Integrating Photon-Based Techniques to Probe Structural and Phonon Dynamics in Bacterial Cellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scanning Electron Microscopy
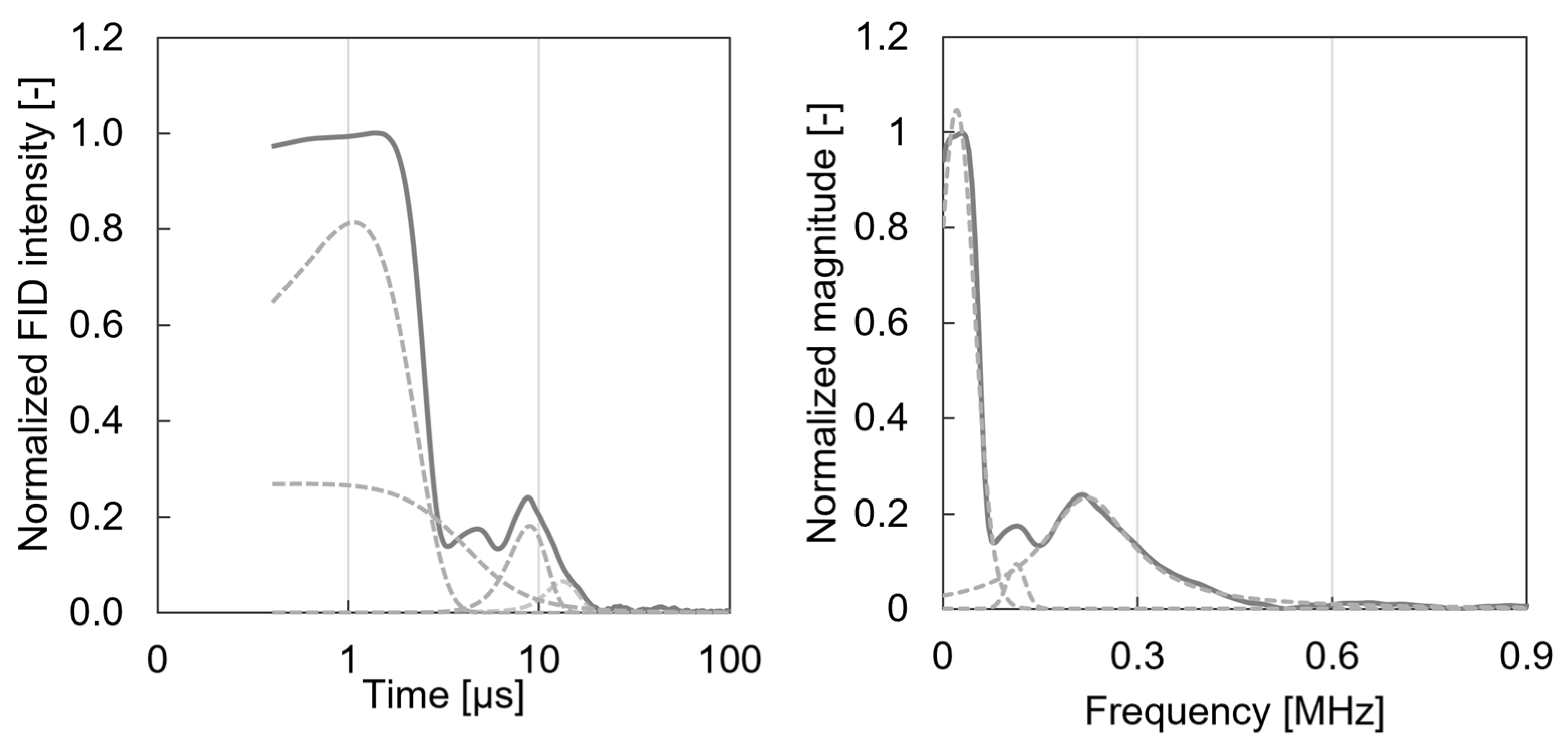
2.2.2. Solid-State Low-Field 1H-NMR Measurements
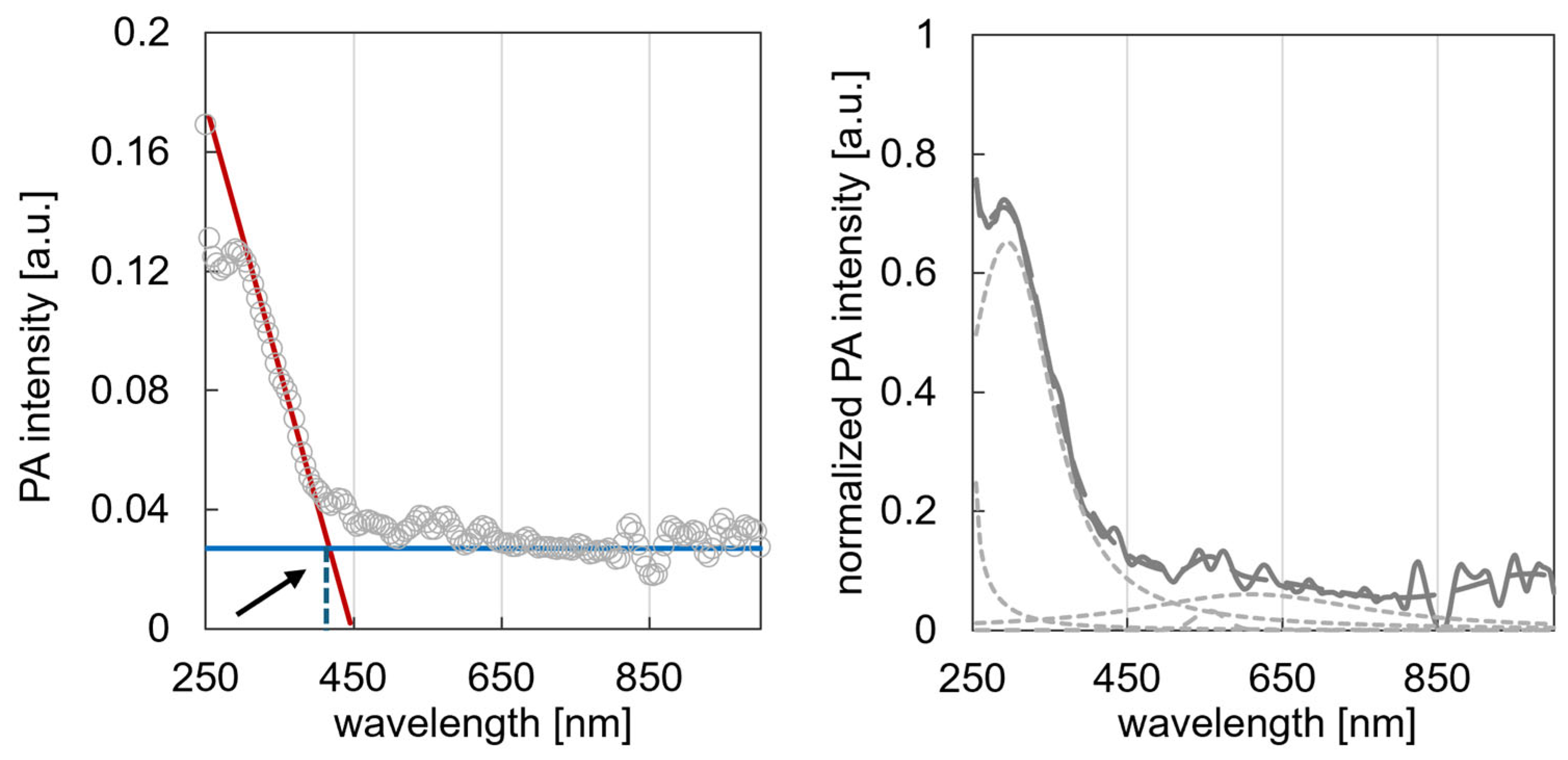
2.2.3. Photoacoustic Spectroscopy
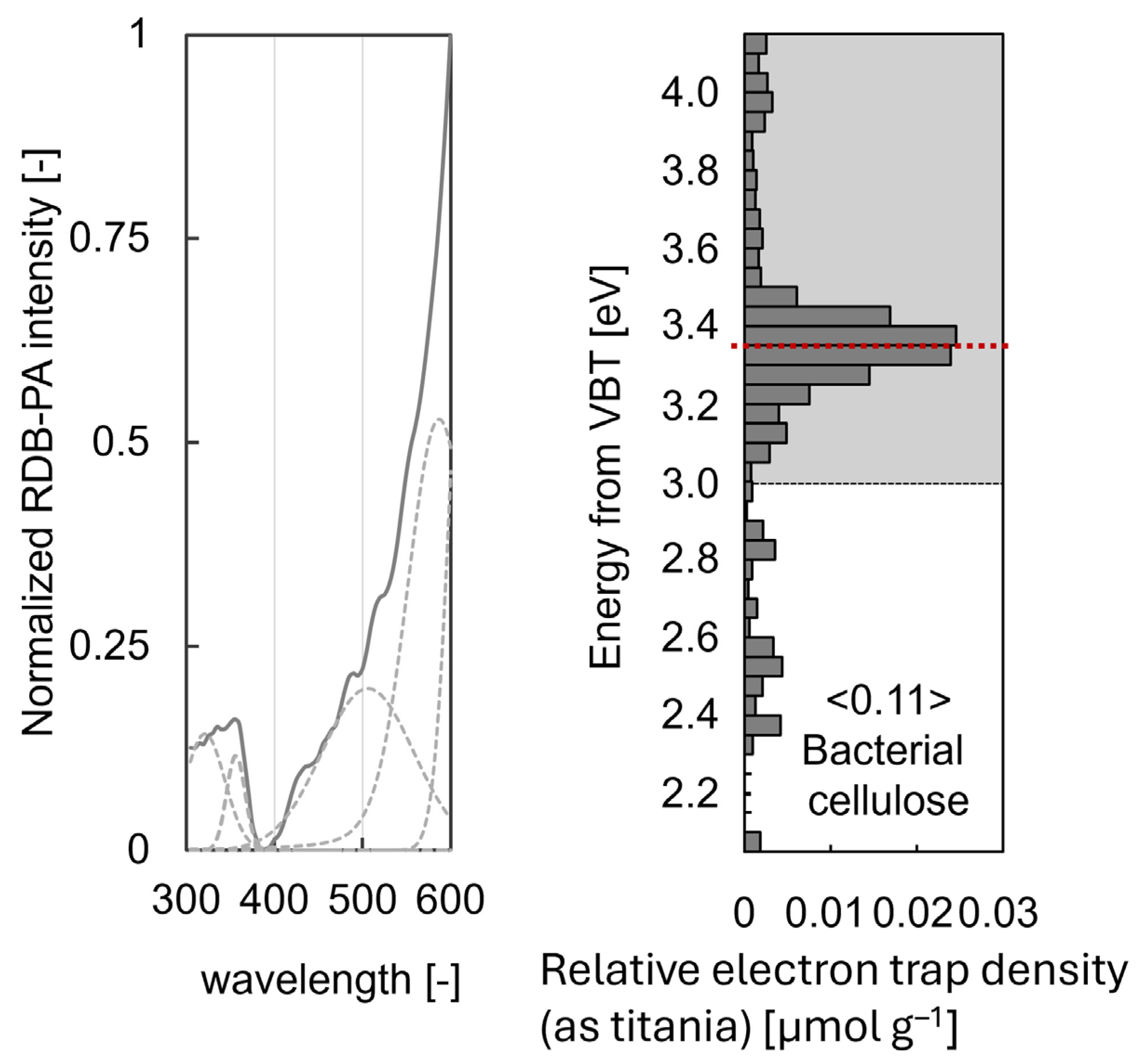
2.2.4. Reversed Double-Beam Photoacoustic Spectroscopy
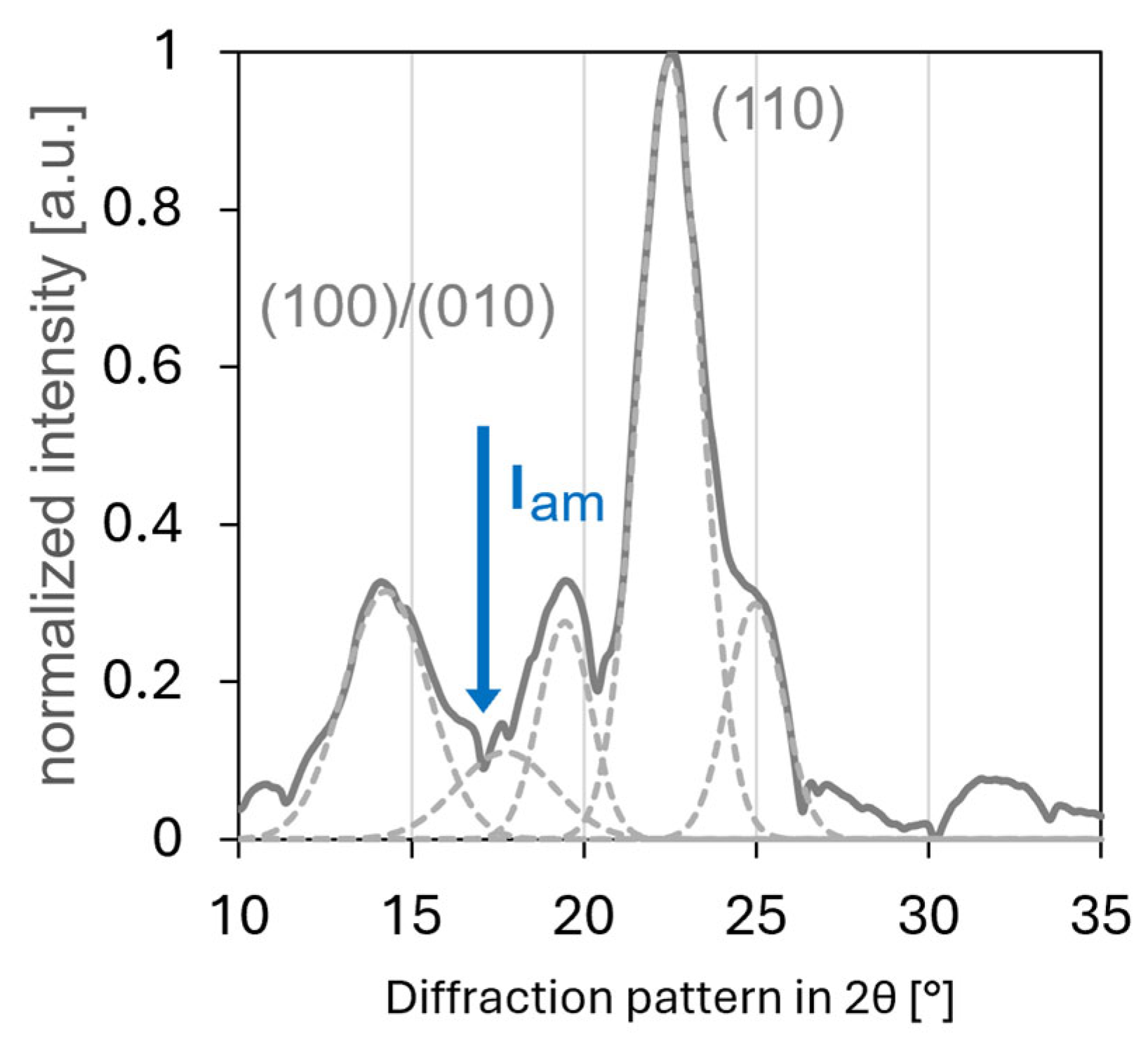
2.2.5. X-Ray Diffraction
3. Results
3.1. SEM Characterisation
3.2. Solid-State Low-Field 1H-NMR Characterisation
3.3. Photoacoustic Characterisation
3.4. RDB-PAS Characterisation
3.5. XRD Characterisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nugroho, D.A.; Sutiarso, L.; Rahayu, E.S.; Masithoh, R.E. Utilizing Real-Time Image Processing for Monitoring Bacterial Cellulose Formation During Fermentation. Agritech 2020, 40, 118–123. [Google Scholar] [CrossRef]
- Hornung, M.; Biener, R.; Schmauder, H.P. Dynamic Modelling of Bacterial Cellulose Formation. Eng. Life Sci. 2009, 9, 342–347. [Google Scholar] [CrossRef]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic Aspects of Bacterial Cellulose Formation in Nata-de-Coco Culture System. Carbohydr. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Muthu, S.S.; Rathinamoorthy, R. Characteristics of Bacterial Cellulose: Textile and Fashion Perspective. In Bacterial Cellulose; Springer: Singapore, 2021. [Google Scholar]
- Sathish, S.K.; Vitta, S. Bacterial Cellulose Based Nanocomposites for Electronic and Energy Applications. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1–5. [Google Scholar]
- Qiu, Z.; Han, Y.; Noori, K.; Chen, Z.; Kashchenko, M.; Lin, L.; Olsen, T.; Li, J.; Fang, H.; Lyu, P.; et al. Evidence for Electron–Hole Crystals in a Mott Insulator. Nat. Mater. 2024, 23, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Csóka, L.; Hosakun, W.; Kolonics, O.; Ohtani, B. Reversed Double-Beam Photoacoustic Spectroscopic Analysis of Photoinduced Change in Absorption of Cellulose Fibres. Sci. Rep. 2022, 12, 14475. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.R. On the Mechanism by Which the Photon Becomes Massive. Nucl. Phys. B 1976, 102, 309–316. [Google Scholar] [CrossRef]
- Alducin, M.; Díez Muiño, R.; Juaristi, J.I. Nonadiabatic Effects in Gas-Surface Dynamics. In Springer Handbook of Surface Science; Rocca, M., Rahman, T.S., Vattuone, L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 929–965. ISBN 978-3-030-46906-1. [Google Scholar]
