Antimicrobial Evaluation of Chlorophyll-Containing Nettle Extract Both in Free Form and Incorporated into Poly-3-Hydroxybutyrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning of Chl-PHB
2.3. Microscopy of Chl-PHB
2.4. Mechanical Properties
2.5. Differential Scanning Calorimetry
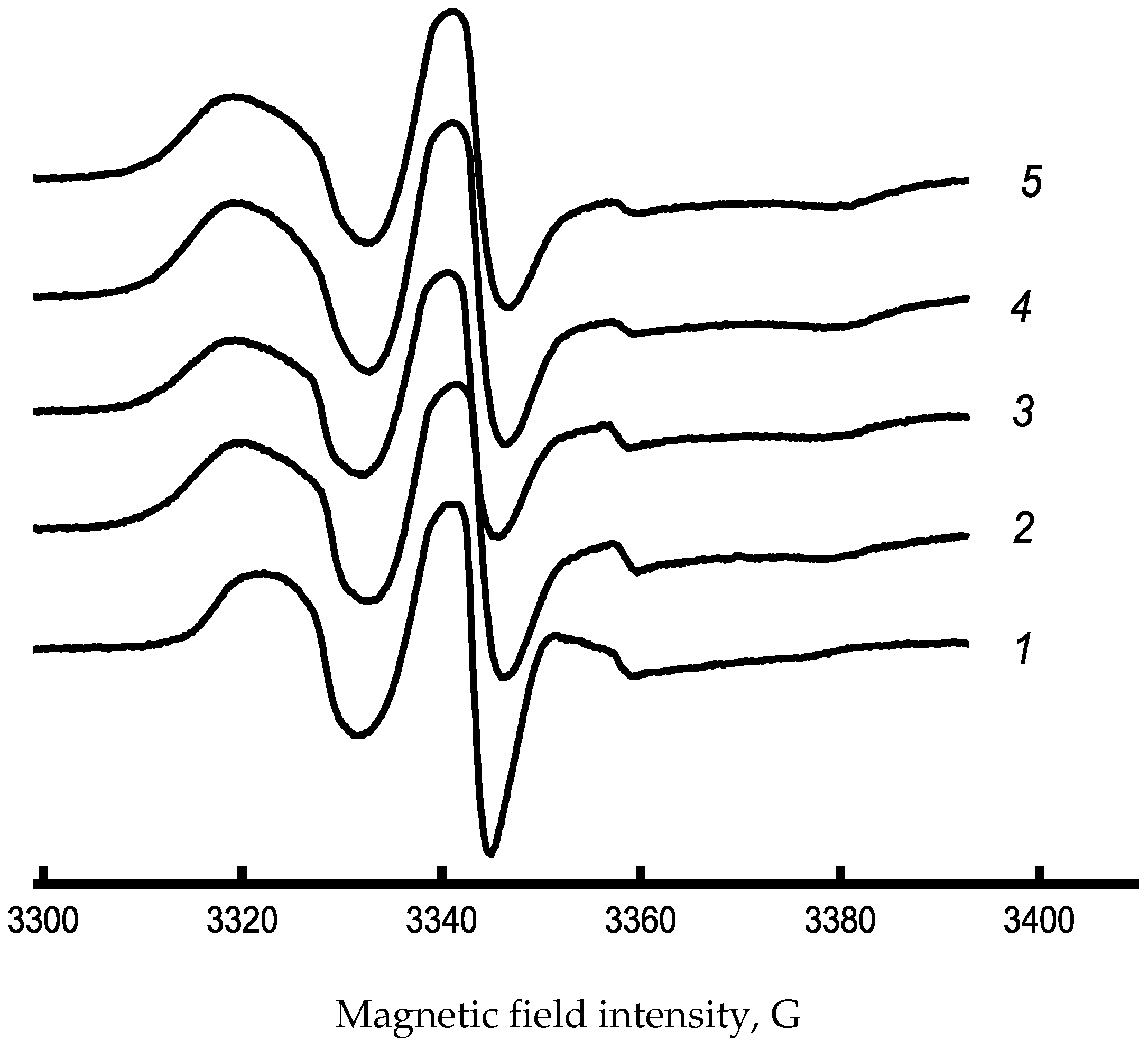
2.6. Electron Paramagnetic Resonance (EPR)
2.7. Fluorescent Spectroscopy
2.8. Stock Solutions Preparation
2.9. Irradiation Source
2.10. In Vitro Chl Release from PHB
2.11. In Vitro Antimicrobial Activity Assessment
2.11.1. Evaluation of the Minimum Inhibitory Concentrations (MICs)
2.11.2. The Microorganism Growth Inhibition Degree Calculation
2.11.3. Inhibition Zone Diameters (IZDs) Determination
2.11.4. Phase Contrast Transmission Electron Microscopy (TEM)
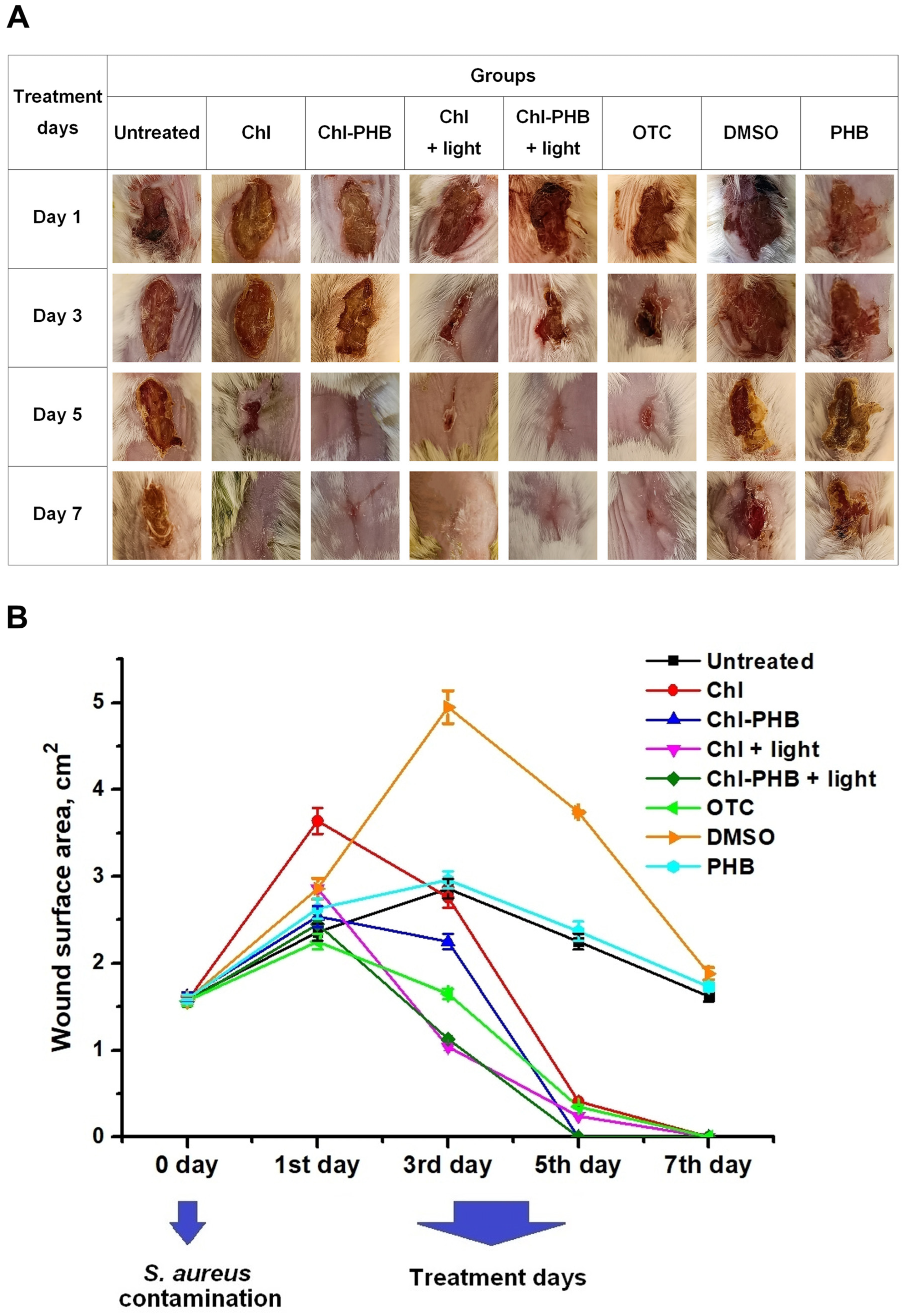
2.12. Assessment of Wound Healing and Antimicrobial Activity in Mouse Model
2.13. Statistical Analysis
3. Results and Discussion
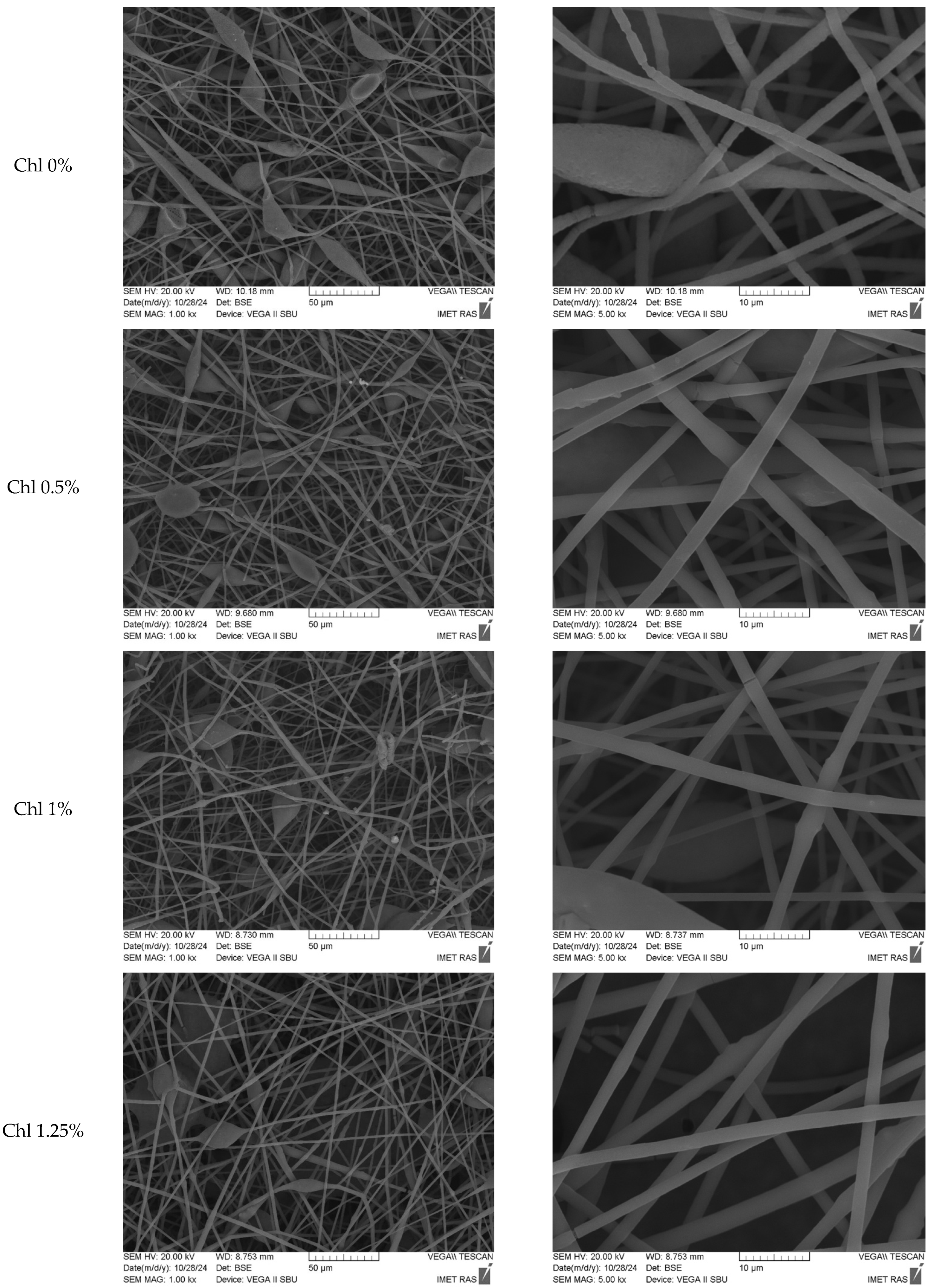
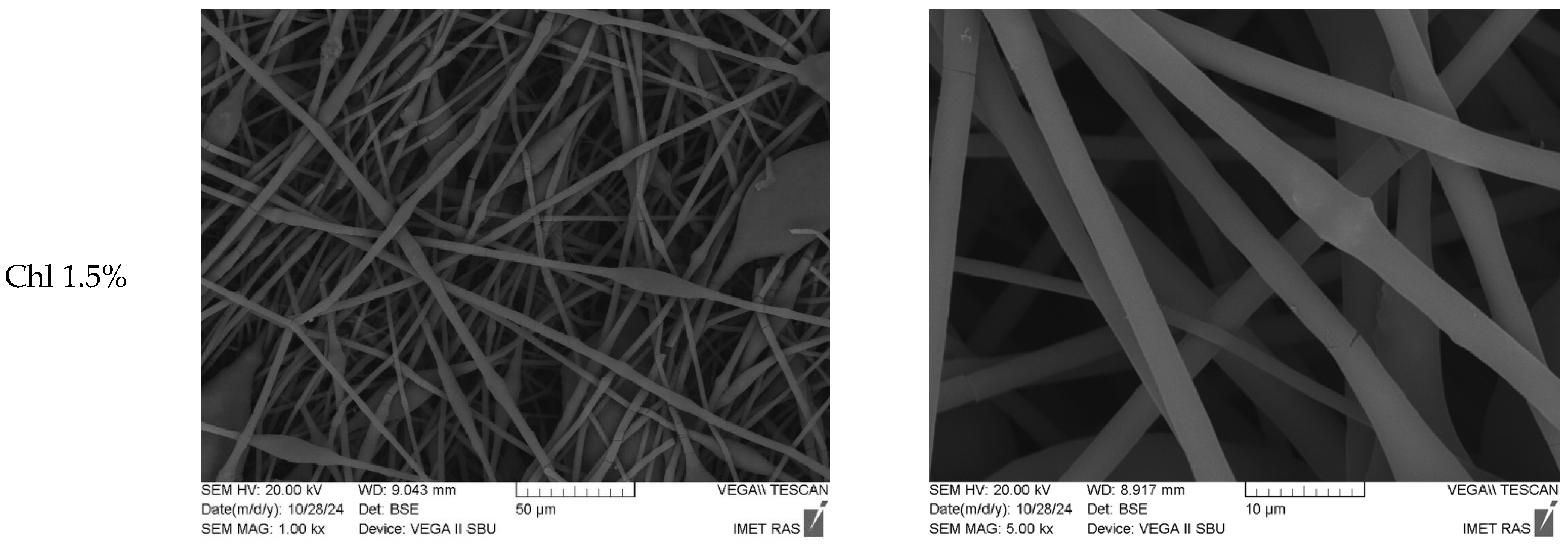
3.1. Characterization of Chl-PHB Matrix
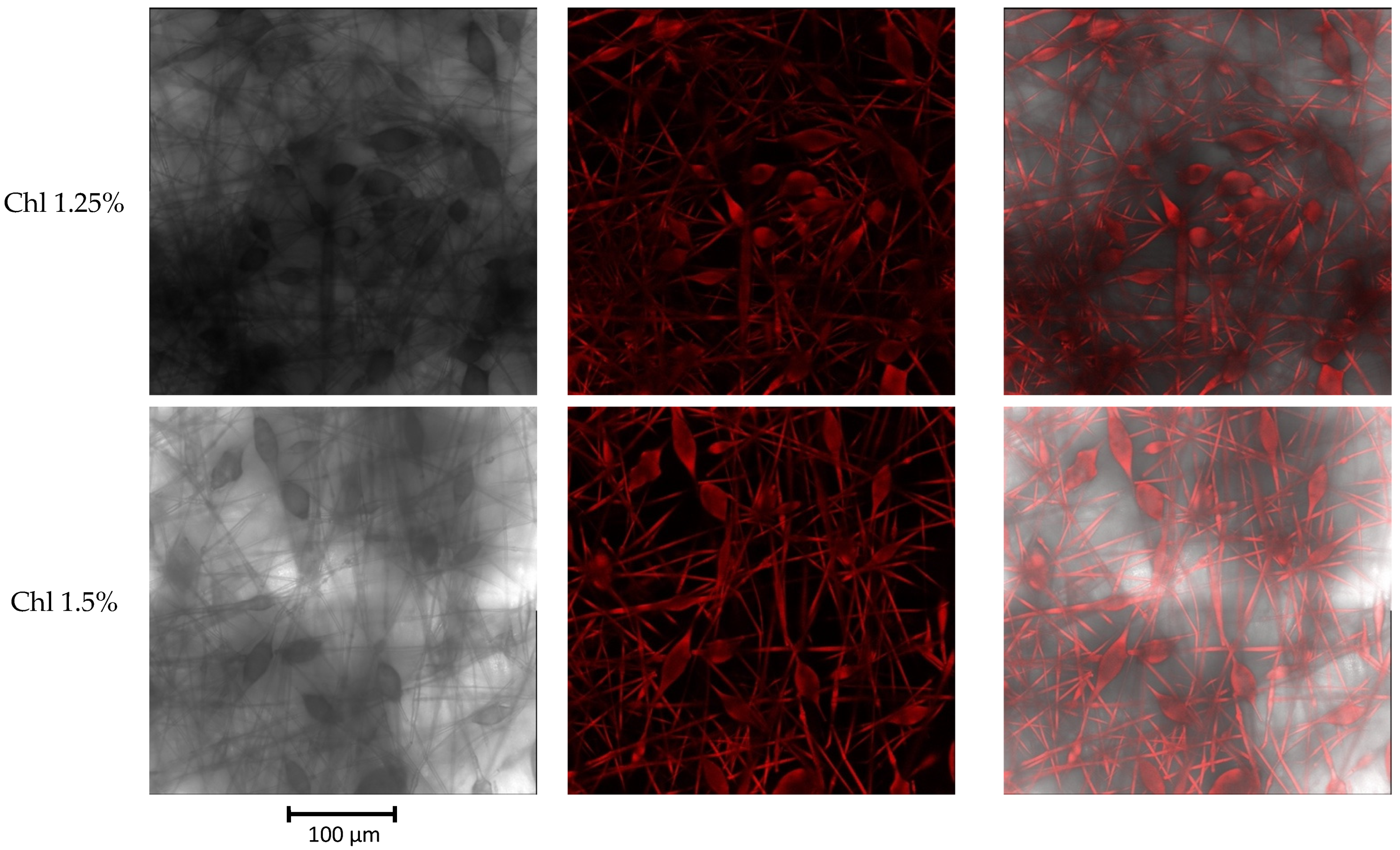
3.2. Fluorescence of Chl-PHB Matrix
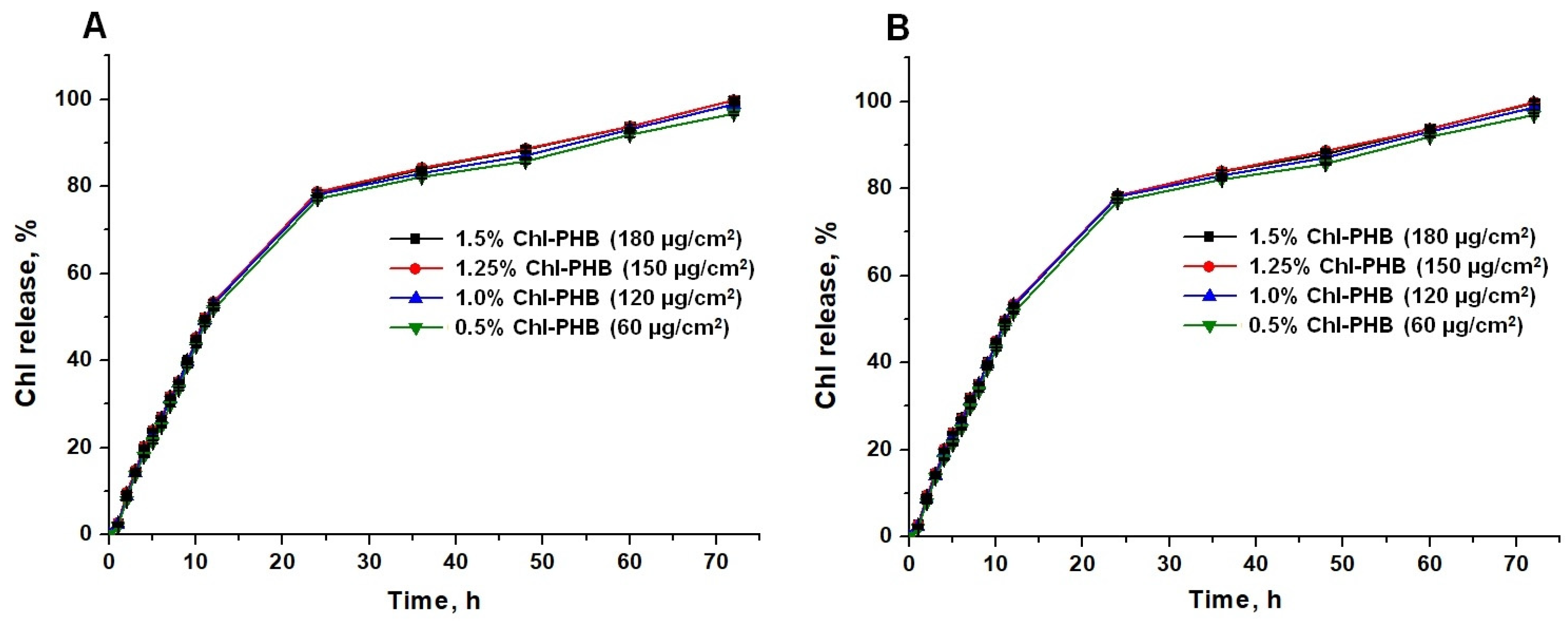
3.3. In Vitro Chl Release from Polymeric Matrix
3.4. In Vitro Antimicrobial Activity of Chl and Chl-PHB
3.4.1. MICs Determination Results
3.4.2. Study of Microorganism Growth Inhibition
3.4.3. Inhibition Zone Diameters (IZDs) Evaluation
3.4.4. Phase Contrast TEM Results
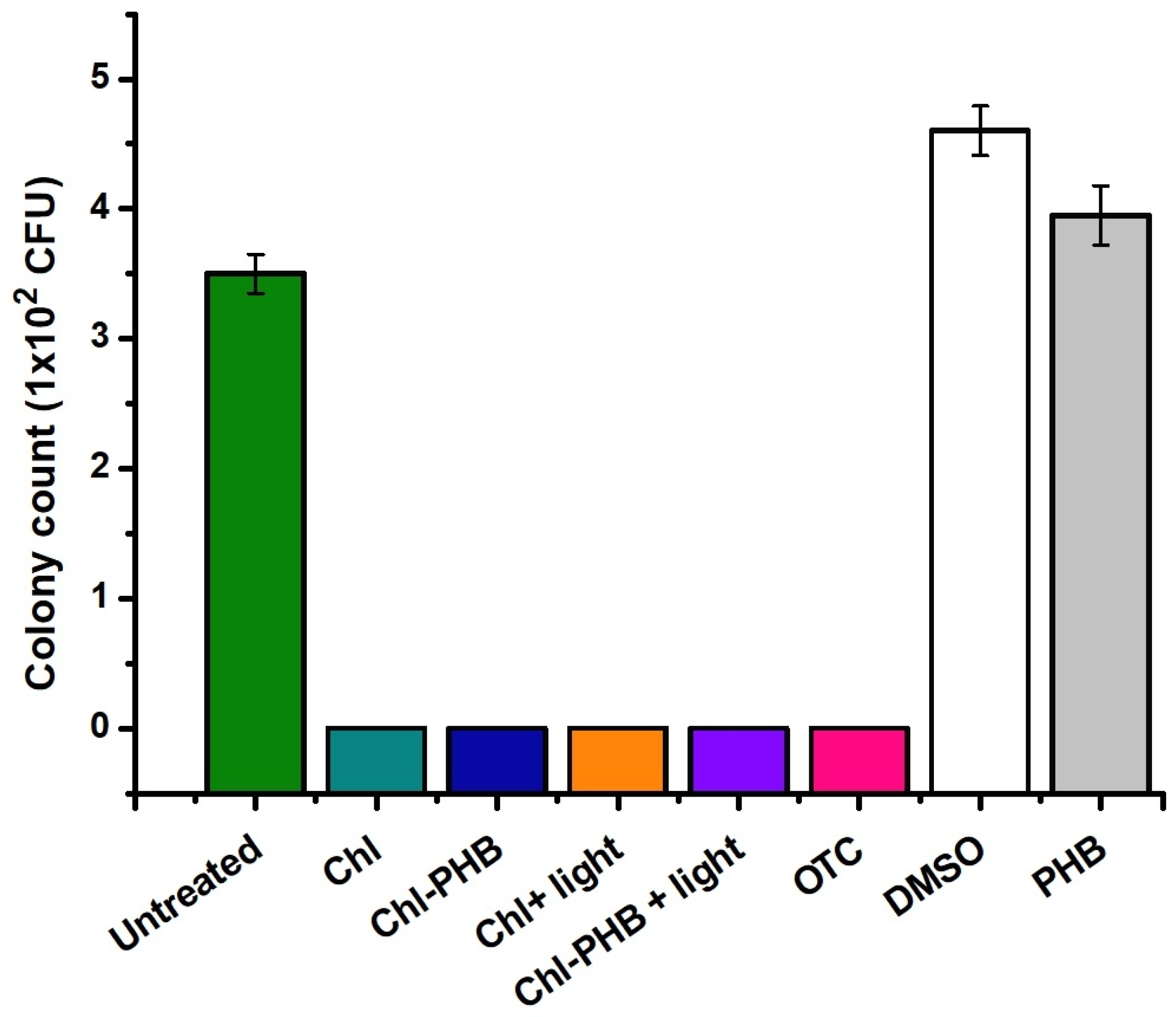
3.5. In Vivo Chl and Chl-PHB Wound Healing Evaluation and Antimicrobial Efficacy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ning, Q.; Deng, Z.; Zhang, M.; You, J. Role of Environmental Stresses in Elevating Resistance Mutations in Bacteria: Phenomena and Mechanisms. Environ. Pollut. 2022, 307, 119603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Zhang, H.; Zhuo, Y.; Weng, Y.; Li, S.; Yu, D. Surfactin-Methylene Blue Complex under LED Illumination for Antibacterial Photodynamic Therapy: Enhanced Methylene Blue Transcellular Accumulation Assisted by Surfactin. Colloids Surf. B Biointerfaces 2021, 207, 111974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Chen, C.; Lu, J.; Liu, C.; Ning, Y.; Lu, F. Berberine@AgNPs@Carboxylated Chitosan Hydrogel Dressing with Immunomodulatory and Anti-Biofilm Properties Promotes Wound Repair in Drug-Resistant Bacterial Infections. Int. J. Biol. Macromol. 2025, 315, 144496. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Sun, M.; Quan, G.; Gong, B.; Kang, Q.; Xiao, L.; Zhu, W.; Wang, H. Preparation of New Natural Hemp Fiber-Based Antibacterial Hydrogel Dressing and Its Performance in Promoting Wound Healing of Bacterial Infection. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137008. [Google Scholar] [CrossRef]
- Imtiaz, M.; Akram, H.; Arshad, B.; Shehzad, A.; Haider, S.; Alam, K.; Khan, S.U.; Mansoor, M.A.; Iqbal, M. Bis-Imidazolium Ionic Liquid and Silver Nanoparticles Mediated Synthesis of Alginate Hydrogels for Bacterial Skin Infection and Wound Healing Applications. Int. J. Biol. Macromol. 2025, 306, 141420. [Google Scholar] [CrossRef]
- Hafez, E.; Shaban, S.M.; Kim, M.-H.; Elbalaawy, A.Y.; Pyun, D.-G.; Kim, D.-H. Fabrication of Activated Carbon Fiber Functionalized Core–Shell Silver Nanoparticles Based in Situ and Low-Cost Technology for Wound Dressings with an Enhanced Antimicrobial Activity and Cell Viability. J. Mol. Liq. 2022, 360, 119561. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, J.; Bai, X.; Yang, Z.; Guo, H.; Wu, L.; Liu, C.; Yu, X.; Du, J. Construction of a Thiophene-Based Conjugated Polymer/TP-PCN S-Scheme to Enhance Visible-Light-Driven Photocatalytic Activity: Promotion of Wound Healing in Super-Resistant Bacterial Infections. J. Hazard. Mater. 2025, 488, 137429. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Hou, S.; Zhang, L.; Liu, X.; Yang, G.; Wang, Y.; Hao, M.; Zhang, W.; Wang, J.; et al. A Light-Responsive Multilayered 3D Porous Ga2O3 Hydrogel for Photocatalytic Antibacterial Therapy Promoting Healing of MDR S. aureus-Infected Wounds. J. Mater. Sci. Technol. 2025, 225, 188–202. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, J.; Xin, L.; Li, Y.; Huang, H.; Miao, W. Photosensitizer-Synergized g-Carbon Nitride Nanosheets with Enhanced Photocatalytic Activity for Eradicating Drug-Resistant Bacteria and Promoting Wound Healing. Chin. Chem. Lett. 2025, 36, 110003. [Google Scholar] [CrossRef]
