Novel Electrospun PVA-PVP-PAAm/TiO2 Nanofibers with Enhanced Optoelectrical, Antioxidant and Antibacterial Performances
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA-PVP-PAAm/TiO2 Nanoparticles
- B: PVA-PVP-PAAm blend
- BT1: PVA-PVP-PAAm with 1 wt.% TiO2
- BT3: PVA-PVP-PAAm with 3 wt.% TiO2
- BT5: PVA-PVP-PAAm with 5 wt.% TiO2
2.3. Characterization of the PVA-PVP-PAAm/TiO2 Nanofibers
3. Results
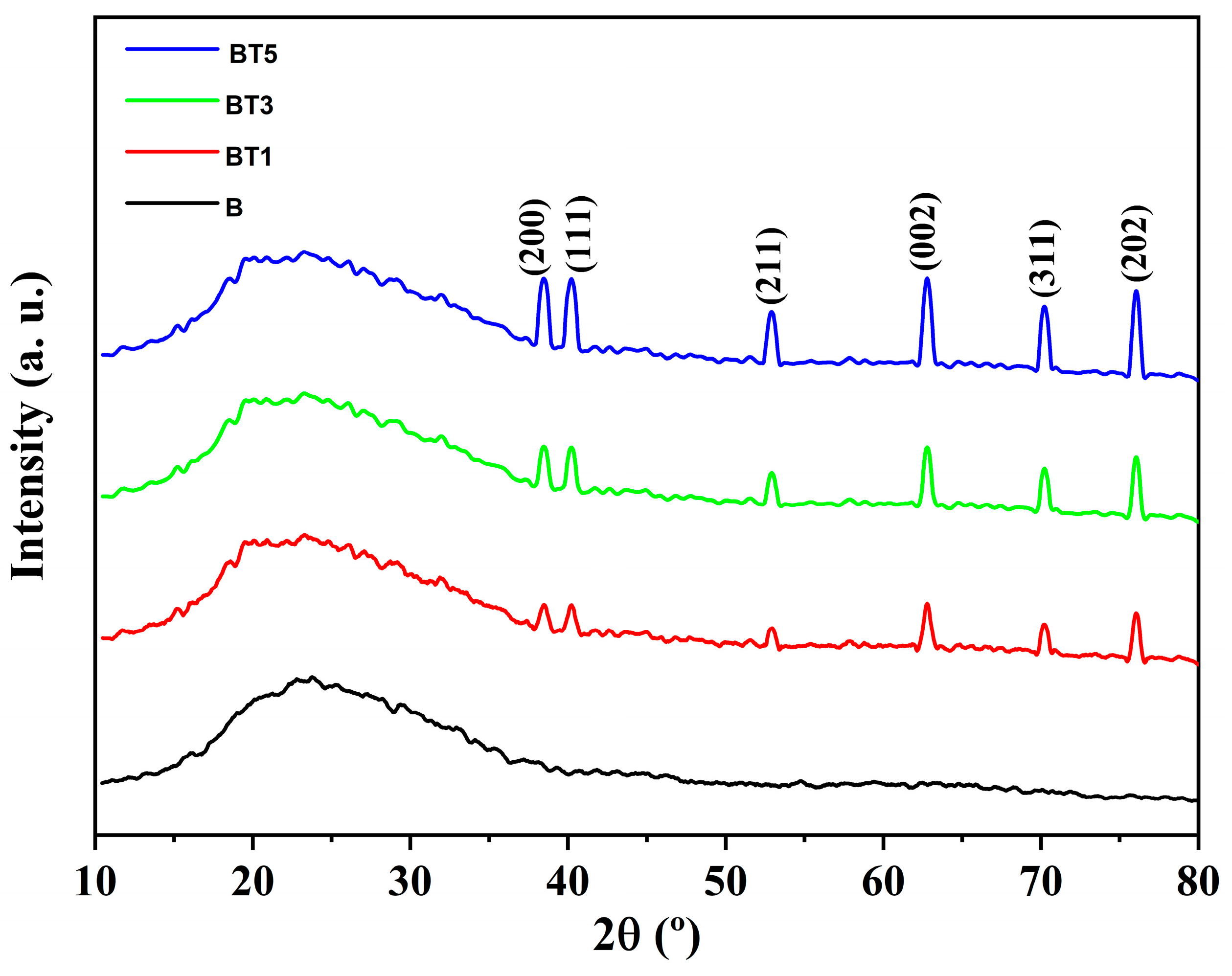
3.1. XRD Investigation
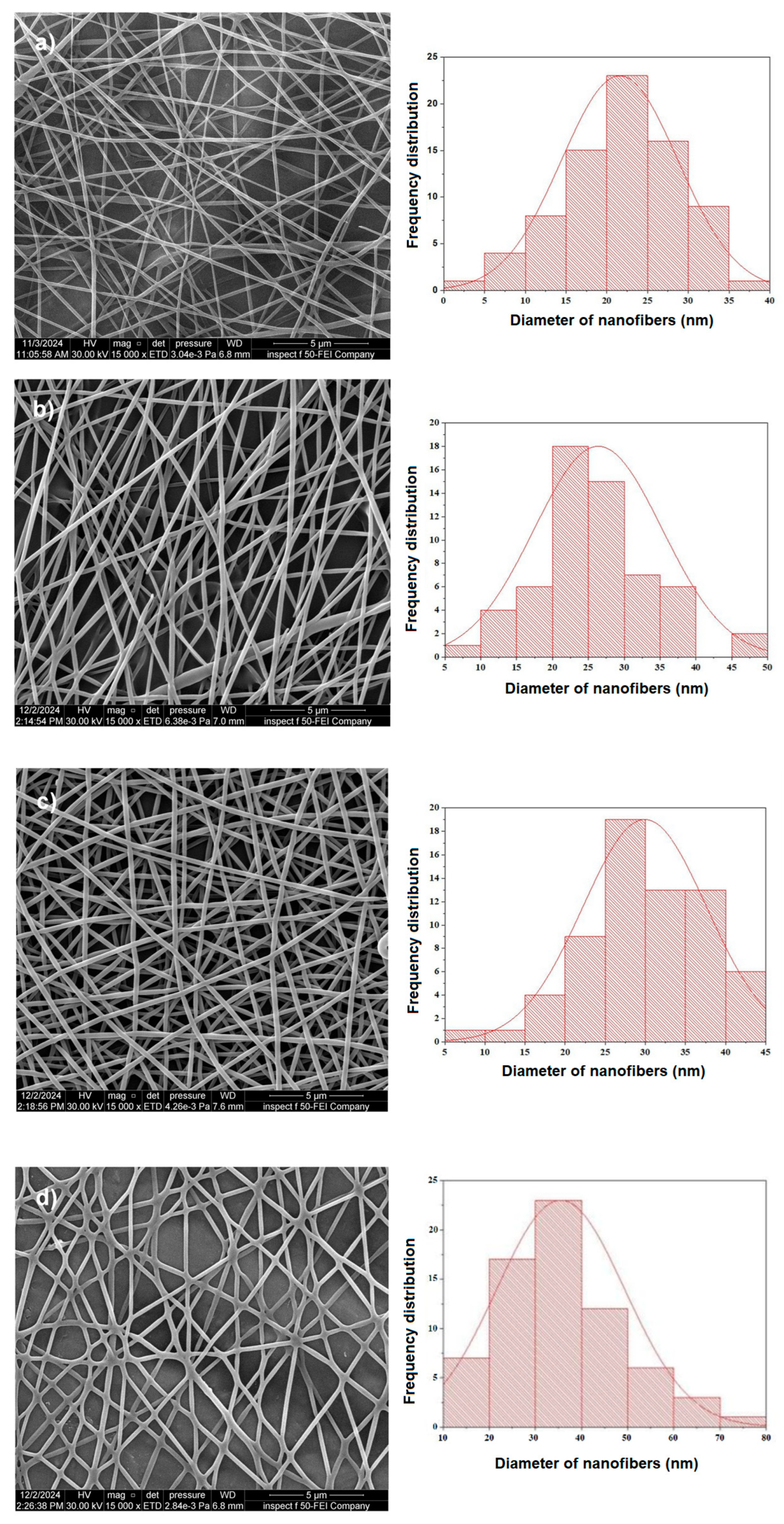
3.2. Fiber Morphology and Diameter
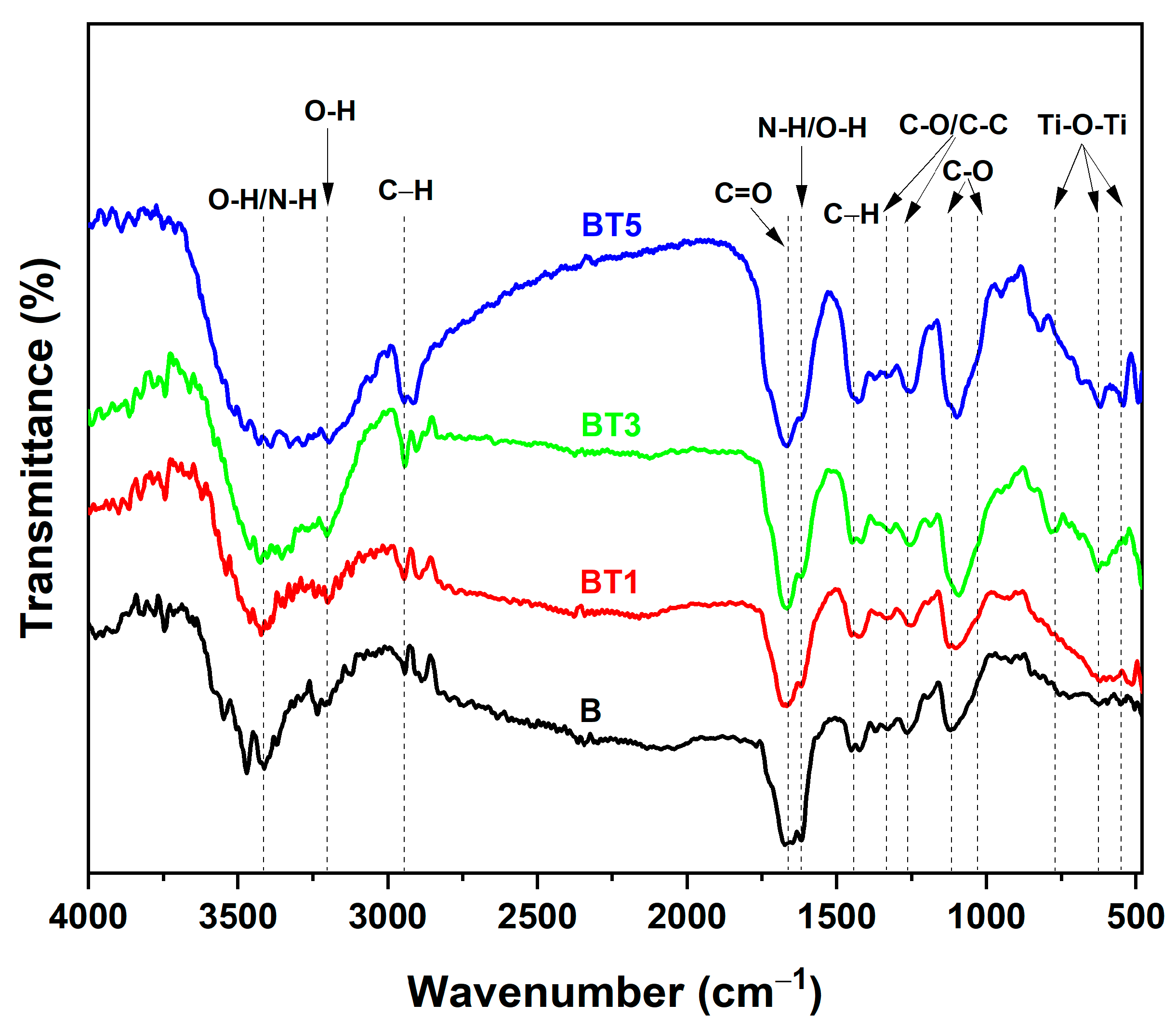
3.3. FTIR Investigation
3.4. UV–Visible Spectroscopy
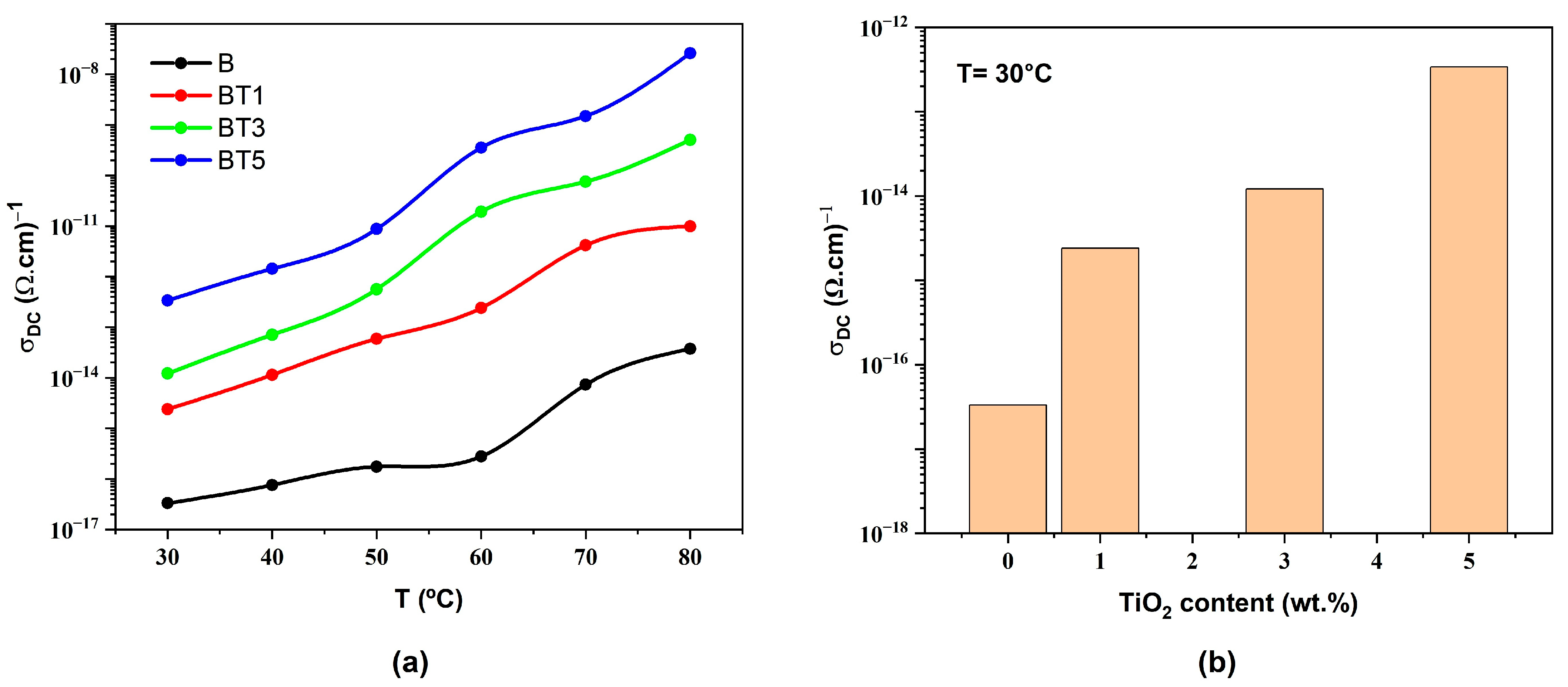
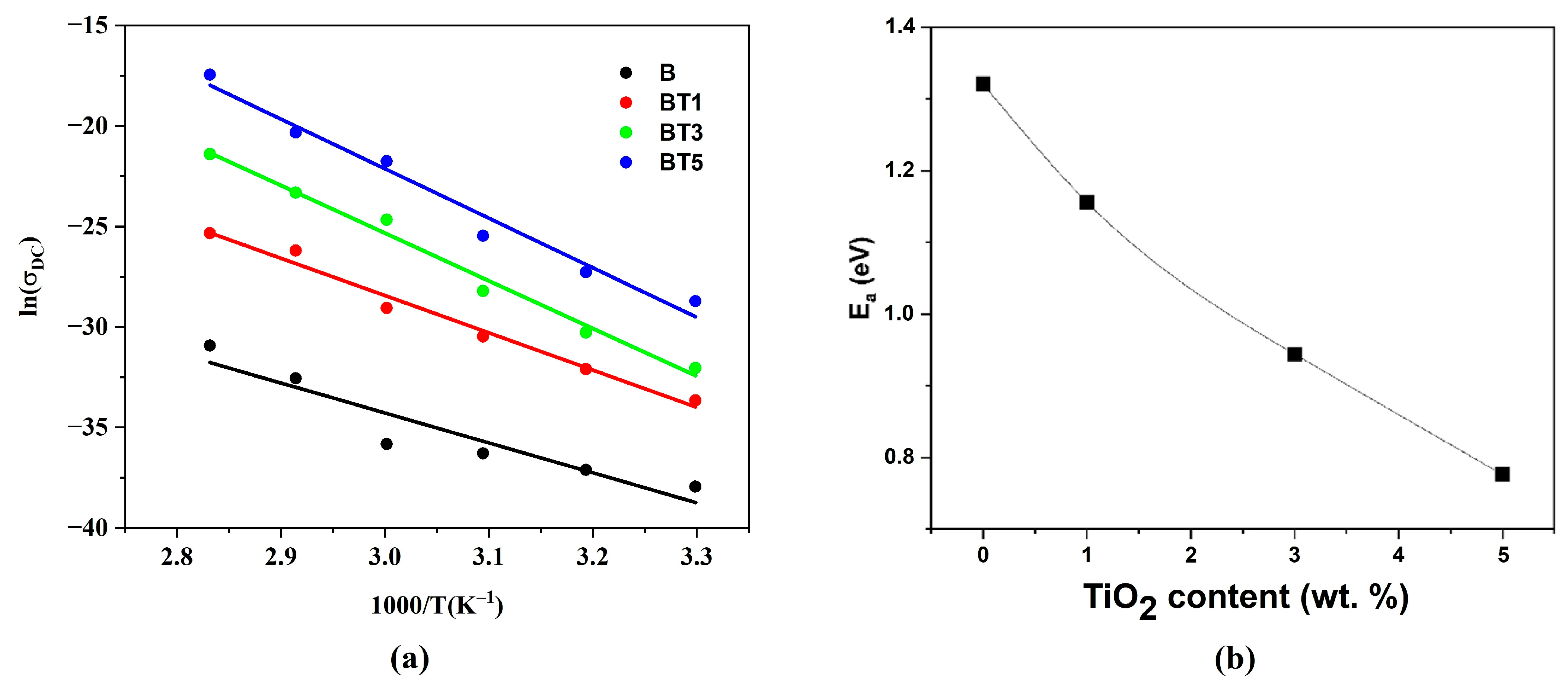
3.5. Electrical Properties of Nanofibers
3.6. Biological Applications
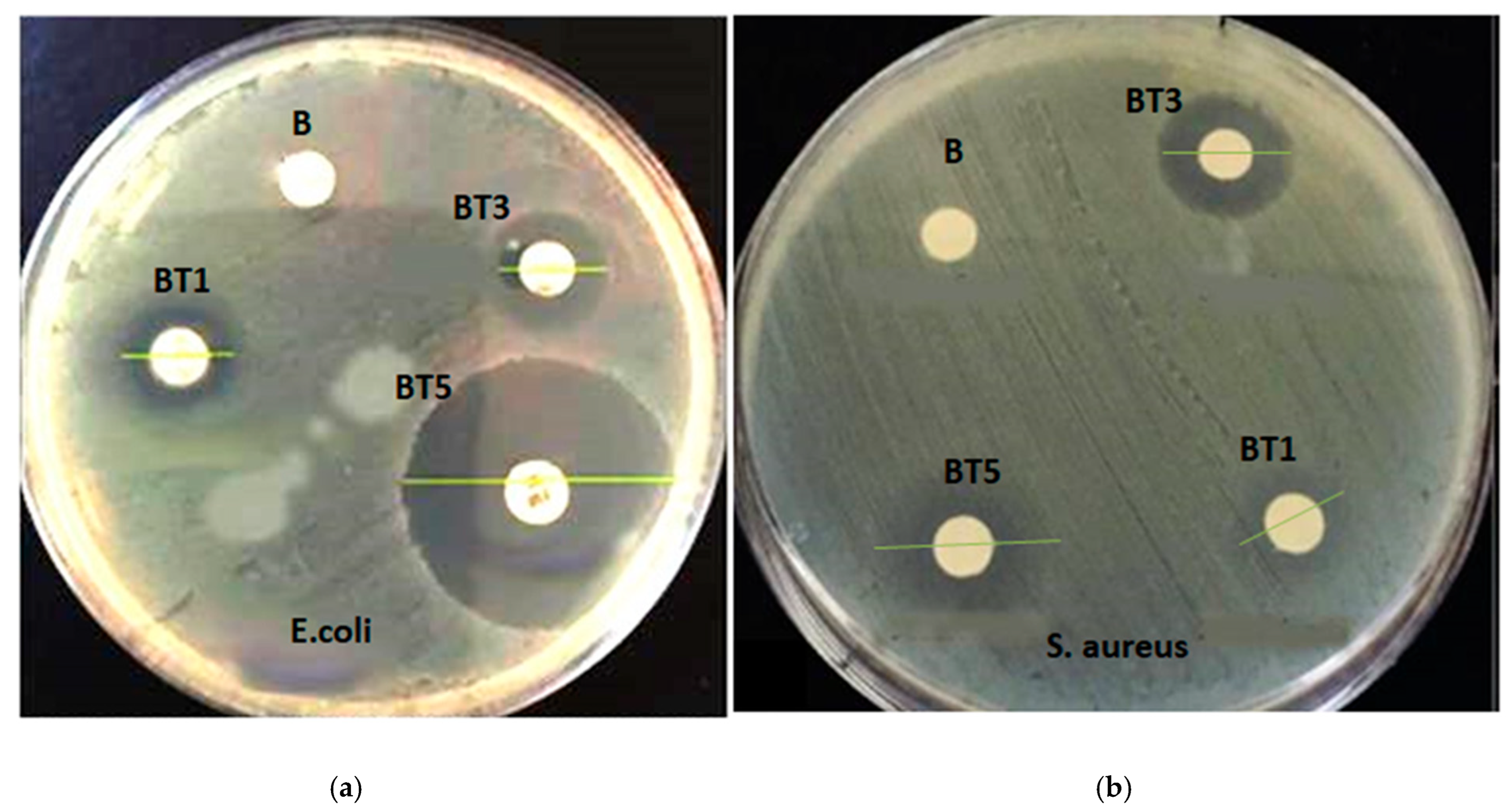
3.6.1. Antibacterial Activity
3.6.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasheed, M.H.; Hashim, F.S.; Abass, K.H. Impact of Ag Nanoparticles on the Spectral and Optical Properties of Electrospun Nanofibrous Poly(Vinyl Alcohol)–Poly(Acrylamide). Int. J. Nanosci. 2023, 22, 23500254. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for Tissue Fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Synthesis and Electrochemical Characterization of Electrospun Biocompatible Poly (ε-Caprolactone) and Polyaniline Nanofiber Composite Electrode Materials for Various Biosensing Applications. Mater. Today Proc. 2023, 80, 1297–1305. [Google Scholar] [CrossRef]
- O′Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of Electrospinning Technique for Biomedical Applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Tsareva, A.D.; Shtol, V.S.; Klinov, D.V.; Ivanov, D.A. Electrospinning for Biomedical Applications: An Overview of Material Fabrication Techniques. Surfaces 2025, 8, 7. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chemie Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing Nanotopography and Integrin–Matrix Interactions to Influence Stem Cell Fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Fabrication and Characterization of PCL/Gelatin/Chitosan Ternary Nanofibrous Composite Scaffold for Tissue Engineering Applications. J. Mater. Sci. 2014, 49, 1076–1089. [Google Scholar] [CrossRef]
- Venkatappa, M.M.; Udagani, C.; Hanume Gowda, S.M.; Venkataramaiah, S.; Casini, R.; Moussa, I.M.; Achur, R.; Sannaningaiah, D.; Elansary, H.O. Green Synthesised TiO2 Nanoparticles-Mediated Terenna Asiatica: Evaluation of Their Role in Reducing Oxidative Stress, Inflammation and Human Breast Cancer Proliferation. Molecules 2023, 28, 5126. [Google Scholar] [CrossRef] [PubMed]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Ragab, H.M.; Rajeh, A. Structural, Thermal, Optical and Conductive Properties of PAM/PVA Polymer Composite Doped with Ag Nanoparticles for Electrochemical Application. J. Mater. Sci. Mater. Electron. 2020, 31, 16780–16792. [Google Scholar] [CrossRef]
- Magesan, P.; Dhanalekshmi, K.I.; Prabha, J.; Umapathy, M.J.; Zhang, X.; Punitha, N.; Kadambary, K.; Sangeetha, K. Photodynamic and Antibacterial Studies of Template-Assisted Fe2O3-TiO2 Nanocomposites. Photodiagn. Photodyn. Ther. 2022, 40, 103064. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.A.M.; Afifi, A.M.; Zuki, F.M.; SalehHudin, H.S. Enhancing Mechanical Properties of Chitosan/PVA Electrospun Nanofibers: A Comprehensive Review. Beilstein J. Nanotechnol. 2025, 16, 286–307. [Google Scholar] [CrossRef]
- Van Etten, E.A.; Ximenes, E.S.; Tarasconi, L.T.; Garcia, I.T.S.; Forte, M.M.C.; Boudinov, H. Insulating Characteristics of Polyvinyl Alcohol for Integrated Electronics. Thin Solid Films 2014, 568, 111–116. [Google Scholar] [CrossRef]
