Recent Advances in Marine-Derived Polysaccharide Hydrogels: Innovative Applications and Challenges in Emerging Food Fields
Abstract
1. Introduction
2. Review Methodology
3. Structure of MPs
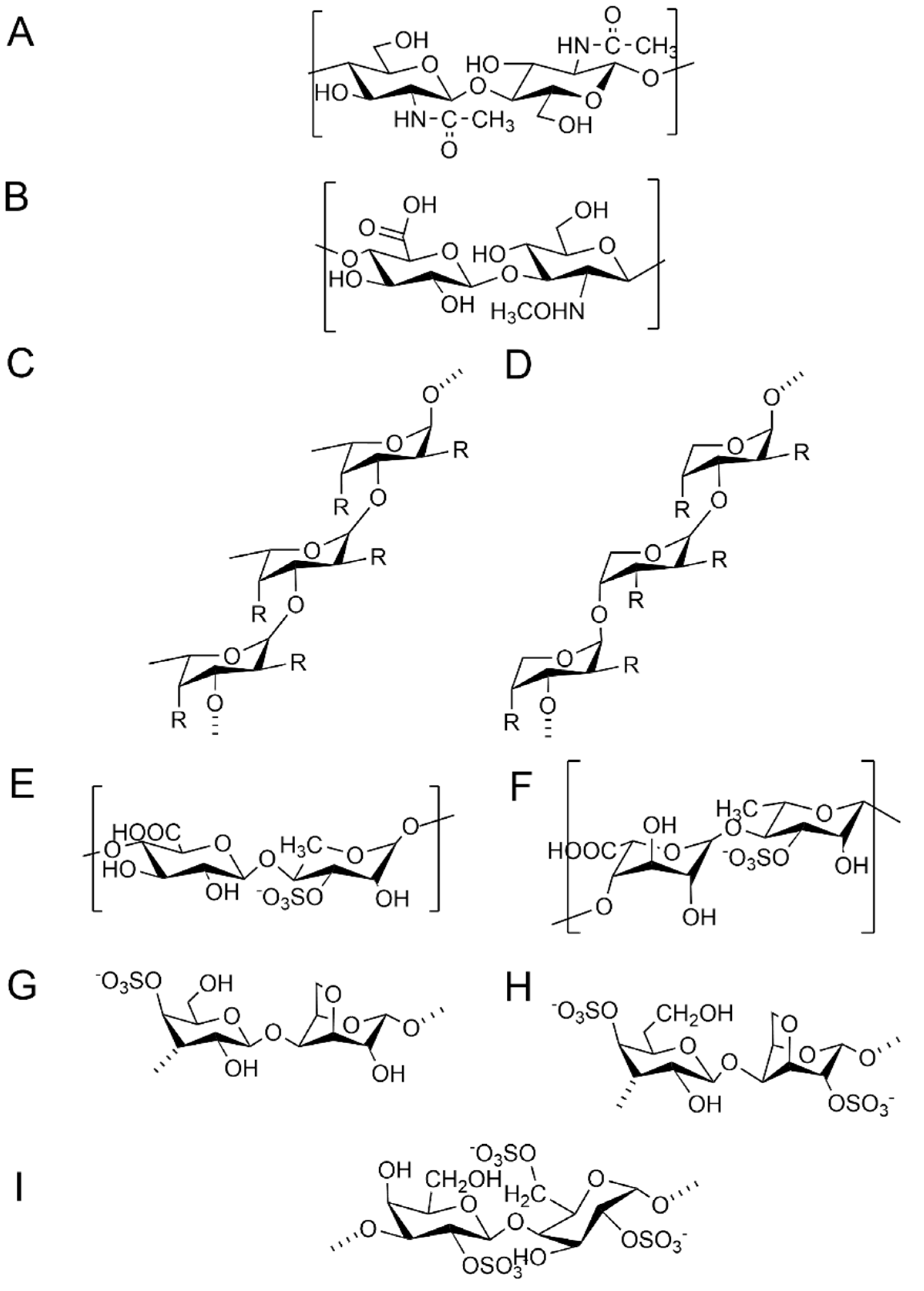
3.1. Monosaccharides and Backbone
3.2. Molecular Weight
3.3. Sulfation Mode
4. Biological Activities of MPs
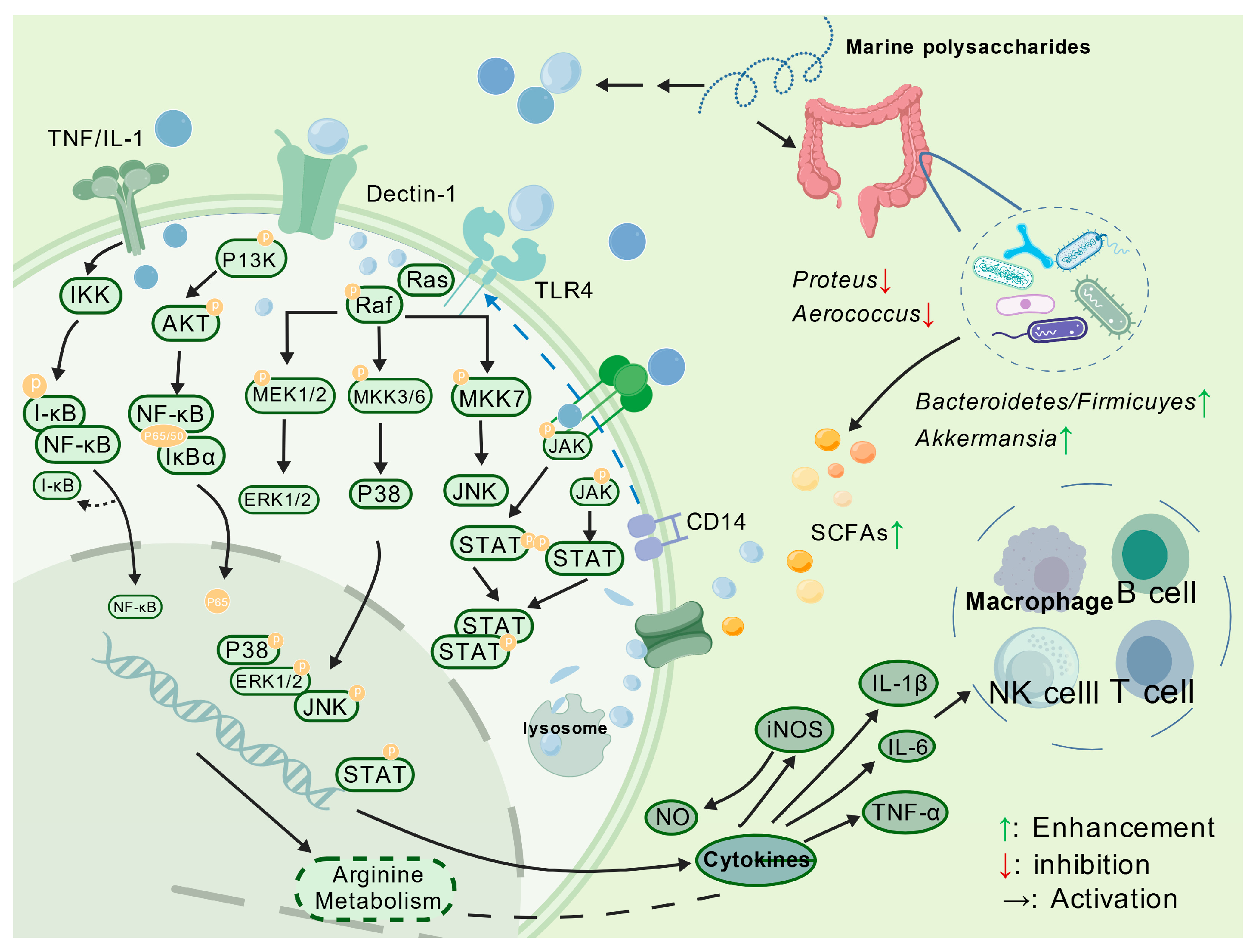
4.1. Immunomodulatory Activity
4.2. Anti-Tumor Activity
4.3. Anti-Obesity Activity
4.4. Anti-Inflammatory Activity
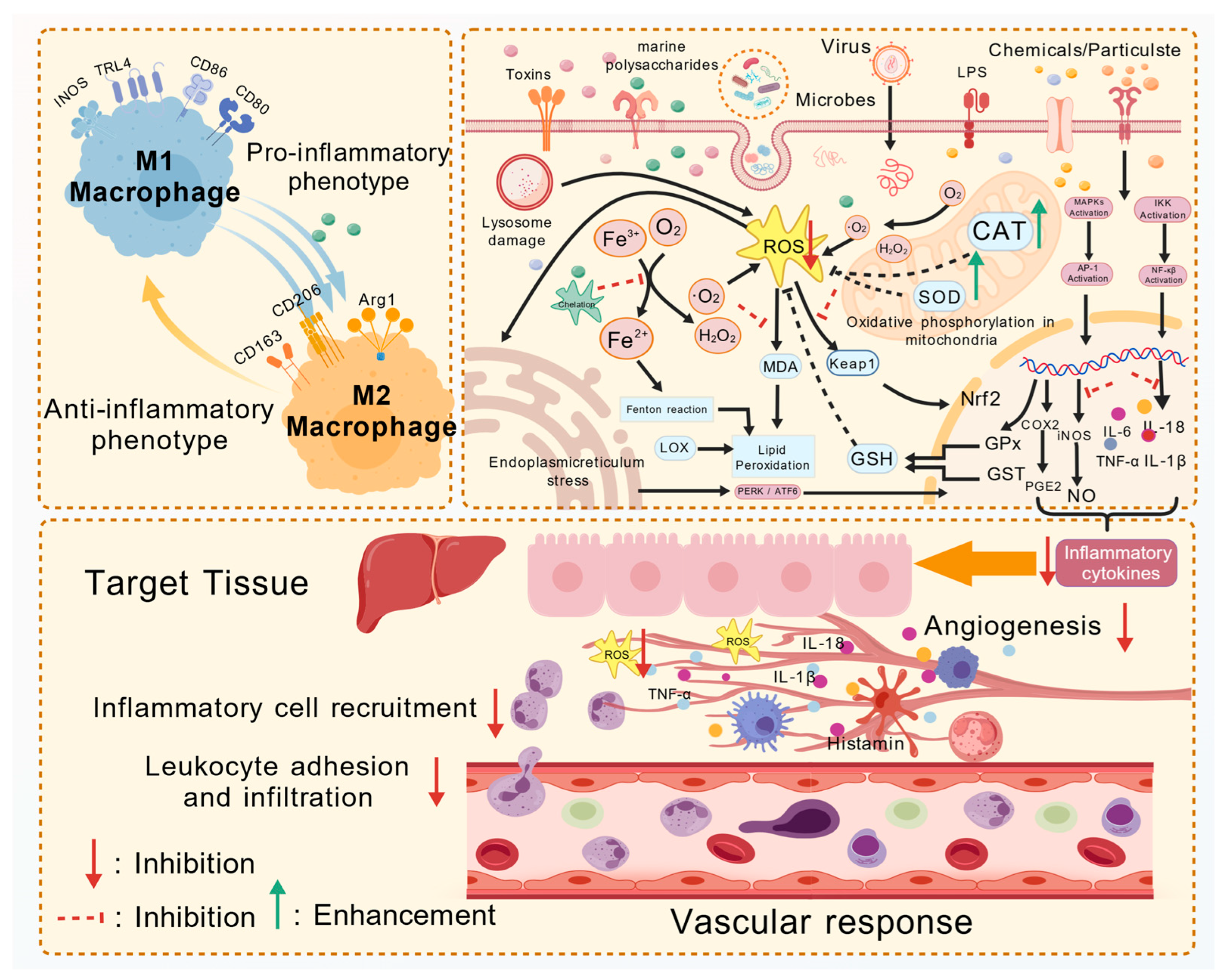
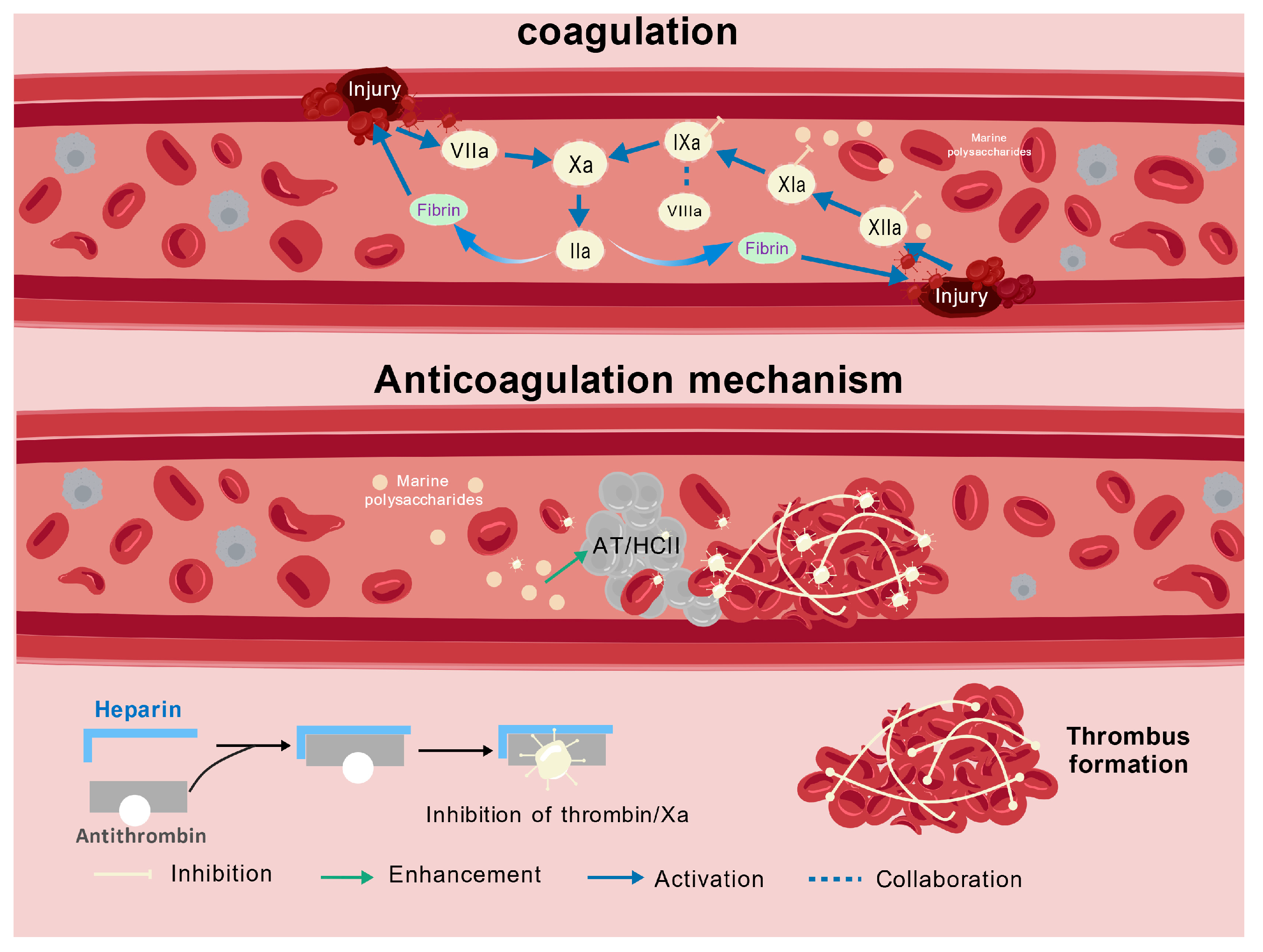
4.5. Anticoagulant Activity
4.6. Antioxidant Activity
5. Toxicity of MPs in Food
6. Representative Marine Polysaccharide-Based Hydrogels
6.1. Chitosan-Based Hydrogel
6.2. Hyaluronic Acid-Based Hydrogel
6.3. Algal Polysaccharide-Based Hydrogel
6.4. Marine Microorganisms Polysaccharides Hydrogel
| Polysaccharide | Structural/Function Components | Hydrogels Types | Crosslinking Type/Interaction Force | Features | Applications | Reference |
|---|---|---|---|---|---|---|
| Chitosan | Pectin; Strawberry extract | Encapsulation; Controlled release | Physical Cross-Linking | Higher stability under acidic conditions. | Functional food products | [83] |
| Tannic acid | Anti-bacterial | Physical Cross-Linking | Edible; Situ rapid cross-linking | Food packaging | [85] | |
| Keratin; Lacticaseibacillus rhamnosus | Encapsulation | Chemical Cross-Linking | Encapsulate probiotics; Effective reusability | Removal mycotoxins from fruit juice | [86] | |
| Ag nanoparticles | Anti-bacterial | Physical Cross-Linking | Eliminate fungal; No residues | Food packaging | [105] | |
| Boric acid group | 3D scaffold | Chemical Cross-Linking | Self-healing and reshaping capabilities; Edible | Cultured meat | [106] | |
| Pomegranate extract | Encapsulation | Chemical Cross-Linking | Excellent stability | Food preservation | [107] | |
| Ethyl cellulose; 1-methylcyclopropene | Humidity responsive | Chemical Cross-Linking | Scavenge ethylene | Food packaging | [108] | |
| Hyaluronic acid | Anti-tumor drug | Laser; Weakly acidic; Overexpressed GSH and haase responsive | Chemical Cross-Linking | In situ forming and injectable | Tissue engineering; Cancer treatment | [90] |
| Modified polyethylene glycol precursor | Electro-responsive | Chemical Cross-Linking | Promotes efficient cell migration | Wound dressing | [91] | |
| Alginate | Agar; Zinc ion; Mxene | Anti-bacterial | Chemical Cross-Linking | Simple preparation; Synergistically acts with photothermal effect. | Wound dressing | [95] |
| Curcumin liposomes; Ag nanoparticles | Reactive oxygen species; (ROS)-responsive | Chemical Cross-Linking | Antioxidant; anti-bacterial; Anti-inflammatory properties; Injectable | Diabetic wound healing | [92] | |
| Poly (vinyl alcohol); Mixed-dye methyl red/bromothymol blue | pH responsive | Physical Cross-Linking | Freeze resistance; Used as a sensor under a basic environment | Food Monitoring | [109] | |
| Phenosafranin | Encapsulation | Chemical Cross-Linking | High selectivity; High sensitivity | Detect the content of nitrite | [110] | |
| Gelatin; Phosphatidylcholine | 3D scaffold | Physical Cross-Linking | Lower cholesterol of cultured meat | Cultured meat | [111] | |
| Cu2+; Tea tree essential oil | 3D scaffold | Chemical Cross-Linking | Broad-spectrum anti-bacterial activity; Moisture responsiveness | Fruit preservation | [112] | |
| Chitosan; Cotton waste | Moisture absorption; Antibacterial | Chemical Cross-Linking | High water absorption rate | Food packaging | [113] | |
| Whey protein | 3D scaffold | Chemical Cross-Linking | Cell adhesion promotion; Edible | Cultured meat | [114] | |
| Carbodiimide chemistries | 3D scaffold | Chemical Cross-Linking | Higher mechanical properties; High cytocompatibility and cell adhesion | Cultured meat | [115] | |
| Agar | Thiabendazole | Fluorescent sensing | Physical Cross-Linking | High sensitivity; High efficiency; excellent selectivity | Pesticide detection | [116] |
| Polyvinyl alcohol; Curcumin | pH responsive | Physical Cross-Linking | Used as a sensor under an acidic environment; Higher color stability | Food Monitoring | [117] | |
| Konjac glucomannan | Ediable | Physical Cross-Linking | Improved springiness and chewiness; Double network | Authentic beef tripe | [118] | |
| Cu nanoparticles and carbon quantum dot doped with nitrogen nanocomplex | Ethylene detection | Physical Cross-Linking | Colorimetric and fluorescent responses; Highly selective | Freshness/Spoilage Monitoring | [119] | |
| Tributyrin | Ediable | Physical Cross-Linking | Harder; more resilient; chewier | Functional food products | [120] | |
| Carrageenan | Konjac glucomannan | 3D scaffold Allow sufficient nutrient diffusion | Physical Cross-Linking | Biocompatibility, food safety; Low cost. Support cell proliferation and allow the formation of tissue-like cell spheres | Cultured meat | [121] |
| Quince seed mucilage; Red cabbage anthocyanin | Encapsulation | Chemical Cross-Linking | pH sensitive | Freshness/Spoilage Monitoring | [122] | |
| Alginate; Rice bran wax/soybean oil | Bigel | Physical Cross-Linking | Mimick butter; Produced shortbread | Fat substitute | [123] | |
| Ulvan | Ag nanoparticles | Anti-bacterial | Chemical Cross-Linking | Anti-bacterial; Adsorb exudate from the wound | Wound dressing | [101] |
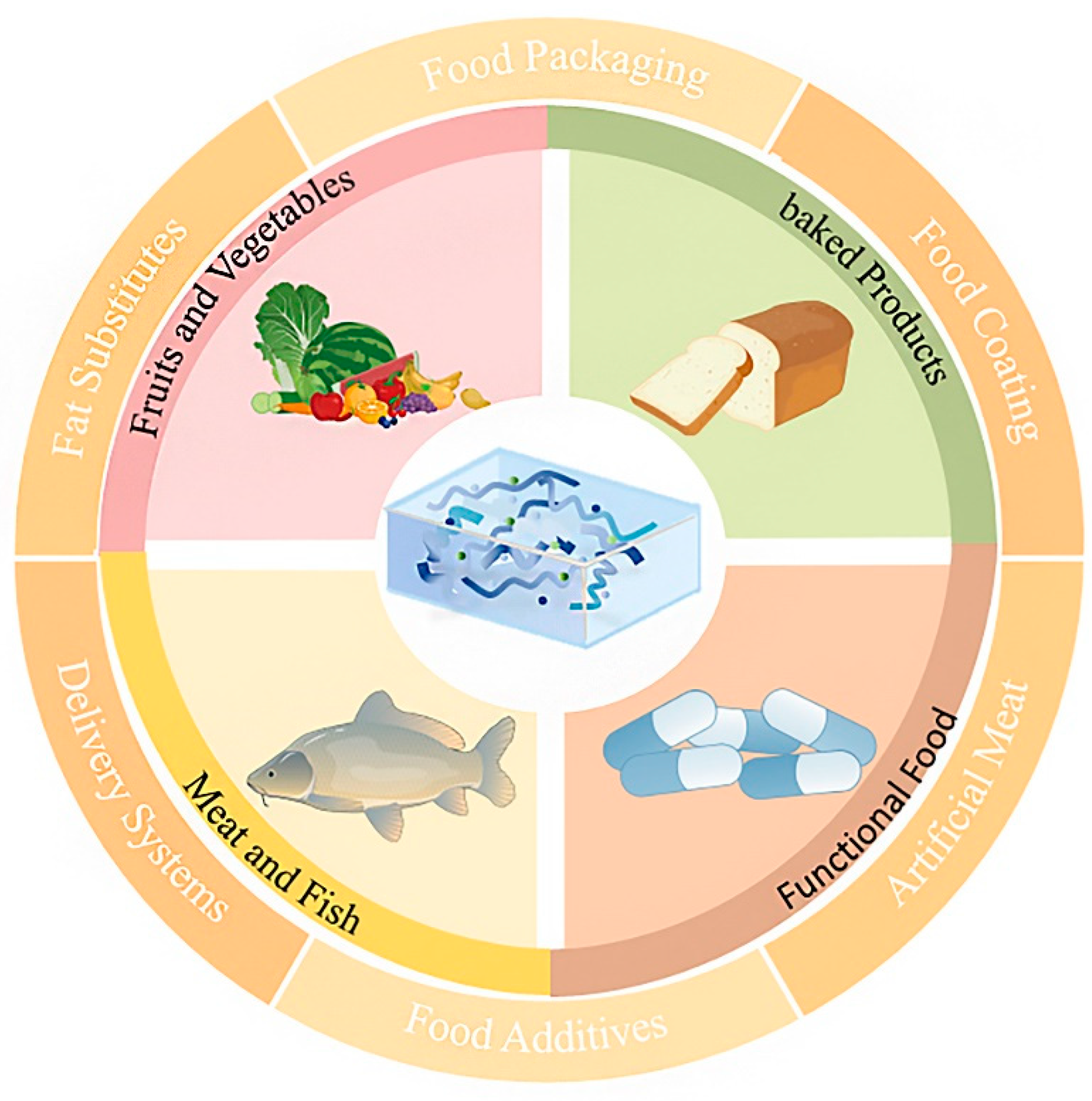
7. Applications of Marine Polysaccharide-Based Hydrogels in the Food Industry
7.1. Fruits and Vegetables
7.2. Meat and Seafood
7.3. Baked Products
7.4. Functional Food
7.5. Biocatalyst
8. Conclusions and Future Prospects
- (1)
- Increase in toxicological studies: Most of the documented bioactivities of MPs are still in the research stage. However, the destruction of marine ecosystems leading to marine organisms (especially algae) can enrich pollutants, such as heavy metals and polycyclic aromatic hydrocarbons. Consequently, the absence of clinical trials, especially toxicological studies including acute toxicity, chronic toxicity, and teratogenicity testing, constitutes a key constraint on the practical application of marine polysaccharides.
- (2)
- Drawbacks of natural polymer hydrogels: Poor mechanical properties, insufficient long-term stability, and weak anti-interference ability of natural polymer hydrogels are key factors limiting their applications. Therefore, the development of safe and efficient cross-linking technologies is urgently required to improve their performance.