- Gonçalves, A.S.C.; Leitão, M.M.; Fernandes, J.R.; Saavedra, M.J.; Pereira, C.; Simões, M.; Borges, A. Photodynamic Activation of Phytochemical-Antibiotic Combinations for Combatting Staphylococcus aureus from Acute Wound Infections. J. Photochem. Photobiol. B 2024, 258, 112978. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, I.; Silva, D.; Conde, T.; Maurício, T.; Cardoso, H.; Pereira, H.; Bartolomeu, M.; Vieira, C.; Domingues, M.R.; Almeida, A. Insight into the Efficiency of Microalgae’ Lipidic Extracts as Photosensitizers for Antimicrobial Photodynamic Therapy against Staphylococcus aureus. J. Photochem. Photobiol. B 2024, 259, 112997. [Google Scholar] [CrossRef] [PubMed]
- Pallavali, R.R.; Avula, S.; Degati, V.L.; Penubala, M.; Damu, A.G.; Durbaka, V.R.P. Data of Antibacterial Activity of Plant Leaves Crude Extract on Bacterial Isolates of Wound Infections. Data Brief. 2019, 24, 103896. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Antioxidant and Antibacterial Properties of Guava Leaf Extracts as Affected by Solvents Used for Prior Dechlorophyllization. J. Food Biochem. 2018, 42, e12600. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Tolaema, A.; Chaikhemarat, P.; Rawdkuen, S. Antioxidant and Anti-Cytotoxicity Effect of Phenolic Extracts from Psidium guajava Linn. Leaves by Novel Assisted Extraction Techniques. Foods 2023, 12, 2336. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant Property of Green Tea Polyphenols Epicatechin and Epigallocatechin-3-Gallate: Implications for Anticancer Properties. Toxicol. Vitr. 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shahidi, S.A.; Safari, R.; Motamedzadegan, A.; Ghorbani-HasanSaraei, A. Evaluation of Stability and Antibacterial Properties of Extracted Chlorophyll from Alfalfa (Medicago sativa L.). Food Chem. Toxicol. 2022, 163, 112980. [Google Scholar] [CrossRef]
- Maekawa, L.E.; Lamping, R.; Marcacci, S.; Maekawa, M.Y.; Nassri, M.R.G.; Koga-Ito, C.Y. Antimicrobial Activity of Chlorophyll-Based Solution on Candida Albicans and Enterococcus Faecalis. Rev. Sul-Bras. De Odontol. 2008, 4, 36–40. [Google Scholar] [CrossRef]
- Gruznov, D.V.; Gruznova, O.A.; Lobanov, A.V.; Shcherbakova, G.S.; Chesnokova, I.P. Antibacterial Activity of Chlorophyll Polymeric Form against Test Cultures S. aureus and E. coli. Proc. BIO Web Conf. 2024, 83, 02001. [Google Scholar] [CrossRef]
- Arslan, E.; Çobanoğlu, Ş.; YAZICI, A. Antimicrobial Activity of Pigments Extracted from Auxenochlorella Protothecoides SC3 against Pseudomonas Aeruginosa. Türk Doğa Ve Fen. Derg. 2021, 10, 163–167. [Google Scholar] [CrossRef]
- Vesenick, D.C.; Aparecida De Paula, N.; Niwa, A.M.; Mantovani, M.S. Evaluation of the Effects of Chlorophyllin on Apoptosis Induction, Inhibition of Cellular Proliferation and MRNA Expression of CASP8, CASP9, APC and $-Catenin. Curr. Res. J. Biol. Sci. 2012, 4, 315–322. [Google Scholar]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Proc. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; Sant’Anna, C. Impact of Culture Conditions on the Chlorophyll Content of Microalgae for Biotechnological Applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Li, X.; Ge, B.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Structure, Functions and Potential Medicinal Effects of Chlorophylls Derived from Microalgae. Mar. Drugs 2024, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Absolute Configuration and the Structure of Chlorophyll. Nature 1967, 216, 151–152. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Wu, W.-B.; Zhang, H.; Liu, H.-C.; Dong, S.-H.; Wu, Y.; Ding, J.; Yue, J.-M. Ivorenoids A-F: Limonoids from Khaya Ivorensis. Tetrahedron 2014, 70, 3570–3575. [Google Scholar] [CrossRef]
