13, 1633–1644. [Google Scholar] [CrossRef]
- Palaniappan, M.; Palanisamy, S.; Khan, R.; H.Alrasheedi, N.; Tadepalli, S.; Murugesan, T.M.; Santulli, C. Synthesis and Suitability Characterization of Microcrystalline Cellulose from Citrus x Sinensis Sweet Orange Peel Fruit Waste-Based Biomass for Polymer Composite Applications. J. Polym. Res. 2024, 31, 105. [Google Scholar] [CrossRef]
- Camarero Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of Thermally Stable Cellulose Nanocrystals by Phosphoric Acid Hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Stricker, A. Polylactones 48. SnOct2-Initiated Polymerizations of Lactide: A Mechanistic Study. Macromolecules 2000, 33, 702–709. [Google Scholar] [CrossRef]
- Rentero, C.; Damián, J.; Medel, A.; Fernández-Millán, M.; Rusconi, Y.; Talarico, G.; Cuenca, T.; Sessini, V.; Mosquera, M.E.G. Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies. Polymers 2022, 14, 2982. [Google Scholar] [CrossRef] [PubMed]
- Rentero, C.; Gaeta, L.; Palenzuela, M.; Sessini, V.; Mosquera, M.E.G. A Greener Process for Poly-L-Lactic Acid Production and Chemical Upcycling under Mild Conditions Using Highly Active Alkali-Metal Based Catalysts. Polymer 2025, 320, 128066. [Google Scholar] [CrossRef]
- Gallegos, C.; Tabernero, V.; García-Valle, F.M.; Mosquera, M.E.G.; Cuenca, T.; Cano, J. Synthesis and Structure of Homo- and Heterometallic Lithium–Magnesium Complexes and Their Reactivity in the ROP of Rac-Lactide. Organometallics 2013, 32, 6624–6627. [Google Scholar] [CrossRef]
- Gallegos, C.; Tabernero, V.; Mosquera, M.E.G.; Cuenca, T.; Cano, J. Comparative Study of Lactide Polymerization with Lithium, Sodium, Potassium, Magnesium, Calcium, and Zinc Azonaphthoxide Complexes. Eur. J. Inorg. Chem. 2015, 2015, 5124–5132. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Tabernero, V.; Cuenca, T.; Mosquera, M.E.G.; Cano, J.; Milione, S. Biodegradable PHB from Rac-β-Butyrolactone: Highly Controlled ROP Mediated by a Pentacoordinated Aluminum Complex. Organometallics 2018, 37, 837–840. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Cuenca, T.; Mosquera, M.E.G.; Milione, S.; Cano, J. Ring-Opening Polymerization (ROP) of Cyclic Esters by a Versatile Aluminum Diphenoxyimine Complex: From Polylactide to Random Copolymers. Eur. Polym. J. 2020, 125, 109527. [Google Scholar] [CrossRef]
- Carlsson, L.; Utsel, S.; Wågberg, L.; Malmström, E.; Carlmark, A. Surface-Initiated Ring-Opening Polymerization from Cellulose Model Surfaces Monitored by a Quartz Crystal Microbalance. Soft Matter 2012, 8, 512–517. [Google Scholar] [CrossRef]
- Lalanne-Tisné, M.; Mees, M.A.; Eyley, S.; Zinck, P.; Thielemans, W. Organocatalyzed Ring Opening Polymerization of Lactide from the Surface of Cellulose Nanofibrils. Carbohydr. Polym. 2020, 250, 116974. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Sluiter, J.; Wolfrum, E.J. Methods for Biomass Compositional Analysis. In Catalysis for the Conversion of Biomass and Its Derivatives; Max-Planck-Gesellschaft zur Förderung der Wissenschaften: Berlin, Germany, 2013; ISBN 978-3-945561-19-5. [Google Scholar]
- DONG, X.M.; REVOL, J.-F.; GRAY, D.G. Effect of Microcrystallite Preparation Conditions on the Formation of Colloid Crystals of Cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow Properties of Microcrystalline Cellulose Suspension Prepared by Acid Treatment of Native Cellulose. Colloids Surf. A Physicochem. Eng. Asp. 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; López, D.; Torre, L.; Kenny, J.M.; Dubois, P.; Raquez, J.M.; Peponi, L. Effect of the Addition of Polyester-Grafted-Cellulose Nanocrystals on the Shape Memory Properties of Biodegradable PLA/PCL Nanocomposites. Polym. Degrad. Stab. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.K.; Lionetto, F.; Nisi, R.; Stoppa, M.; Licciulli, A. Sustainable Production of Stiff and Crystalline Bacterial Cellulose from Orange Peel Extract. Sustainability 2022, 14, 2247. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Ngoc Lieu, L.; Thuy Thi Thanh, T.; Linh Tran Khanh, V. Characterization of Green-Extracted Orange Peel Pectin. J. Sci. Technol. 2020, 142A, 43–46. Available online: https://jst.vn/index.php/old/article/view/539 (accessed on 29 July 2025).