- (3)
- Modern computational methods: Based on extensive structural and activity data of marine polysaccharides, artificial intelligence (AI), bioinformatics, and chemoinformatics can contribute to predicting the structure and function of MPs, analyzing the interactions between polysaccharides and proteins/lipids, simulating the interactions between MPs and biological targets, and further screening target polysaccharides.
- (4)
- Multi-omics: Multi-omics integration (e.g., proteomics, metabolomics, and genomics) can better elucidate the metabolic pathways and interactions between hydrogels and bioactive components in vivo, which will facilitate delving into the underlying molecular mechanisms.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | marine-derived polysaccharides |
| CS | chitosan |
| HA | hyaluronic acid |
| Glc | glucose |
| Gal | galactose |
| Man | mannose |
| Fuc | fucose |
| GalA | galacturonic acid |
| GlcA | glucuronic acid |
| IdoA | iduronic acid |
| LMW | low molecular weight |
| HMW | high molecular weight |
| APTT | activated partial thromboplastin time |
| TT | thrombin time |
| VEGF | vascular endothelial growth factors |
| IL-1/6/10 | interleukin-1/6/10 |
| IL-1β | interleukin—1Β |
| TNF-α | tumor necrosis factor-A |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2’-azinobis-(3-ethylbenzthiazoline-6-sulphonate) |
| CAT | catalase |
| SOD | superoxide dismutase |
| GSH-PX | glutathione peroxidase |
| Bax/Bcl | Bcl2-associated X |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa-B |
| TLR2/3/4 | toll-like receptor 2/4 |
| SCFAs | short-chain fatty acids |
| Dectin-1 | dendritic cell-associated C-type lectin receptor |
| SR | scavenger receptor |
| ERK 1/2 | extracellular-regulated kinase 1/2 |
| p38 | P38 mitogen-activated protein kinase |
| JNK | C-Jun N-terminal kinase |
| JAK2 | Janus kinase |
| PI3K-AKT | phosphatidylinositol 3-kinase/Akt |
| ER | endoplasmic reticulum |
| ROS | reactive oxygen species |
| GM | gut microbiota |
| MAPK | mitogen-activated protein kinase |
| JAK-STAT | The Janus kinase-signal transducer and activator of transcription |
| NF-κb | nuclear factor-kappa B |
| AT | antithrombin |
| HC-II | heparin cofactor II |
| SA | sodium alginate |
References
- Guan, X.; Wang, F.; Zhou, B.; Sang, X.; Zhao, Q. The nutritional function of active polysaccharides from marine animals: A review. Food Biosci. 2024, 58, 103693. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Kannappan, P.; Abdullah, N.; Ahmed, A.B.A. Cardioprotective activity of polysaccharides derived from marine algae: An overview. Trends Food Sci. Technol. 2013, 30, 98–104. [Google Scholar] [CrossRef]
- Jeewon, R.; Aullybux, A.A.; Puchooa, D.; Nazurally, N.; Alrefaei, A.F.; Zhang, Y. Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications. Mar. Drugs 2023, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gu, Q.; Yu, X. Effect of in vitro simulated digestion on the physicochemical properties and pancreatic lipase inhibitory activity of fucoidan. J. Sci. Food Agric. 2024, 104, 4157–4164. [Google Scholar] [CrossRef]
- George, A.M.; Chakraborty, K.; Paulose, S.K.; Bose, C.; Dhara, S. Anti-coagulant effects of α-(1 → 3)/(1 → 4) linked sulfated galactofucan from brown macroalga Sargassum plagiophyllum (C. Agardh) in human umbilical vein endothelial cells. Food Biosci. 2024, 62, 105467. [Google Scholar] [CrossRef]
- Chen, R.; Hao, Y.; Francesco, S.; Mao, X.; Huang, W.C. A chitosan-based antibacterial hydrogel with injectable and self-healing capabilities. Mar. Life Sci. Technol. 2024, 6, 115–125. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.-W.; Ahn, G.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.-J. In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum. Mar. Drugs 2021, 19, 277. [Google Scholar] [CrossRef]
- Allapitchai, J.F.; Pitchai, A.; Ramasamy, P. Isolation and Free Radical Scavenging Ability of Linear Polysaccharides From Cuttlebone of Sepia prashadi. Cureus J. Med. Sci. 2024, 16, e60163. [Google Scholar] [CrossRef]
- Geng, H.; Chen, M.; Guo, C.; Wang, W.; Chen, D. Marine polysaccharides: Biological activities and applications in drug delivery systems. Carbohydr. Res. 2024, 538, 109071. [Google Scholar] [CrossRef]
- Yang, M.; Deng, Q.; Chen, R.; Sun, Y.; Zhou, X.; Chen, H. Extraction, Purification, Structural Characteristics, and Biological Activities of Seaweed Polysaccharides: A Review. Starch-Starke 2024, 77, 2400029. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, J.; Liu, W.; Mei, J.; Xie, J. Characterization of Sodium Alginate—Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging. Polymers 2022, 14, 731. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lan, W.; Xie, J. Chitosan grafted gallic acid /polyvinyl alcohol-based hydrogel, developed through the freeze-thaw method, holds promise for use as a fresh-water absorbent pad. Int. J. Biol. Macromol. 2025, 320, 146078. [Google Scholar] [CrossRef]
- Roheen, T.; Ramzan, R.; Nadeem, M.; Atif, F.A.; Munir, M.; Qureshi, T.M. Synthesis and Characterization of CMC/PAM-Amy Hydrogel and Its Efficacy in Apple Juice Clarification. Processes 2024, 12, 2264. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, D.; Sun, Z.; Liu, F.; Zhang, D.; Wang, D. Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydrocoll. 2022, 127, 107546. [Google Scholar] [CrossRef]
- Dahal, P.; Janaswamy, S. Hydrocolloid-based nutraceutical delivery systems: Potential of kappa-carrageenan hydrogel beads for sustained release of curcumin. Food Res. Int. 2024, 183, 114223. [Google Scholar] [CrossRef]
- Moncada, D.; Rico, M.; Montero, B.; Rodriguez-Llamazares, S.; Feijoo-Bandin, S.; Gualillo, O.; Lago, F.; Aragon-Herrera, A.; Salavagione, H.; Pettinelli, N.; et al. Injectable hybrid hydrogels physically crosslinked based on carrageenan and green graphene for tissue repair. Int. J. Biol. Macromol. 2023, 235, 123777. [Google Scholar] [CrossRef]
- Luo, S.; Hu, C.Y.; Xu, X. Ammonia-responsive chitosan/polymethacrylamide double network hydrogels with high-stretchability, fatigue resistance and anti-freezing for real-time chicken breast spoilage monitoring. Int. J. Biol. Macromol. 2024, 268, 131617. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Jastrzębska, A.; Apjok, R.; Petrus, M.; Mihaly Cozmuta, L.; Peter, A.; Nicula, C. Immobilization of baker’s yeast in the alginate-based hydrogels to impart sensorial characteristics to frozen dough bread. Food Biosci. 2021, 42, 101143. [Google Scholar] [CrossRef]
