Exploring the Properties of Organometallic Lactone-Containing Poly(benzofuran-co-arylacetic Acid): Traditional Synthesis Versus Mechanosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of Polyaryacetic Acid PAAA
2.1.2. General Procedure for Traditional In-Solution Synthesis of Organometallic Polymers
2.1.3. General Procedure for Mechanochemistry Synthesis of Organometallic Polymers
2.2. Methods
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization of Polymeric Ligands
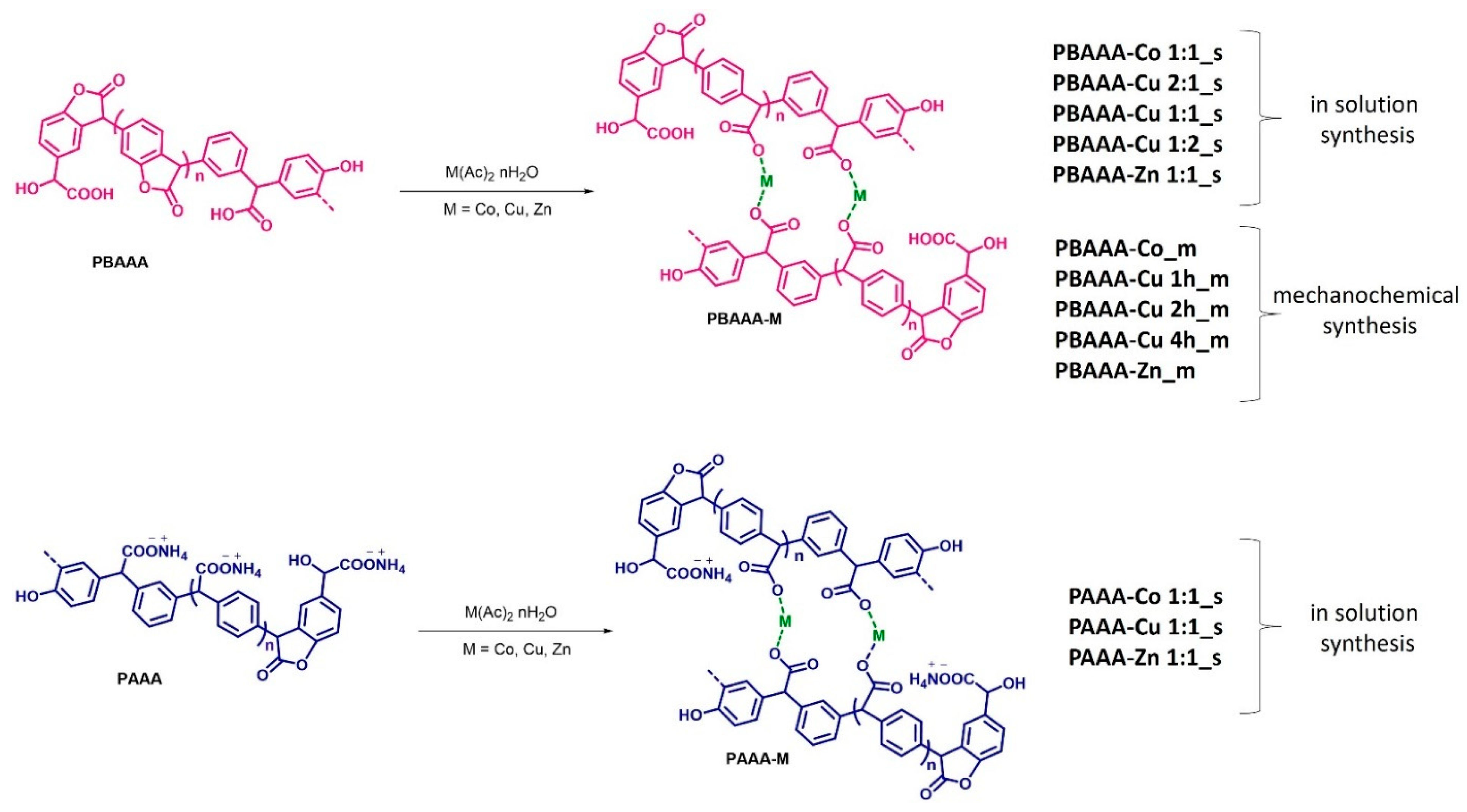
3.1.1. Synthesis of Polymeric Ligands
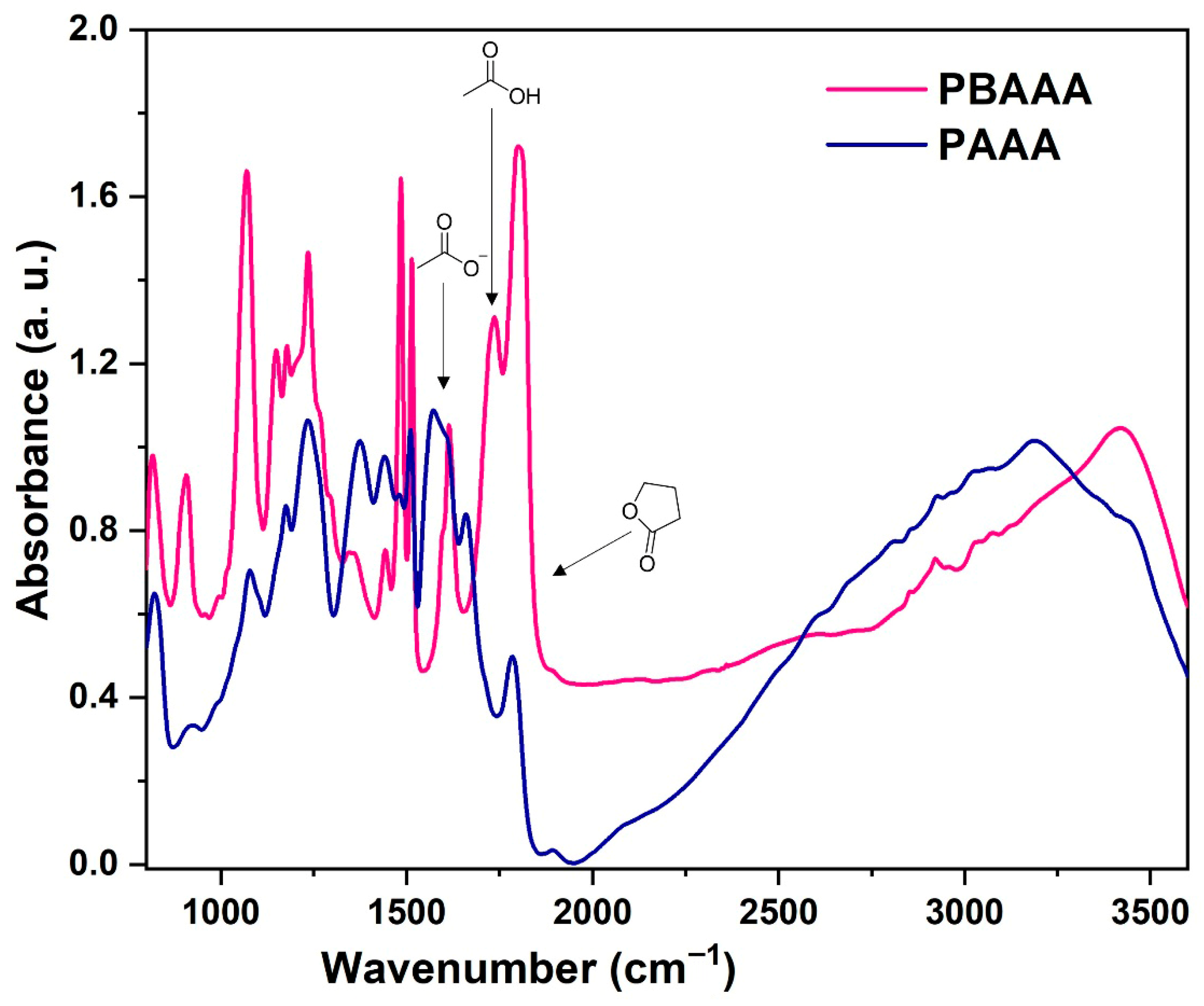
3.1.2. FTIR Spectroscopy of Polymeric Ligands
3.1.3. Powder XRD of Polymeric Ligands
3.1.4. UV-Vis Spectroscopy of Polymeric Ligands
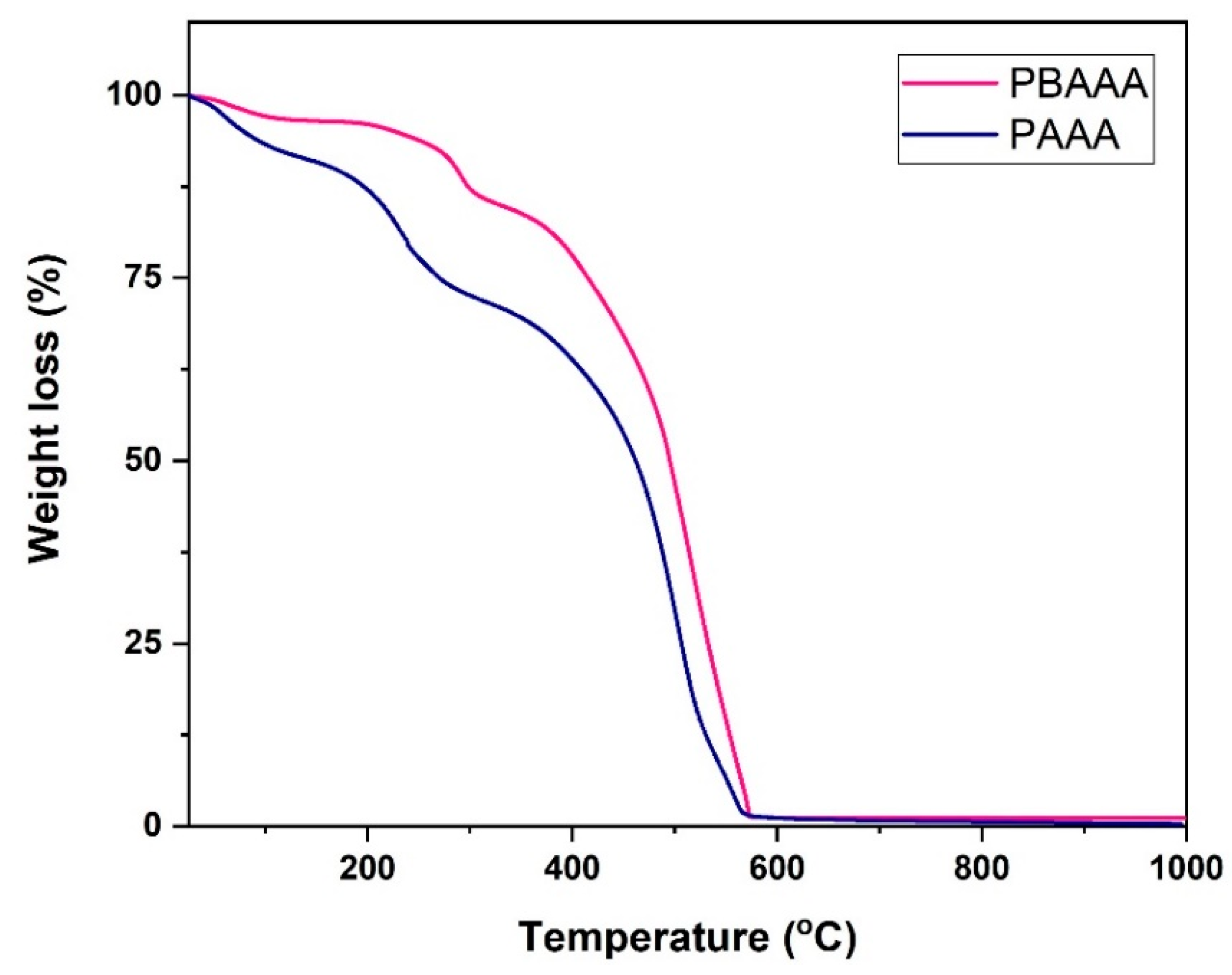
3.1.5. TGA of Polymeric Ligands
3.2. Synthesis and Characterization of Organometallic Polymers
3.2.1. Synthesis of Organometallic Polymers
3.2.2. FTIR Spectroscopy of Organometallic Polymers
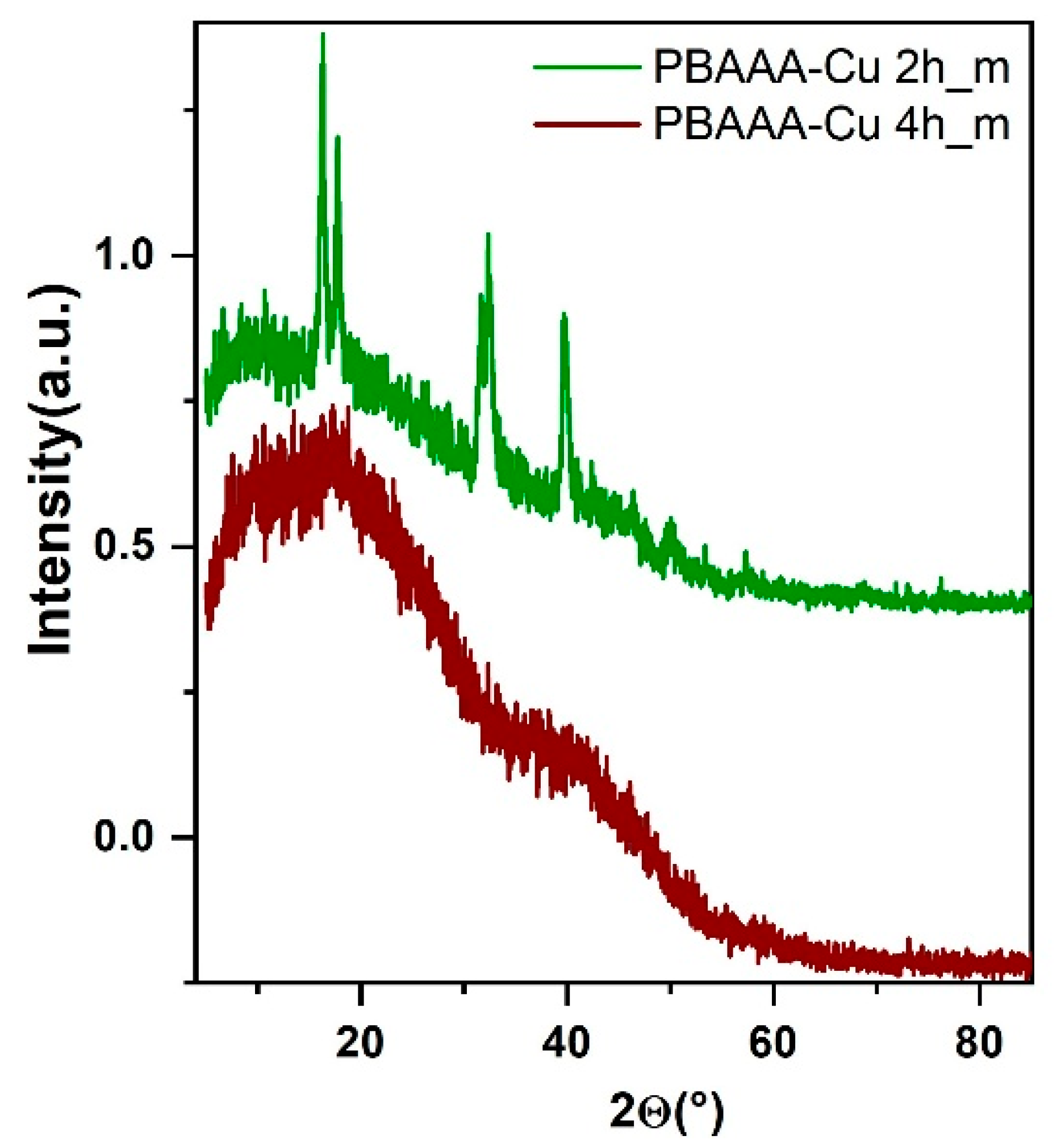
3.2.3. Powder XRD of Organometallic Polymers
3.2.4. Thermogravimetric Analysis of Organometallic Polymers
3.2.5. Thermal Conductivity of Organometallic Polymers
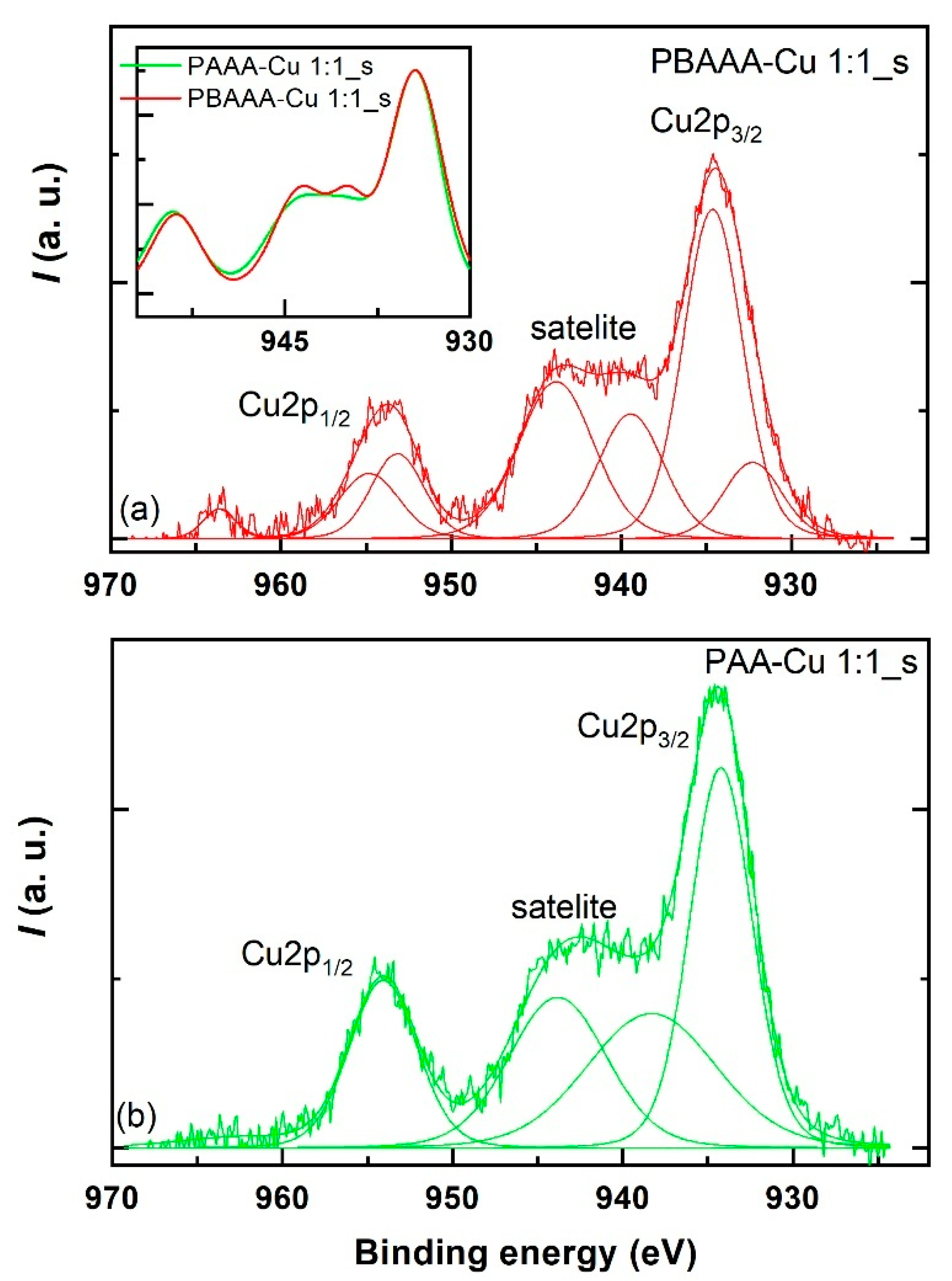
3.2.6. X-Ray Photoelectron Spectroscopy of Organometallic Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Yang, K.; Feng, Y.; Liang, L.; Chi, M.; Zhang, Z.; Chen, X. Recent advances in thermal-conductive insulating polymer composites with various fillers. Compos. Part A 2024, 178, 107998. [Google Scholar] [CrossRef]
