Effect of Degradation During Multiple Primary Mechanical Recycling Processes on the Physical Properties and Biodegradation of Commercial PLA-Based Water Bottles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and PLA Commercial Water Bottle Preforms
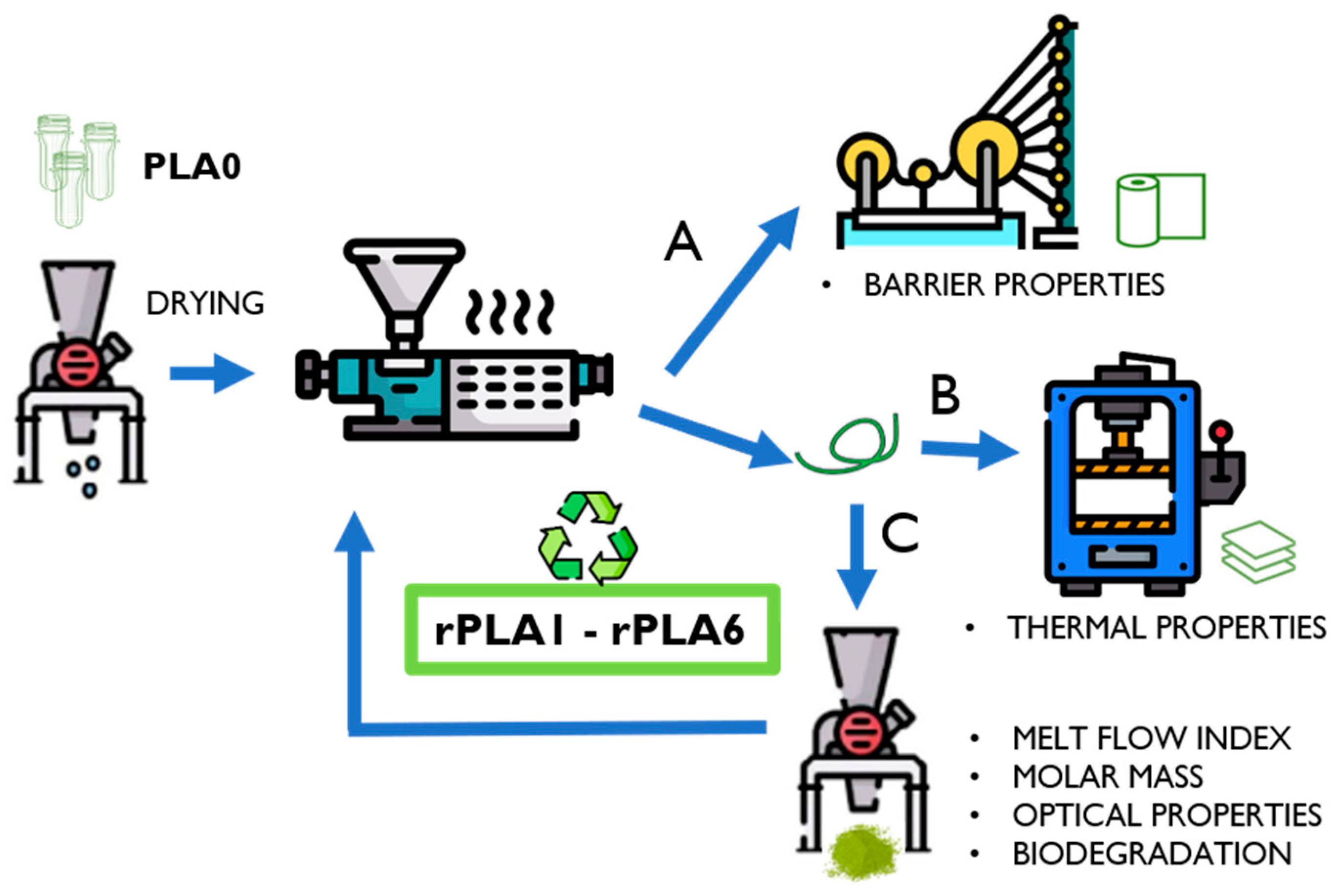
2.2. Reprocessing Cycles and Samples: PLA0 to rPLA6
2.3. Characterization of Reprocessed PLA-Based Water Bottles
2.3.1. Structural Characterization
2.3.2. Molar Mass Distribution (MMD)
2.3.3. Melt Flow Index (MFI) Analysis
2.3.4. Optical Characterization
2.3.5. Thermal Properties
2.3.6. Barrier Properties
2.4. Biodegradation Studies
2.5. Statistical Analysis
3. Results
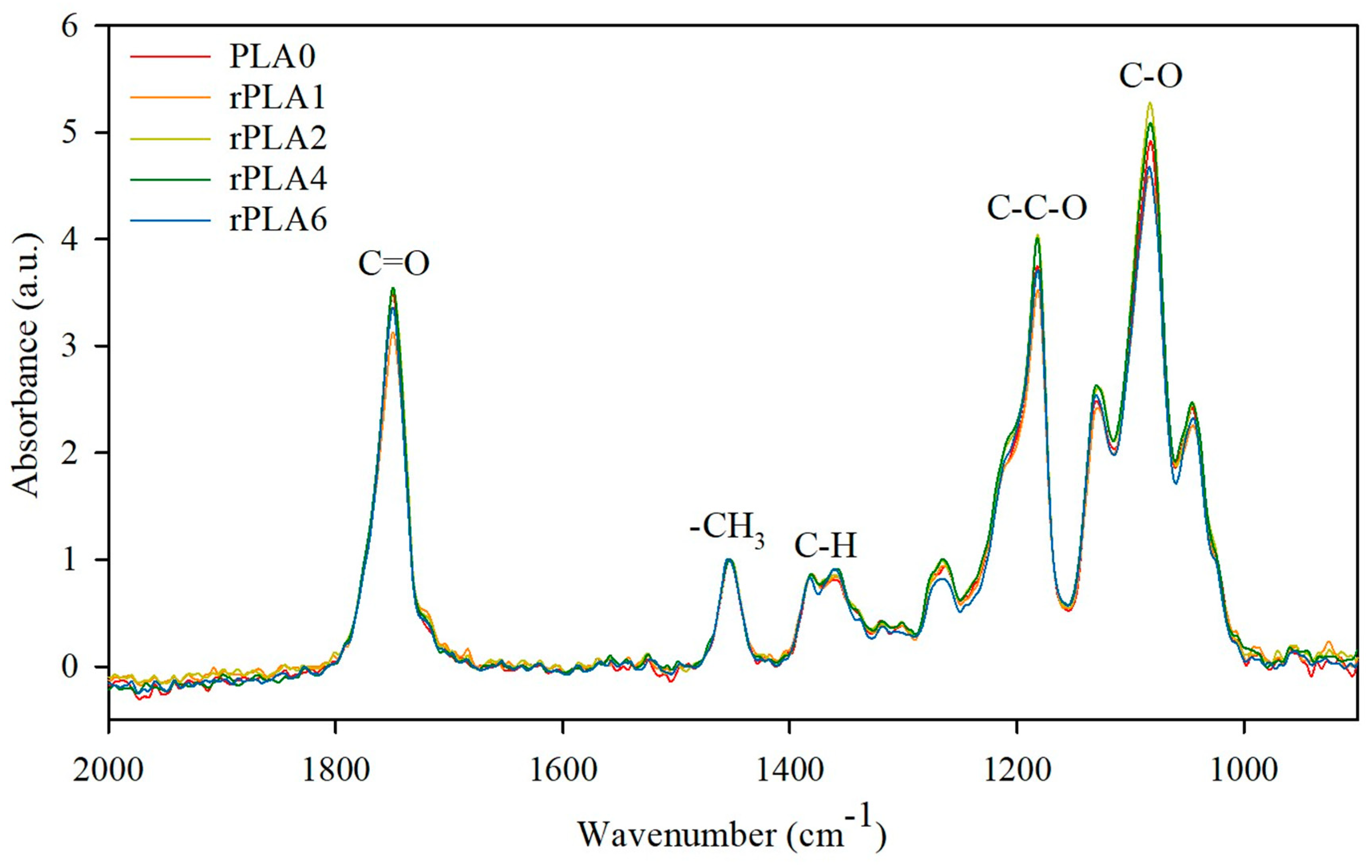
3.1. Structural and Optical Properties After Multiple Mechanical Recycling
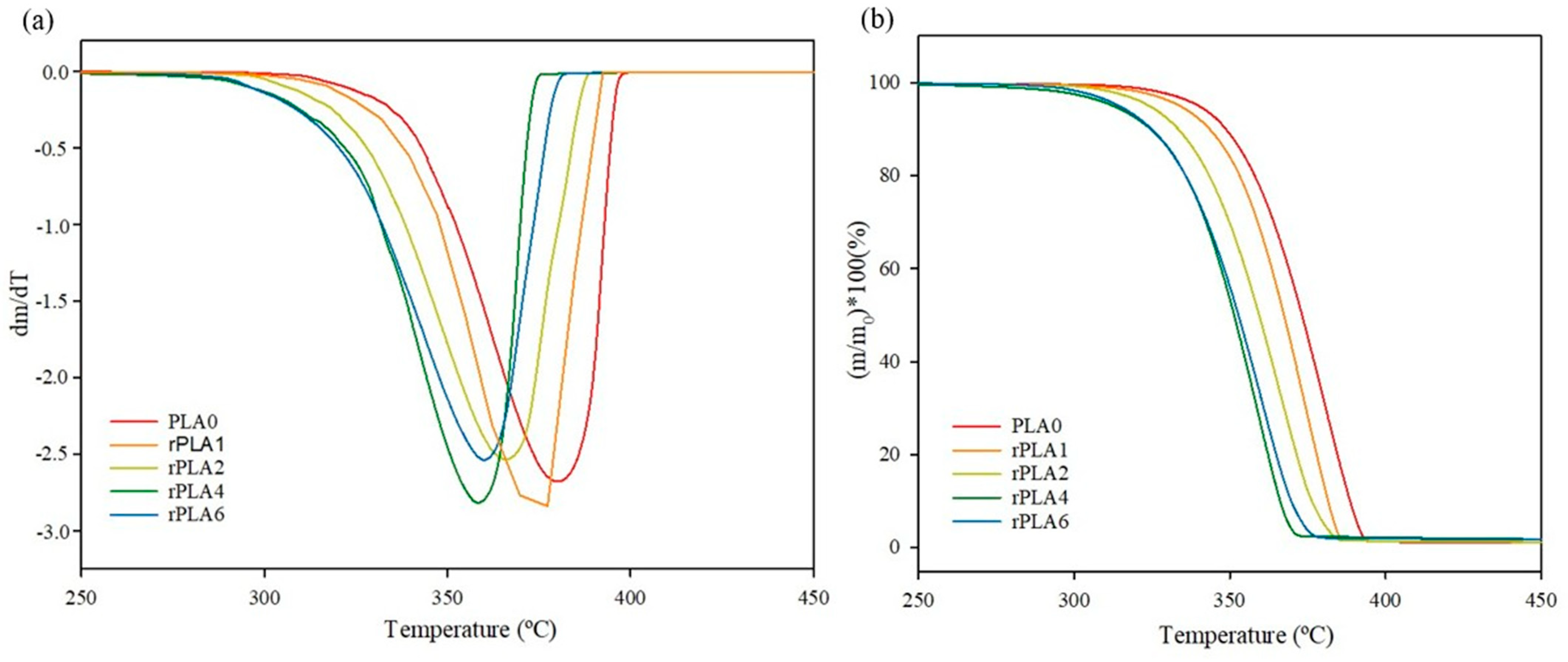
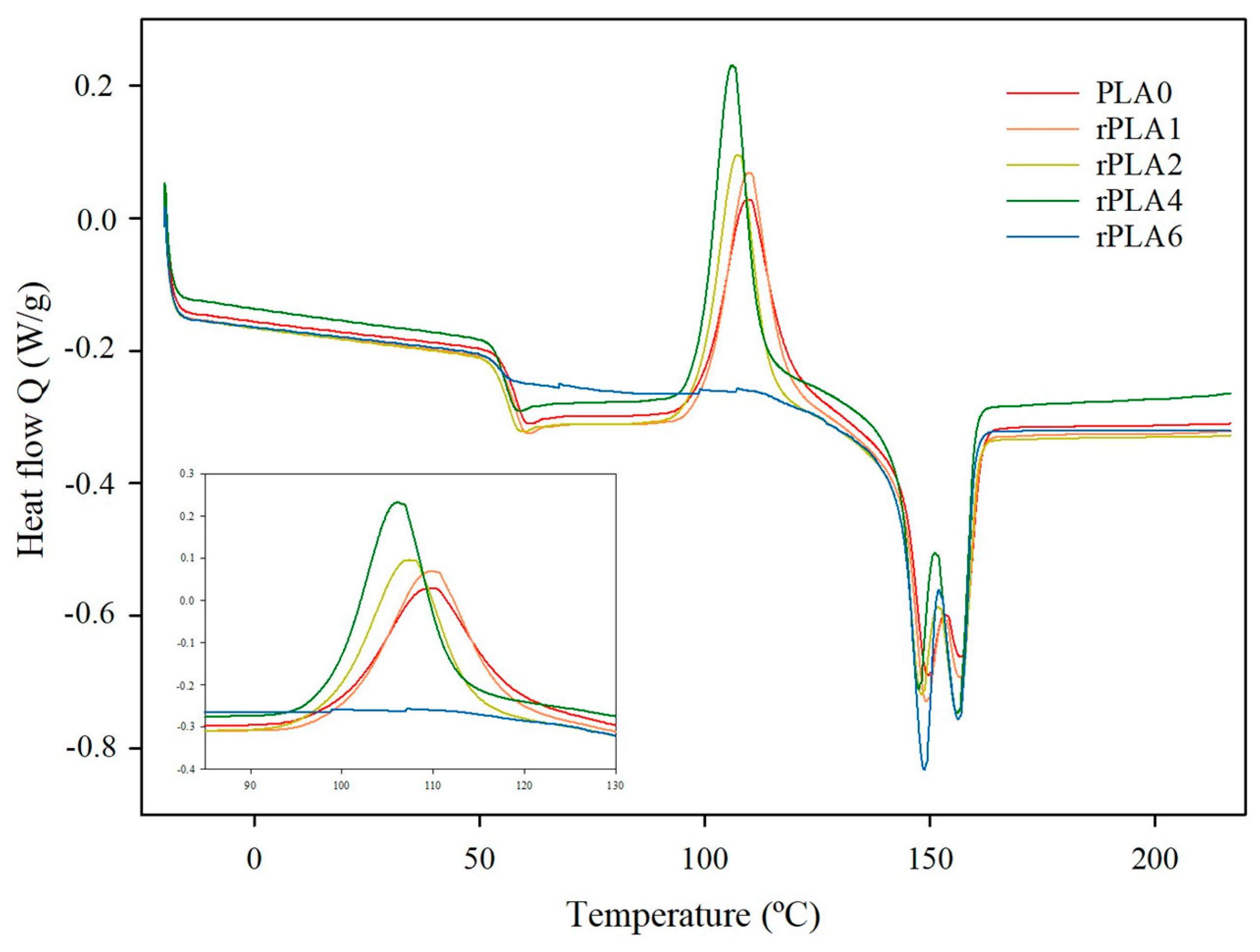
3.2. Thermal Properties
3.3. Barrier Properties
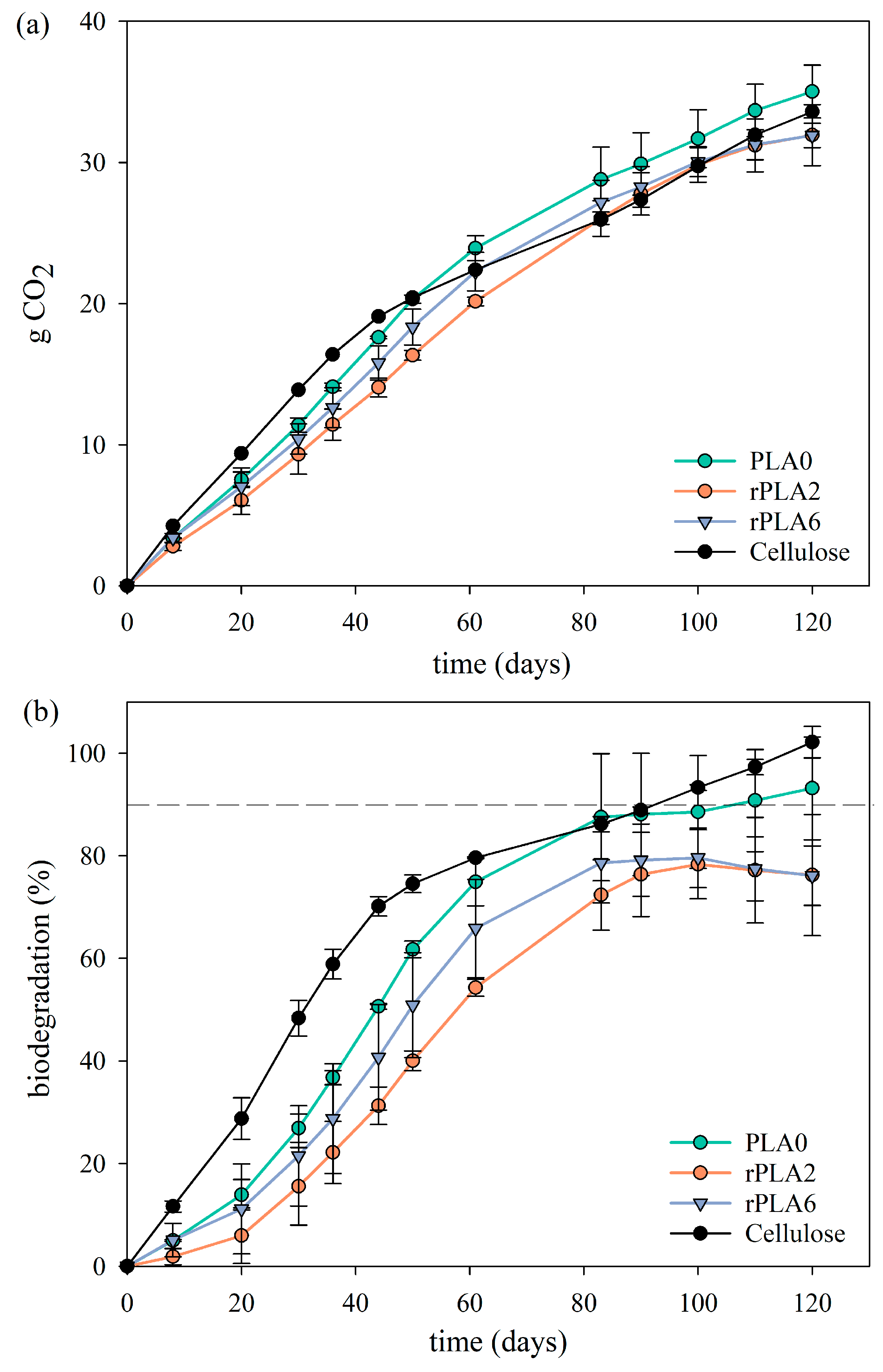