- Qasem, J.R.; Abu-Blan, H.A. Fungicidal Activity of Some Common Weed Extracts against Different Plant Pathogenic Fungi. J. Phytopathol. 1996, 144, 157–161. [Google Scholar] [CrossRef]
- Sarkar, G.; Jatar, N.; Goswami, P.; Cyriac, R.; Suthindhiran, K.; Jayasri, M.A. Combination of Different Marine Algal Extracts as Biostimulant and Biofungicide. J. Plant Nutr. 2018, 41, 1163–1171. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging Nettle, Urtica dioica L.: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Abd-Nikfarjam, B.; Abbasi, M.; Memarzadeh, M.; Farzam, S.A.; Jamshidian, A.; Dolati-Somarin, A. Therapeutic Efficacy of Urtica dioica and Evening Primrose in Patients with Rheumatoid Arthritis: A Randomized Double-Blind, Placebo-Controlled Clinical Trial. J. Herb. Med. 2022, 32, 100556. [Google Scholar] [CrossRef]
- Bonetti, G.; Tedeschi, P.; Meca, G.; Bertelli, D.; Mañes, J.; Brandolini, V.; Maietti, A. In Vitro Bioaccessibility, Transepithelial Transport and Antioxidant Activity of Urtica dioica L. Phenolic Compounds in Nettle Based Food Products. Food Funct. 2016, 7, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, R.; Qamar, H.M.-U.; Khan, S.; Salma, U.; Khan, T.; Shah, A.J. Mechanisms Underlying the Antihypertensive Properties of Urtica dioica. J. Transl. Med. 2016, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Biregani, Z.M.; Hassanloofard, Z.; Salami, M. Nettle (Urtica dioica L.) as a Functional Bioactive Food Ingredient: Applications in Food Products and Edible Films, Characterization, and Encapsulation Systems. Trends Food Sci. Technol. 2024, 147, 104421. [Google Scholar] [CrossRef]
- Shabani, M.; Ghorbani-HasanSaraei, A.; Shariatifar, N.; Savadkoohi, F.; Shahidi, S.A. Effect of Urtica dioica L. Essential Oil (Forms of Free and Nanoliposome) on Some Inoculated Pathogens (Escherichia coli and Listeria monocytogenes) in Minced Camel Meat. Food Chem. X 2023, 20, 101050. [Google Scholar] [CrossRef]
- Albogami, S. Urtica dioica Leaf Extract Attenuates Oxidative Stress Induced by Environment Pollutant Benzo[a] Pyrene in Mouse Testicular Tissues. Open Biotechnol. J. 2023, 1–14. [Google Scholar] [CrossRef]
- Moula, N.; Sadoudi, A.; Touazi, L.; Leroy, P.; Geda, F. Effects of Stinging Nettle (Urtica dioica) Powder on Laying Performance, Egg Quality, and Serum Biochemical Parameters of Japanese Quails. Anim. Nutr. 2019, 5, 410–415. [Google Scholar] [CrossRef]
- Abbasinia, S.; Monfared-Hajishirkiaee, R.; Ehtesabi, H. Nanocomposite Sponge Based on Sodium Alginate, Polyvinyl Alcohol, Carbon Dots, and Woolly Hedge Nettle Antibacterial Extract for Wound Healing. Ind. Crops Prod. 2024, 214, 118554. [Google Scholar] [CrossRef]
- Alizadeh-Otaghvar, H.R.; Moradi, F.; Sadigh, N.; Raoofi, A.; Darabi, S.; Rustamzadeh, A.; Ghadimi, T.; Rezaie, M.J.; Seidkhani, E.; Ahadi, R.; et al. Silk Fibroin and Nettle Extract Promote Wound Healing in a Rat Model: A Histological and Morphometrical Study. Acta Histochem. 2022, 124, 151930. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mohammadnabi, S. Characterizing the Novel Surfactant-Stabilized Nanoemulsions of Stinging Nettle Essential Oil: Thermal Behaviour, Storage Stability, Antimicrobial Activity and Bioaccessibility. J. Mol. Liq. 2016, 224, 1332–1340. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Characterization of Active Films of Chitosan Containing Nettle Urtica dioica L. Extract: Spectral and Water Properties, Microstructure, and Antioxidant Activity. Int. J. Biol. Macromol. 2023, 253, 127318. [Google Scholar] [CrossRef] [PubMed]
- Belykh, D.V.; Zainullina, E.N.; Startseva, O.M. 1H NMR Quantitative Determination of Chlorophyll a in the Spirulina Biomass. Macroheterocycles 2020, 13, 260–268. [Google Scholar] [CrossRef]
- Alkarri, S.; Bin Saad, H.; Soliman, M. On Antimicrobial Polymers: Development, Mechanism of Action, International Testing Procedures, and Applications. Polymers 2024, 16, 771. [Google Scholar] [CrossRef]
- Volova, T.G.; Nekrasov, Y.P.; Shishatskaya, E.I.; Puzyr’, A.P.; Gordeev, S.A. Characteristics of Products Based on Polyoxyalkanoates—Degradable Natural Polyesters. Int. Polym. Sci. Technol. 2003, 30, 9–13. [Google Scholar] [CrossRef]