- Vallejos, S.; Trigo-López, M.; Arnaiz, A.; Miguel, Á.; Muñoz, A.; Mendía, A.; García, J.M. From Classical to Advanced Use of Polymers in Food and Beverage Applications. Polymers 2022, 16, 4954. [Google Scholar] [CrossRef] [PubMed]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Doyle, L.; Weidlich, I.; Maio, E.D. Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective. Materials 2022, 15, 6212. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, K.; Bhatnagar, N. Conductive polymers: A multipurpose material for protecting coating. Prog. Org. Coat. 2024, 187, 108083. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A.D. Conducting polymers and their inorganic composites for advanced Li-ion batteries: A review. RSC Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials. J. Phys. Chem. B 2001, 105, 8475–8491. [Google Scholar] [CrossRef]
- Heeger, A.J. Nobel Lecture: Semiconducting and metallic polymers: The fourth generation of polymeric materials. Rev. Mod. Phys. 2021, 73, 681. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.S.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A Review on Polyaniline: Synthesis, Properties, Nanocomposites, and Electrochemical Applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Wang, L.-X.; Li, X.-G.; Yang, Y.-L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Song, W.; Liu, Z. Controllable Synthesis of Conducting Polypyrrole Nanostructures. Phys. Chem. B 2006, 110, 1158–1165. [Google Scholar] [CrossRef]
- Thanasamy, D.; Jesuraj, D.; Kannan, S.K.K.; Avadhanam, V. A novel route to synthesis polythiophene with great yield and high electrical conductivity without post doping process. Polymer 2019, 175, 32–40. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhou, J.; Song, B.; Jiang, Z.; Lee, E.M.Y.; Huberman, S.; Gleason, K.K.; Chen, G. Molecular engineered conjugated polymer with high thermal conductivity. Sci. Adv. 2018, 4, 3031. [Google Scholar] [CrossRef]