- Aravantinos-Zafiris, G.; Oreopoulou, V.; Tzia, C.; Thomopoulos, C.D. Fibre Fraction from Orange Peel Residues after Pectin Extraction. LWT-Food Sci. Technol. 1994, 27, 468–471. [Google Scholar] [CrossRef]
- Salmén, L.; Stevanic, J.S. Effect of Drying Conditions on Cellulose Microfibril Aggregation and “Hornification”. Cellulose 2018, 25, 6333–6344. [Google Scholar] [CrossRef]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and Characterization of Cellulose Nanocrystal Extracted from Ramie Fibers by Sulfuric Acid Hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef] [PubMed]
- Whba, F.; Mohamed, F.; Idris, M.I.; Yahya, M.S. Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI. Appl. Sci. 2023, 13, 6316. [Google Scholar] [CrossRef]
- Bouramdane, Y.; Fellak, S.; El Mansouri, F.; Boukir, A. Impact of Natural Degradation on the Aged Lignocellulose Fibers of Moroccan Cedar Softwood: Structural Elucidation by Infrared Spectroscopy (ATR-FTIR) and X-Ray Diffraction (XRD). Fermentation 2022, 8, 698. [Google Scholar] [CrossRef]
- Hurtubise, F.G.; Krassig, H. Classification of Fine Structural Characteristics in Cellulose by Infared Spectroscopy. Use of Potassium Bromide Pellet Technique. Anal. Chem. 1960, 32, 177–181. [Google Scholar] [CrossRef]
- Åkerholm, M.; Hinterstoisser, B.; Salmén, L. Characterization of the Crystalline Structure of Cellulose Using Static and Dynamic FT-IR Spectroscopy. Carbohydr. Res. 2004, 339, 569–578. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Latticed Type. Part I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Santana, R.M.C.; Zattera, A.J. Materials Produced from Plant Biomass. Part II: Evaluation of Crystallinity and Degradation Kinetics of Cellulose. Mater. Res. 2012, 15, 421–427. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of the Nanofabrication by Acid Hydrolysis of Bacterial Cellulose Nanowhiskers. Carbohydr. Polym. 2011, 85, 228–236. [Google Scholar] [CrossRef]
- Cesarino, I.; Carnietto, M.B.; Bronzato, G.R.F.; Leao, A.L. Fabrication of Pineapple Leaf Fibers Reinforced Composites. In Pineapple Leaf Fibers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 265–277. [Google Scholar]
- Nada, A.-A.M.A.; Kamel, S.; El-Sakhawy, M. Thermal Behaviour and Infrared Spectroscopy of Cellulose Carbamates. Polym. Degrad. Stab. 2000, 70, 347–355. [Google Scholar] [CrossRef]