- Li, Y.; Lin, M.; Davenport, J.W. Ab Initio Studies of Cellulose I: Crystal Structure, Intermolecular Forces, and Interactions with Water. J. Phys. Chem. C 2011, 115, 11533–11539. [Google Scholar] [CrossRef]
- Zhang, M.; Geng, Z.; Yu, Y. Density Functional Theory (DFT) Study on the Dehydration of Cellulose. Energy Fuels 2011, 25, 2664–2670. [Google Scholar] [CrossRef]
- Srivastava, D.; Kuklin, M.S.; Ahopelto, J.; Karttunen, A.J. Electronic Band Structures of Pristine and Chemically Modified Cellulose Allomorphs. Carbohydr. Polym. 2020, 243, 116440. [Google Scholar] [CrossRef]
- Haensel, T.; Reinmöller, M.; Lorenz, P.; Beenken, W.J.D.; Krischok, S.; Ahmed, S.I.U. Valence Band Structure of Cellulose and Lignin Studied by XPS and DFT. Cellulose 2012, 19, 1005–1011. [Google Scholar] [CrossRef]
- Shen, F.; Zhou, Y.; Ma, J.; Zheng, J.; Wang, J.; Chen, Z.; Xu, J. Tunable Kerker Scattering in a Self-Coupled Polaritonic Metasurface. Laser Photonics Rev. 2024, 18, 2300584. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Bacterial Cellulose: Recent Progress in Production and Industrial Applications. World J. Microbiol. Biotechnol. 2022, 38, 86. [Google Scholar] [CrossRef]