- Ikizer, B.; Lawton, C.W.; Orbey, N. Poly(para-phenylene) fibers—Characterization and preliminary data for conversion to carbon fiber. Polymer 2021, 228, 123945. [Google Scholar] [CrossRef]
- Skotheim, T.A. Hand Book of Conducting Polymers; Marcell Dekker: New York, NY, USA, 1986; Volume 1, p. 254. [Google Scholar]
- González-Tejera, M.J.; Sánchez de la Blanca, E.; Carrillo, I. Polyfuran conducting polymers: Synthesis, properties, and applications. Synth. Met. 2008, 158, 165–189. [Google Scholar] [CrossRef]
- Sheberla, D.; Patra, S.; Wijsboom, Y.H.; Sharma, S.; Sheynin, Y.; Haj-Yahia, A.-E.; Barak, A.H.; Gidron, O.; Bendikov, M. Conducting polyfurans by electropolymerization of oligofurans. Chem. Sci. 2015, 6, 360–371. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef]
- Singh, V.; Bougher, T.L.; Weathers, A.; Cai, Y.; Bi, K.; Pettes, M.T.; McMenamin, S.A.; Lv, W.; Resler, D.P.; Gattuso, T.R.; et al. High thermal conductivity of chain-oriented amorphous polythiophene. Nat. Nanotechnol. 2014, 9, 384–390. [Google Scholar] [CrossRef]
- Xiao, Z.; Ishii, M.; Takeya, J.; Ariga, K.; Yamashita, Y. Chemical doping of a semicrystalline polymeric semiconductor realizing high stability and work function. J. Mater. Chem. C 2024, 12, 12739–12746. [Google Scholar] [CrossRef]
- Ispas, G.; Filip, X.; Caspari, A.; Nan, A. Incorporation of magnetic nanoparticles into polytartaric acid as a key for enhancing thermal conductivity. J. Alloys Compd. 2025, 1010, 178209. [Google Scholar] [CrossRef]
- Nan, A.; Filip, X.; Liebscher, J. Reaction of Lactone-Containing Poly(benzofuran-co-arylacetic acid) with Diamines to Cross-Linked Products of Improved Thermal Conductivity. Molecules 2024, 29, 6020. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T. Recent progress in graphene based polymer nanocomposites. Cogent Chem. 2020, 6, 1833476. [Google Scholar] [CrossRef]
