A Comparison Study on Polysaccharides Extracted from Citrus reticulata Blanco cv. Tankan Peel Using Five Different Methods: Structural Characterization and Immunological Competence
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
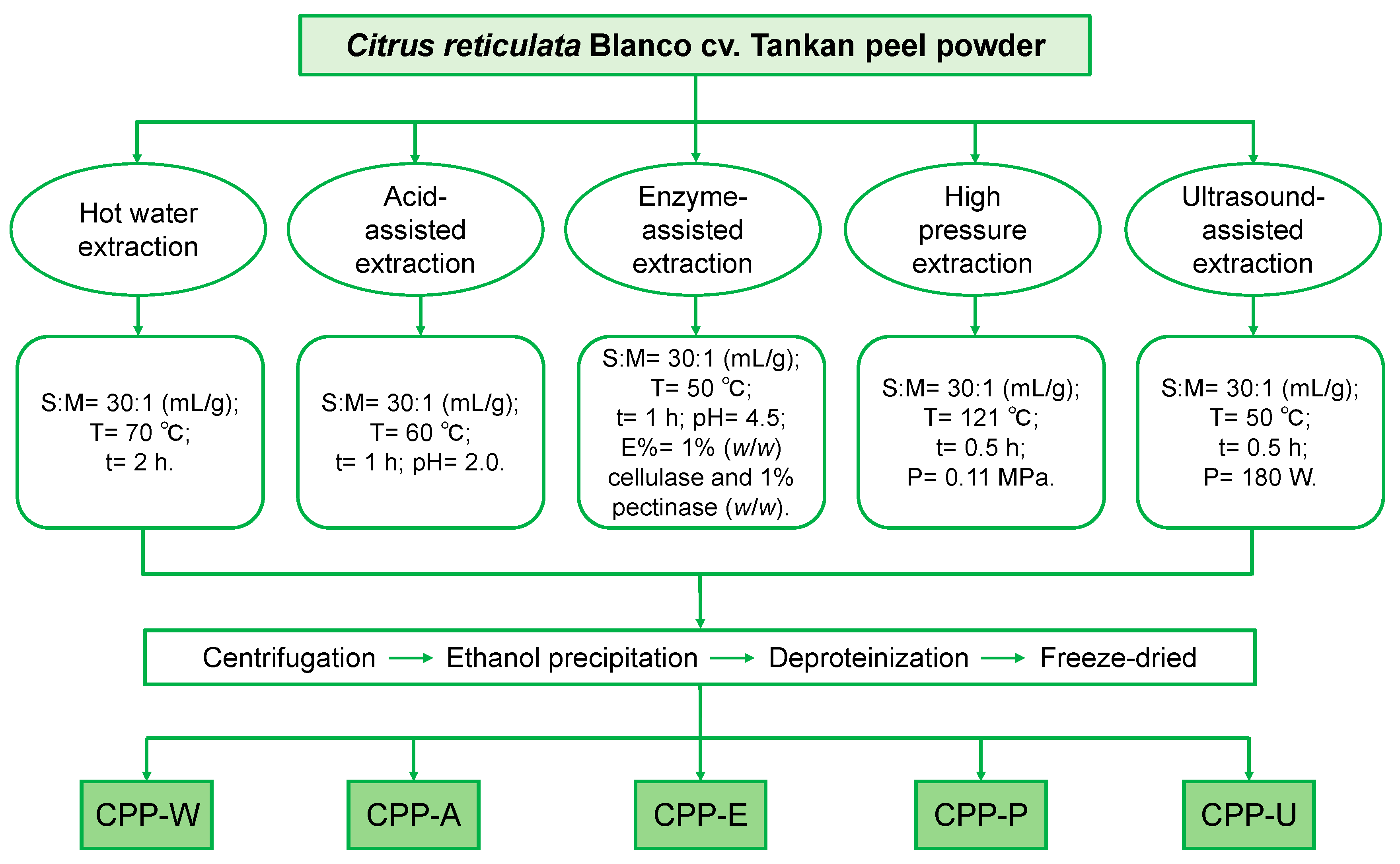
2.2. Preparation of Five Different CPPs
2.3. General Chemical Properties
2.4. Molecular Weight Analysis
2.5. Monosaccharide Composition Analysis
2.6. Molecular Morphology Observation
2.7. Fourier Transform Infrared Spectrometer (FT-IR) Analysis
2.8. Nuclear Magnetic Resonance (NMR) Analysis
2.9. Cell Viability Analysis
2.10. Immune Agents Determination
2.11. Western Blot
2.12. Statistical Analysis
3. Results and Discussion
3.1. Comparative Analysis of Polysaccharide Yield Among Five Different Methods
3.2. Physical–Chemical Characteristics of the CPPs Extracted by Five Different Methods
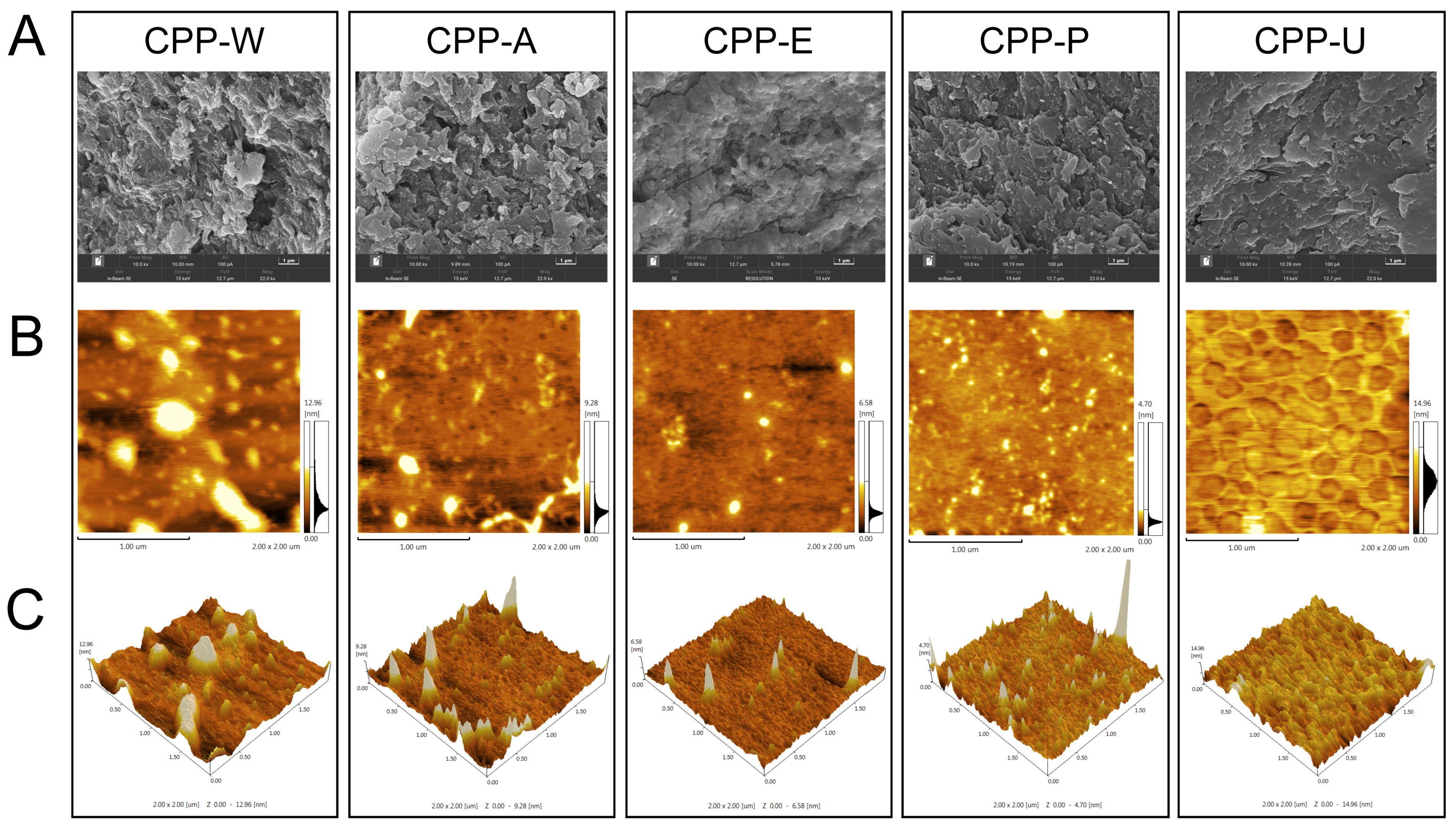
3.3. Surface Morphology of the CPPs Extracted by Five Different Methods
3.4. Monosaccharide Compositions of the CPPs Extracted by Five Different Methods
3.5. FT-IR Spectroscopy of the CPPs Extracted by Five Different Methods
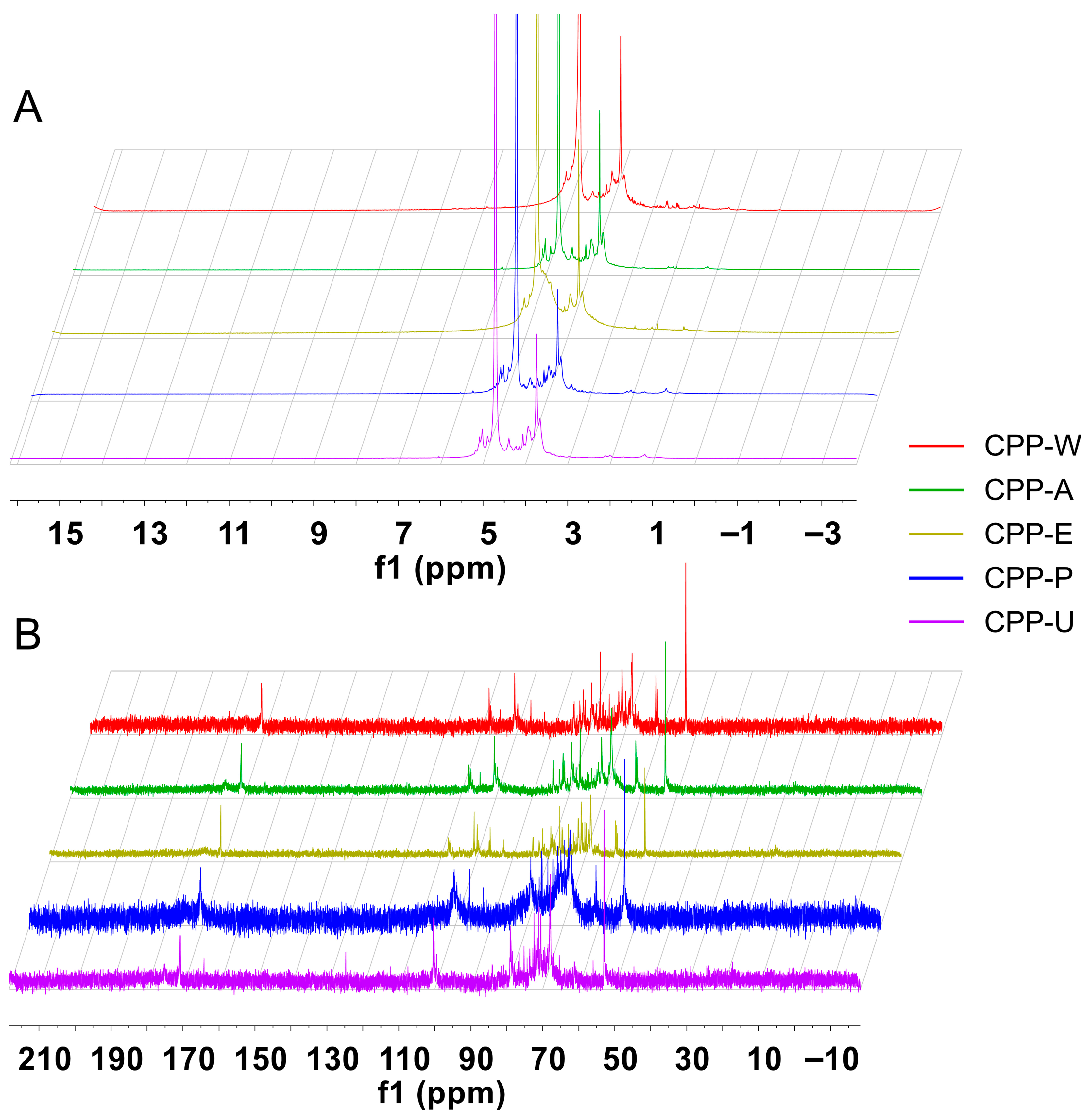
3.6. NMR Spectra of the CPPs Extracted by Five Different Methods
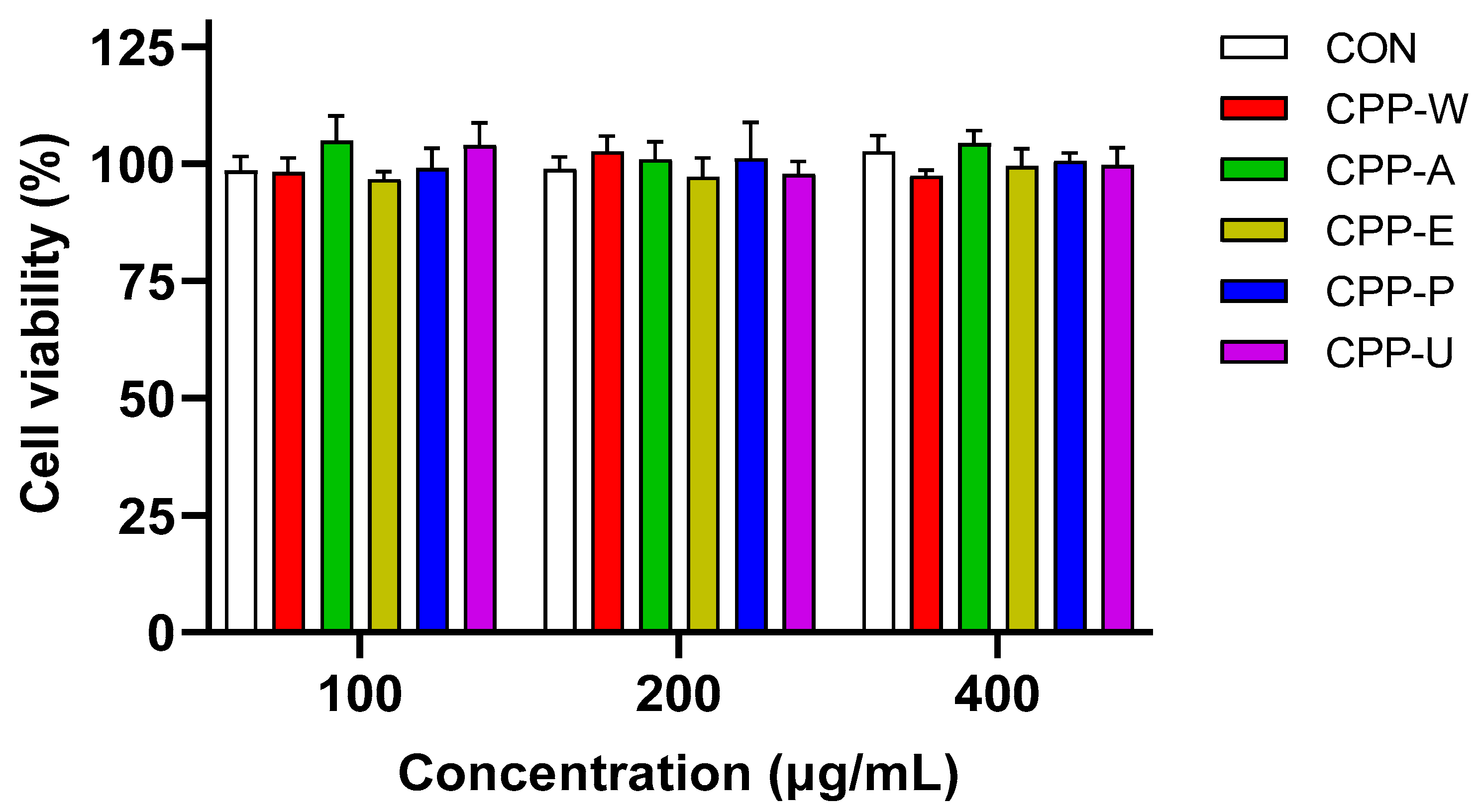
3.7. Effects of the CPPs Extracted by Five Different Methods on RAW264.7 Cell Viability
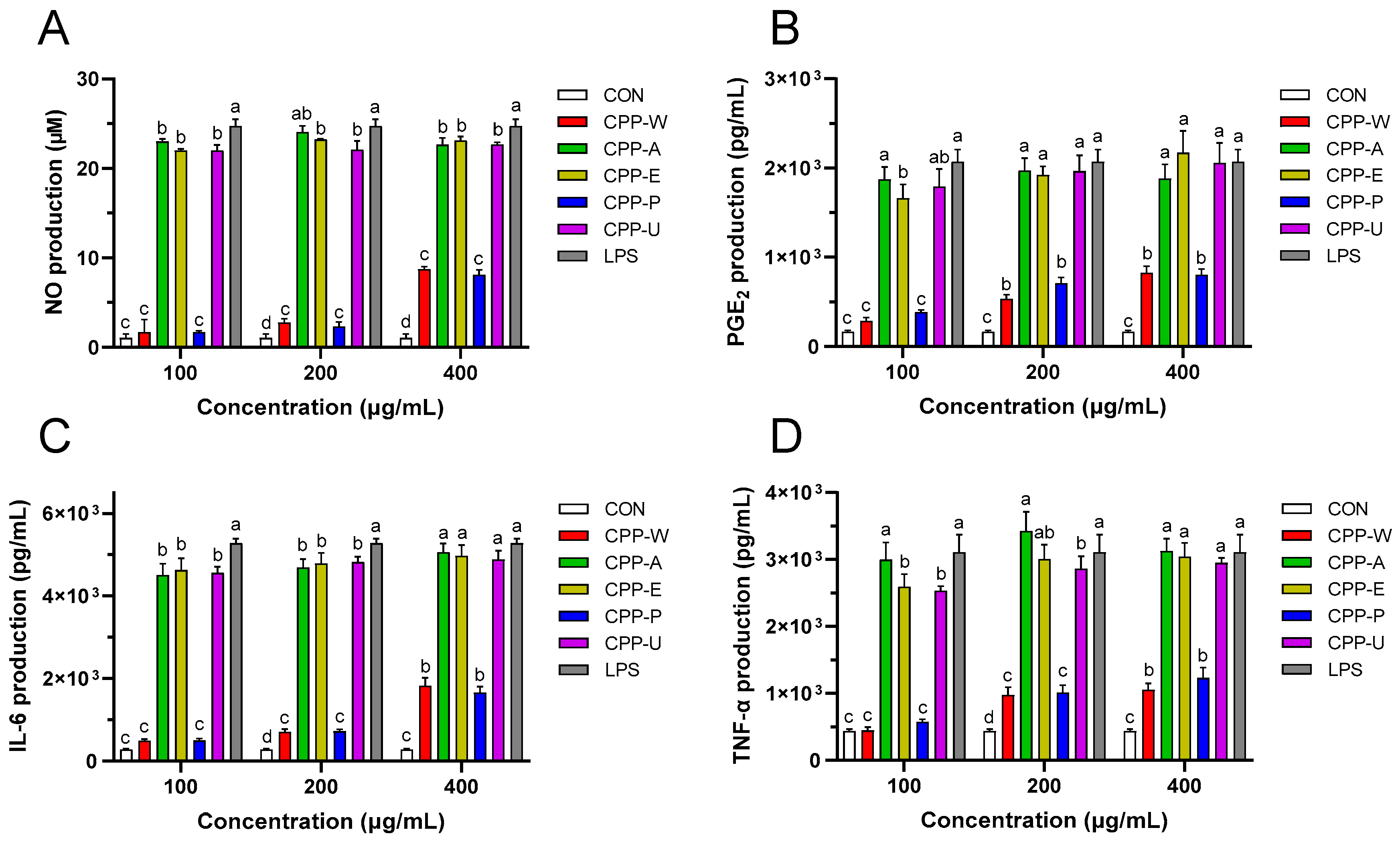
3.8. Effects of the CPPs Extracted by Five Different Methods on Immune Agents in RAW264.7 Cells
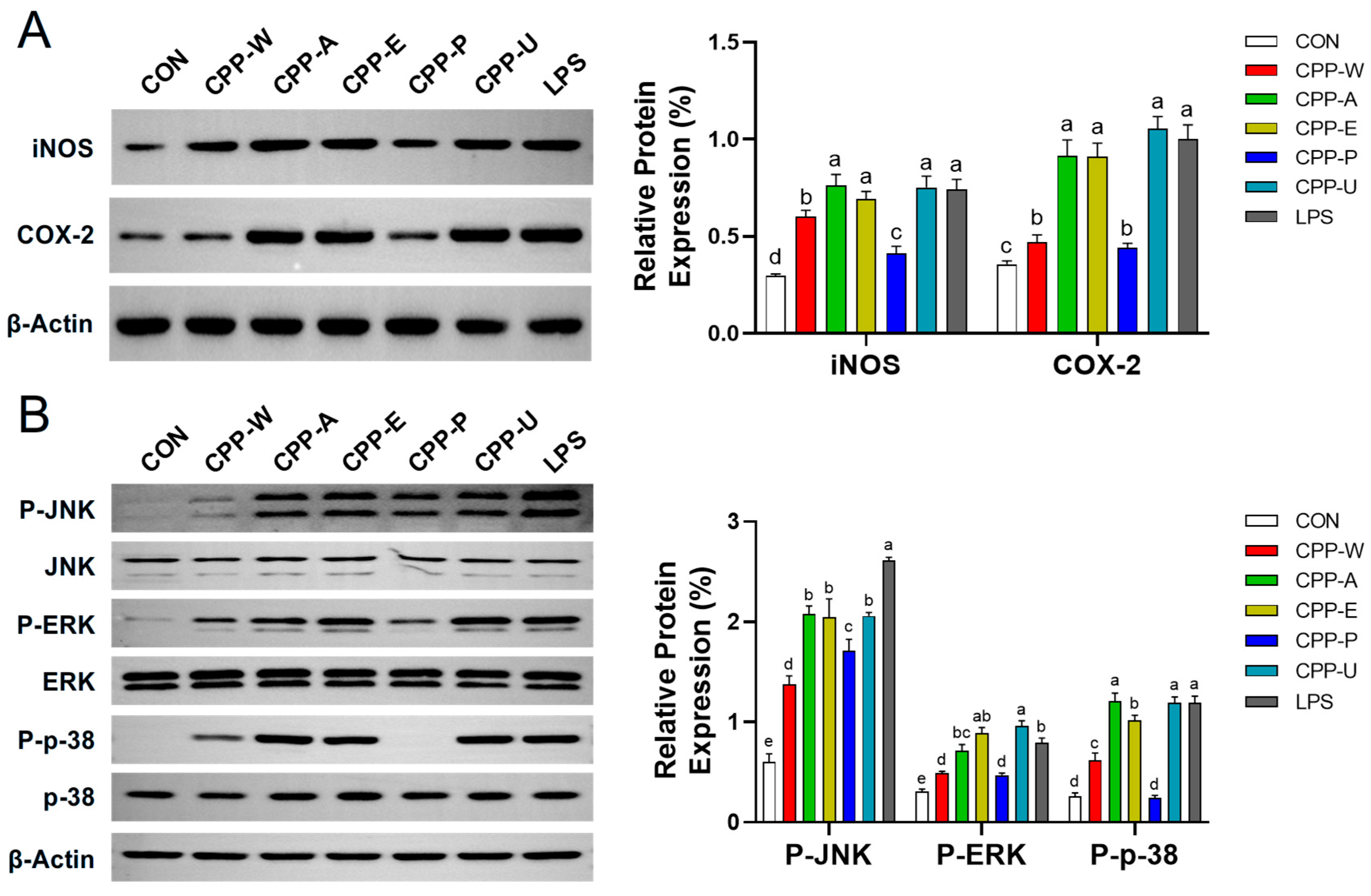
3.9. Effects of the CPPs Extracted by Five Different Methods on Immune-Specific Protein Expression in RAW264.7 Cells