- Wan, W.; Wang, Y.; Xie, D.; Su, J.; Luo, J.; Liao, C.; Ouyang, Y. Sandwich-Architected MXene@TiOx/PVA Composite Films with Augmented Dielectric Performance and Energy Storage Capability. Polym. Adv. Technol. 2025, 36, e70241. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun Polyvinylpyrrolidone (PVP) Hydrogels Containing Hydroxycinnamic Acid Derivatives as Potential Wound Dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Pan, Y.; Yu, M.; Lin, X.; Mondal, A.K. Biocompatible, Injectable, and Self-Healing Poly( N -Vinylpyrrolidone)/Carboxymethyl Cellulose Hydrogel for Drug Release. ACS Omega 2024, 9, 5854–5861. [Google Scholar] [CrossRef]
- Sorkhabi, T.S.; Samberan, M.F.; Ostrowski, K.A.; Zajdel, P.; Stempkowska, A.; Gawenda, T. Electrospinning of Poly (Acrylamide), Poly (Acrylic Acid) and Poly (Vinyl Alcohol) Nanofibers: Characterization and Optimization Study on the Effect of Different Parameters on Mean Diameter Using Taguchi Design of Experiment Method. Materials 2022, 15, 5876. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, F.; Jin, D.; Fang, S.; Wang, Y. Eco-Friendly Fabrication of Hydrophobic and Breathable Nanofibrous Membranes via Molecularly Engineered WPU/PAM Composites. Nanoscale Adv. 2025. [Google Scholar] [CrossRef]
- Humphries, R.M.; Kircher, S.; Ferrell, A.; Krause, K.M.; Malherbe, R.; Hsiung, A.; Burnham, C.-A.D. The Continued Value of Disk Diffusion for Assessing Antimicrobial Susceptibility in Clinical Laboratories: Report from the Clinical and Laboratory Standards Institute Methods Development and Standardization Working Group. J. Clin. Microbiol. 2018, 56, 10-1128. [Google Scholar] [CrossRef]
- Silva, F.; Veiga, F.; Cardoso, C.; Dias, F.; Cerqueira, F.; Medeiros, R.; Cláudia Paiva-Santos, A. A Rapid and Simplified DPPH Assay for Analysis of Antioxidant Interactions in Binary Combinations. Microchem. J. 2024, 202, 110801. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Aldulaimi, N.R.; Al-Bermany, E. Tuning the Bandgap and Absorption Behaviour of the Newly-Fabricated Ultrahigh Molecular Weight Polyethylene Oxide-Polyvinyl Alcohol/Graphene Oxide Hybrid Nanocomposites. Polym. Polym. Compos. 2022, 30, 09673911221112196. [Google Scholar] [CrossRef]