3.4. Biodegradation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva Pens, C.J.; Klug, T.V.; Stoll, L.; Izidoro, F.; Flores, S.H.; de Oliveira Rios, A. Poly (Lactic Acid) and Its Improved Properties by Some Modifications for Food Packaging Applications: A Review. Food Packag. Shelf Life 2024, 41, 101230. [Google Scholar] [CrossRef]
- Huang, S.; Dong, Q.; Che, S.; Li, R.; Tang, K.H.D. Bioplastics and Biodegradable Plastics: A Review of Recent Advances, Feasibility and Cleaner Production. Sci. Total Environ. 2025, 969, 178911. [Google Scholar] [CrossRef] [PubMed]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the Influence of the Reprocessing Cycles on the Final Properties of Polylactide Pieces Obtained by Injection Molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef]
- Majgaonkar, P.; Hanich, R.; Malz, F.; Brüll, R. Chemical Recycling of Post-Consumer PLA Waste for Sustainable Production of Ethyl Lactate. Chem. Eng. J. 2021, 423, 129952. [Google Scholar] [CrossRef]
- Dedieu, I.; Peyron, S.; Gontard, N.; Aouf, C. The Thermo-Mechanical Recyclability Potential of Biodegradable Biopolyesters: Perspectives and Limits for Food Packaging Application. Polym. Test. 2022, 111, 107620. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Bikiaris, D.N. Biobased Plastics for the Transition to a Circular Economy. Mater. Lett. 2024, 362, 136174. [Google Scholar] [CrossRef]
- Cosate de Andrade, M.F.; Souza, P.M.S.; Cavalett, O.; Morales, A.R. Life Cycle Assessment of Poly(Lactic Acid) (PLA): Comparison Between Chemical Recycling, Mechanical Recycling and Composting. J. Polym. Environ. 2016, 24, 372–384. [Google Scholar] [CrossRef]
- Rebolledo-Leiva, R.; Ladakis, D.; Ioannidou, S.-M.; Koutinas, A.; Moreira, M.T.; González-García, S. Attributional and Consequential Life Cycle Perspectives of Second-Generation Polylactic Acid: The Benefits of Integrating a Recycling Strategy. J. Clean. Prod. 2023, 420, 138354. [Google Scholar] [CrossRef]
- Maga, D.; Hiebel, M.; Thonemann, N. Life Cycle Assessment of Recycling Options for Polylactic Acid. Resour. Conserv. Recycl. 2019, 149, 86–96. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A. Recycling of Bioplastic Waste: A Review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar] [CrossRef]
