Enhanced Mechanical Robustness of Sprayed Cellulose Nanofibril Coatings Through Internal Crosslinking with Boric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Methods
2.3. Characterization Techniques
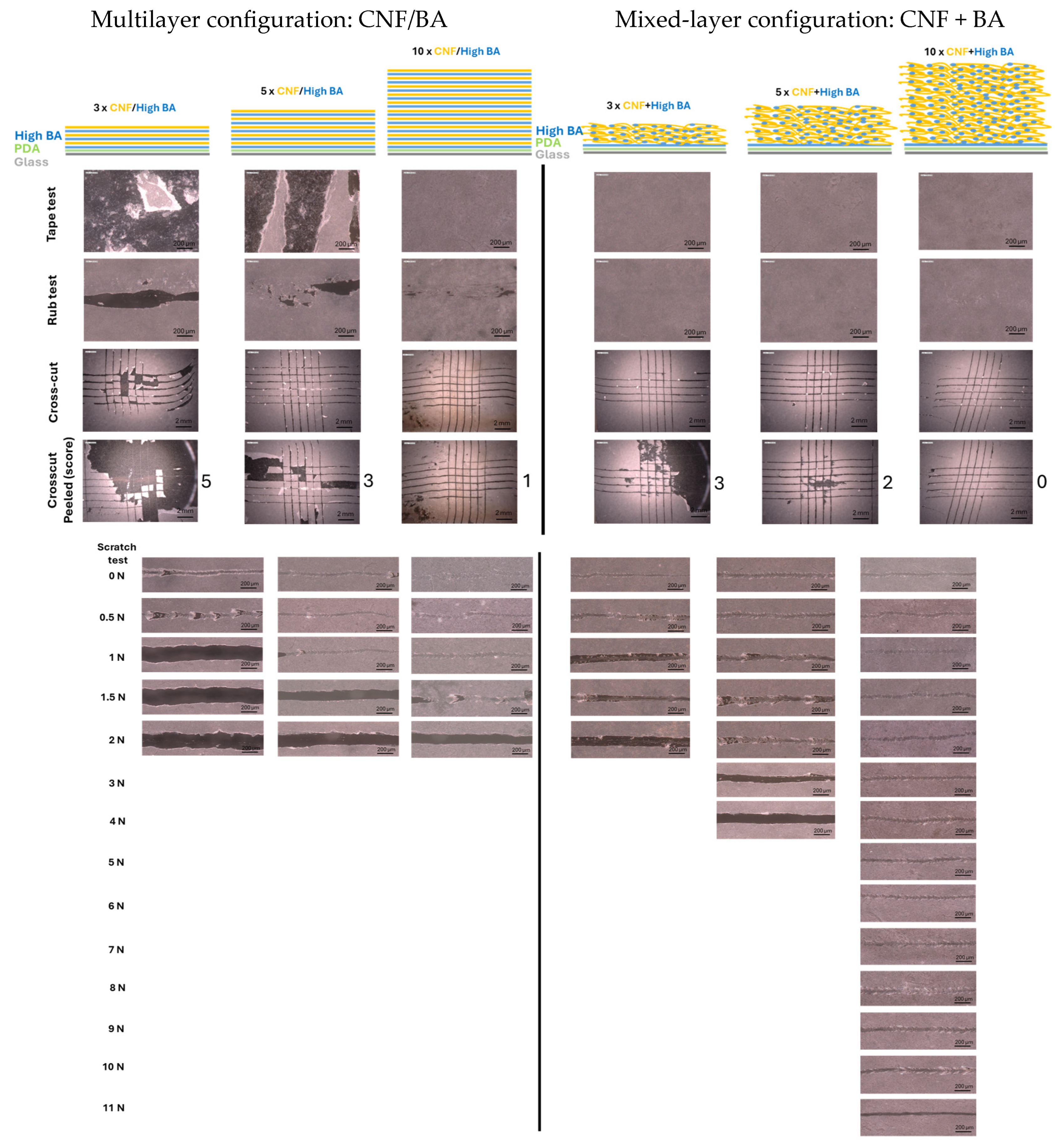
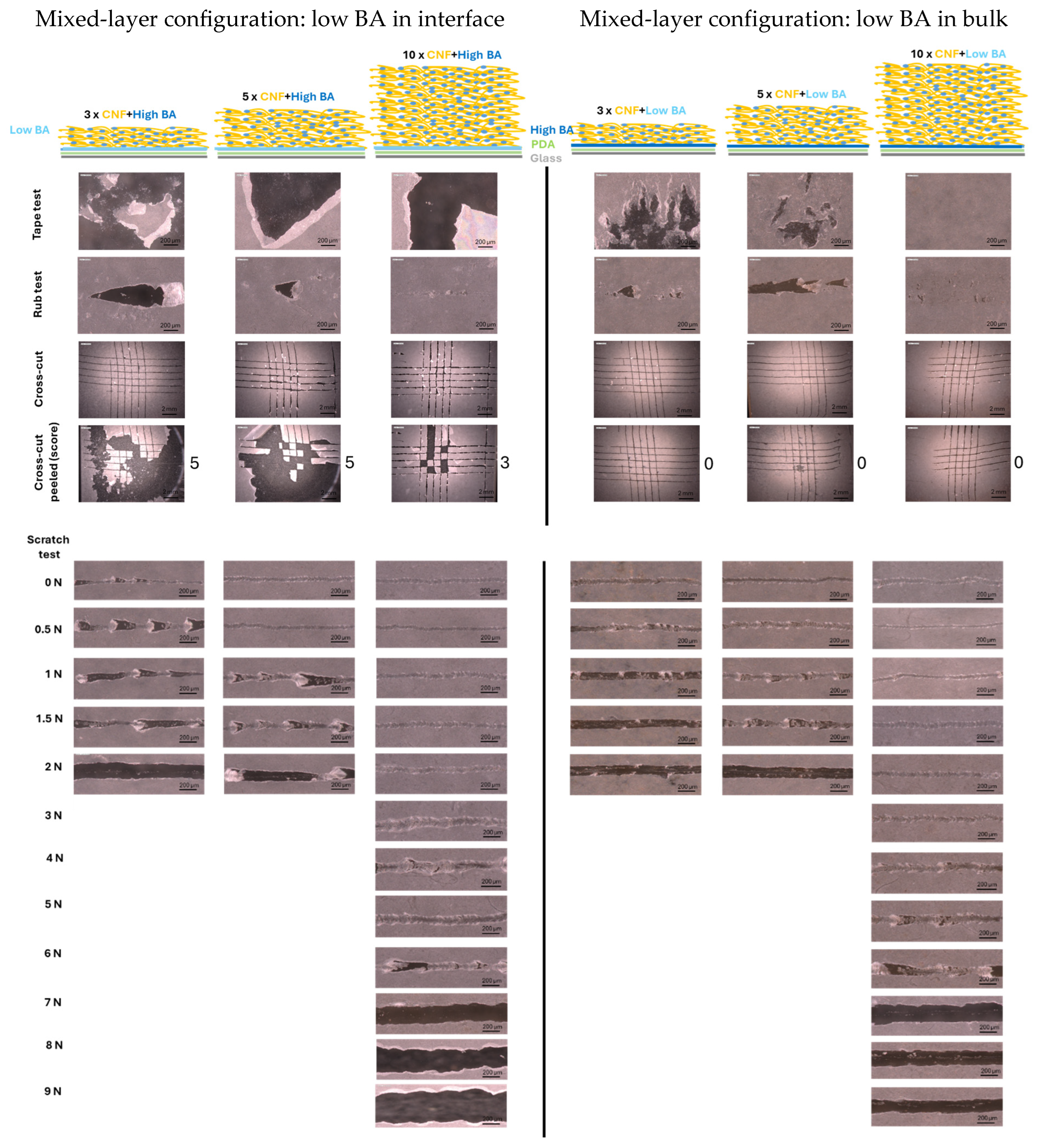
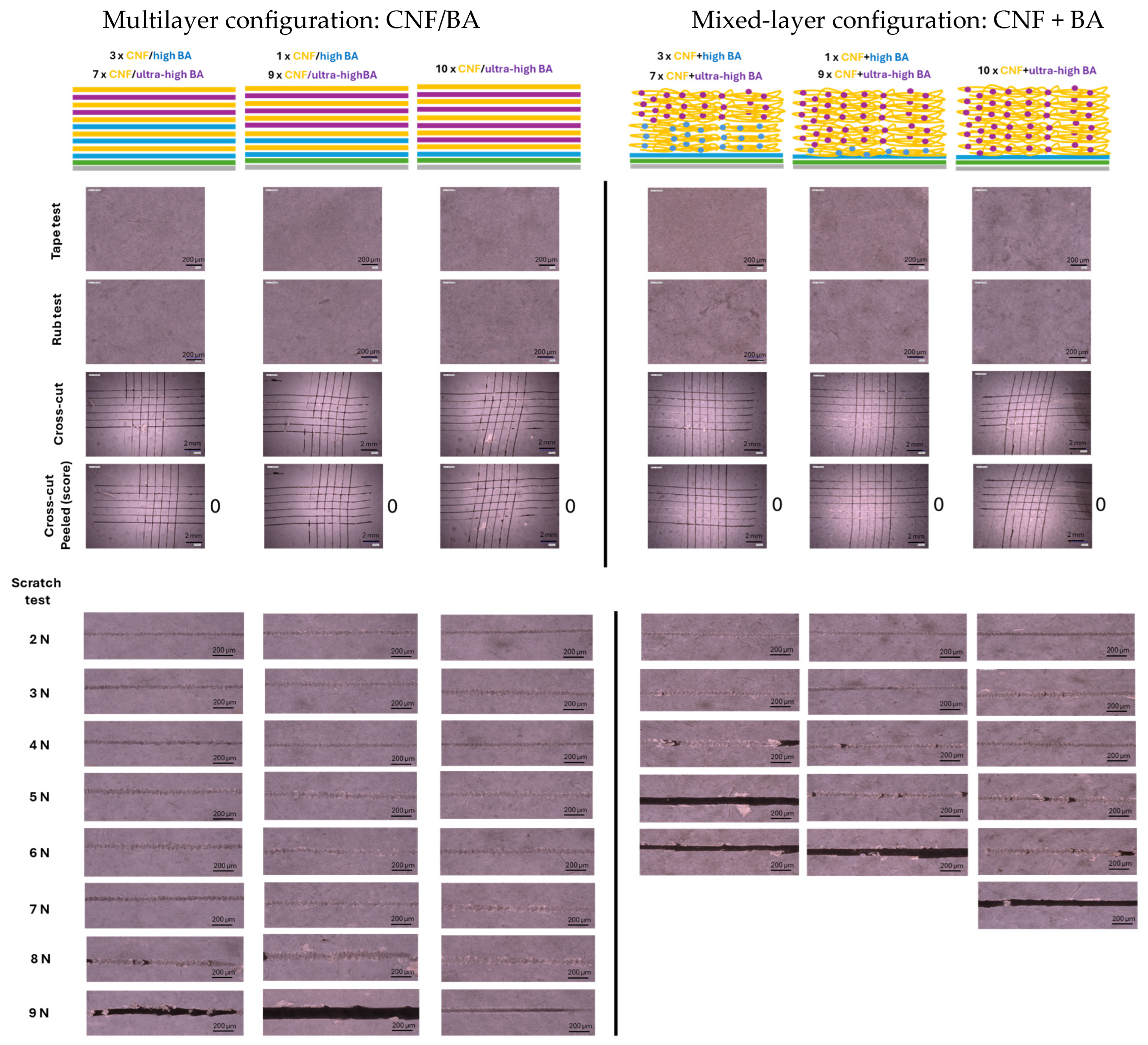
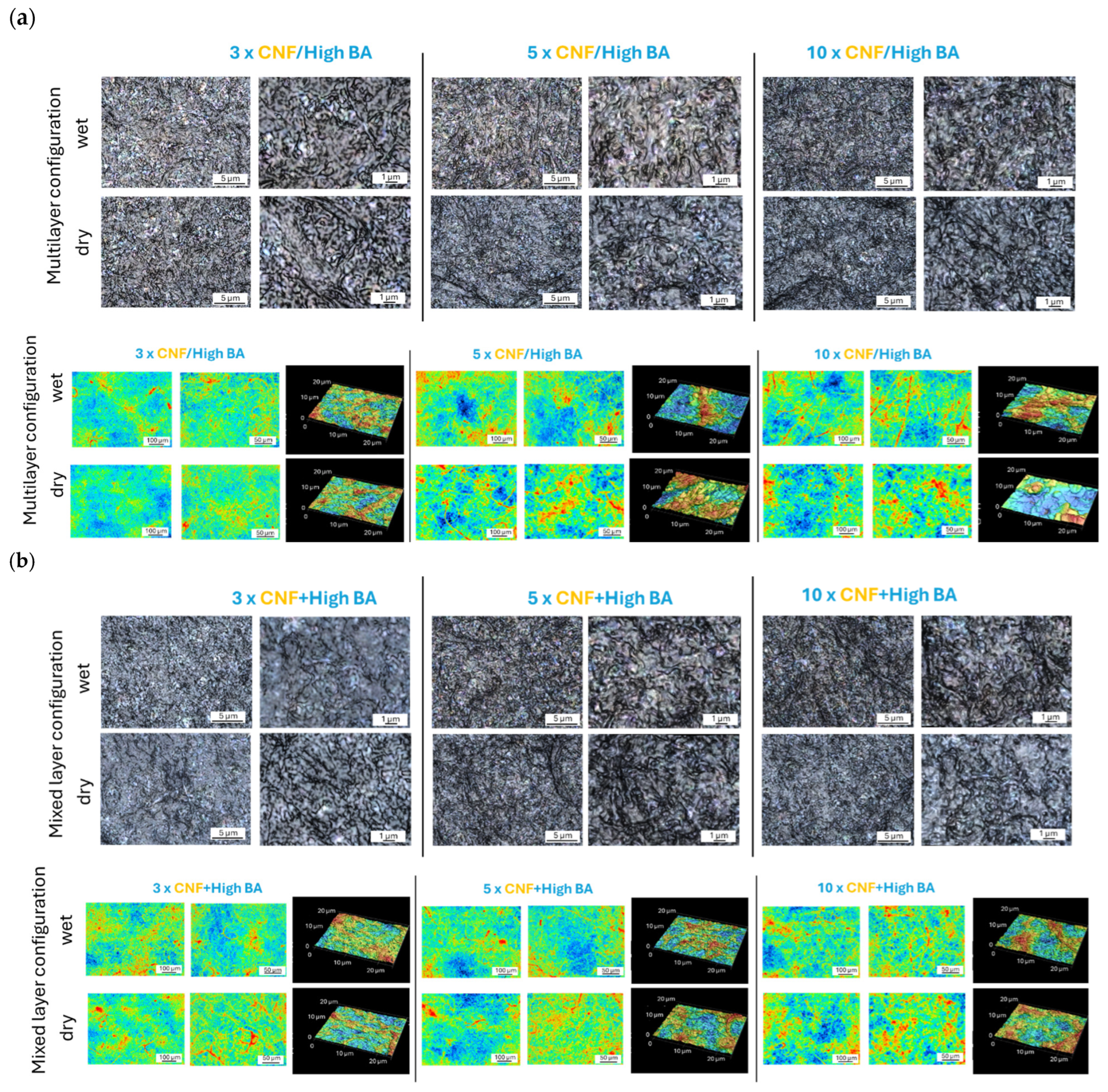
3. Results
3.1. Mechanical Testing of Coatings
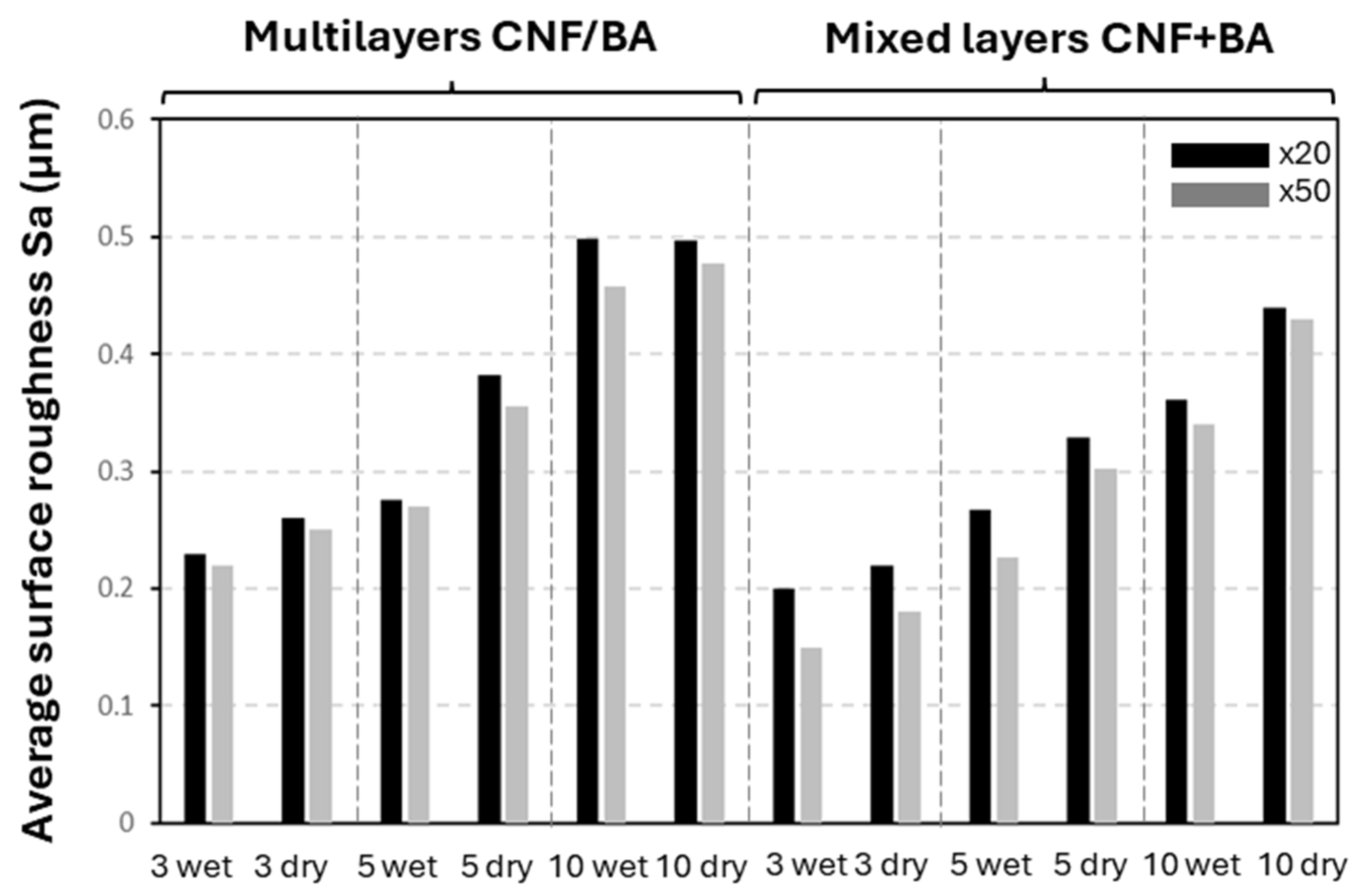
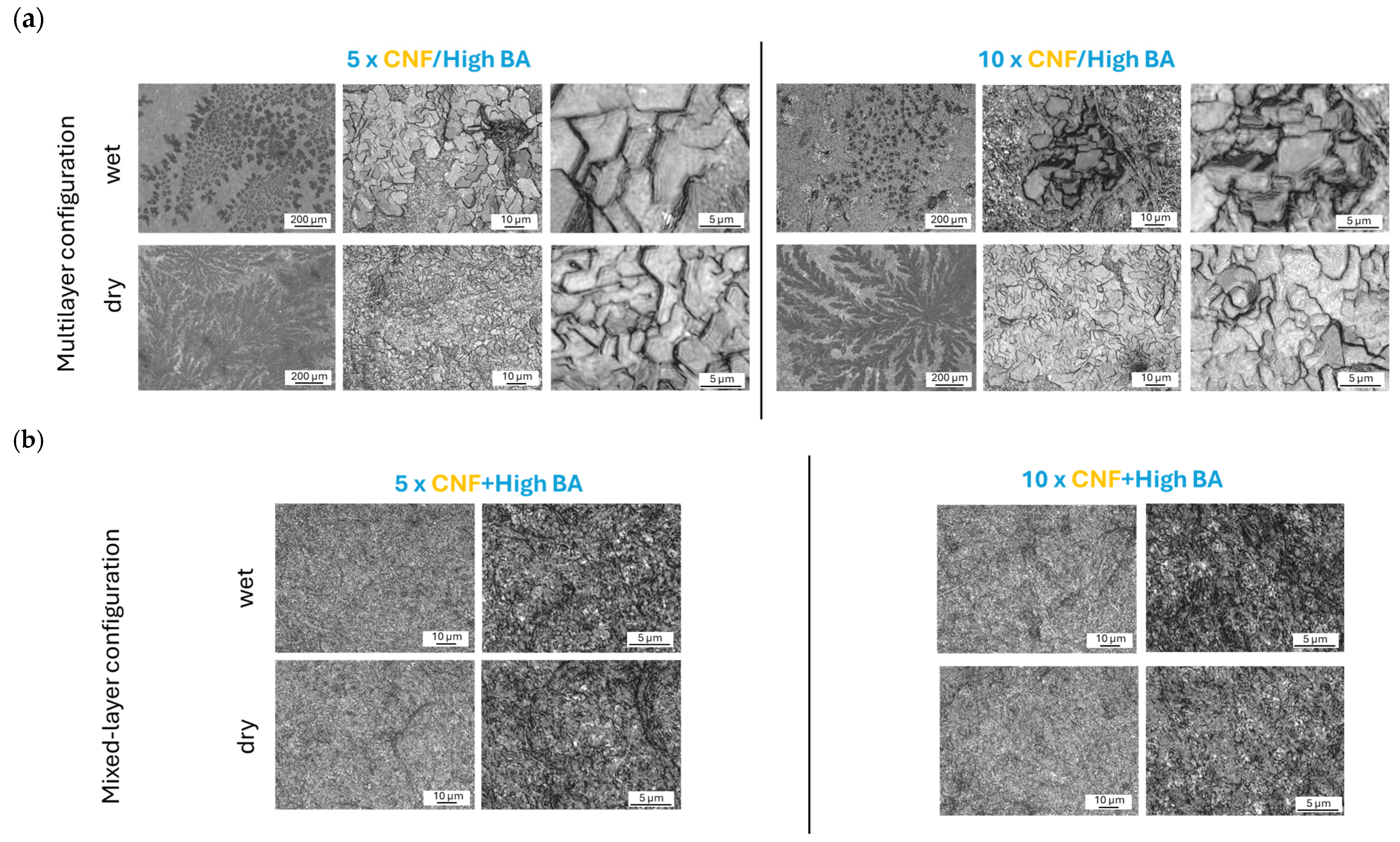
3.2. Morphology and Surface Roughness
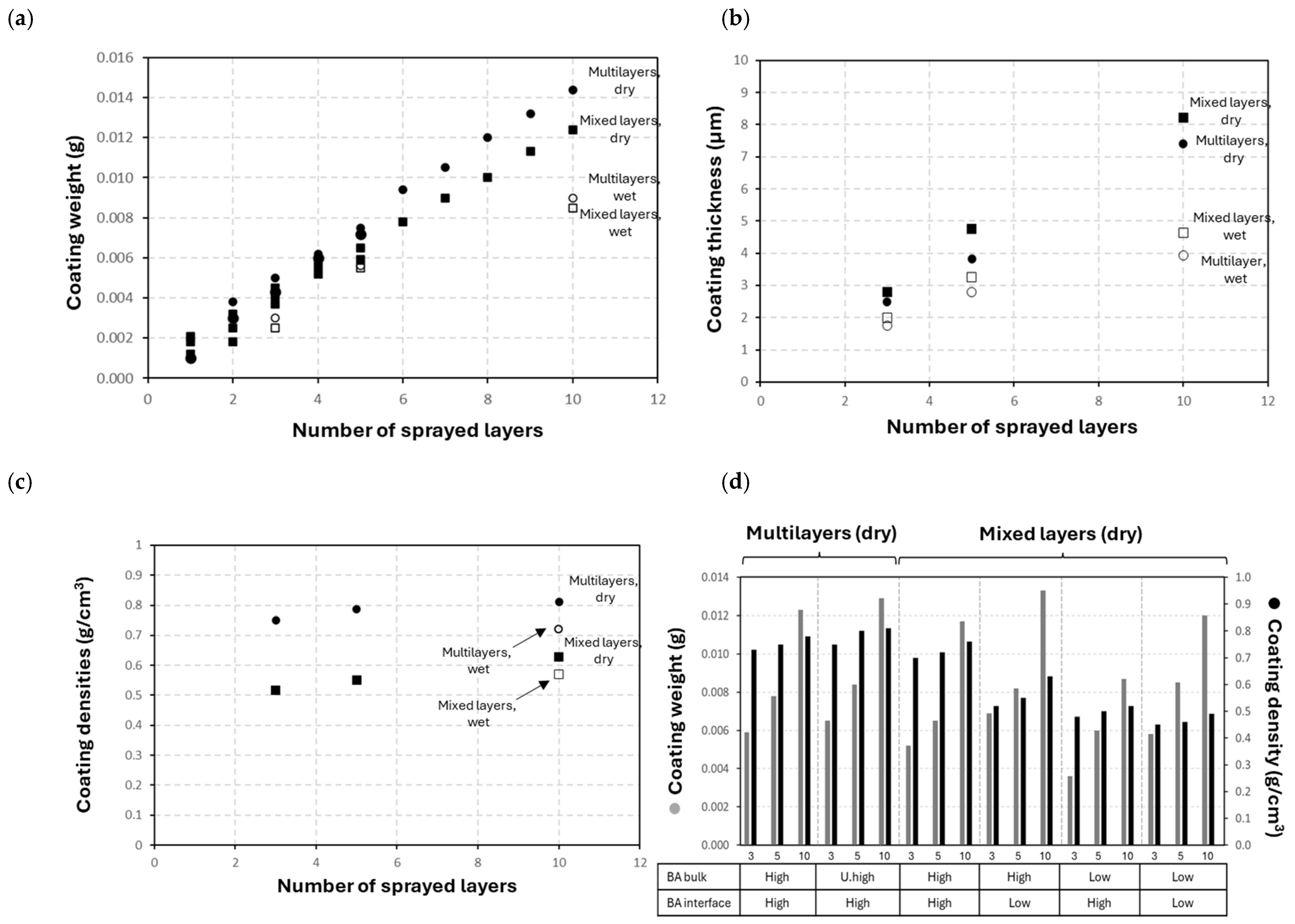
3.3. Physical Coating Properties
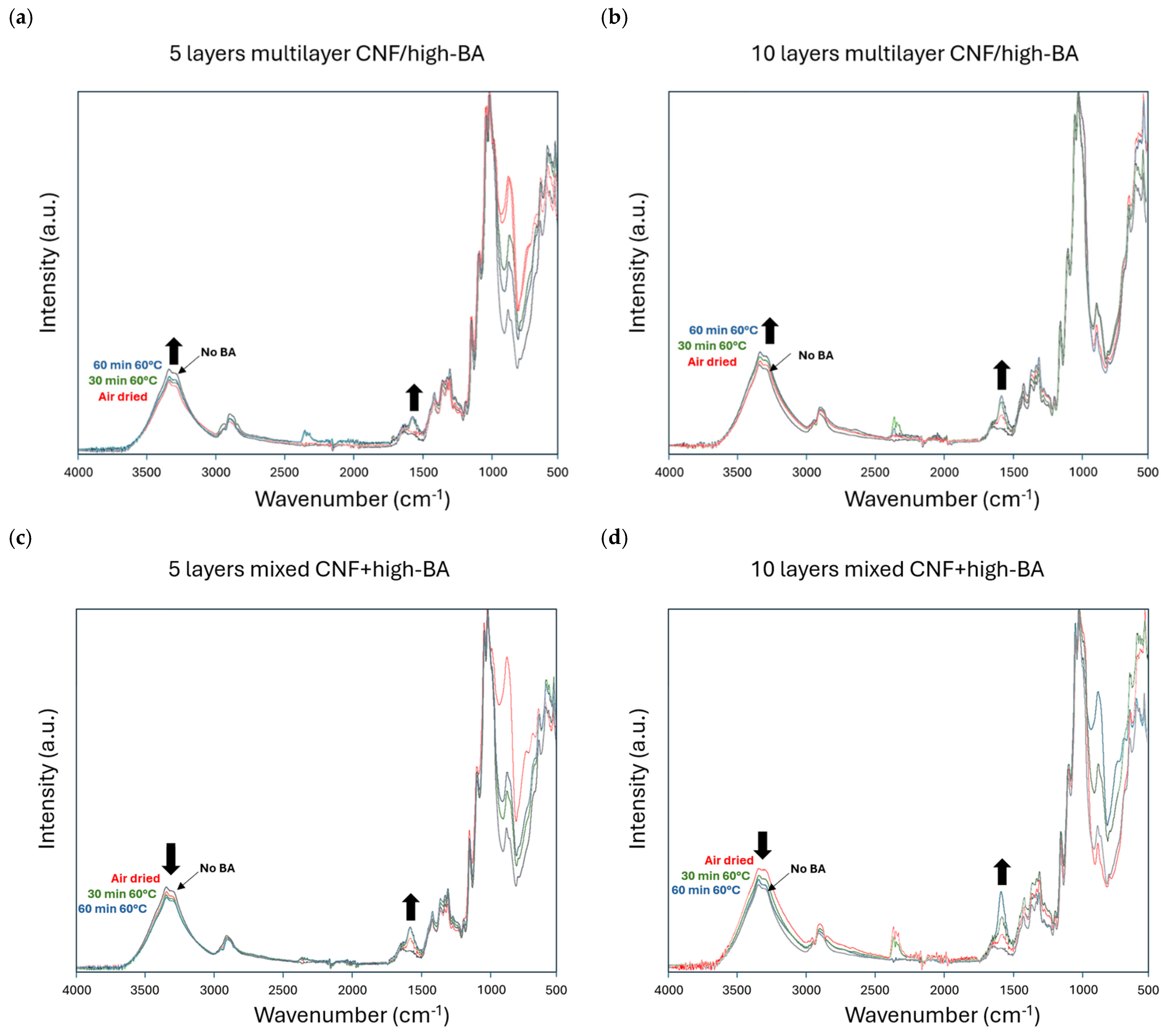
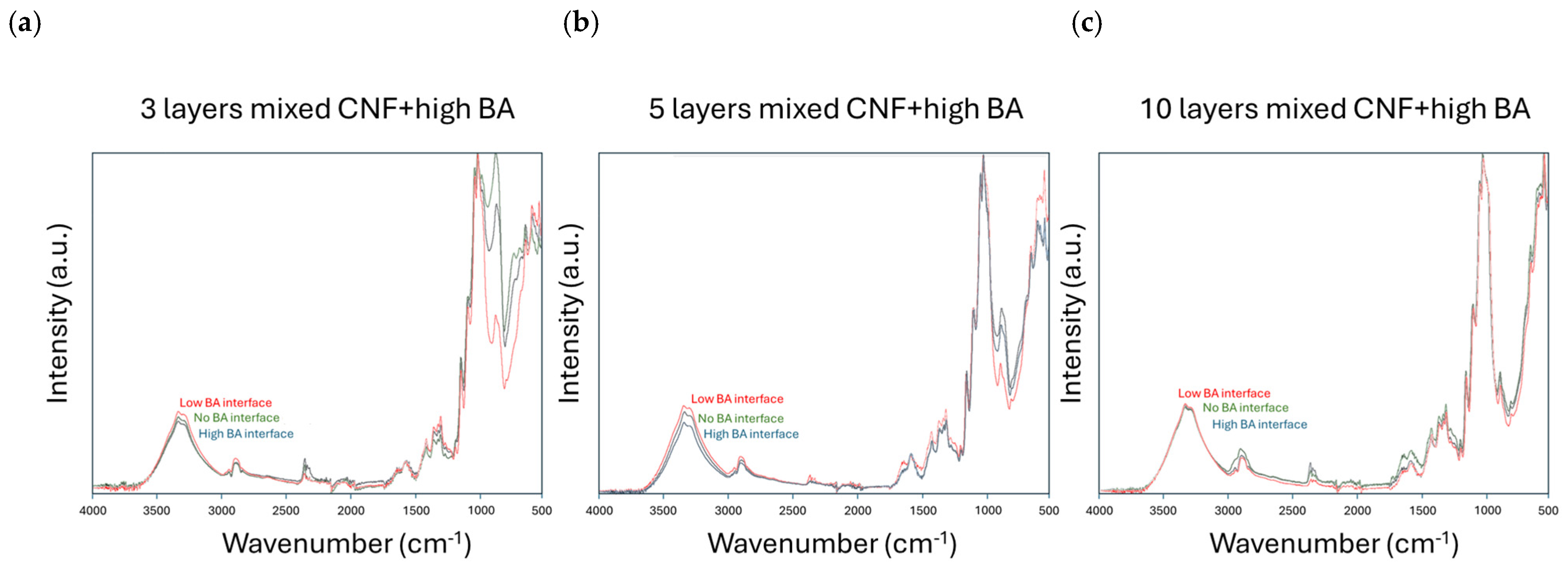
3.4. Chemical Coating Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, K.; Chandrasekar, N.; Balaji, R. Nanocellulose as a sustainable nanomaterial for films and coating layers via spray-coating and applications. In Interaction of Nanomaterials with Living Cells; Sheikh, F.A., Majeed, S., Beigh, M.A., Eds.; Springer: Singapore, 2023; pp. 485–556. [Google Scholar]