- Obulisamy, P.K.; Mehariya, S. Polyhydroxyalkanoates from Extremophiles: A Review. Bioresour. Technol. 2021, 325, 124653. [Google Scholar] [CrossRef]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyhydroxybutyrate (PHB): A Standout Biopolymer for Environmental Sustainability. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2019; Volume 4. [Google Scholar]
- Quispe, M.M.; Villanueva, M.E.; Copello, G.J.; López, O.V.; Villar, M.A. Films of Poly(Hydroxybutyrate) (PHB) and Copper with Antibacterial Activity. Polymers 2023, 15, 2907. [Google Scholar] [CrossRef]
- Rech, C.R.; da Silva Brabes, K.C.; Bagnara e Silva, B.E.; Bittencourt, P.R.S.; Koschevic, M.T.; da Silveira, T.F.S.; Martines, M.A.U.; Caon, T.; Martelli, S.M. Biodegradation of Eugenol-Loaded Polyhydroxybutyrate Films in Different Soil Types. Case Stud. Chem. Environ. Eng. 2020, 2, 100014. [Google Scholar] [CrossRef]
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun Antimicrobial Films of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef]
- Beran, M.; Horna, A.; Vorisek, V.; Berkova, E.; Korinkova, R.; Trousil, V.; Hrubanova, M. Antimicrobial Polyhydroxybutyrate Submicron Fiber Mat Loaded with Extract of Hypericum Perforatum. J. Plant Biotechnol. 2022, 49, 257–270. [Google Scholar] [CrossRef]
- Heidarkhan Tehrani, A.; Zadhoush, A.; Karbasi, S.; Sadeghi-Aliabadi, H. Scaffold Percolative Efficiency: In Vitro Evaluation of the Structural Criterion for Electrospun Mats. J. Mater. Sci. Mater. Med. 2010, 21, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Zhang, K.; EL-Newehy, M.; Abdulhameed, M.M.; Mo, X.; Cao, L.; Wang, Y. Application of Electrospinning and 3D-Printing Based Bilayer Composite Scaffold in the Skull Base Reconstruction during Transnasal Surgery. Colloids Surf. B Biointerfaces 2025, 245, 114337. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Asl, M.A.; Karbasi, S.; Beigi-Boroujeni, S.; Benisi, S.Z.; Saeed, M. Polyhydroxybutyrate-Starch/Carbon Nanotube Electrospun Nanocomposite: A Highly Potential Scaffold for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 223, 524–542. [Google Scholar] [CrossRef]
- Raza, Z.A.; Noor, S.; Khalil, S. Recent Developments in the Synthesis of Poly(Hydroxybutyrate) Based Biocomposites. Biotechnol. Prog. 2019, 35, e2855. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Synthetic-Based Blended Electrospun Scaffolds in Tissue Engineering Applications. J. Mater. Sci. 2022, 57, 4020–4079. [Google Scholar] [CrossRef]
- Mohammadalipour, M.; Behzad, T.; Karbasi, S.; Mohammadalipour, Z. Optimization and Characterization of Polyhydroxybutyrate/Lignin Electro-Spun Scaffolds for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 317–334. [Google Scholar] [CrossRef]
- Cherpinski, A.; Szewczyk, P.K.; Gruszczyński, A.; Stachewicz, U.; Lagaron, J.M. Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique. Nanomaterials 2019, 9, 262. [Google Scholar] [CrossRef]
- Tyubaeva, P.; Varyan, I.; Krivandin, A.; Shatalova, O.; Karpova, S.; Lobanov, A.; Olkhov, A.; Popov, A. The Comparison of Advanced Electrospun Materials Based on Poly(-3-Hydroxybutyrate) with Natural and Synthetic Additives. J. Funct. Biomater. 2022, 13, 23. [Google Scholar] [CrossRef]
- Severyukhina, A.N.; Petrova, N.V.; Smuda, K.; Terentyuk, G.S.; Klebtsov, B.N.; Georgieva, R.; Bäumler, H.; Gorin, D.A. Photosensitizer-Loaded Electrospun Chitosan-Based Scaffolds for Photodynamic Therapy and Tissue Engineering. Colloids Surf. B Biointerfaces 2016, 144, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H. Polymeric Nanomaterials for Efficient Delivery of Antimicrobial Agents. Pharmaceutics 2021, 13, 2108. [Google Scholar] [CrossRef]
- Havlíček, K.; Svobodová, L.; Bakalova, T.; Lederer, T. Influence of Electrospinning Methods on Characteristics of Polyvinyl Butyral and Polyurethane Nanofibres Essential for Biological Applications. Mater. Des. 2020, 194, 108898. [Google Scholar] [CrossRef]