- Wang, Y.; Astruc, D.; Abd-El-Aziz, A.S. Metallopolymers for advanced sustainable applications. Chem. Soc. Rev. 2019, 48, 558–636. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Rend, L.; Tang, C. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef]
- Wu, P.-T.; Bull, T.; Kim, F.S.; Luscomb, C.K.; Jenekhe, S.A. Organometallic Donor–Acceptor Conjugated Polymer Semiconductors: Tunable Optical, Electrochemical, Charge Transport, and Photovoltaic Properties. Macromolecules 2009, 42, 671–681. [Google Scholar] [CrossRef]
- Eloi, J.-C.; Chabanne, L.; Whittell, G.R.; Manners, I. Metallopolymers with emerging applications. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Napierała, S.; Kubicki, M.; Wałęsa-Chorab, M. Toward Electrochromic Metallopolymers: Synthesis and Properties of Polyazomethines Based on Complexes of Transition-Metal Ions. Inorg. Chem. 2021, 60, 14011–14021. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.B.; Bielawski, C.W.; Cowley, A.H. Design, Synthesis, and Study of Main Chain Poly(N-Heterocyclic Carbene) Complexes: Applications in Electrochromic Devices. J. Am. Chem. Soc. 2010, 132, 10184–10194. [Google Scholar] [CrossRef]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, and future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Liu, S.; Zhao, Q.; Huang, W. Stimuli–responsive metallopolymers. Coord. Chem. Rev. 2016, 319, 180–195. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, L.; Li, Q.; Yue, C.; Han, B.; Liu, K.; Li, H. Multi-Functional Lanthanide Metallopolymer: Self-Healing and Photo-Stimuli-Responsive Dual-Emitting Luminescence for Diverse Applications. Adv. Mater. 