- Bakky, M.A.H.; Tran, N.T.; Zhang, Y.; Li, S. Utilization of marine macroalgae-derived sulphated polysaccharides as dynamic nutraceutical components in the feed of aquatic animals: A review. Aquac. Res. 2022, 53, 5787–5808. [Google Scholar] [CrossRef]
- Mubuchi, A.; Katsumoto, S.; Tsuboi, M.; Ishikawa, H.; Nomura, Y.; Higashi, K.; Miyata, S. Isolation and structural characterization of bioactive glycosaminoglycans from the green-lipped mussel Perna canaliculus. Biochem. Biophys. Res. Commun. 2022, 612, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, Z.; Liu, Y.; Cheong, K.L.; Teng, B.; Khan, B.M. Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri. Mar. Drugs 2020, 18, 429. [Google Scholar] [CrossRef] [PubMed]
- Berezhnaya, Y.D.; Kazachenko, A.S.; Kazachenko, A.S.; Malyar, Y.N.; Borovkova, V.S. Sulfation of Various Polysaccharide Structures: Different Methods and Perspectives. Chemistry 2024, 6, 640–665. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.-J.; Chen, C.-W.; Dong, C.-D. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical Modification of Polysaccharides: A Review of Synthetic Approaches, Biological Activity and the Structure-Activity Relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef]
- Bhadja, P.; Tan, C.Y.; Ouyang, J.M.; Yu, K. Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells. Polymers 2016, 8, 188. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, T.; Xu, J.; Xi, W.; Shang, E.; Xiao, P.; Duan, J.-a. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef]
- Lu, C.; Gu, Q.; Yu, X. The preparation and anti-atherosclerotic effects of different low-molecular weights fucoidan. Food Biosci. 2024, 58, 103755. [Google Scholar] [CrossRef]
- Hu, S.; Chen, S.; Zhu, H.; Du, M.; Jiang, W.; Liu, Y.; Gao, X.; Su, L.; Xu, Y. Low Molecular Weight, 4-O-Sulfation, and Sulfation at Meta-Fucose Positively Promote the Activities of Sea Cucumber Fucoidans on Improving Insulin Resistance in HFD-Fed Mice. Mar. Drugs 2021, 20, 37. [Google Scholar] [CrossRef]
- Li, X.; Shen, A.; Xiao, M.; Li, S.; Yang, W. New insights on health benefits, interactions with food components and potential application of marine-derived sulfated polysaccharides: A review. Int. J. Biol. Macromol. 2025, 294, 139516. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, A.L.; Aguiar, J.A.K.; Correa da Silva, F.S.; Michelacci, Y.M. Do chondroitin sulfates with different structures have different activities on chondrocytes and macrophages? Int. J. Biol. Macromol. 2017, 103, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Khongthong, S.; Theapparat, Y.; Roekngam, N.; Tantisuwanno, C.; Otto, M.; Piewngam, P. Characterization and immunomodulatory activity of sulfated galactan from the red seaweed Gracilaria fisheri. Int. J. Biol. Macromol. 2021, 189, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Son, S.-U.; Shin, K.-S. Low-molecular-weight ulvan prepared from the Korean seaweed Ulva pertusa enhances immune responses via signaling pathways, short chain fatty acids, and G-protein coupled receptors. Int. J. Biol. Macromol. 2025, 318, 145143. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Kravchenko, A.O.; Sergeeva, N.V.; Kalinovsky, A.I.; Glazunov, V.P.; Bogdanovich, L.N.; Yermak, I.M. Effect of red seaweed sulfated galactans on initial steps of complement activation in vitro. Carbohydr. Polym. 2021, 254, 117251. [Google Scholar] [CrossRef]
- Wassie, T.; Lu, Z.; Duan, X.; Xie, C.; Gebeyew, K.; Yumei, Z.; Yin, Y.; Wu, X. Dietary Enteromorpha Polysaccharide Enhances Intestinal Immune Response, Integrity, and Caecal Microbial Activity of Broiler Chickens. Front. Nutr. 2021, 8, 783819. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Ai, Y.; Han, Z.; Li, M.; Qi, Y.; Zhao, Q.; Li, Z. Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice. Foods 2021, 10, 2478. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Dwan, C.; De Medts, J.; Duysburgh, C.; Rotsaert, C.; Marzorati, M. Undaria pinnatifida Fucoidan Enhances Gut Microbiome, Butyrate Production, and Exerts Anti-Inflammatory Effects in an In Vitro Short-Term SHIME® Coupled to a Caco-2/THP-1 Co-Culture Model. Mar. Drugs 2025, 23, 242. [Google Scholar] [CrossRef]
- Peng, B.; Liu, Y.; Lin, Y.; Kraithong, S.; Mo, L.; Gao, Z.; Huang, R.; Zhang, X. A New Exopolysaccharide of Marine Coral-Associated Aspergillus pseudoglaucus SCAU265: Structural Characterization and Immunomodulatory Activity. J. Fungi 2023, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1901–1929. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, J.-Y.; Yang, C.; Song, G.; Lim, W. Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis. Mar. Drugs 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Liu, M.; Wei, B.; Tang, Q.; Wang, Y.; Xue, C. Fucoidan exerts antitumor effects by regulating gut microbiota and tryptophan metabolism. Int. J. Biol. Macromol. 2025, 300, 140334. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-Derived from Brown Algae Suppresses the Growth of Ovarian Cancer Cells via Mitochondrial Dysfunction and ER Stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef]
- Liu, G.; Shan, Y.; Sun, C. A Novel Exopolysaccharide, Highly Prevalent in Marine Spongiibacter, Triggers Pyroptosis to Exhibit Potent Anticancer Effects. FASEB J. 2025, 39, e70758. [Google Scholar] [CrossRef]
- Liu, M.; Li, F.; Feng, S.; Guo, J.; Yu, J.; Zou, S.; Gao, X.; Wei, Y. Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum. Foods 2024, 13, 3552. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, L.; Ke, H.; Jiang, S.; Zeng, M. Oyster (Crassostrea gigas) polysaccharide ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat diet fed mice. Int. J. Biol. Macromol. 2022, 216, 916–926. [Google Scholar] [CrossRef]
- Lee, H.-G.; Jayawardhana, H.H.A.C.K.; Yang, F.; Nagahawaththa, D.P.; Liyanage, N.M.; Song, K.-M.; Choi, Y.-S.; Lee, S.-H.; Jeon, Y.-J.; Kang, M.-C. Anti-obesity effects of fucoidan from Sargassum thunbergii in adipocytes and high fat diet induced obese mice through inhibiting adipogenic specific transcription factor. Food Sci. Hum. Wellness 2024, 13, 1608–1616. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine algae: A treasure trove of bioactive anti-inflammatory compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ward, F.; Deyab, M.A.; Al-Zahrani, M.; Touliabah, H.E. Chemical Composition, Antioxidant, and Antitumor Activity of Fucoidan from the Brown Alga Dictyota dichotoma. Molecules 2023, 28, 7175. [Google Scholar] [CrossRef] [PubMed]