- Tyubaeva, P.M.; Varyan, I.A.; Romanov, R.R.; Merzlikin, V.A.; Gruznova, O.A.; Gruznov, D.V.; Popov, N.I.; Shcherbakova, G.S.; Shuteeva, E.N.; Chesnokova, I.P.; et al. Electrospinning of Poly-3-Hydroxybutyrate Fibers Loaded with Chlorophyll for Antibacterial Purposes. Polymers 2024, 16, 3221. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, A.V.; Kobzev, G.I.; Davydov, K.S.; Komissarov, G.G. Generation of Reactive Oxygen Species under Singlet Oxygen Photosensitivity by Chlorophyll and Its Analogs. Russ. J. Phys. Chem. B 2014, 8, 277–283. [Google Scholar] [CrossRef]
- Tyubaeva, P.M.; Gasparyan, K.G.; Fedotov, A.Y.; Lobzhanidze, P.V.; Baranov, O.V.; Egorov, A.A.; Sirotinkin, V.P.; Komlev, V.S.; Olkhov, A.A. Development of Nonwoven Fibrous Materials Based on Poly-3-Hydroxybutyrate with a High Content of α-Tricalcium Phosphate. Polymers 2023, 15, 3167. [Google Scholar] [CrossRef]
- Pradhan, S.; Dikshit, P.K.; Moholkar, V.S. Production, Ultrasonic Extraction, and Characterization of Poly (3-Hydroxybutyrate) (PHB) Using Bacillus Megaterium and Cupriavidus Necator. Polym. Adv. Technol. 2018, 29, 2392–2400. [Google Scholar] [CrossRef]
- Safari, S.; van de Ven, T.G.M. Effect of Crystallization Conditions on the Physical Properties of a Two-Layer Glassine Paper/Polyhydroxybutyrate Structure. J. Mater. Sci. 2015, 50, 3686–3696. [Google Scholar] [CrossRef]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Gnatowski, M.; Kowalczuk, M.; Jedliński, Z. Polymer Blends of Natural Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and a Synthetic Atactic Poly(3-Hydroxybutyrate). Characterization and Biodegradation Studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Liang, Z.; Freed, J.H. An Assessment of the Applicability of Multifrequency ESR to Study the Complex Dynamics of Biomolecules. J. Phys. Chem. B 1999, 103, 6384–6396. [Google Scholar] [CrossRef]
- Sezer, D.; Freed, J.H.; Roux, B. Simulating Electron Spin Resonance Spectra of Nitroxide Spin Labels from Molecular Dynamics and Stochastic Trajectories. J. Chem. Phys. 2008, 128, 165106. [Google Scholar] [CrossRef]
- García-Sánchez, M.A.; Serratos, I.N.; Sosa, R.; Tapia-Esquivel, T.; González-García, F.; Rojas-González, F.; Tello-Solís, S.R.; Palacios-Enriquez, A.Y.; Esparza Schulz, J.M.; Arrieta, A. Chlorophyll a Covalently Bonded to Organo-Modified Translucent Silica Xerogels: Optimizing Fluorescence and Maximum Loading. Molecules 2016, 21, 961. [Google Scholar] [CrossRef]
- Tyubaeva, P.M.; Varyan, I.A.; Nikolskaya, E.D.; Mollaeva, M.R.; Yabbarov, N.G.; Sokol, M.B.; Chirkina, M.V.; Popov, A.A. Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin. Nanomaterials 2023, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Kundin, J.I. A New Way to Size up a Wound. Am. J. Nurs. 1989, 89, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Vanheusden, C.; Vanminsel, J.; Reddy, N.; Samyn, P.; D’Haen, J.; Peeters, R.; Ethirajan, A.; Buntinx, M. Fabrication of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Fibers Using Centrifugal Fiber Spinning: Structure, Properties and Application Potential. Polymers 2023, 15, 1181. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Perdiguero, M.; Fiori, S.; Kenny, J.M.; Peponi, L. Biodegradable Electrospun PLA-PHB Fibers Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2020, 179, 109226. [Google Scholar] [CrossRef]
- Borisova, I.; Stoilova, O.; Manolova, N.; Rashkov, I. Modulating the Mechanical Properties of Electrospun PHB/PCL Materials by Using Different Types of Collectors and Heat Sealing. Polymers 2020, 12, 693. [Google Scholar] [CrossRef]
- El-hadi, A.M.; Al-Jabri, F.Y. Influence of Electrospinning Parameters on Fiber Diameter and Mechanical Properties of Poly(3-Hydroxybutyrate) (PHB) and Polyanilines (PANI) Blends. Polymers 2016, 8, 97. [Google Scholar] [CrossRef]
- Thanh, N.H.; Olekhnovich, R.; Sitnikova, V.; Kremleva, A.; Snetkov, P.; Uspenskaya, M. PHB/PEG Nanofiber Mat Obtained by Electrospinning and Their Performances. Technologies 2023, 11, 48. [Google Scholar] [CrossRef]
- P, D. CRC Handbook of Chemistry and Physics. J. Mol. Struct. 1992, 268, 320. [Google Scholar] [CrossRef]
- Karpova, S.G.; Chumakova, N.A.; Lobanov, A.V.; Olkhov, A.A.; Vetcher, A.A.; Iordanskii, A.L. Evaluation and Characterization of Ultrathin Poly(3-Hydroxibutirate) Fibers Loaded with Tetraphenylporphyrin and Its Complexes with Fe(III) and Sn(IV). Polymers 2022, 14, 610. [Google Scholar] [CrossRef]