3.10. Effects of the CPPs Extracted by Five Different Methods on MAPK Pathway in RAW264.7 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Zhang, H.; Du, W.; Qian, L.; Xu, Y.; Huang, Y.; Xiong, Q.; Li, H.; Yuan, J. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge. Int. J. Biol. Macromol. 2020, 150, 1011–1019. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yin, Y.; Xiao, S.; Zhang, R.; Yang, Y.; Li, L.; Xu, H.; Zhang, X.; Hu, P. Chemical structure elucidation and functional activities comparison of two polysaccharides purified from Citrus reticulata Blanco peels. Chem. Biol. Technol. Agric. 2024, 11, 37. [Google Scholar] [CrossRef]
- Nair, S.A.; Sr, R.K.; Nair, A.S.; Baby, S. Citrus peels prevent cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Liu, M.; Chen, X.; Huang, F.; Li, G.; Wu, Y. Study on chemical composition, antimicrobial activity and antioxidant ability of essential oil from Chaozhou citrus peel. J. Hanshan Norm. Univ. 2014, 35, 61–67. [Google Scholar]
- Liang, X.; Liang, G.; Qiu, Y.; Xiao, G.; Wang, Q.; Peng, J. Effects of Citrus reticulate Blanco cv. Bonkan peels polysaccharides on set yogurt quality during refrigerated storage life. Food Res. Dev. 2024, 45, 60–66. [Google Scholar]
- Tang, W.; Liu, D.; Yin, J.; Nie, S. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Song, T.; Xu, Z.; Wang, M. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. Food Chem. 2024, 445, 138752. [Google Scholar] [CrossRef]
- Geng, X.; Guo, D.; Wu, B.; Wang, W.; Zhang, D.; Hou, S.; Bau, T.; Lei, J.; Xu, L.; Cheng, Y.; et al. Effects of different extraction methods on the physico-chemical characteristics and biological activities of polysaccharides from Clitocybe squamulosa. Int. J. Biol. Macromol. 2024, 259, 129234. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Ruan, Y.; Wen, C.; Ge, M.; Qian, Y.; Ma, B. Physicochemical properties and biological activities of polysaccharides from the peel of Dioscorea opposita Thunb. extracted by four different methods. Food Sci. Hum. Wellness 2023, 12, 130–139. [Google Scholar] [CrossRef]