- Sun, C.; Wei, S.; Tan, H.; Huang, Y.; Zhang, Y. Progress in Upcycling Polylactic Acid Waste as an Alternative Carbon Source: A Review. Chem. Eng. J. 2022, 446, 136881. [Google Scholar] [CrossRef]
- Ramos-Hernández, T.; Robledo-Ortíz, J.R.; González-López, M.E.; del Campo, A.S.M.; González-Núñez, R.; Rodrigue, D.; Pérez Fonseca, A.A. Mechanical Recycling of PLA: Effect of Weathering, Extrusion Cycles, and Chain Extender. J. Appl. Polym. Sci. 2023, 140, e53759. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Rehovsky, C.; Bajwa, S.G.; Vahidi, G. Deterioration in the Physico-Mechanical and Thermal Properties of Biopolymers Due to Reprocessing. Polymers 2019, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, J.; Rowe, S.; Howard, T.; Connolly, S.; Doran, C.; Devine, D.M.; Gately, N.M.; Chyzna, V.; Portela, A.; Bezerra, G.S.N.; et al. Effect of Mechanical Recycling on the Mechanical Properties of PLA-Based Natural Fiber-Reinforced Composites. J. Compos. Sci. 2023, 7, 141. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Gaspar, G.; Dadras Chomachayi, M.; Jalali-Arani, A.; Lozano-Pérez, A.A.; Cenis, J.L.; de la Orden, M.U.; Pérez, E.; Martínez Urreaga, J.M. Influence of Addition of Organic Fillers on the Properties of Mechanically Recycled PLA. Environ. Sci. Pollut. Res. 2021, 28, 24291–24304. [Google Scholar] [CrossRef]
- Velghe, I.; Buffel, B.; Vandeginste, V.; Thielemans, W.; Desplentere, F. Review on the Degradation of Poly(Lactic Acid) during Melt Processing. Polymers 2023, 15, 2047. [Google Scholar] [CrossRef]
- Wang, W.; Ye, G.; Fan, D.; Lu, Y.; Shi, P.; Wang, X.; Bateer, B. Photo-Oxidative Resistance and Adjustable Degradation of Poly-Lactic Acid (PLA) Obtained by Biomass Addition and Interfacial Construction. Polym. Degrad. Stab. 2021, 194, 109762. [Google Scholar] [CrossRef]
- ISO 1133-1:2012; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method. 2022. Available online: https://www.iso.org/standard/83905.html (accessed on 15 September 2025).