- Hong, S.G.; Gau, T.K.; Huang, S.C. Enhancement of the Crystallization and Thermal Stability of Polyhydroxybutyrate by Polymeric Additives. J. Therm. Anal. Calorim. 2011, 103, 967–975. [Google Scholar] [CrossRef]
- Li, J.; Zhu, B.; He, Y.; Inoue, Y. Thermal and Infrared Spectroscopic Studies on Hydrogen-Bonding Interaction between Poly(3-Hydroxybutyrate) and Catechin. Polym. J. 2003, 35, 384–392. [Google Scholar] [CrossRef]
- Liu, C.; Jia, M.; Qu, J.; Noda, I.; Chase, D.B.; Street, R.; Rabolt, J.F. Intermolecular Hydrogen Bonding between Poly[(R)-3-Hydroxybutyrate] (PHB) and Pseudoboehmite and Its Effect on Crystallization of PHB. ACS Appl. Polym. Mater. 2020, 2, 4762–4769. [Google Scholar] [CrossRef]
- Zilberman, M.; Egozi, D.; Shemesh, M.; Keren, A.; Mazor, E.; Baranes-Zeevi, M.; Goldstein, N.; Berdicevsky, I.; Gilhar, A.; Ullmann, Y. Hybrid Wound Dressings with Controlled Release of Antibiotics: Structure-Release Profile Effects and in Vivo Study in a Guinea Pig Burn Model. Acta Biomater. 2015, 22, 155–163. [Google Scholar] [CrossRef]
- Du, M.; Liu, J.; Wang, F.; Bi, L.; Ma, C.; Song, M.; Jiang, G. A Sustained-Release Microcarrier Effectively Prolongs and Enhances the Antibacterial Activity of Lysozyme. J. Environ. Sci. 2023, 129, 128–138. [Google Scholar] [CrossRef]
- Egozi, D.; Baranes-Zeevi, M.; Ullmann, Y.; Gilhar, A.; Keren, A.; Matanes, E.; Berdicevsky, I.; Krivoy, N.; Zilberman, M. Biodegradable Soy Wound Dressings with Controlled Release of Antibiotics: Results from a Guinea Pig Burn Model. Burns 2015, 41, 1459–1467. [Google Scholar] [CrossRef]
- Tamahkar, E.; Özkahraman, B.; Süloğlu, A.K.; İdil, N.; Perçin, I. A Novel Multilayer Hydrogel Wound Dressing for Antibiotic Release. J. Drug Deliv. Sci. Technol. 2020, 58, 101536. [Google Scholar] [CrossRef]
- Skorb, E.V.; Möhwald, H.; Andreeva, D.V. How Can One Controllably Use of Natural ΔpH in Polyelectrolyte Multilayers? Adv. Mater. Interfaces 2017, 4, 1600282. [Google Scholar] [CrossRef]
- Hashem, H.R.; Amin, B.H.; Yosri, M. Investigation of the Potential Roles of Adipose Stem Cells and Substances of Natural Origin in the Healing Process of E. coli Infected Wound Model in Rats. Tissue Cell 2023, 85, 102214. [Google Scholar] [CrossRef]
- Montgomery, C.P.; David, M.Z.; Daum, R.S. Host Factors That Contribute to Recurrent Staphylococcal Skin Infection. Curr. Opin. Infect. Dis. 2015, 28, 253–258. [Google Scholar] [CrossRef]
- Acosta Pedemonte, N.B.; Rocchetti, N.S.; Villalba, J.; Lerman Tenenbaum, D.; Settecase, C.J.; Bagilet, D.H.; Colombo, L.G.; Gregorini, E.R. Bacillus Cereus Bacteremia in a Patient with an Abdominal Stab Wound. Rev. Argent. Microbiol. 2020, 52, 115–117. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic Therapy in the Treatment of Microbial Infections: Basic Principles and Perspective Applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Sperandio, F.; Huang, Y.-Y.; Hamblin, M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic Therapy with Fullerenes in Vivo: Reality or a Dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Shebelski, S.D.; Hesje, C.K. Killing of Streptococcus Pneumoniae by Azithromycin, Clarithromycin, Erythromycin, Telithromycin and Gemifloxacin Using Drug Minimum Inhibitory Concentrations and Mutant Prevention Concentrations. Int. J. Antimicrob. Agents 2015, 45, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A.N.; Scarbrough, B.; Saleh, R.; Hovey, J.; Ari, F.; Goyal, S.; Chi, R.J.; Troutman, J.M.; Vivero-Escoto, J.L. Influence of Cationic Meso-Substituted Porphyrins on the Antimicrobial Photodynamic Efficacy and Cell Membrane Interaction in Escherichia coli. Int. J. Mol. Sci. 2019, 20, 134. [Google Scholar] [CrossRef]
- Baek, K.H.; Choi, J.; Jung, S.; Jeon, Y.; Kwon, J.H.; Lee, S. In Vitro Antimicrobial Photodynamic Therapy for Methicillin-Resistant Staphylococcus aureus Using Flexible Organic Light-Emitting Diode. Photodiagnosis Photodyn. Ther. 2025, 53, 104613. [Google Scholar] [CrossRef]