- Focher, B.; Palma, M.T.; Canetti, M.; Torri, G.; Cosentino, C.; Gastaldi, G. Structural Differences between Non-Wood Plant Celluloses: Evidence from Solid State NMR, Vibrational Spectroscopy and X-Ray Diffractometry. Ind. Crops Prod. 2001, 13, 193–208. [Google Scholar] [CrossRef]
- Froix, M.F.; Nelson, R. The Interaction of Water with Cellulose from Nuclear Magnetic Resonance Relaxation Times. Macromolecules 1975, 8, 726–730. [Google Scholar] [CrossRef]
- Ali, M.; Apperley, D.C.; Eley, C.D.; Emsley, A.M.; Harris, R.K. A Solid-State NMR Study of Cellulose Degradation. Cellulose 1996, 3, 77–90. [Google Scholar] [CrossRef]
- Capitani, D.; Emanuele, M.C.; Bella, J.; Segre, A.L.; Attanasio, D.; Focher, B.; Capretti, G. 1H NMR Relaxation Study of Cellulose and Water Interaction in Paper. Tappi J. 1999, 82. [Google Scholar]
- Gopal, P.M.; Suganya Priyadharshini, G.; Suyambulingam, I.; Divakaran, D.; Kavimani, V.; Sanjay, M.R.; Siengchin, S. Exfoliation and Physicochemical Characterization of Novel Biomass-Based Microcrystalline Cellulose Derived from Millettia Pinnata Leaf. Biomass Convers. Biorefin. 