- Lengowski, E.C.; Bonfatti Júnior, E.A.; Coelho Simon, L.; Bolzon de Muniz, G.I.; Sulato de Andrade, A.; Neves Leite, A.; Souza de Miranda Leite, E.L. Nanocellulose coating on kraft paper. Coatings 2023, 13, 1705. [Google Scholar] [CrossRef]
- Tozluoglu, A.; Ates, S.; Durmaz, E.; Setkaya, S.; Arslan, R.; Ozcelik, O.; Candan, Z. Nanocellulose in paper and board coating. In Emerging Nanomaterials; Taghiyari, H.R., Morrell, J.J., Husen, A., Eds.; Springer: Cham, Germany, 2022; pp. 197–298. [Google Scholar]
- Qi, S.; Kiratzis, I.; Adoni, P.; Tuekprakhon, A.; Hill, H.J.; Stamataki, Z.; Nabi, A.; Waugh, D.; Rodriguez, J.R.; Clarke, S.M.; et al. Porous cellulose thin films as sustainable and effective antimicrobial surface coatings. ACS Appl. Mater. Interf. 2023, 15, 20638–20648. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sharma, N.; Managala, M.; Rajkhowa, R.; Allardyce, B.; Shastri, Y.; Agrawal, R. Nanocellulose: Resources, physio-chemical properties, current uses and future applications. Front. Nanotechnol. 2021, 3, 747329. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure—Properties relationship at the single fibril level. Nature Commun. 2015, 6, 7564. [Google Scholar] [CrossRef]