- Xu, G.; Liao, A.; Huang, J.; Zhang, J.; Thakur, K.; Wei, Z. Evaluation of structural, functional, and anti-oxidant potential of differentially extracted polysaccharides from potatoes peels. Int. J. Biol. Macromol. 2019, 129, 778–785. [Google Scholar] [CrossRef]
- Yan, J.; Chen, T.; Wang, Z.W.; Wang, C.; Liu, C.; Li, L. Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chem. 2022, 389, 133083. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.; Liu, W.; Su, Y.; Han, Q.; Zhao, L.; Zhang, Q.; Lin, D.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Shang, H.; Chen, S.; Li, R.; Zhou, H.; Wu, H.; Song, H. Influences of extraction methods on physicochemical characteristics and activities of Astragalus cicer L. polysaccharides. Process Biochem. 2018, 73, 220–227. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; You, L.; Fu, X. Chemical property and impacts of different polysaccharide fractions from Fructus Mori. on lipolysis with digestion model in vitro. Carbohydr. Polym. 2017, 178, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Z.; Wang, H.; Meng, Y.; Sun, Y.; Yi, Y. Study on the structure and lipid-lowering activity of different components of lotus root polysaccharides. Food Res. Int. 2025, 203, 115801. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhu, J.; Liu, T.; Bi, S.; Hu, X.; Chen, Z.; Song, L.; Lv, W.; Yu, R. Structural characterization and biological activities of a novel polysaccharide from cultured Cordyceps militaris and Its sulfated derivative. J. Agric. Food Chem. 2015, 63, 3464–3471. [Google Scholar] [CrossRef]