- Khatua, C.; Chinya, I.; Saha, D.; Das, S.; Sen, R.; Dhar, A. Modified Clad Optical Fibre Coated With PVA/TiO2 Nano Composite for Humidity Sensing Application. Int. J. Smart Sens. Intell. Syst. 2015, 8, 1424–1442. [Google Scholar] [CrossRef]
- Alawi, A.I.; Al-Bermany, E. Newly Fabricated Ternary PAAm-PVA-PVP Blend Polymer Doped by SiO2: Absorption and Dielectric Characteristics for Solar Cell Applications and Antibacterial Activity. Silicon 2023, 15, 5773–5789. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Brnawi, S.Z.; Kamal, A.M.; Albassam, A.A.; Heiba, Z.K.; Mohamed, M.B. Structural, Optical, and Electrical Parameters of Doped PVA/PVP Blend with TPAI or THAI Salt. Polymers 2023, 15, 2661. [Google Scholar] [CrossRef]
- Rasheed, M.H.; Kadhim, Q.S.; Mohaimeed, A.A.; Alsaedi, R.J. Synthesis and Evaluation Structural, Thermal and Electrical Properties for PCL/TiO2 Nanocomposites. Trans. Electr. Electron. Mater. 2025, 26, 37–47. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, X.; Yang, Z.; Ji, H. Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials. Polymers 2021, 13, 866. [Google Scholar] [CrossRef]
- Alkabsh, A. Modification of Structural, Optical, and Electrical Properties of PVA/PVP Blend Filled by Nanostructured Titanium Dioxide for Optoelectronic Applications. ECS J. Solid State Sci. Technol. 2024, 13, 093006. [Google Scholar] [CrossRef]
- Elkalashy, S.I.; Khater, S.I.; Zaki, M.F. Boosting of Structural, Thermal, Linear, and Nonlinear Optical Properties of PVA/PVP Blend Using Titanium Dioxide Filler. Polym. Eng. Sci. 2024, 64, 4321–4331. [Google Scholar] [CrossRef]