2024, 14, 20189–20199. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Digel, I.; Akimbekov, N.; Rogachev, E.; Pogorelova, N. Bacterial Cellulose Produced by Medusomyces Gisevii on Glucose and Sucrose: Biosynthesis and Structural Properties. Cellulose 2023, 30, 11439–11453. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Y.; Wen, Z.; Luo, Z.; Qiao, Y.; Guo, L. Bacterial Cellulose Nanofibrous Aerogels Grafted with Citric Acid for Absorption and Separation of Protein. Cellulose 2024, 31, 349–361. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, X.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Scalable Fabrication of Electrospun Nanofibrous Membranes Functionalized with Citric Acid for High-Performance Protein Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 11819–11829. [Google Scholar] [CrossRef] [PubMed]
- Bingül, N.D.; Öz, Y.E.; Morçimen, Z.G.; Gürsoy, G.E.; Tekkaptan, B.; Hameş, E.E. Hydrolyzed Bacterial Cellulose as a UV Radiation Barrier. Cellulose 2024, 31, 9759–9776. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza Filho, M.d.S.M.; de Freitas Rosa, M. Bacterial Cellulose Nanocrystals Produced under Different Hydrolysis Conditions: Properties and Morphological Features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Drosos, A.; Kordopati, G.G.; Anastasopoulos, C.; Zafeiropoulos, J.; Koutinas, A.A.; Kanellaki, M. Comparative Study and Characterization of Water-Treated Bacterial Cellulose Produced by Solid or Liquid Inoculum of Komagateibacter Sucrofermentans. Cellulose 2024, 31, 5545–5573. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csóka, L.; Ohtani, B. Integrating Photon-Based Techniques to Probe Structural and Phonon Dynamics in Bacterial Cellulose. Polymers 2025, 17, 2544. https://doi.org/10.3390/polym17182544
Csóka L, Ohtani B. Integrating Photon-Based Techniques to Probe Structural and Phonon Dynamics in Bacterial Cellulose. Polymers. 2025; 17(18):2544. https://doi.org/10.3390/polym17182544
Chicago/Turabian StyleCsóka, Levente, and Bunsho Ohtani. 2025. "Integrating Photon-Based Techniques to Probe Structural and Phonon Dynamics in Bacterial Cellulose" Polymers 17, no. 18: 2544. https://doi.org/10.3390/polym17182544
APA StyleCsóka, L., & Ohtani, B. (2025). Integrating Photon-Based Techniques to Probe Structural and Phonon Dynamics in Bacterial Cellulose. Polymers, 17(18), 2544. https://doi.org/10.3390/polym17182544