- Navneet; Martinez, M.M.; Joye, I.J. Heat-Treated Bean Flour: Exploring Techno-Functionality via Starch-Protein Structure-Function Analysis. Food. Hydrocoll. 2024, 157, 110416. [Google Scholar] [CrossRef]
- Romani, A.; Perusin, L.; Ciurnelli, M.; Levi, M. Characterization of PLA Feedstock after Multiple Recycling Processes for Large-Format Material Extrusion Additive Manufacturing. Mater. Today Sustain. 2024, 25, 100636. [Google Scholar] [CrossRef]
- ISO 2528:2017; Sheet Materials—Determination of Water Vapour Transmission Rate (WVTR)—Gravimetric (Dish) Method. 2017. Available online: https://www.iso.org/standard/72382.html (accessed on 15 September 2025).
- ISO 14855-2:2018; Determination of the Ultimate aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide. Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test. 2018. Available online: https://www.iso.org/standard/72046.html (accessed on 15 September 2025).
- Palsikowski, P.A.; Kuchnier, C.N.; Pinheiro, I.F.; Morales, A.R. Biodegradation in Soil of PLA/PBAT Blends Compatibilized with Chain Extender. J. Polym. Environ. 2018, 26, 330–341. [Google Scholar] [CrossRef]
- Oh, J.; Park, S.B.; Cha, C.; Hwang, D.K.; Park, S.A.; Park, J.; Oh, D.X.; Jeon, H.; Koo, J.M. Structural Evaluation of Poly(Lactic Acid) Degradation at Standardized Composting Temperature of 58 Degrees. Chemosphere 2024, 354, 141729. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Tsagkalias, I.; Vouvoudi, E.C.; Achilias, D.S. Development of Bio-Composites with Enhanced Antioxidant Activity Based on Poly(Lactic Acid) with Thymol, Carvacrol, Limonene, or Cinnamaldehyde for Active Food Packaging. Polymers 2021, 13, 3652. [Google Scholar] [CrossRef] [PubMed]
- Salah, L.S.; Ouslimani, N.; Danlée, Y.; Beltrán, F.R.; Huynen, I.; de la Orden, M.U. Investigation of Mechanical Recycling Effect on Electromagnetic Properties of Polylactic Acid (PLA)–Nanoclay Nanocomposites: Towards a Valorization of Recycled PLA Nanocomposites. Compos. Part C Open Access 2023, 10, 100339. [Google Scholar] [CrossRef]
- Garcia Gonçalves, L.M.; Rocha Rigolin, T.; Maia Frenhe, B.; Prado Bettini, S.H. On the Recycling of a Biodegradable Polymer: Multiple Extrusion of Poly (Lactic Acid). Mater. Res. 2020, 23, 20200274. [Google Scholar] [CrossRef]
- Velásquez, E.; Guerrero Correa, M.; Garrido, L.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Food Packaging Plastics: Identification and Recycling. In Recent Developments in Plastic Recycling; Parameswaranpillai, J., Mavinkere Rangappa, S., Gulihonnehalli Rajkumar, A., Siengchin, S., Eds.; Springer: Singapore, 2021; pp. 311–343. ISBN 978-981-16-3627-1. [Google Scholar]
- Beltrán, F.R.; Infante, C.; de la Orden, M.U.; Martínez Urreaga, J. Mechanical Recycling of Poly(Lactic Acid): Evaluation of a Chain Extender and a Peroxide as Additives for Upgrading the Recycled Plastic. J. Clean. Prod. 2019, 219, 46–56. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.; Kim, I.; Hwang, K.; Kim, N. Thermal and Mechanical Degradation of Recycled Polylactic Acid Filaments for Three-Dimensional Printing Applications. Polymers 2022, 14, 5385. [Google Scholar] [CrossRef]
- Botta, L.; Scaffaro, R.; Sutera, F.; Mistretta, M.C. Reprocessing of PLA/Graphene Nanoplatelets Nanocomposites. Polymers 2017, 10, 18. [Google Scholar] [CrossRef]