2024, 36, 2405164. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Bandyopadhyay, A. Tethering smartness to the metal containing polymers—Recent trends in the stimuli-responsive metal containing polymers. J. Organomet. Chem. 2021, 956, 122129. [Google Scholar] [CrossRef]
- Nan, A.; Bunge, A.; Cîrcu, M.; Petran, A.; Hădade, N.D.; Filip, X. Poly(benzofuran-co-arylacetic acid)—A new type of highly functionalized polymers. Polym. Chem. 2017, 8, 3504–3514. [Google Scholar] [CrossRef]
- Zhou, J.; Hsu, T.-G.; Wang, J. Mechanochemical Degradation and Recycling of Synthetic Polymers. Angew. Chem. Int. Ed. 2023, 62, e202300768. [Google Scholar] [CrossRef]
- Aydonat, S.; Hergesell, A.H.; Seitzinger, C.L.; Lennarz, R.; Chang, G.; Sievers, C.; Meisner, J.; Vollmer, I.; Göstl, R. Leveraging mechanochemistry for sustainable polymer degradation. Polym. J. 2024, 56, 249–268. [Google Scholar] [CrossRef]
- Lazarescu, A.; Shova, S.; Bartolome, J.; Alonso, P.; Arauzo, A.; Balu, A.M.; Simonov, Y.A.; Gdaniec, M.; Turta, C.; Filotih, G.; et al. Heteronuclear (Co–Ca, Co–Ba) 2,3-pyridinedicarboxylate complexes: Synthesis, structure and physico-chemical properties. Dalton Trans. 2011, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Terenti, N.; Nedelko, N.; Melnic, E.; Preda, S.; Fruth, V.; Latuch, J.; Kravtsov, V.C.; Slawska-Waniewska, A.; Lazarescu, A.; Lozan, V. Heterometallic Co–Sr–2,3-pyridinedicarboxylate complex and its related perovskite oxide: Synthesis, structural characterization and magnetic properties. J. Solid State Chem. 2024, 331, 124538. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Su, P.; Jiang, Z.; Yang, Q.; Li, C. Preparation of Zn–Co–O mixed-metal oxides nanoparticles through a facile coordination polymer based process. RSC Adv. 2013, 3, 4081–4085. [Google Scholar] [CrossRef]