- Peng, J.; Wen, W.; Liang, G.; Huang, W.; Qiu, Z.; Wang, Q.; Xiao, G. Camellia oleifera shells polyphenols inhibit advanced glycation end-products (AGEs) formation and AGEs-induced inflammatory response in RAW264.7 macrophages. Ind. Crops Prod. 2023, 197, 116589. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef]
- Bai, C.; Chen, R.; Chen, Y.; Bai, H.; Sun, H.; Li, D.; Wu, W.; Wang, Y.; Gong, M. Plant polysaccharides extracted by high pressure: A review on yields, physicochemical, structure properties, and bioactivities. Int. J. Biol. Macromol. 2024, 263, 129939. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Fraeye, I.; Duvetter, T.; Verlent, I.; Ndaka Sila, D.; Hendrickx, M.; Van Loey, A. Comparison of enzymatic de-esterification of strawberry and apple pectin at elevated pressure by fungal pectinmethylesterase. Innov. Food Sci. Emerg. Technol. 2007, 8, 93–101. [Google Scholar] [CrossRef]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Liu, X.; Liu, J.; Fan, W.; Li, Y.; et al. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason. Sonochem. 2019, 51, 249–257. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, B.; Xu, Y.; Yang, J.; Song, L.; Bi, S.; Chen, Y.; Zhu, J.; Wen, Y.; Yu, R. Structural characterization and immunoregulatory activity of a new polysaccharide from Citrus medica L. var. sarcodactylis. RSC Adv. 2019, 9, 6603–6612. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, F.; Zhang, R.; Liu, F.; Peng, Q.; Wang, M. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: Purification and structural characterization. Food Chem. 2019, 274, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ji, X.; Wang, M.; Yin, S.; Peng, Q. An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao: Structural characterizations and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 607–615. [Google Scholar] [CrossRef]