- Morales, J.; Rodrigue, D. The Effect of Reprocessing and Moisture on Polyamide Recycling: A Focus on Neat, Composites, and Blends. Macromol. Mater. Eng. 2025, 310, 2400304. [Google Scholar] [CrossRef]
- Verberckmoes, A.; Fernandez, E.; Martins, C.; Reyes, P.; Cardon, L.; D’hooge, D.R.; Edeleva, M. Morphological Analysis of Mechanically Recycled Blends of High Density Polyethylene and Polypropylene with Strong Difference in Melt Flow Index. Polymer 2024, 300, 126999. [Google Scholar] [CrossRef]
- Velásquez, E.; de Dicastillo, C.L.; Rojas, A.; Garrido, L.; Pérez, C.J.; Lira, M.; Guarda, A.; Galotto, M.J. Multiple Mechanical Recycling of a Post-Industrial Flexible Polypropylene and Its Nanocomposite with Clay: Impact on Properties for Food Packaging Applications. Food Packag. Shelf Life 2024, 43, 101272. [Google Scholar] [CrossRef]
- Plouzeau, M.; Belyamani, I.; Fatyeyeva, K.; Marais, S.; Kobzar, Y.; Cauret, L. Recyclability of Poly(Hydroxybutyrate-Co-Hydroxyhexanoate) (PHBH) for Food Packaging Applications. Food Packag. Shelf Life 2023, 40, 101170. [Google Scholar] [CrossRef]
- Martin-Perez, L.; Contreras, C.; Chiralt, A.; Gonzalez-Martinez, C. Active Polylactic Acid (PLA) Films Incorporating Almond Peel Extracts for Food Preservation. Molecules 2025, 30, 1988. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Arrieta, M.P.; Gaspar, G.; de la Orden, M.U.; Urreaga, J.M. Effect of Lignocellulosic Nanoparticles Extracted from Yerba Mate (Ilex Paraguariensis) on the Structural, Thermal, Optical and Barrier Properties of Mechanically Recycled Poly(Lactic Acid). Polymers 2020, 12, 1690. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J.; Rytlewski, P.; Moraczewski, K.; Stepczyńska, M.; Karasiewicz, T. Characterisation of Multi-Extruded Poly(Lactic Acid). Polym Test. 2009, 28, 412–418. [Google Scholar] [CrossRef]
- Muñoz-Shugulí, C.; Rodríguez-Mercado, F.; Guarda, A.; Galotto, M.J.; Jiménez, A.; Garrigós, M.C.; Ramos, M. Release and Disintegration Properties of Poly(Lactic Acid) Films with Allyl Isothiocyanate-β-Cyclodextrin Inclusion Complexes for Active Food Packaging. Molecules 2024, 29, 5859. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, L.; Lan, L.; Dan, Y.; Jiang, L.; Huang, Y. Effect of Poly(Vinyl Alcohol)-g-Poly(Lactic Acid) on the Oxygen Barrier Performance of Poly(Lactic Acid)-Based Film. Int. J. Biol. Macromol. 2025, 290, 138819. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Lorenzo, V.; Acosta, J.; de la Orden, M.U.; Martínez Urreaga, J. Effect of Simulated Mechanical Recycling Processes on the Structure and Properties of Poly(Lactic Acid). J. Environ. Manag. 2018, 216, 25–31. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, J.; Limsukon, W.; Bher, A.; Auras, R. Impact of Hydrolysis Pretreatment on the Compostability of Biodegradable Poly(Caprolactone) and Poly(Lactic Acid) Films. RSC Appl. Polym. 2025, 3, 711–721. [Google Scholar] [CrossRef]
- Kim, M.N.; Park, S.T. Degradation of Poly(L-Lactide) by a Mesophilic Bacterium. J. Appl. Polym. Sci. 2010, 117, 67–74. [Google Scholar] [CrossRef]
| Sample | MFI (g 10 min−1) | Ð | ||
|---|---|---|---|---|
| PLA0 | 10.27 ± 0.20 d | 88,998 ± 1782 a | 186,702 ± 2587 a | 2.10 ± 0.02 ab |