- Anguluri, K.; Sharma, B.; Bagherpour, S.; Calpena, A.C.; Halbaut, L.; Amabilino, D.B.; Kaur, G.; Chaudhary, G.R.; Pérez-García, L. Supramolecular Gels for Antimicrobial Photodynamic Therapy against E. coli and S. aureus. Photodiagnosis Photodyn. Ther. 2025, 52, 104529. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Jin, Y.; Li, Z.; Deng, T.; Su, T.; Zheng, A.; Cao, L. Photodynamic and Photothermal Bacteria Targeting Nanosystems for Synergistically Combating Bacteria and Biofilms. J. Nanobiotechnol. 2025, 23, 40. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C.; Azambuja, N.; Fregnani, E.R.; Rodriguez, H.M.H.; Hamblin, M.R.; Suzuki, H.; Ribeiro, M.S. Effects of Photodynamic Therapy on Gram-Positive and Gram-Negative Bacterial Biofilms by Bioluminescence Imaging and Scanning Electron Microscopic Analysis. Photomed. Laser Surg. 2013, 31, 519–525. [Google Scholar] [CrossRef]
- Junior, R.C.D.S.; Campanholi, K.D.S.S.; Rando, F.D.S.; Gonçalves, R.S.; Lazarin-Bidóia, D.; de Morais, F.A.P.; Silva, A.P.A.D.S.; Nakamura, C.V.; Pozza, M.S.D.S.; Caetano, W. Antimicrobial Photoinactivation Approach Based on Safranine-O Loaded F127 Copolymeric Micelles for Control of Gram-Negative and Gram-Positive Bacteria. Dyes Pigment. 2022, 197, 109900. [Google Scholar] [CrossRef]
- Jakovija, A.; Chtanova, T. Skin Immunity in Wound Healing and Cancer. Front. Immunol. 2023, 14, 1060258. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in Veterinary Medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef]
- King, J.M.; Kulhankova, K.; Stach, C.S.; Vu, B.G.; Salgado-Pabón, W. Phenotypes and Virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 Clonal Lineages. mSphere 2016, 1, e00071-16. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- Küpper, H.; Dědic, R.; Svoboda, A.; Hála, J.; Kroneck, P.M.H. Kinetics and Efficiency of Excitation Energy Transfer from Chlorophylls, Their Heavy Metal-Substituted Derivatives, and Pheophytins to Singlet Oxygen. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 107–113. [Google Scholar] [CrossRef]
- Kustov, A.V.; Belykh, D.V.; Smirnova, N.L.; Venediktov, E.A.; Kudayarova, T.V.; Kruchin, S.O.; Khudyaeva, I.S.; Berezin, D.B. Synthesis and Investigation of Water-Soluble Chlorophyll Pigments for Antimicrobial Photodynamic Therapy. Dyes Pigment. 2018, 149, 553–559. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial Properties of Porphyrins. Expert Opin. Investig. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef]
- Bueno, M.S.; Miñambres, G.G.; Bongioanni, A.; Chattah, A.K.; Aiassa, V.; Longhi, M.R.; Garnero, C. Exploring Solid Forms of Oxytetracycline Hydrochloride. Int. J. Pharm. 2020, 585, 119496. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Leng, J.; Li, K.; Xu, K.; Lin, C.; Yuan, Z.; Zhang, R.; Wang, D.; Tao, B.; Huang, T.J.; et al. A Multifunctional Hydrogel Coating to Direct Fibroblast Activation and Infected Wound Healing via Simultaneously Controllable Photobiomodulation and Photodynamic Therapies. Biomaterials 2021, 278, 121164. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; He, C.; Ling, J. Electrospun Fibers Based Anisotropic Silk Fibroin Film with Photodynamic Antibacterial Therapy for S. aureus Infected Wound Healing. Int. J. Biol. Macromol. 2024, 254, 127685. [Google Scholar] [CrossRef]
- Chepurnaya, J.L.; Melkonyan, G.G.; Gul’muradova, N.T.; Sorokin, A.A. The Effect of Photodynamic Therapy on the Wound Process Dynamics in Patients with Purulent Hand Diseases. Biomed. Photonics 2021, 10, 4–17. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Otterbach, A.; Lamprecht, A. Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches. Pharmaceutics 2021, 13, 320. [Google Scholar] [CrossRef]
- Tarrand, J.J.; LaSala, P.R.; Han, X.Y.; Rolston, K.V.; Kontoyiannis, D.P. Dimethyl Sulfoxide Enhances Effectiveness of Skin Antiseptics and Reduces Contamination Rates of Blood Cultures. J. Clin. Microbiol. 2012, 50, 1552–1557. [Google Scholar] [CrossRef]






| No | Groups | Preparation Dose, µg | Description |
|---|---|---|---|
| 1 | Control | - | Intact animals |
| 2 | Untreated | - | No treatment |