- D’Acierno, F.; Hamad, W.Y.; Michal, C.A.; Maclachlan, M.J. Thermal Degradation of Cellulose Filaments and Nanocrystals. Biomacromolecules 2020, 21, 3374–3386. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Aryasena, R.; Kusmono; Umami, N. Production of Cellulose Nanocrystals Extracted from Pennisetum Purpureum Fibers and Its Application as a Lubricating Additive in Engine Oil. Heliyon 2022, 8, e11315. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, M.G.; Fernández-Pérez, A.; Oviedo, C.; Reyes, G.; Reyes-Contreras, P. Relationship between Structural Characteristics of Cellulose Nanocrystals Obtained from Kraft Pulp. Nanomaterials 2020, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Eloi, J.C.; Rowlandson, J.; Eichhorn, S.J. Phosphorylated Cellulose Nanocrystals: Optimizing Production by Decoupling Hydrolysis and Surface Modification. Carbohydr. Polym. 2024, 325, 121560. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Vahabi, H.; Janbaz, S.; Darafsheh, A.; Mazur, T.R.; Ramakrishna, S. 4D Printing of Shape Memory Polylactic Acid (PLA). Polymer 2021, 230, 124080. [Google Scholar] [CrossRef]
- Leonés, A.; Peponi, L.; Lieblich, M.; Benavente, R.; Fiori, S. In Vitro Degradation of Plasticized PLA Electrospun Fiber Mats: Morphological, Thermal and Crystalline Evolution. Polymers 2020, 12, 2975. [Google Scholar] [CrossRef]
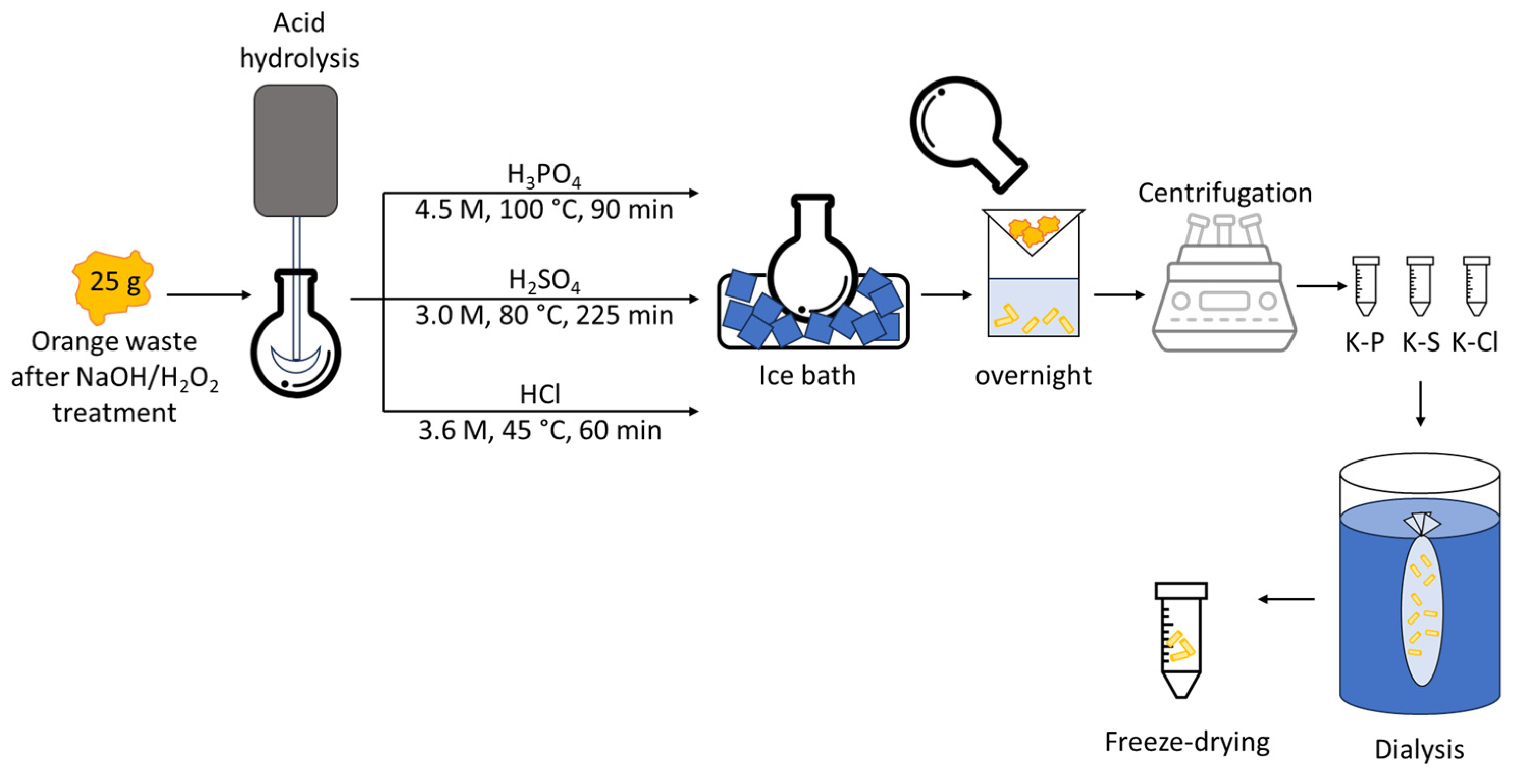
| Code | Acid | M (mol/L) | Temp. (°C) | Time (min) | Reference |
|---|---|---|---|---|---|
| K-P | H3PO4 | 4.5 | 100 | 90 | Adapted from [13] |
| K-S | H2SO4 | 3.0 | 80 | 225 | Adapted from [25] |
| K-Cl | HCl | 3.6 | 45 | 60 | Adapted from [26] |
| Compounds (g/100 g Dry Weight) | ||
|---|---|---|
| Extractives | Water | 52 ± 7 |
| Ethanol | 8 ± 1 | |
| Sugars in water extractives | Sucrose | Nd |
| Glucose | 9.8 ± 0.9 | |
| Fructose | 9.9 ± 0.5 | |
| Xylose | 0.3 ± 0.0 | |
| Galactose | 0.14 ± 0.0 | |
| Arabinose | <0.1 | |
| Mannose | <0.1 | |
| Glucan | Glucose | 10 ± 1 |
| Hemicellulose (7.9%) | Xylose | 1.5 ± 0.04 |
| Galactose | 4.1 ± 0.5 | |
| Arabinose | 4.6 ± 0.3 | |
| Mannose | 1 ± 0.3 | |
| Lignin | Acid-insoluble residue | 1.9 ± 0.4 |
| Acid-soluble residue | 1.7 ± 0.3 | |
| Ash | 3.81 ± 0.0 | |
| Code | IR Crystallinity Ratio | Crystallinity Index (%) | |
|---|---|---|---|
| 1372 cm−1/2900 cm−1 (TCI) | 1429 cm−1/897 cm−1 (LOI) | (Acryst/Atotal) · 100 | |
| K-Cellulose | 0.50 | 0.85 | - |
| K-P | 0.81 | 0.88 | 28.6 |
| K-S | 0.86 | 1.51 | 35.2 |
| K-Cl | 0.79 | 1.00 | 26.0 |