- Dörschmann, P.; Kopplin, G.; Thalenhorst, T.; Seeba, C.; Ullah, S.F.; Srivastava, V.; Roider, J.; Klettner, A. Influence of a Very High-Molecular Weight Fucoidan from Laminaria hyperborea on Age-Related Macular Degeneration-Relevant Pathomechanisms in Ocular Cell Models. Mar. Drugs 2025, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Yang, W.; Li, L.; Zhu, Y.; Yang, Y.; Ni, H.; Jiang, Z.; Zheng, M. In vitro fermentation of seaweed polysaccharides and tea polyphenol blends by human intestinal flora and their effects on intestinal inflammation. Food Funct. 2023, 14, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Lakhrem, M.; Eleroui, M.; Boujhoud, Z.; Feki, A.; Dghim, A.; Essayagh, S.; Hilali, S.; Bouhamed, M.; Kallel, C.; Deschamps, N.; et al. Anti-Vasculogenic, Antioxidant, and Anti-Inflammatory Activities of Sulfated Polysaccharide Derived from Codium tomentosum: Pharmacokinetic Assay. Pharmaceuticals 2024, 17, 672. [Google Scholar] [CrossRef]
- Jegani, K.T.; Balde, A.; Nazeer, R.A. A review on anti-inflammatory and antioxidant peptides derived from marine organisms: Mechanism of action and therapeutic applications. Food Biosci. 2025, 63, 105745. [Google Scholar] [CrossRef]
- Qiu, M.; Huang, S.; Luo, C.; Wu, Z.; Liang, B.; Huang, H.; Ci, Z.; Zhang, D.; Han, L.; Lin, J. Pharmacological and clinical application of heparin progress: An essential drug for modern medicine. Biomed. Pharmacother. 2021, 139, 111561. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhang, A.; Shao, X.; Liu, T.; Tang, B.; Fang, G. Strategies for sustained release of heparin: A review. Carbohydr. Polym. 2022, 294, 119793. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, W.; Fang, S.; Zhao, J.; Wang, W.; Zhou, S.; Wang, T.; Fang, X.; Chen, X.; Awais, M.; et al. Low-Molecular-Weight Fucoidan Inhibits Thromboinflammation and Ameliorates Deep Vein Thrombosis via Targeting S100A8/A9. Mar. Drugs 2025, 23, 180. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.E.; Rjabovs, V.; Hammami, R.; Critchley, A.T.; Tuvikene, R.; Hincke, M.T. Immunomodulation by xylan and carrageenan-type polysaccharides from red seaweeds: Anti-inflammatory, wound healing, cytoprotective, and anticoagulant activities. Int. J. Biol. Macromol. 2024, 260, 129433. [Google Scholar] [CrossRef]
- Maurya, A.K.; Ahmed, H.A.; DeWitt, A.; Shami, A.A.; Misra, S.K.; Pomin, V.H. Structure and Binding Properties to Blood Co-Factors of the Least Sulfated Galactan Found in the Cell Wall of the Red Alga Botryocladia occidentalis. Mar. Drugs 2024, 22, 81. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ali, M.S.; Anuradha, V.; Abinaya, R.; Ananthalakshmi, J.S.; Yogananth, N. Antioxidant Potential of Fucose Isolated from the Marine Macroalgae Padina gymnospora. Biosci. Biotechnol. Res. Commun. 2021, 14, 1302–1308. [Google Scholar] [CrossRef]
- Perumal, P.K.; Huang, C.-Y.; Singhania, R.R.; Patel, A.K.; Haldar, D.; Chen, C.-w.; Dong, C.-D. In vitro evaluation of antioxidant potential of polysaccharides and oligosaccharides extracted from three different seaweeds. J. Food Sci. Technol. 2023, 61, 1481–1491. [Google Scholar] [CrossRef]
- Wang, F.; Kong, L.-M.; Xie, Y.-Y.; Wang, C.; Wang, X.-L.; Wang, Y.-B.; Fu, L.-L.; Zhou, T. Purification, structural characterization, and biological activities of degraded polysaccharides from Porphyra yezoensis. J. Food Biochem. 2021, 45, e13661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, Y.; Zhang, Z.; Li, C.; Mei, L.; Hou, R.; Liu, X.; Jiang, H. Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity. Polymers 2024, 16, 867. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Han, F.; Yuan, Z.; Zhu, Z.; Liu, C.; Tu, Z.; Guo, Q.; Zhao, R.; Zhang, W.; Wang, H.; et al. Fucoidan-loaded nanofibrous scaffolds promote annulus fibrosus repair by ameliorating the inflammatory and oxidative microenvironments in degenerative intervertebral discs. Acta Biomater. 2022, 148, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Dörschmann, P.; Apitz, S.; Hellige, I.; Neupane, S.; Alban, S.; Kopplin, G.; Ptak, S.; Fretté, X.; Roider, J.; Zille, M.; et al. Evaluation of the Effects of Fucoidans from Fucus Species and Laminaria hyperborea against Oxidative Stress and Iron-Dependent Cell Death. Mar. Drugs 2021, 19, 557. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, S.; Chen, K.; Liu, L.; Wang, X.; Zhang, B.; Hu, L.; Liu, S.; Zhao, T.; Qi, H. High-sulfated derivative of polysaccharide from Ulva pertusa improves Adriamycin-induced nephrotic syndrome by suppressing oxidative stress. Food Funct. 2023, 14, 9167–9180. [Google Scholar] [CrossRef]
- Qin, L.; Xu, H.; He, Y.; Liang, C.; Wang, K.; Cao, J.; Qu, C.; Miao, J. Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea antarctica. Mar. Drugs 2022, 20, 223. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, W.; Wang, H.; Yao, L.; He, Z.; Sun, M.; Feng, T.; Yu, C.; Yue, H. Flash extraction of ulvan polysaccharides from marine green macroalga Ulva linza and evaluation of its antioxidant and gut microbiota modulation activities. Int. J. Biol. Macromol. 2024, 262, 130174. [Google Scholar] [CrossRef]
- He, Y.-L.; An, M.; Liu, H.; Jia, R.; Zhong, S.; Hong, P.; Song, B.; Gao, P.; Qian, Z.-J. Antioxidant extracellular polysaccharide EPSLAN4 from marine-derived Limosilactobacillus fermentum LAN4 potential to against inflammation by inhibiting MAPK/NF-κB signaling pathway. Int. J. Biol. Macromol. 2025, 321, 146267. [Google Scholar] [CrossRef]
- Aramsangtienchai, P.; Sirirak, T.; Seesan, P.; Singrakphon, W.; Padungkasem, J.; Srisook, K.; Watanachote, J. Sulfated polysaccharide from Phyllophorella kohkutiensis: Extraction, purification, and bioactivity assessment. Food Chem. X 2025, 29, 102772. [Google Scholar] [CrossRef]
- Xiao, T.; Yu, Z.; Yang, H.; You, J.; Wu, X. Marine polysaccharides hydrogel with encapsulated mesalazine for the treatment of ulcerative colitis: Integrative effects on inflammation, microbiota, and mucosal repair. Colloids Surf. B Biointerfaces 2025, 253, 114722. [Google Scholar] [CrossRef]
- Rajan, D.K.; Zhang, L.; Li, H.; Li, J.; Di, X.; Zhang, S. Purification and characterization of alginate extracted from Sargassum hemiphyllum and its antioxidant and wound healing efficacy. Food Biosci. 2024, 62, 105228. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.E.; Sooäär, A.; Rjabovs, V.; Critchley, A.T.; Hincke, M.T.; Tuvikene, R. Extraction and functional characterization of fucoidans and alginates from Ecklonia maxima: A focus on skin, immune, and intestinal health. Food Hydrocoll. 2025, 159, 110668. [Google Scholar] [CrossRef]