- Chen, S.; Xue, M.; Li, Y.; Pan, Y.; Zhu, L.; Qiu, S. Rational design and synthesis of NixCo3−xO4 nanoparticles derived from multivariate MOF-74 for supercapacitors. J. Mater. Chem. A 2015, 3, 20145–20152. [Google Scholar] [CrossRef]
- Wei, X.; Ma, R.; Luo, T. Thermal Conductivity of Polyelectrolytes with Different Counterions. J. Phys. Chem. C 2020, 124, 4483–4488. [Google Scholar] [CrossRef]
- Lee, G.S.; Lee, H.S.; Kim, N.; Shin, H.G.; Hwang, Y.H.; Lee, S.J.; Kim, J.G. Mechanochemical Synthesis of Ionic Polymers: Solid-State Ball-Milling Polymerization for Unrestricted Solubility Enabling Copolymerization of Immiscible Monomers. Macromolecules 2024, 57, 9408–9418. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.G. Mechanochemical synthesis of poly(trimethylene carbonate)s: An example of rate acceleration. Beilstein J. Org. Chem. 2019, 15, 963–970. [Google Scholar] [CrossRef]
- Thorwirth, R.; Bernhardt, F.; Stolle, A.; Ondruschka, B.; Asghari, J. Switchable Selectivity during Oxidation of Anilines in a Ball Mill. Chem.-Eur. J. 2010, 16, 13236–13242. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2010, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ioffe, M.S.; Borod’ko, Y.G. Dependence of satellite intensity in X-ray photoelectron spectra of Cu(II) complexes on the spin density on copper. J. Electron Spectrosc. Relat. Phenom. 1977, 11, 235–238. [Google Scholar] [CrossRef]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO. J. Appl. Phys. 1982, 53, 1173. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef] [PubMed]




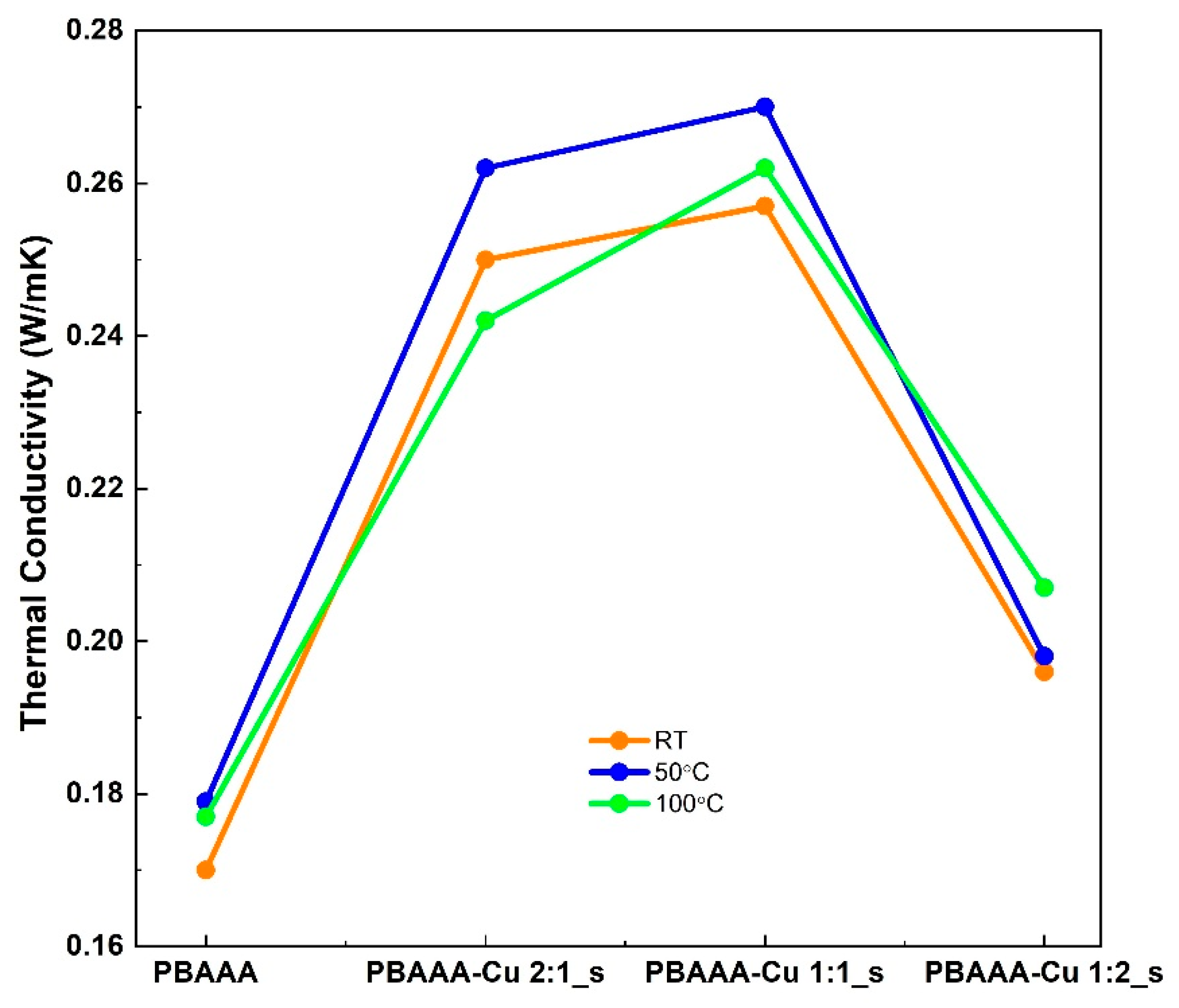