| Sample | Tmax1 (°C) | Weight Loss (%) | Tmax2 (°C) | Weight Loss (%) | Char Residue at 500 °C (%) |
|---|---|---|---|---|---|
| K-P | 259 | 13.1 | 346 | 42.1 | 25.7 |
| K-S | 262 | 14.9 | 339 | 38.0 | 26.2 |
| K-Cl | 257 | 14.0 | 345 | 41.2 | 16.5 |
| Sample | Zeta Potential (mV) |
|---|---|
| K-P | −27.5 ± 1.9 |
| K-S | −28.8 ± 1.7 |
| K-Cl | −26.2 ± 1.0 |
| Entry | Sample | LLA/Cellulose Weight Ratio | [LLA] M (mol/L) | Conv. a (%) | Mw, exp b (kDa) | Mn, exp b (kDa) | Đ b |
|---|---|---|---|---|---|---|---|
| 1 | K-P | 50:1 | 0.85 | 55 | 203.3 | 185.3 | 1.09 |
| 67.1 | 48.8 | 1.07 | |||||
| 2 | K-S | 50:1 | 0.85 | 43 | 144.9 | 134.5 | 1.07 |
| 52.5 | 48.8 | 1.07 | |||||
| 3 | K-Cl | 50:1 | 0.85 | 24 | 65.0 | 55.4 | 1.10 |
| 14.6 | 13.3 | 1.09 |
| 2nd Heating | TGA | |||||
|---|---|---|---|---|---|---|
| Sample | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) | Tmax (°C) | Grafted PLLA (wt%) |
| PLLA (60 kDa) | 60 | 99 | 173 | 17.2 | 326 | - |
| K-P-PLLA | 61 | 107 | 174 | 5.7 | 328 | 94 |
| K-S-PLLA | 61 | 113 | 172 | 3.8 | 327 | 93 |
| K-Cl-PLLA | 61 | 108 | 171 | 10.6 | 320 | 90 |
| Sample | E (MPa) | σ (MPa) | εbreak (%) |
|---|---|---|---|
| PLLA (60 kDa) | 886.5 ± 7.6 | 16.1 ± 3.6 | 1.9 ± 0.5 |
| K-P-PLLA | 784.7 ± 107.1 | 9.5 ± 5.0 | 2.4 ± 0.2 |
| K-S-PLLA | 537.2 ± 80.9 | 9.6 ± 2.9 | 1.5 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonés, A.; Sánchez-Solís, C.; Medel, A.; García-Aparicio, M.P.; Mosquera, M.E.G.; Sessini, V. Upcycling Orange-Based Waste into Functional CNCs for Greener L-Lactide Ring-Opening Polymerization. Polymers 2025, 17, 2605. https://doi.org/10.3390/polym17192605
Leonés A, Sánchez-Solís C, Medel A, García-Aparicio MP, Mosquera MEG, Sessini V. Upcycling Orange-Based Waste into Functional CNCs for Greener L-Lactide Ring-Opening Polymerization. Polymers. 2025; 17(19):2605. https://doi.org/10.3390/polym17192605
Chicago/Turabian StyleLeonés, Adrián, Cayetano Sánchez-Solís, Asier Medel, Maria P. García-Aparicio, Marta E. G. Mosquera, and Valentina Sessini. 2025. "Upcycling Orange-Based Waste into Functional CNCs for Greener L-Lactide Ring-Opening Polymerization" Polymers 17, no. 19: 2605. https://doi.org/10.3390/polym17192605
APA StyleLeonés, A., Sánchez-Solís, C., Medel, A., García-Aparicio, M. P., Mosquera, M. E. G., & Sessini, V. (2025). Upcycling Orange-Based Waste into Functional CNCs for Greener L-Lactide Ring-Opening Polymerization. Polymers, 17(19), 2605. https://doi.org/10.3390/polym17192605