- Chen, C.; Shao, Y.; Tao, Y.; Wen, H. Optimization of dynamic microwave-assisted extraction of Armillaria polysaccharides using RSM, and their biological activity. LWT-Food Sci. Technol. 2015, 64, 1263–1269. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Tian, C.; Luo, J.; Zheng, C.; Zhan, J. Polysaccharide extraction from Sphallerocarpus gracilis roots by response surface methodology. Int. J. Biol. Macromol. 2016, 88, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Chen, B.; Hou, Y.; Wen, Y.; Gan, L.; Jin, J.; Li, C.; Wu, P.; Li, D.; et al. Ultrasonic assisted extraction, characterization and gut microbiota-dependent anti-obesity effect of polysaccharide from Pericarpium Citri Reticulatae ‘Chachiensis’. Ultrason. Sonochem. 2023, 95, 106383. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wei, Z.; He, X.; Ling, D.; Qin, M.; Yi, P.; Liu, G.; Li, L.; Li, C.; Sun, J. A comparison study on polysaccharides extracted from banana flower using different methods: Physicochemical characterization, and antioxidant and antihyperglycemic activities. Int. J. Biol. Macromol. 2024, 264, 130459. [Google Scholar] [CrossRef]
- Jiang, J.; Kong, F.; Li, N.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Liang, L.; Ao, L.; Ma, T.; Ni, Y.; Liao, X.; Hu, X.; Song, Y. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018, 106, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lai, M.; Ng, L. Effects of different extraction temperatures on the physicochemical properties of bioactive polysaccharides from Grifola frondosa. Food Chem. 2017, 220, 400–405. [Google Scholar] [CrossRef]
- Liao, Z.; Zeng, R.; Hu, L.; Maffucci, K.G.; Qu, Y. Polysaccharides from tubers of Bletilla striata: Physicochemical characterization, formulation of buccoadhesive wafers and preliminary study on treating oral ulcer. Int. J. Biol. Macromol. 2019, 122, 1035–1045. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Huang, Q.; You, L.; Liu, R.; Zhao, M.; Fu, X.; Luo, Z. A comparison study on polysaccharides extracted from Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, J.; Yin, J.; Nie, S.; Xie, M. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, H.; Wang, Z. Ultrasonic degradation of Polysaccharides from Auricularia auricula and the antioxidant activity of their degradation products. LWT 2019, 113, 108266. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Ma, L.; Liu, A. Polysaccharides from the peels of Citrus aurantifolia induce apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2017, 101, 680–689. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Shu, X.; Du, H.; Li, N.; Wang, J. Extraction, characterization and immunological activity of polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Lei, L.; Li, F.; Tang, Y.; Yuan, Y.; Zhang, Y.; Wu, S.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264.7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Yu, J.; Hu, M.; Wang, Y.; Zhang, Q.; Xu, W.; Su, W. Extraction, partial characterization and bioactivity of polysaccharides from Senecio scandens Buch.-Ham. Int. J. Biol. Macromol. 2018, 109, 535–543. [Google Scholar] [CrossRef]
- Foley, E.; O’Farrell, P.H. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev. 2003, 17, 115–125. [Google Scholar] [CrossRef]
- Martínez-Colón, G.J.; Moore, B.B. Prostaglandin E2 as a regulator of immunity to pathogens. Pharmacol. Ther. 2018, 185, 135–146. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting cytokines in inflammatory bowel disease. Sci. Transl. Med. 2022, 14, eabq4473. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhou, P.; Wang, X.; Zhao, R.; Wang, Y. Comparative characterization and immunomodulatory activities of polysaccharides extracted from the Radix of Platycodon grandiflorum with different extraction methods. Molecules 2022, 27, 4759. [Google Scholar] [CrossRef]