| Sample | Temperature Range (°C) | Mass Loss (%) | Decomposition Temperature (°C) | Residue |
|---|---|---|---|---|
| PBAAA-Co 1:1_s | 25–200 | 11.2 | 893 | 10.6% |
| 201–450 | 77.6 | |||
| 450–893 | 0.6 | |||
| PBAAA-Cu 2:1_s | 25–150 | 7 | 485 | 14.1% |
| 151–310 | 29.0 | |||
| 311–490 | 49.9 | |||
| PBAAA-Cu 1:1_s | 25–150 | 6.5 | 478 | 18.5% |
| 151–320 | 29.3 | |||
| 321–475 | 45.7 | |||
| PBAAA-Cu 1:2_s | 25–208 | 12.7 | 483 | 18.6% |
| 209–300 | 22.7 | |||
| 301–485 | 46.0 | |||
| PBAAA-Zn 1:1_s | 25–150 | 7.5 | 520 | 14.4% |
| 151–420 | 28.6 | |||
| 421–520 | 49.5 |
| Sample | Temperature Range (°C) | Mass Loss (%) | Decomposition Temperature (°C) | Residue |
|---|---|---|---|---|
| PAAA-Co 1:1_s | 25–130 | 15.0 | 455 (890) | 18.5% |
| 131–245 | 8.5 | |||
| 246–455 | 56.8 | |||
| 455–490 | 1.2 | |||
| PAAA-Cu 1:1_s | 25–127 | 4.5 | 495 | 26.0% |
| 128–220 | 9.9 | |||
| 221–320 | 11.0 | |||
| 321–370 | 16.7 | |||
| 371–495 | 31.9 | |||
| PAAA-Zn 1:1_s | 25–198 | 8.1 | 545 | 17.7% |
| 151–545 | 74.2 |
| Sample | Temperature Range (°C) | Mass Loss (%) | Decomposition Temperature (°C) | Residue |
|---|---|---|---|---|
| PBAAA-Co 1h_m | 25–126 | 3.7 | 860 | 15.3% |
| 127–290 | 20.8 | |||
| 246–455 | 57.1 | |||
| 456–860 | 3.1 | |||
| PBAAA-Cu 1h_m | 25–126 | 5.5 | 870 | 15.6% |
| 127–308 | 16.9 | |||
| 309–480 | 59.9 | |||
| 371–870 | 2.1 | |||
| PBAAA-Cu 2h_m | 25–125 | 8.1 | 883 | 10.3% |
| 151–320 | 35.8 | |||
| 321–480 | 44.6 | |||
| 481–883 | 1.2 | |||
| PBAAA-Cu 4h_m | 25–179 | 8.6 | 929 | 6.6% |
| 180–339 | 10.8 | |||
| 340–417 | 11.9 | |||
| 418–587 | 54.6 | |||
| 588–929 | 7.4 | |||
| PBAAA-Zn 1h_m | 25–172 | 10.1 | 992 | 6.6% |
| 173–354 | 11.3 | |||
| 355–437 | 22.8 | |||