- Din, N.A.S.; Ishak, A.A.; Maarof, S.; Abdul Rahman, H.; Sofian-Seng, N.S.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Potential anti-obesity effects of fucoidan from Malaysian brown seaweed (Sargassum binderi) by in vivo study. Int. J. Food Sci. Technol. 2024, 59, 3402–3411. [Google Scholar] [CrossRef]
- Chung, H.-J.; Jeun, J.; Houng, S.-J.; Jun, H.-J.; Kweon, D.-K.; Lee, S.-J. Toxicological Evaluation of Fucoidan from Undaria pinnatifida In Vitro and In Vivo. Phytother. Res. 2010, 24, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.A.; Aloufi, A.S.; Parveen, B. Essential bioactive competence of laminarin (beta-glucan)/ laminaran extracted from Padina tetrastromatica and Sargassum cinereum biomass. Environ. Res. 2024, 252, 118836. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, P.A.; Palaniselvam, S.; Jayachandran, J.; Senthil, A.; Valliappan, V.; Ramachandran, S. Toxicity minimization of carrageenan via isoliquiritigenin grafting: A zebrafish model study. Results Chem. 2024, 10, 101728. [Google Scholar] [CrossRef]
- Jiang, J.L.; Zhang, W.Z.; Ni, W.X.; Shao, J.W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, K.; Zhang, M.; Zhou, Q.; Ji, W.; Yao, Z.; Li, D. Pharmacokinetics, tissue distribution, and subacute toxicity of oral carrageenan in mice. Int. J. Biol. Macromol. 2024, 266, 130725. [Google Scholar] [CrossRef]
- Song, C.; Chen, Z.; Wang, L.; Wang, N.; Tian, J.; Ai, C.; Song, S. Low-molecular-weight carrageenan prepared by photocatalysis-freeze-thaw circle and its in vivo distribution after oral administration. Food Hydrocoll. 2025, 162, 111027. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef] [PubMed]
- Tsirtsidou, K.; Zou, Y.; Robbens, J.; Raes, K. Pectin-chitosan hydrogels with modified properties for the encapsulation of strawberry phenolic compounds. Food Chem. 2025, 463, 141236. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shen, M.; Luo, Y.; Wu, T.; Chen, X.; Wang, Y.; Xie, J. Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends Food Sci. Technol. 2021, 110, 822–832. [Google Scholar] [CrossRef]
- Xie, F.; Liu, M.; Feng, X.; He, Z.; Chen, Q.; Zhou, J.; Cai, J. Tannic acid one-step induced quaternized chitin-based edible and easy-cleaning coatings with multifunctional preservation for perishable products. Food Hydrocoll. 2025, 159, 110636. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Mabrook, G.; Hathi, Z.; Mettu, S.; Banat, F.; Taher, H. Lacticaseibacillus rhamnosus encapsulated cross-linked Keratin-Chitosan hydrogel for removal of patulin from apple juice. Food Chem. 2024, 454, 139619. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S. Polysaccharide-Based Multifunctional Hydrogel Bio-Adhesives for Wound Healing: A Review. Gels 2023, 9, 138. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, L.; Wan, W.; Zhang, C.; Liu, Y.; Luo, L.; Zhu, T.; Zhang, W. Preparation and characterization of mussel-inspired dual-crosslinked hydrogels based on hydroxypropyl chitosan. J. Porous Mater. 2023, 31, 611–624. [Google Scholar] [CrossRef]
- Gorgol, D.; Mrlik, M.; Mikulka, F.; Vichova, Z.; Mahelova, L.; Ilcikova, M.; Minarik, A. Smart Biopolymer Scaffolds Based on Hyaluronic Acid and Carbonyl Iron Microparticles: 3D Printing, Magneto-Responsive, and Cytotoxicity Study. ACS Appl. Bio Mater. 2024, 7, 7483–7493. [Google Scholar] [CrossRef]
- Fan, S.; Liu, Q.; Dong, J.; Ai, X.; Li, J.; Huang, W.; Sun, T. In situ forming an injectable hyaluronic acid hydrogel for drug delivery and synergistic tumor therapy. Heliyon 2024, 10, e32135. [Google Scholar] [CrossRef]
- Castrejon-Comas, V.; Mataro, N.; Resina, L.; Zanuy, D.; Nunez-Aulina, Q.; Sanchez-Moran, J.; Enshaei, H.; Arnau, M.; Munoz-Galan, H.; Worch, J.C.; et al. Electro-responsive hyaluronic acid-based click-hydrogels for wound healing. Carbohydr. Polym. 2025, 348, 122941. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Wu, G.; Zhu, Z.; Zheng, H.; Sun, X.; Heng, Y.; Pan, S.; Xiu, H.; Zhang, J.; et al. Hyaluronic Acid-Based Reactive Oxygen Species-Responsive Multifunctional Injectable Hydrogel Platform Accelerating Diabetic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2302626. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Al-Ghzawi, B.; BaniAmer, F. Exploring the Formulation and Approaches of Injectable Hydrogels Utilizing Hyaluronic Acid in Biomedical Uses. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 3869387. [Google Scholar] [CrossRef]
- Jing, X.; Sun, Y.; Ma, X.; Hu, H. Marine polysaccharides: Green and recyclable resources as wound dressings. Mater. Chem. Front. 2021, 5, 5595–5616. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, Q.; Hu, Y.; He, J.; Wang, H.; Deng, C.; Li, D. MXene/zinc ion embedded agar/sodium alginate hydrogel for rapid and efficient sterilization with photothermal and chemical synergetic therapy. Talanta 2024, 266, 125101. [Google Scholar] [CrossRef]
- Liu, W.; Mei, J.; Xie, J. Effect of locust bean gum-sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition. Int. J. Biol. Macromol. 2021, 170, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fang, S.; Xie, Y.; Mei, J.; Xie, J. Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage. Coatings 2024, 14, 338. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Gao, X.; Mei, J.; Xie, J. Effect of Active Coatings Containing Lippa citriodora Kunth. Essential Oil on Bacterial Diversity and Myofibrillar Proteins Degradation in Refrigerated Large Yellow Croaker. Polymers 2021, 13, 1787. [Google Scholar] [CrossRef] [PubMed]
- Naghib, S.M.; Matini, A.; Amiri, S.; Ahmadi, B.; Mozafari, M.R. Exploring the potential of polysaccharides-based injectable self-healing hydrogels for wound healing applications: A review. Int. J. Biol. Macromol. 2024, 282, 137209. [Google Scholar] [CrossRef]
- Ghahtan, N.; Dehghan, N.; Ullah, M.; Khoradmehr, A.; Habibi, H.; Nabipour, I.; Baghban, N. From seaweed to healing: The potential of fucoidan in wound therapy. Nat. Prod. Res. 2024, 39, 1345–1358. [Google Scholar] [CrossRef]