| 3 | Chl | 360 | Chl treatment |
| 4 | Chl-PHB | Chl-PHB treatment | |
| 5 | Chl + light | Chl treatment + irradiation | |
| 6 | Chl-PHB + light | Chl-PHB treatment + irradiation | |
| 7 | OTC | OTC antibiotic treatment | |
| 8 | DMSO | - | DMSO treatment |
| 9 | PHB | - | Chl-free PHB treatment |
| Content of Chl, % | Strength, N/mm2 ± S.D., n = 10 | Young’s Modulus, MPa ± S.D., n = 10 | Elongation at Break, % ± S.D., n = 10 |
|---|---|---|---|
| 0 | 0.24 ± 0.01 | 0.03 ± 0.01 | 10.0 ± 0.9 |
| 0.5 | 0.79 ± 0.06 | 0.50 ± 0.05 | 1.6 ± 0.6 |
| 1.0 | 0.65 ± 0.08 | 0.37 ± 0.03 | 2.4 ± 0.3 |
| 1.25 | 0.46 ± 0.06 | 0.11 ± 0.02 | 4.1 ± 0.4 |
| 1.50 | 0.37 ± 0.06 | 0.40 ± 0.03 | 2.4 ± 0.4 |
| Content of Chl, % | 1 Heating | 2 Heating | ||||
|---|---|---|---|---|---|---|
| Tm, °C | ∆H, J/g | χ, % | Tm, °C | ∆H, J/g | χ, % | |
| 0 | 176.4 | 78.4 | 52.6 | 173.5 | 80.5 | 54.0 |
| 0.5 | 163.3 | 44.6 | 29.9 | 158.3 | 38.7 | 26.0 |
| 1.0 | 163.5 | 48.4 | 32.5 | 159.3 | 46.8 | 31.4 |
| 1.25 | 160.2 | 32.3 | 21.7 | 155.7 | 13.2 | 8.9 |
| 1.50 | 156.2 | 25.1 | 16.8 | - | - | - |
| 1 cooling | 2 cooling | |||||
| Tc, °C | ∆H, J/g | χ, % | Tc, °C | ∆H, J/g | χ, % | |
| 0 | 73.4 | 60.6 | - | 69.1 | 59.5 | - |
| 0.5 | 102.9 | 0.5 | - | 98.5 | 0.6 | - |
| 1.0 | 102.6 | 0.5 | - | 96.0 | 0.6 | - |
| 1.25 | 103.1 | 0.5 | - | 97.5 | 0.5 | - |
| 1.50 | 109.0 | 0.4 | - | 102.0 | 0.3 | - |
| Content of Chl, % | Correlation Time, s × 10−10 |
|---|---|
| 0 | 76.9 |
| 0.5 | 127.1 |
| 1.0 | 109.3 |
| 1.25 | 240.3 |
| 1.50 | 216.8 |
| Microorganism | Chl | Chl-PHB | ||
|---|---|---|---|---|
| No Irradiation | Irradiation * | No Irradiation | Irradiation * | |
| S. aureus | 12.25 ± 0.30 | 6.13 ± 0.22 | 12.15 ± 0.40 | 5.75 ± 0.20 |
| E. coli | 36.50 ± 1.40 | 18.25 ± 0.65 | 34.5 ± 1.20 | 17.5 ± 0.75 |
| B. cereus | 5.93 ± 0.26 | 1.43 ± 0.07 | 5.5 ± 0.15 | 1.25 ± 0.05 |
| Preparations | Reduction in Microorganism Growth, % | |||||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | B. cereus | ||||
| MIC | 2 × MIC | MIC | 2 × MIC | MIC | 2 × MIC | |
| Chl | 91.93 ± 0.43 | 99.68 ± 0.09 | 90.76 ± 0.85 | 99.28 ± 0.34 | 93.55 ± 0.52 | 99.69 ± 0.25 |
| Chl + Irradiation | 92.32 ± 0.52 | 99.72 ± 0.25 | 91.36 ± 0.93 | 99.35 ± 0.52 | 94.81 ± 1.32 | 99.74 ± 0.18 |
| Chl-PHB | 92.67 ± 0.75 | 99.75 ± 0.12 | 92.42 ± 1.15 | 99.47 ± 0.37 | 95.44 ± 1.21 | 99.82 ± 0.15 |
| Chl-PHB + Irradiation | 94.43 ± 0.64 | 99.88 ± 0.10 | 93.68 ± 1.42 | 99.79 ± 0.20 | 96.85 ± 0.12 | 99.89 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyubaeva, P.M.; Varyan, I.A.; Obydennyi, S.I.; Merzlikin, V.A.; Karpova, S.G.; Gruznova, O.A.; Gruznov, D.V.; Shuteeva, E.N.; Kuvshinchikov, N.N.; Popov, N.I.; et al. Antimicrobial Evaluation of Chlorophyll-Containing Nettle Extract Both in Free Form and Incorporated into Poly-3-Hydroxybutyrate. Polymers 2025, 17, 2507. https://doi.org/10.3390/polym17182507
Tyubaeva PM, Varyan IA, Obydennyi SI, Merzlikin VA, Karpova SG, Gruznova OA, Gruznov DV, Shuteeva EN, Kuvshinchikov NN, Popov NI, et al. Antimicrobial Evaluation of Chlorophyll-Containing Nettle Extract Both in Free Form and Incorporated into Poly-3-Hydroxybutyrate. Polymers. 2025; 17(18):2507. https://doi.org/10.3390/polym17182507
Chicago/Turabian StyleTyubaeva, Polina M., Ivetta A. Varyan, Sergei I. Obydennyi, Vasily A. Merzlikin, Svetlana G. Karpova, Olga A. Gruznova, Dmitry V. Gruznov, Ekaterina N. Shuteeva, Nikolay N. Kuvshinchikov, Nikolay I. Popov, and et al. 2025. "Antimicrobial Evaluation of Chlorophyll-Containing Nettle Extract Both in Free Form and Incorporated into Poly-3-Hydroxybutyrate" Polymers 17, no. 18: 2507. https://doi.org/10.3390/polym17182507
APA StyleTyubaeva, P. M., Varyan, I. A., Obydennyi, S. I., Merzlikin, V. A., Karpova, S. G., Gruznova, O. A., Gruznov, D. V., Shuteeva, E. N., Kuvshinchikov, N. N., Popov, N. I., Lobanov, A. V., Abramov, I. A., Sergeev, A. P., Zagaynova, A. V., & Olkhov, A. A. (2025). Antimicrobial Evaluation of Chlorophyll-Containing Nettle Extract Both in Free Form and Incorporated into Poly-3-Hydroxybutyrate. Polymers, 17(18), 2507. https://doi.org/10.3390/polym17182507