- Alsaad, A.; Al Dairy, A.R.; Ahmad, A.; Qattan, I.A.; Al Fawares, S.; Al-Bataineh, Q. Synthesis and Characterization of Polymeric (PMMA-PVA) Hybrid Thin Films Doped with TiO2 Nanoparticles Using Dip-Coating Technique. Crystals 2021, 11, 99. [Google Scholar] [CrossRef]
- Ghazi, R.A.; Al-Mayalee, K.H.; Al-Bermany, E.; Hashim, F.S.; Albermany, A.K.J. Impact of Polymer Molecular Weights and Graphene Nanosheets on Fabricated PVA-PEG/GO Nanocomposites: Morphology, Sorption Behavior and Shielding Application. AIMS Mater. Sci. 2022, 9, 584–603. [Google Scholar] [CrossRef]
- Khaleel, M.R.; Hashim, F.S.; Alkhayatt, A.H.O. Impact of PH and ZnO NPs on the Fabricated PVA-PVP/ZnO Nanofibers: Morphology, Absorption Behavior, Anticancer, and Antibacterial Activity. Ceram. Int. 2024, 50, 40161–40170. [Google Scholar] [CrossRef]
- Rajeswari, N.; Selvasekarapandian, S.; Karthikeyan, S.; Nithya, H.; Sanjeeviraja, C. Lithium Ion Conducting Polymer Electrolyte Based on Poly (Vinyl Alcohol)—Poly (Vinyl Pyrrolidone) Blend with LiClO 4. Int. J. Polym. Mater. 2012, 61, 1164–1175. [Google Scholar] [CrossRef]
- Bhogi, A.; Srinivas, B.; Padmavathi, P.; Venkataramana, K.; Ganta, K.K.; Shareefuddin, M.; Kistaiah, P. Absorption Spectrum Fitting Method (ASF), DASF Method and Structural Studies of Li2O–SrO–B2O3–MnO Quaternary Glass System. Opt. Mater. 2022, 133, 112911. [Google Scholar] [CrossRef]
- Hajer, S.; Khaldi, O.; Dahri, A.; Abdelmoula, N.; Hammami, I.; Pedro Fernandes Graça, M.; Benzarti, Z. Influence of (Co+Al) Co−doping on Structural, Micro-Structural, Optical and Electrical Properties of Nanostructured Zinc Oxide. Ceram. Int. 2024, 50, 44151–44164. [Google Scholar] [CrossRef]
- Mohamad, A.H.; Saeed, S.R.; Abdullah, O.G. Synthesis of Very-Fine PbS Nanoparticles Dispersed Homogeneously in MC Matrix: Effect of Concentration on the Structural and Optical Properties of Host Polymer. Mater. Res. Express 2019, 6, 115332. [Google Scholar] [CrossRef]