- Li, K.; Cui, L.; Cao, Y.; Li, S.; Shi, L.; Qin, X.; Du, Y. UHPLC Q-exactive MS-based serum metabolomics to explore the effect mechanisms of immunological activity of Astragalus polysaccharides with different molecular weights. Front. Pharmacol. 2020, 11, 595692. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 1, 78. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mo, J.; Xiang, W.; Shi, X.; Guo, L.; Li, Y.; Bao, Y.; Zheng, L. Immunoregulatory effects of Tetrastigma hemsleyanum polysaccharide via TLR4-mediated NF-κB and MAPK signaling pathways in Raw264.7 macrophages. Biomed. Pharmacother. 2023, 161, 114471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases—Regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; Cui, L.; Zhang, Y.; Waterhouse, G.I.N.; Sun-Waterhouse, D.; Ma, C.; Kang, W. Flammulina velutipes polysaccharide exerts immunomodulatory function involving RSAD2 to regulate the NF-κB/MAPK signaling pathway in RAW264.7 macrophage cells and in mouse spleen cells. Int. J. Biol. Macromol. 2025, 309, 142985. [Google Scholar] [CrossRef]

| Samples | CPP-W | CPP-A | CPP-E | CPP-P | CPP-U |
|---|---|---|---|---|---|
| Polysaccharide yield (%) | 2.13 ± 0.02 e | 3.66 ± 0.05 d | 9.34 ± 0.02 b | 11.75 ± 0.05 a | 4.88 ± 0.03 c |
| Sulfuric radical (%) | 4.20 ± 0.03 d | 1.64 ± 0.01 e | 4.57 ± 0.03 c | 6.28 ± 0.03 a | 5.85 ± 0.01 b |
| Uronic acid (%) | 2.88 ± 0.18 e | 6.91 ± 0.05 c | 7.52 ± 0.29 b | 11.65 ± 0.26 a | 5.18 ± 0.26 d |
| Protein (%) | 1.02 ± 0.02 b | 0.71 ± 0.03 c | 1.00 ± 0.02 b | 1.48 ± 0.04 a | 1.49 ± 0.00 a |
| Mw (kDa) | 5908 | 1788 | 5264 | 11,267 | 4535 |
| Wavenumber (cm−1) | Assignment | Vibration Mode | References |
|---|---|---|---|