| 438–512 | 9.2 | |||
| 513–992 | 40.8 |
| Ligand | Counter Ion | |||
|---|---|---|---|---|
| Co2+ | Cu2+ | Zn2+ | ||
| PBAAA | Ionic radius | 0.74 | 0.65 | 0.6 |
| CN | 6 | 5 | 4 | |
| PAAA | Ionic radius | 0.74 | 0.57 | 0.6 |
| CN | 6 | 4 | 4 | |
| Sample | Species | Peak | Binding Energy (eV) | Spin–Orbit Splitting (eV) | At Conc. (at%) |
|---|---|---|---|---|---|
| PBAAA-Cu 1:1_s | Cu+ | 3/2 | 932.3 | 20.8 | 17 |
| 1/2 | 953.1 | ||||
| Cu2+ | 3/2 | 934.6 | 20.2 | 82 | |
| 1/2 | 954.8 | ||||
| PAAA-Cu 1:1_s | Cu2+ | 3/2 | 934.2 | 19.9 | 100 |
| 1/2 | 954.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, T.; Nan, A.; Dan, M.; Miclǎuş, M.; Terenti, N. Exploring the Properties of Organometallic Lactone-Containing Poly(benzofuran-co-arylacetic Acid): Traditional Synthesis Versus Mechanosynthesis. Polymers 2025, 17, 2511. https://doi.org/10.3390/polym17182511
Radu T, Nan A, Dan M, Miclǎuş M, Terenti N. Exploring the Properties of Organometallic Lactone-Containing Poly(benzofuran-co-arylacetic Acid): Traditional Synthesis Versus Mechanosynthesis. Polymers. 2025; 17(18):2511. https://doi.org/10.3390/polym17182511
Chicago/Turabian StyleRadu, Teodora, Alexandrina Nan, Monica Dan, Maria Miclǎuş, and Natalia Terenti. 2025. "Exploring the Properties of Organometallic Lactone-Containing Poly(benzofuran-co-arylacetic Acid): Traditional Synthesis Versus Mechanosynthesis" Polymers 17, no. 18: 2511. https://doi.org/10.3390/polym17182511
APA StyleRadu, T., Nan, A., Dan, M., Miclǎuş, M., & Terenti, N. (2025). Exploring the Properties of Organometallic Lactone-Containing Poly(benzofuran-co-arylacetic Acid): Traditional Synthesis Versus Mechanosynthesis. Polymers, 17(18), 2511. https://doi.org/10.3390/polym17182511