- Ali, A.I.; Son, J.Y.; Ammar, A.H.; Abdel Moez, A.; Kim, Y.S. Optical and Dielectric Results of Y0.225Sr0.775CoO3±δ Thin Films Studied by Spectroscopic Ellipsometry Technique. Results Phys. 2013, 3, 167–172. [Google Scholar] [CrossRef]
- Badawi, A.; Alsufyani, S.J.; Alharthi, S.S.; Althobaiti, M.G.; Alkathiri, A.A.; Almurayshid, M.; Alharbi, A.N. Impact of Gamma Irradiation on the Structural, Linear and Nonlinear Optical Properties of Lead Oxide Incorporated PVA/Graphene Blend for Shielding Applications. Opt. Mater. 2022, 127, 112244. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I. Electrical Properties of Nanowires and Nanofibers. In Handbook of Nanofibers; Springer International Publishing: Cham, Germany, 2018; pp. 1–62. [Google Scholar]
- Ahmad, M.S.; Zihilif, A.M.; Martuscelli, E.; Ragosta, G.; Scafora, E. The Electrical Conductivity of Polypropylene and Nickel-coated Carbon Fiber Composite. Polym. Compos. 1992, 13, 53–57. [Google Scholar] [CrossRef]
- Jensen, F. Activation Energies and the Arrhenius Equation. Qual. Reliab. Eng. Int. 1985, 1, 13–17. [Google Scholar] [CrossRef]
- Pandey, M.; Joshi, G.M.; Deshmukh, K.; Ahmad, J. Impedance Spectroscopy And Conductivity Studies of CdCl2 Doped Polymer Electrolyte. Adv. Mater. Lett. 2015, 6, 165–171. [Google Scholar] [CrossRef]
- Benzarti, Z.; Saadi, H.; Abdelmoula, N.; Hammami, I.; Fernandes Graça, M.P.; Alrasheedi, N.; Louhichi, B.; Seixas de Melo, J.S. Enhanced Dielectric and Photocatalytic Properties of Li-Doped ZnO Nanoparticles for Sustainable Methylene Blue Degradation with Reduced Lithium Environmental Impact. Ceram. Int. 2025. [Google Scholar] [CrossRef]
- Fartode, P.A.; Yawale, S.S.; Yawale, S.P. Study of Transport and Electrical Properties of PEO:PVP:NaClO2 Based Polymer Electrolyte. Int. J. Chem. Phys. Sci. 2015, 4, 60–64. [Google Scholar]