- Sulastri, E.; Lesmana, R.; Zubair, M.S.; Abdelwahab Mohammed, A.F.; Elamin, K.M.; Wathoni, N. Ulvan/Silver nanoparticle hydrogel films for burn wound dressing. Heliyon 2023, 9, e18044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Xu, B.; Xiang, W.; Li, A.; Li, T. Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology. Mar. Drugs 2024, 22, 498. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Davila, C.E.; Solis-Andrade, K.I.; Olvera-Sosa, M.; Palestino, G.; Rosales-Mendoza, S. Core-shell chitosan/Porphyridium-exopolysaccharide microgels: Synthesis, properties, and biological evaluation. Int. J. Biol. Macromol. 2023, 246, 125655. [Google Scholar] [CrossRef]
- Fillaudeau, A.; Desdouits, M.; Sinquin, C.; Colliec-Jouault, S.; Zykwinska, A.; Cuenot, S. Thermoresponsive and cytocompatible infernan-based hydrogels exhibiting poroelastic properties. Appl. Mater. Today 2025, 42, 102544. [Google Scholar] [CrossRef]
- He, W.; Zhu, Y.; Chen, Y.; Shen, Q.; Hua, Z.; Wang, X.; Xue, P. Inhibitory Effect and Mechanism of Chitosan-Ag Complex Hydrogel on Fungal Disease in Grape. Molecules 2022, 27, 1688. [Google Scholar] [CrossRef]
- Hao, L.T.; Lee, S.; Hwang, D.S.; Jeon, H.; Park, J.; Kim, H.J.; Oh, D.X. Self-Healing Scaffolding Technology with Strong, Reversible Interactions under Physiological Conditions for Engineering Marbled Cultured Meat. ACS Appl. Mater. Interfaces 2025, 17, 31881–31897. [Google Scholar] [CrossRef]
- Farasati Far, B.; Jahanbakhshi, M.; Jameie, L.; Zolfigol, F.; Taromi, P.; Ertas, Y.N. Chitosan-graft-Pomegranate Extract Hydrogel: A Dual-Functional Pad for Antibacterial and Antioxidant Enhancement for Shelf Life Extension in Food Packaging. ACS Appl. Polym. Mater. 2024, 6, 9545–9558. [Google Scholar] [CrossRef]
- Wu, W.; Ni, X.; Shao, P.; Gao, H. Novel packaging film for humidity-controlled manipulating of ethylene for shelf-life extension of Agaricus bisporus. LWT 2021, 145, 111331. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, J.; Li, S.; Lin, S.; Tu, Y.; Gui, X. Colorimetric hydrogel indicators based on polyvinyl alcohol/sodium alginate for visual food spoilage monitoring. Int. J. Food Sci. Technol. 2022, 57, 6867–6880. [Google Scholar] [CrossRef]
- Liang, F.; Huang, Y.; Miao, J.; Lai, K. A simple and efficient alginate hydrogel combined with surface-enhanced Raman spectroscopy for quantitative analysis of sodium nitrite in meat products. Analyst 2024, 149, 1518–1526. [Google Scholar] [CrossRef]
- Liu, S.; Hua, S.; Gu, X.; Cai, P.; Zhou, Y.; Wang, Y.; Zhou, M.; Shan, T. Production of sodium alginate-gelatin composite hydrogel-based 3D cultured fat with low cholesterol and high polyunsaturated fatty acids. Food Hydrocoll. 2024, 154, 110156. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, L.; Liu, J.; Yu, K.; Yu, W.; Jiang, H.; Chen, X.; Peng, S.; Zhong, J.; Liu, W. Metal-organic frameworks-based moisture responsive essential oil hydrogel beads for fresh-cut pineapple preservation. Food Chem. 2024, 451, 139440. [Google Scholar] [CrossRef]
- Ahmad, F.; Hassan, A.; Mushtaq, B.; Azam, F.; Ahmad, S.; Rasheed, A.; Nawab, Y. A biodegradable alginate/chitosan hydrogel based nonwoven from pre-consumer cotton waste and virgin wool for food packaging. Ind. Crops Prod. 2024, 216, 118795. [Google Scholar] [CrossRef]
- Foley, C.; Floreani, R.A. Impact of whey protein on the physico-mechanical properties of alginate dialdehyde scaffolds and cell adhesion for cultivated meat applications. Food Hydrocoll. 2025, 170, 111749. [Google Scholar] [CrossRef]
- Tahir, I.; Floreani, R. Dual-Crosslinked Alginate-Based Hydrogels with Tunable Mechanical Properties for Cultured Meat. Foods 2022, 11, 2829. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, K.; Yan, B. Food and environmental safety monitoring platform based on Tb(III) functionalized HOF hybrids for ultrafast detection of thiabendazole and 2-chlorophenol. Talanta 2024, 272, 125829. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M.; et al. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Zhao, Y.; Fang, Y. Agarose/konjac glucomannan double network hydrogels to mimic the texture of beef tripe. Food Hydrocoll. 2023, 135, 108173. [Google Scholar] [CrossRef]
- Lohrasbi Nejad, S.; Shekarchizadeh, H. An agar hydrogel-CuNPs/N@CQDs dual-mode colorimetric/fluorescent indicator for non-destructive monitoring of banana ripening. Food Chem. 2025, 473, 143098. [Google Scholar] [CrossRef]
- Sun, G.; Li, B.; Li, Y.; McClements, D.J. Construction of biopolymer-based hydrogel beads for encapsulation, retention, and colonic delivery of tributyrin: Development of functional beverages (fortified bubble tea). Food Res. Int. 2024, 197, 115165. [Google Scholar] [CrossRef]
- Gu, X.; Hua, S.; Huang, Y.; Liu, S.; Wang, Y.; Zhou, M.; Shan, T. κ-Carrageenan/konjac glucomannan composite hydrogel-based 3D porcine cultured meat production. Food Hydrocoll. 2024, 151, 109765. [Google Scholar] [CrossRef]
- Forghani, S.; Almasi, H. Characterization and performance evaluation of colorimetric pH-sensitive indicator based on Ҡ-carrageenan/quince seed mucilage hydrogel as freshness/spoilage monitoring of rainbow trout fillet. Food Chem. 2024, 457, 140072. [Google Scholar] [CrossRef] [PubMed]
- Nutter, J.; Shi, X.; Lamsal, B.; Acevedo, N.C. Plant-based bigels as a novel alternative to commercial solid fats in short dough products: Textural and structural properties of short dough and shortbread. Food Biosci. 2023, 54, 102865. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, Z.; Wu, D. Fabrication of dual-layered microfluidic-blow-spinning nanofiber films with antibacterial and antioxidant functions for food packaging. Food Qual. Saf. 2025, 9, fyaf030. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive review of natural based hydrogels as an upcoming trend for food packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Awad, S.A.; Al-Kubaisi, A.A.; Mukhlif, M.Y.; Khalaf, E.M.; Fahad, M.M. In Vitro and Molecular Docking Characterization of Polyvinyl Alcohol/Ag NPs-Capparis spinosa (CS)/Chitosan Hydrogels as Films Antibacterial Surface Contamination. Polym. Eng. Sci. 2025. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, L.; Yu, J.; Liu, L.; Goksen, G.; Shao, P. High-sensitivity intelligent packaging films harnessing rose anthocyanins and hydrophilic silica aerogel for visual food freshness monitoring. Food Qual. Saf. 2024, 8, fyad051. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Lin, Z.; Yan, X.; Sun, C.; Yao, D. Carbon dots-based stimuli-responsive hydrogel for in-situ detection of thiram on fruits and vegetables. Food Chem. 2024, 460, 140405. [Google Scholar] [CrossRef]