- Nishiyama, Y. Molecular interactions in nanocellulose assembly. Phil. Trans. R. Soc. A 2017, 376, 20170027. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K. Spray-coated cellulose nanofiber films: Preparation, characterization and application. In Nanocellulose—Sources, Preparations and Applications; Kazi, S.M., Ed.; Intechopen: London, UK, 2024; pp. 130–179. [Google Scholar]
- Pacaphol, K.; Aht-Ong, D. The influences of silanes on interfacial adhesion and surface properties of nanocellulose film coating on glass and aluminum substrates. Surf. Coat. Technol. 2017, 320, 70–81. [Google Scholar] [CrossRef]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for paper and textile coating: The importance of surface chemistry. ChemPlusChem 2022, 87, e202200204. [Google Scholar] [CrossRef]
- Farooq, A.; Memon, H.; Farooq, A.; Wang, Z. (Eds.) Nanocellulose in textile applications. In Deep Eutectic Solvents in the Textile Industry; Springer: Singapore, 2024; pp. 75–88. [Google Scholar]
- Saremi, R.; Borodinov, N.; Laradji, A.M.; Sharma, S.; Luzinov, I.; Minko, S. Adhesion and stability of nanocellulose coatings on flat polymer films and textiles. Molecules 2020, 25, 3238. [Google Scholar] [CrossRef]
- Liang, L.; Bhagia, S.; Li, M.; Huang, C.; Ragauskas, A.J. Cross-linked nanocellulosic materials and their applications. ChemSusChem 2020, 13, 78–87. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Xie, Y.; Zhu, E.; Shoi, Z.; Yang, Q.; Xiong, C. Doubly cross-linked nanocellulose hydrogels with excellent mechanical properties. Cellulose 2019, 26, 8645–8654. [Google Scholar] [CrossRef]
- Hossain, L.; Raghuwanshi, V.S.; Tanner, J.; Wu, C.M.; Kleinerman, O.; Cohen, Y.; Garnier, G. Structure and swelling of cross-linked nanocellulose foams. J. Coll. Interf. Sci. 2020, 568, 234–244. [Google Scholar] [CrossRef]
- Zulfiana, D.; Karimah, A.; Anita, S.H.; Masruchin, N.; Wijaya, K.; Suryanegara, L.; Fatriasari, W.; Fudholi, A. Antimicrobial Imperata cylindrica paper coated with anionic nanocellulose crosslinked with cationic ions. Int. J. Biol. Macromol. 