- Saadi, H.; Benzarti, Z.; Mourad, S.; Sanguino, P.; Hadouch, Y.; Mezzane, D.; Abdelmoula, N.; Khemakhem, H. Electrical Conductivity Improvement of (Fe + Al) Co-Doped ZnO Nanoparticles for Optoelectronic Applications. J. Mater. Sci. Mater. Electron. 2022, 33, 8065–8085. [Google Scholar] [CrossRef]
- Faraz Ahmer, M.; Hameed, S. Studies on the Electrical Conductivity Measurement of Organic/Organic Composite Polyvinyl Alcohol/Polyaniline (PVA/PANI). Int. J. Adv. Res. Electr. 2014, 3, 12731–12736. [Google Scholar] [CrossRef]
- Kahdim, Q.S.; Benzarti, Z.; Mousa, M.H.; Rasheed, M.H.; Abdelmoula, N.; Khalfallah, A. Enhancing the Multifunctional Properties of Polycaprolactone/Chitosan Films with Zirconium Dioxide Nanoparticles for Biomedical and Flexible Optoelectronic Applications. RSC Adv. 2025, 15, 31788. [Google Scholar] [CrossRef]
- Zeinab, B.; Buthaina, J.; Rafik, K. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve it. Molecules 2023, 25, 1340. [Google Scholar]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef]
- Saeed, A.; Guizani, I.; Hanash, F.E.; Asnag, G.M.; Al-Harthi, A.M.; Alwafi, R.; Qahtan, T.F.; Morsi, M.A.; Assran, A.S. Enhancing Optical, Structural, Thermal, Electrical Properties, and Antibacterial Activity in Chitosan/Polyvinyl Alcohol Blend with ZnO Nanorods: Polymer Nanocomposites for Optoelectronics and Food/Medical Packaging Applications. Polym. Bull. 2024, 81, 11645–11670. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Bryk, R.; Lima, C.D.; Erdjument-Bromage, H.; Tempst, P.; Nathan, C. Metabolic Enzymes of Mycobacteria Linked to Antioxidant Defense by a Thioredoxin-Like Protein. Science 2002, 295, 1073–1077. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, H.; Fu, Q. Recent Progress on PEDOT:PSS Based Polymer Blends and Composites for Flexible Electronics and Thermoelectric Devices. Mater. Chem. Front. 2020, 4, 3130–3152. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.H.; Kumar Srinivasan, D. Nanoparticle-Based Therapeutic Approach for Diabetic Wound Healing. Nanomaterials 2020, 10, 1234. [Google Scholar] [CrossRef] [PubMed]