- Kollia, E.; Kopsacheili, A.; Birlia, C.; Nikolaou, D.; Avdylaj, L.; Giannakas, A.E.; Salmas, C.E.; Tomasevic, I.; Brennan, C.; Proestos, C. Chitosan-zinc oxide thyme oil-based edible antimicrobial hydrogel coatings: Application on pork and tofu sausages. Int. J. Food Sci. Technol. 2025, 60, vvae096. [Google Scholar] [CrossRef]
- Jang, S.; Son, S.U.; Kim, J.; Kim, H.; Lim, J.; Seo, S.B.; Kang, B.; Kang, T.; Jung, J.; Seo, S.; et al. Polydiacetylene-based hydrogel beads as colorimetric sensors for the detection of biogenic amines in spoiled meat. Food Chem. 2023, 403, 134317. [Google Scholar] [CrossRef]
- Yogeswari, M.S.; Selamat, J.; Jambari, N.N.; Khatib, A.; Mohd Amin, M.H.; Murugesu, S. Metabolomics for quality assessment of poultry meat and eggs. Food Qual. Saf. 2024, 8, fyae004. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Xu, Y.; Yin, J.; Hu, J. A 3D-printable gelatin/alginate/ε-poly-l-lysine hydrogel scaffold to enable porcine muscle stem cells expansion and differentiation for cultured meat development. Int. J. Biol. Macromol. 2024, 271, 131980. [Google Scholar] [CrossRef] [PubMed]
- Melzener, L.; Spaans, S.; Hauck, N.; Pötgens, A.J.G.; Flack, J.E.; Post, M.J.; Doğan, A. Short-Stranded Zein Fibers for Muscle Tissue Engineering in Alginate-Based Composite Hydrogels. Gels 2023, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cho, I.; Heo, S.; Han, K.; Baek, Y.-J.; Sim, W.-S.; Jeong, D.-W. Metabolomic insights of cultured meat compared to conventional meat. Sci. Rep. 2025, 15, 15668. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, S.; Choi, B.; Choi, W.; Lee, H.; Lee, J.M.; Lee, S.T.; Yoo, K.H.; Han, D.; Bang, G.; et al. Cultured meat with enriched organoleptic properties by regulating cell differentiation. Nat. Commun. 2024, 15, 77. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, D.; Sun, L.; Ye, X.; Cao, J.; Li, H.; Liu, X. Investigation into the fabrication of plant-based simulant connective tissue utilizing algae polysaccharide-derived hydrogel. Int. J. Biol. Macromol. 2024, 273, 133126. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, K.; Lee, Y.; Yang, K.; Choe, D.; Roh, Y.H. Thermoresponsive semi-interpenetrating gelatin-alginate networks for encapsulation and controlled release of scent molecules. Int. J. Biol. Macromol. 2022, 208, 1096–1105. [Google Scholar] [CrossRef]
- Steinkellner, C.; Ohlmeyer, M.; Franke, K. Effect of Margarine Replacement by a Low-Saturated Fat Bigel on Puff Pastry Quality. Food Bioprocess Technol. 2024, 18, 3981–3992. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zheng, X.; Yang, Y.; Xiao, D.; Zhang, H.; Ai, B.; Sheng, Z. Chitosan inhibits advanced glycation end products formation in chemical models and bakery food. Food Hydrocoll. 2022, 128, 107600. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Liu, Y.; Cui, B. Fabrication of kappa-carrageenan hydrogels with cinnamon essential oil/hydroxypropyl-beta-cyclodextrin composite: Evaluation of physicochemical properties, release kinetics and antimicrobial activity. Int. J. Biol. Macromol. 2021, 170, 593–601. [Google Scholar] [CrossRef]
- Lasta, E.L.; da Silva Pereira Ronning, E.; Dekker, R.F.H.; da Cunha, M.A.A. Encapsulation and dispersion of Lactobacillus acidophilus in a chocolate coating as a strategy for maintaining cell viability in cereal bars. Sci. Rep. 2021, 11, 20550. [Google Scholar] [CrossRef]
- Ren, F.; Kang, R.; Song, T.; Lv, S.; Zhang, H.; Wang, J. Preparation, structural characterization, and functional properties of wheat gluten amyloid fibrils-chitosan double network hydrogel as delivery carriers for ferulic acid. Int. J. Biol. Macromol. 2024, 277, 134282. [Google Scholar] [CrossRef]
- Sahin, O.I.; Uzuner, K.; Dundar, A.N.; Parlak, M.E.; Gul, L.B.; Dagdelen, A.F.; Saricaoglu, F.T.; Simsek, S. Functional properties and bioaccessibility of alginate based phycocyanin-honey hydrogels. LWT 2023, 184, 115099. [Google Scholar] [CrossRef]
- AziziShafa, M.; Akhondzadeh Basti, A.; Sharifan, A.; Khanjari, A. Reformulation of traditional Iranian food (Doeeneh) using probiotics: Bifidobacterium animalis subsp. lactis BB-12, Lactobacillus acidophilus LA-5, Lacticaseibacillus rhamnosus LGG, and inulin and its effect on diabetic and non-diabetic rats. Food Qual. Saf. 2023, 7, fyad028. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Phimolsiripol, Y.; Mousavi Khaneghah, A. A comprehensive review on the utilization of biopolymer hydrogels to encapsulate and protect probiotics in foods. Int. J. Biol. Macromol. 2024, 254, 127907. [Google Scholar] [CrossRef]
- Dwamena, A.K.; Woo, S.H.; Kim, C.S. Enzyme immobilization on porous chitosan hydrogel capsules formed by anionic surfactant gelation. Biotechnol. Lett. 2020, 42, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Salehipour, M.; Rezaei, S.; Yazdani, M.; Mogharabi-Manzari, M. Recent advances in preparation of polymer hydrogel composites and their applications in enzyme immobilization. Polym. Bull. 2022, 80, 5861–5896. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Wang, L.; Wang, B.; Zeng, X.; Ren, B. Functionalized Chitosan and Alginate Composite Hydrogel-Immobilized Laccase with Sustainable Biocatalysts for the Effective Removal of Organic Pollutant Bisphenol A. Catalysts 2024, 14, 304. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Rashidi, M.; Tabibiazar, M.; Mohammadi, M.; Pezeshki, A.; Ghorbani, M. Recent Advances of Macromolecular Hydrogels for Enzyme Immobilization in the Food Products. Adv. Pharm. Bull. 2021, 12, 309–318. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Nada, D.; Radwan, R.A.; Mohamed, S.A.; Gohary, N.A.E.L. Optimization of catalytic properties of Mucor racemosus lipase through immobilization in a biocompatible alginate gelatin hydrogel matrix for free fatty acid production: A sustainable robust biocatalyst for ultrasound-assisted olive oil hydrolysis. 3 Biotech 2022, 12, 285. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, M.; Fang, Y.; Wu, Y.; Liu, Y.; Zhao, Y.; Xu, J. Recent advancements in encapsulation of chitosan-based enzymes and their applications in food industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 11044–11062. [Google Scholar] [CrossRef]
- Tavassoli, M.; Abedi-Firoozjah, R.; Bahramian, B.; Hashemi, M.; Noori, S.M.A.; Oladzadabbasabadi, N.; Nagdalian, A.; Jafari, S.M. Glutaraldehyde cross-linking for improving the techno-functional properties of biopolymeric food packaging films; a comprehensive review. Food Chem. 2025, 478, 143740. [Google Scholar] [CrossRef]