| rPLA1 | 14.77 ± 0.30 c | 74,463 ± 1410 b | 155,217 ± 1178 b | 2.08 ± 0.04 a |
| rPLA2 | 14.59 ± 0.09 c | 72,292 ± 1907 c | 152,676 ± 2094 c | 2.11 ± 0.03 c |
| rPLA4 | 15.81 ± 0.09 b | 62,652 ± 1503 d | 132,388 ± 1960 d | 2.11 ± 0.02 d |
| rPLA6 | 15.30 ± 0.37 a | 53,972 ± 453 e | 113,942 ± 1355 e | 2.11 ± 0.01 bc |
| Sample | L* | a* | b* | ΔE* | |
|---|---|---|---|---|---|
| PLA0 |  | 71.1 ± 0.1 a | −34.8 ± 0.1 e | 34.4 ± 0.1 a | - |
| rPLA1 |  | 66.7 ± 0.2 b | −31.7 ± 0.2 d | 32.4 ± 0.3 b | 5.8 ± 0.4 d |
| rPLA2 |  | 63.6 ± 0.1 c | −24.5 ± 0.4 c | 30.4 ± 1.0 c | 13.4 ± 0.7 c |
| rPLA4 |  | 60.6 ± 0.3 d | −16.9 ± 0.1 b | 27.2 ± 0.2 d | 22.1 ± 0.1 b |
| rPLA6 |  | 47.3 ± 0.1 e | −9.30 ± 0.1 a | 25.7 ± 0.6 e | 35.9 ± 0.1 a |
| Sample | Tg (°C) | Tcc (°C) | Tm1 (°C) | Tm2 (°C) | ΔHcc (J/g) | ΔHm (J/g) | χc (%) | Tonset (°C) | Tdeg (°C) |
|---|---|---|---|---|---|---|---|---|---|
| PLA0 | 57.9 ± 0.4 a | 108.4 ± 2.0 ab | 149.0 ± 0.7 b | 156.8 ± 0.8 d | 26.4 ± 0.7 a | 31.6 ± 0.4 c | 5.6 ± 1.1 b | 356.6 ± 2.3 c | 376.6 ± 4.3 b |
| rPLA1 | 57.9 ± 0.3 a | 110.0 ± 0.3 a | 149.1 ± 0.1 b | 156.6 ± 0.3 c | 28.1 ± 0.6 a | 31.7± 0.5 c | 3.8 ± 1.2 b | 350.6 ± 1.9 bc | 374.2 ± 2.9 b |
| rPLA2 | 56.7 ± 0.2 a | 107.3 ± 0.2 ab | 156.3 ± 0.1 a | 156.3 ± 0.1 b | 25.7 ± 1.4 a | 33.4 ± 0.1 b | 8.2 ± 1.4 b | 342.6 ± 6.0 ab | 369.1 ± 6.0 ab |
| rPLA4 | 56.5 ± 0.4 a | 106.2 ± 0.2 b | 156.0 ± 0.1 a | 156.0 ± 0.2 a | 28.4 ± 2.1 a | 34.8 ± 1.1 b | 6.9 ± 3.5 b | 337.3 ± 4.6 a | 357.8 ± 8.7 a |
| rPLA6 | 54.8 ± 1.0 b | - | 148.6 ± 0.3 b | 156.2 ± 0.3 b | - | 36.7 ± 0.7 a | 39.5 ± 0.7 a | 337.9 ± 6.4 a | 359.7 ± 9.4 a |
| Sample | WVP (kg m m−2 s−1 Pa −1)·10−15 | OP (cc m m−2 day−1 Pa−1)·10−7 | ||
|---|---|---|---|---|
| RH 50% | RH 90% | RH 50% | RH 90% | |
| rPLA1 | 4.1 ± 0.9 | 5.7 ± 0.6 | 2.1 ± 0.4 | 24.8 ± 4.7 |
| rPLA2 | 5.3 ± 0.6 | 6.8 ± 0.3 | 5.3 ± 0.9 | 225.7 ± 33.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Shugulí, C.; Morán, D.; Velásquez, E.; López-Vilariño, J.M.; López-de-Dicastillo, C. Effect of Degradation During Multiple Primary Mechanical Recycling Processes on the Physical Properties and Biodegradation of Commercial PLA-Based Water Bottles. Polymers 2025, 17, 2542. https://doi.org/10.3390/polym17182542
Muñoz-Shugulí C, Morán D, Velásquez E, López-Vilariño JM, López-de-Dicastillo C. Effect of Degradation During Multiple Primary Mechanical Recycling Processes on the Physical Properties and Biodegradation of Commercial PLA-Based Water Bottles. Polymers. 2025; 17(18):2542. https://doi.org/10.3390/polym17182542
Chicago/Turabian StyleMuñoz-Shugulí, Cristina, Diana Morán, Eliezer Velásquez, José Manuel López-Vilariño, and Carol López-de-Dicastillo. 2025. "Effect of Degradation During Multiple Primary Mechanical Recycling Processes on the Physical Properties and Biodegradation of Commercial PLA-Based Water Bottles" Polymers 17, no. 18: 2542. https://doi.org/10.3390/polym17182542
APA StyleMuñoz-Shugulí, C., Morán, D., Velásquez, E., López-Vilariño, J. M., & López-de-Dicastillo, C. (2025). Effect of Degradation During Multiple Primary Mechanical Recycling Processes on the Physical Properties and Biodegradation of Commercial PLA-Based Water Bottles. Polymers, 17(18), 2542. https://doi.org/10.3390/polym17182542