| 3351 | O-H | Stretching vibration | [31] |
| 2930 | C-H | Stretching vibration | [31] |
| 1733 | C=O, methyl-esterified | Stretching vibration | [32] |
| 1592 | C=O, galacturonic acid | Stretching vibration | [33] |
| 1230 | S=O, sulfate | Asymmetric stretching vibration | [33] |
| 1090 | pyranose ring/C-O-C | Stretching vibration | [34] |
| 896 | α-glycosidic linkage | Deformation vibration | [34] |
| 830 | α-pyranose | Ring vibration | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Chen, G.; Lin, Z.; Guo, S.; Zeng, Y.; Wang, Q.; Yang, W.; Li, J. A Comparison Study on Polysaccharides Extracted from Citrus reticulata Blanco cv. Tankan Peel Using Five Different Methods: Structural Characterization and Immunological Competence. Polymers 2025, 17, 2554. https://doi.org/10.3390/polym17182554
Peng J, Chen G, Lin Z, Guo S, Zeng Y, Wang Q, Yang W, Li J. A Comparison Study on Polysaccharides Extracted from Citrus reticulata Blanco cv. Tankan Peel Using Five Different Methods: Structural Characterization and Immunological Competence. Polymers. 2025; 17(18):2554. https://doi.org/10.3390/polym17182554
Chicago/Turabian StylePeng, Jinming, Guangwei Chen, Ziyuan Lin, Shaoxin Guo, Yue Zeng, Qin Wang, Wenhua Yang, and Jun Li. 2025. "A Comparison Study on Polysaccharides Extracted from Citrus reticulata Blanco cv. Tankan Peel Using Five Different Methods: Structural Characterization and Immunological Competence" Polymers 17, no. 18: 2554. https://doi.org/10.3390/polym17182554
APA StylePeng, J., Chen, G., Lin, Z., Guo, S., Zeng, Y., Wang, Q., Yang, W., & Li, J. (2025). A Comparison Study on Polysaccharides Extracted from Citrus reticulata Blanco cv. Tankan Peel Using Five Different Methods: Structural Characterization and Immunological Competence. Polymers, 17(18), 2554. https://doi.org/10.3390/polym17182554