- D′Almeida, A.P.; Gonçalves, L.R.B.; de Albuquerque, T.L.; Fernandez-Lafuente, R.; da Silva, I.J. Alcalase immobilization in iota-carrageenan-matrix hydrogel beads derived from the macroalga Solieria filiformis. Enzym. Microb. Technol. 2025, 188, 110636. [Google Scholar] [CrossRef]
- Ferdiansyah, R.; Abdassah, M.; Zainuddin, A.; Rachmaniar, R.; Chaerunisaa, A.Y. Effects of Alkaline Solvent Type and pH on Solid Physical Properties of Carrageenan from Eucheuma cottonii. Gels 2023, 9, 397. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Zhang, K.; Zeng, J.; Yin, H.; Zheng, W.; Li, R.; Ding, A.; Chen, S.; Liu, Y.; Wu, W.; et al. Immobilization on magnetic PVA/SA@Fe3O4 hydrogel beads enhances the activity and stability of neutral protease. Enzym. Microb. Technol. 2022, 157, 110017. [Google Scholar] [CrossRef]
- Eom, T.; Isanapong, J.; Kumnorkaew, P.; Pornwongthong, P. Cellulase entrapment in calcium alginate hydrogel beads for improved reusability and hydrolysis of cellulosic biomass. Cellulose 2025, 32, 839–852. [Google Scholar] [CrossRef]
- Fraile-Gutierrez, I.; Iglesias, S.; Acosta, N.; Revuelta, J. Chitosan-based oral hydrogel formulations of β-galactosidase to improve enzyme supplementation therapy for lactose intolerance. Int. J. Biol. Macromol. 2024, 255, 127755. [Google Scholar] [CrossRef] [PubMed]
| Polysaccharides | Source/Species | Main Monosaccharide | Backbone | Substitution Position of Sulfate Group | Biological Activity | Structural Features | References |
|---|---|---|---|---|---|---|---|
| Sulfated Fucoidan | Durvillaea antarctica (Brown Alga) | L-Fucose, Xylose, Galactose, Mannose | (1→3)-α-L-Fucose, (1→4)-α-L-Fucose | C-4((1→3)-Fucose), C-2((1→4)-F Fucose), C-6((1→4)-Galactose) | Immunomodulatory activity (macrophage/NK cell activation, lymphocyte↑, NO↑) | Highly sulfated (>20%), no glucose, terminal residues (β-D-Xylp-(1→) and (β-D-Galp-(1→) | [68] |
| Sulfated Fucoidan | Fucus vesiculosus (Brown Alga) | L-Fucose | α-(1→3)-L-Fucose, α-(1→4)-L-Fucose | C-2/C-4 positions of Fucose | Anti-ovarian cancer activity (sub-G1 phase↓, caspase-3/9↑, PI3K/Akt↓, MAPK↓, Anti-angiogenesis) | Highly sulfated | [43] |
| Sulfated Fucoidan | Laminaria japonica (kelp) | L-Fucose, D-Galactose, D-Mannose | — | C-4 position of Fucose | Anti-obesity activity (pancreatic lipase↓) | Highly sulfated (>25%), negatively charged, high digestive resistanc | [5] |
| Laminarin | Laminaria digitata | D-Glucose | β-(1→3)-D-Glucos, β-(1→6)-linked glucose branches | Anti-ovarian cancer activity (cell cycle arrest, mitochondrial function, apoptosis↑, Ca2+ homeostasis, PI3K/Akt↓, MAPK↓) | LMW, single monosaccharide, lacking modified groups | [45] | |
| Sulfated Galactan, | Gracilaria fisheri (Red Alga) | D-Galactose, D-Glucose, D-Xylose | 1,3-β-D-Galactopyranose, 1,4-α-L-anhydrogalactopyranose | C-6 position of 1,4-α-L-galactose | Immunomodulatory activity (Pro-Inflammatory Cytokine↑, iNOS↑, Dectin-1↑, Macrophage activity↑) | High galactose purity, highly sulfated, HMW, O-methylation | [35] |
| Enteromorpha prolifera | Rhamnose, Glucuronic acid | α- and β-(1,4)-linked monosaccharides | C-3 position of rhamnose | Immunomodulatory activity (pro-inflammatory cytokines, Caecal microbiota modulation) | LMW | [38] | |
| Patinopecten yessoensis (Scallop) | — | — | — | Immunostimulatory activity (Immunoglobulin↑) Bacteroides/Firmicutes↑, TLR↑) | No sulfate groups, not hydrolyzed by human digestive enzymes, prebiotic function | [48] | |
| Sulfated Polysaccharide | Sargassum fulvellum (Brown Alga) | L-Fucose, D-Galactose, D-Galactose, D-Xylose | — | — | Anti-inflammatory activity (iNOS↓, COX-2↓, Pro-inflammatory cytokines↓) | Moderately sulfated (>1%) | [8] |
| Ulvan | Ulva linza (Green Alga) | L-Rhamnose, D-Glucuronic Acid | β-D-GluA-(1→4)-α-L-Rhamnose, α-L-IdoA-(1→4)-α-L-Rha3S | C-3 of L-Rhamnose | Antioxidant activity (DPPH↓, ABTS↓, ROS↓, Microbiota Modulation, SCFAs↑) | High-content rhamnose and glucuronic acid, HMW | [69] |
| Extracellular Polysaccharide | Limosilactobacillus | Glucose, Mannose, Galactose | — | — | Antioxidant activity (DPPH↓) Anti-inflammatory Activity (iNOS↓, COX-2↓) Immunomodulatory activity (NO↑, IL-6↑, TNF-α↑) | LMW, minor glucuronic acid and galacturonic acid | [70] |
| Sulfated Chondroitin | Phyllophorella kohkutiensis (Sea Cucumber) | D-Glucuronic Acid, D-Galactose, D-Glucosamine, N-Acetylgalactosamine, L-Fucose | β-D-Glucuronic Acid-(1→3)-β-D-N-Acetylgalactosamine | C-4 position of Fucose, C-2 /C-4 position of Fucose, C-6 position of N-Acetylgalactosamine | Antioxidant activity (DPPH↓, ABTS↓, FRAR↑) | High-content fucose, moderate sulfated (>10%) | [71] |
| Aminopolysaccharide | Agelas aff. Nemoechinata | Mannose, N-Acetylglucosamine, N-Acetylgalactosamine, Galactose, Fucose | α-(1→2)-Mannose/α-(1→6)-N-Acetylgalactosamine | — | Anti-liver cancer activity (MAPK/mTOR/TNF/Hippo, umor angiogenesis↓, Mitochondrial apoptosis pathway↑, Migration and invasion↓) | HMW, high-Content aminoglycoside, multi-type sidechains | |
| Oxidized Fucoidan | Brown algal | Fucose | (1→3)-α-Fucose, (1→4)-α--Fucose | Antioxidant activity (ROS↓, Pro-inflammatory cytokines↓) | Aldehyde group | [72] | |
| Carboxymethyl Chitosan | Crustaceans | Glucosamine | β-(1→4)-Glucosamine | Mucosal adhesion↑ | C-6 hydroxylation or amination with carboxymethylation | ||
| Sulfated Galactan | Botryocladia occidentalis | D-Galactose, 3, 6-Anhydrogalactose | α-(1→4)-galactose, β-(1→3) galactose | C-2 or C4 positions of Galactose | Antioxidant activity (binding to heparin cofactor II) | Low sulfation, methylation | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Xie, J.; Mei, J. Recent Advances in Marine-Derived Polysaccharide Hydrogels: Innovative Applications and Challenges in Emerging Food Fields. Polymers 2025, 17, 2553. https://doi.org/10.3390/polym17182553
Yi X, Xie J, Mei J. Recent Advances in Marine-Derived Polysaccharide Hydrogels: Innovative Applications and Challenges in Emerging Food Fields. Polymers. 2025; 17(18):2553. https://doi.org/10.3390/polym17182553
Chicago/Turabian StyleYi, Xinge, Jing Xie, and Jun Mei. 2025. "Recent Advances in Marine-Derived Polysaccharide Hydrogels: Innovative Applications and Challenges in Emerging Food Fields" Polymers 17, no. 18: 2553. https://doi.org/10.3390/polym17182553
APA StyleYi, X., Xie, J., & Mei, J. (2025). Recent Advances in Marine-Derived Polysaccharide Hydrogels: Innovative Applications and Challenges in Emerging Food Fields. Polymers, 17(18), 2553. https://doi.org/10.3390/polym17182553