2020, 164, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Deng, X.; Dong, Y.; Zhou, Z.; Zhang, Y.; Yu, J.; Cai, J.; Zhang, Y. High-strength, transparent and superhydrophobic nanocellulose/nanochitin membranes fabricated via crosslinking of nanofibers and coating F-SiO2 suspensions. Carbohydr. Polym. 2020, 237, 116694. [Google Scholar] [CrossRef]
- Jeon, H.; Son, J.H.; Lee, J.; Park, S.B.; Ju, S.; Oh, D.X.; Koo, J.M.; Park, J. Preparation of a nanocellulose/nanochitin coating on a poly(lactic acid) film for improved hydrolysis resistance. Int. J. Biol. Macromol. 2024, 254, 127790. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Rana, H.; Singh, N.; Goswami, S. Guargum/nanocellulose based novel crosslinked antimicrobial film with enhanced barrier and mechanical properties for food packaging. J. Environ. Chem. Eng. 2023, 11, 109254. [Google Scholar] [CrossRef]
- Dai, L.; Long, Z.; Chen, J.; An, X.; Cheng, D.; Khan, A.; Ni, Y. Robust guar gum/cellulose nanofibrils multilayer films with good barrier properties. ACS Appl. Mater. Interf. 2017, 9, 5477–5485. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Sugiyama, J.; Wolfgang, G. Cellulosic nanocomposites. I. Thermally deformable cellulosic hexanoates from heterogeneous reaction. J. Appl. Polym. Sci. 2000, 78, 2242–2253. [Google Scholar] [CrossRef]
- Vuoti, S.; Talja, R.; Johansson, L.; Heikkinen, H.; Tammelin, T. Solvent impact on esterification and film formation ability of nanofibrillated cellulose. Cellulose 2013, 20, 2359–2370. [Google Scholar] [CrossRef]
- De Cuadro, P.; Belt, T.; Kontturi, K.; Kontturi, E.; Vuorinen, T.; Hughes, M. Cross-linking of cellulose and poly(-ethylene glycol) with citric acid. React. Func. Polym. 2015, 90, 21–24. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Luner, P.; Caluwe, P. Mechanism of cross-linking of paper with polyfunctional carboxylic acids. J. Appl. Polym. Sci. 1995, 58, 1523–1534. [Google Scholar] [CrossRef]
- Quellmalz, A.; Mihranyan, A. Citric acid cross-linked nanocellulose-based paper for size-exclusion nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef]