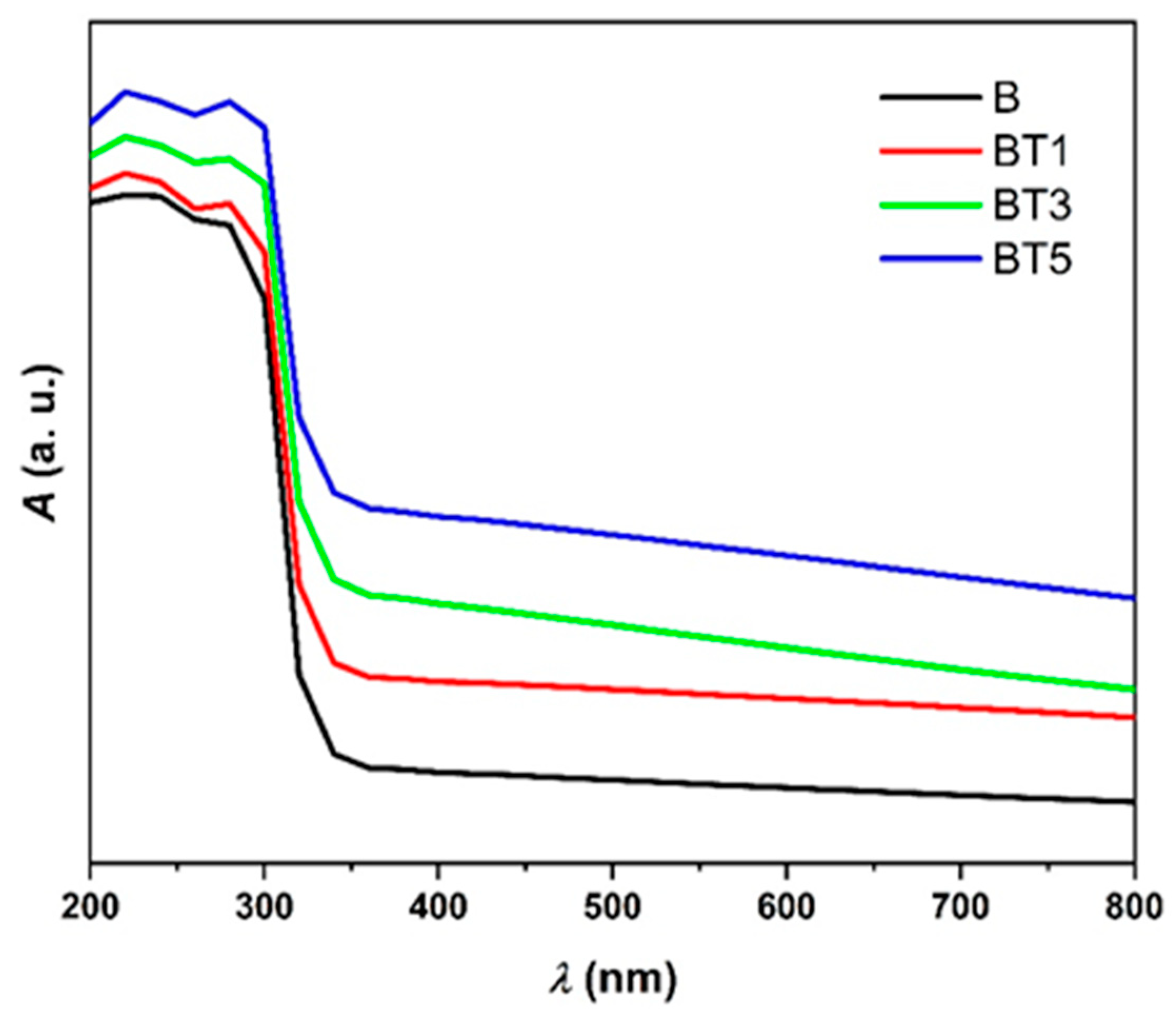

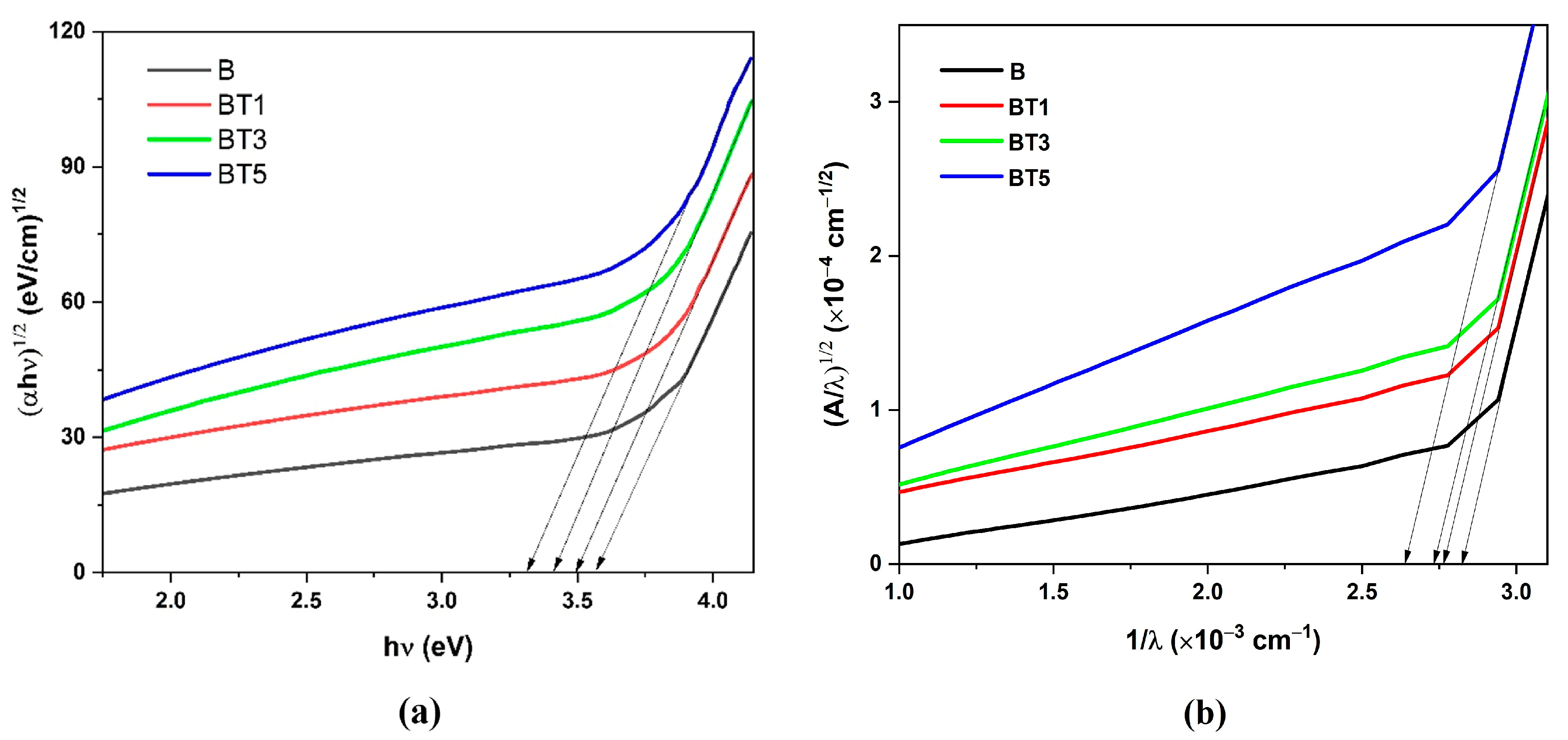

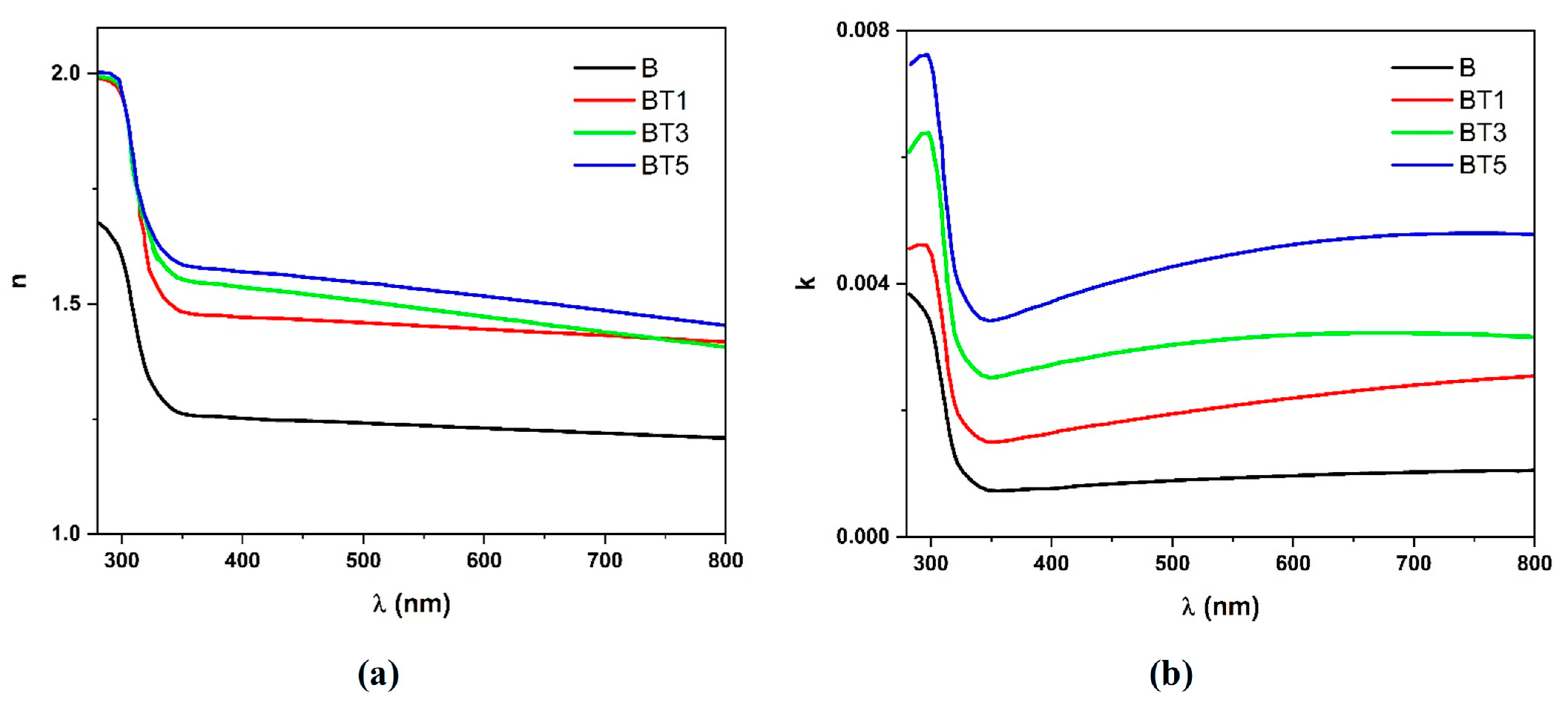
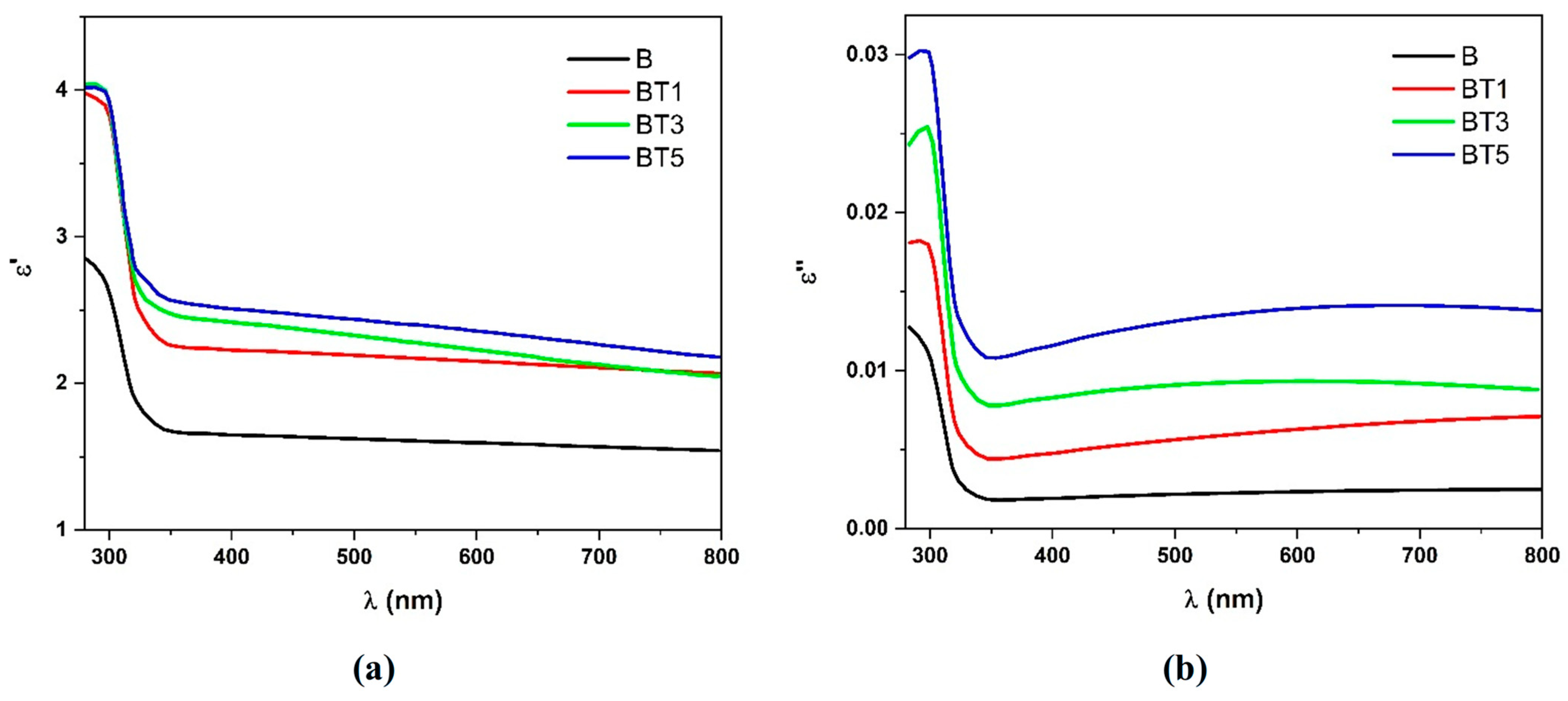
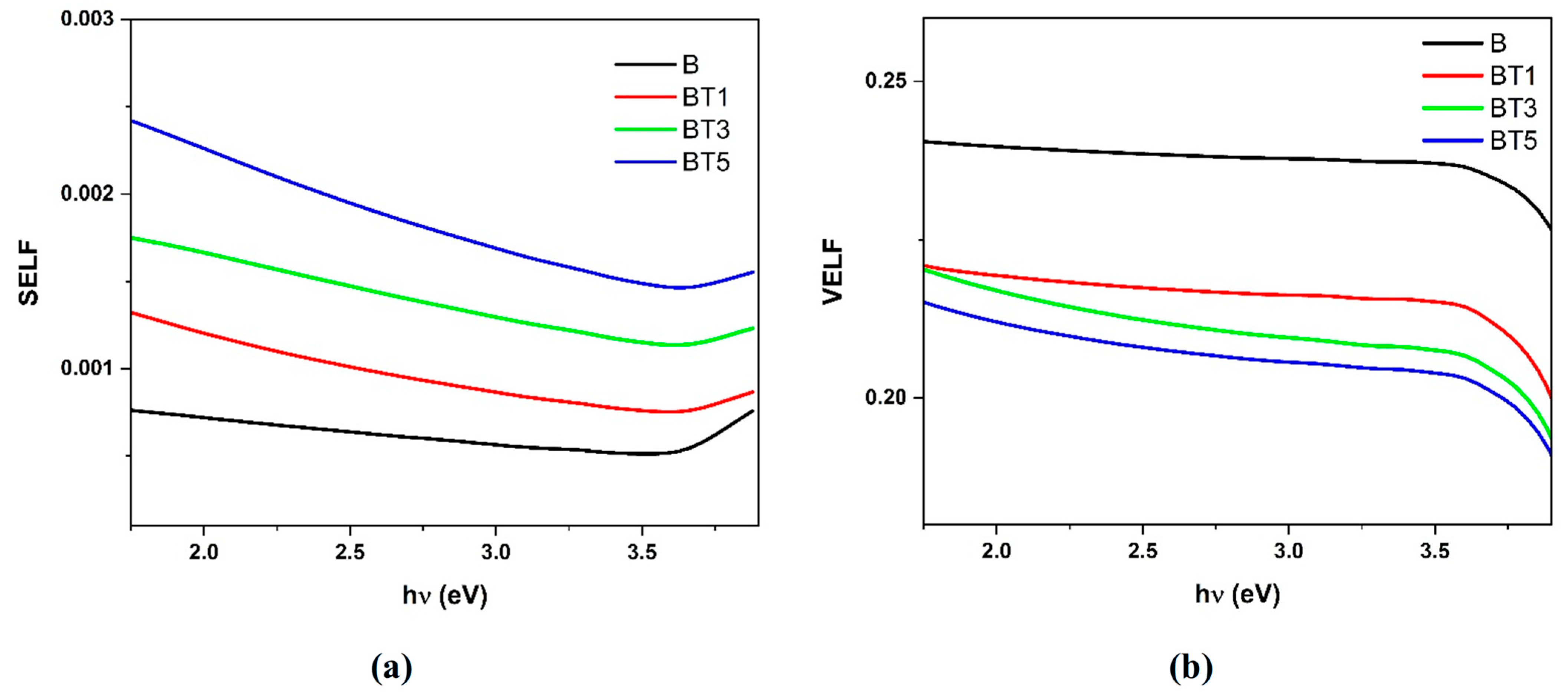

| Sample | Tauc Relation | ASF Method | |
|---|---|---|---|
| B | 3.575 | 3.499 | 0.208 |
| BT1 | 3.501 | 3.429 | 0.265 |
| BT3 | 3.421 | 3.391 | 0.417 |
| BT5 | 3.320 | 3.276 | 0.507 |
| Sample | E. coli. Diameter of Inhibitory Zone (mm) | S. aureus Diameter of Inhibitory Zone (mm) |
|---|---|---|
| B | 0 | 0 |
| BT1 | 7.5 ± 0.2 | 7.3 ± 0.2 |
| BT3 | 8.2 ± 0.2 | 7.7 ± 0.2 |
| BT5 | 18.2 ± 0.2 | 11.6 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, M.H.; Mousa, M.H.; Kadhim, Q.S.; Abdelmoula, N.; Khalfallah, A.; Benzarti, Z. Novel Electrospun PVA-PVP-PAAm/TiO2 Nanofibers with Enhanced Optoelectrical, Antioxidant and Antibacterial Performances. Polymers 2025, 17, 2487. https://doi.org/10.3390/polym17182487
Rasheed MH, Mousa MH, Kadhim QS, Abdelmoula N, Khalfallah A, Benzarti Z. Novel Electrospun PVA-PVP-PAAm/TiO2 Nanofibers with Enhanced Optoelectrical, Antioxidant and Antibacterial Performances. Polymers. 2025; 17(18):2487. https://doi.org/10.3390/polym17182487
Chicago/Turabian StyleRasheed, Maher Hassan, Mohanad H. Mousa, Qasim Shakir Kadhim, Najmeddine Abdelmoula, Ali Khalfallah, and Zohra Benzarti. 2025. "Novel Electrospun PVA-PVP-PAAm/TiO2 Nanofibers with Enhanced Optoelectrical, Antioxidant and Antibacterial Performances" Polymers 17, no. 18: 2487. https://doi.org/10.3390/polym17182487
APA StyleRasheed, M. H., Mousa, M. H., Kadhim, Q. S., Abdelmoula, N., Khalfallah, A., & Benzarti, Z. (2025). Novel Electrospun PVA-PVP-PAAm/TiO2 Nanofibers with Enhanced Optoelectrical, Antioxidant and Antibacterial Performances. Polymers, 17(18), 2487. https://doi.org/10.3390/polym17182487