- Sirvio, J.; Kolehmainen, A.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Biocomposite cellulose-alginate films: Promising packaging materials. Food Chem. 2014, 151, 343–351. [Google Scholar] [CrossRef]
- Csiszár, E.; Herceg, I.; Fekete, E. Effect of heating and citric acid on the performance of cellulose nanocrystal thin films. Polymers 2023, 15, 1698. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Edberg, J.; Gueskine, V.; Vagin, M.; Say, M.G.; Erlandsson, J.; Wagberg, L.; Engquist, I.; Berggren, M. The effect of crosslinking on ion transport in nanocellulose-based membranes. Carbohydr. Polym. 2022, 278, 118938. [Google Scholar] [CrossRef]
- Cherian, R.M.; Tharayil, A.; Varghese, R.T.; Antony, T.; Kargarzadeh, H.; Chirayil, C.J.; Thomas, S. A review on the emerging applications of nano-cellulose as advanced coatings. Carbohydr. Polym. 2022, 282, 119123. [Google Scholar] [CrossRef]
- Hossain, L.; Ledesma, R.M.; Tanner, J.; Garnier, G. Effect of crosslinking on nanocellulose superabsorbent biodegradability. Carbohydr. Polym. Technol. Appl. 2022, 3, 100199. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Tayeb, P.; Joyce, M.; Tyagi, P.; Kehoe, M.; Dimic-Misic, K.; Pal, L. Rheology of nanocellulose-rich aqueous suspensions: A review. BioResources 2017, 12, 9556–9661. [Google Scholar] [CrossRef]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Hossain, L.; Raghuwanshi, V.S.; Tanner, J.; Garnier, G. Modulating nanocellulose hydrogels and cryogels strength by crosslinking and blending. Coll. Surf. A 2021, 630, 127608. [Google Scholar] [CrossRef]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and mechanical properties of plasticized and cross-linked nanocellulose coatings for paper packaging applications. Cellulose 2017, 24, 30969–33980. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Song, L.; Ren, L.; Xu, X.; Lu, S. Swelling resistance and water-induced shape memory performances of sisal cellulose nanofibers/polyethylene glycol/citric acid nanocellulose papers. J. Nanomater. 2019, 1, 4304532. [Google Scholar] [CrossRef]
- Hadrup, H.; Fredriksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Reg. Tox. Pharm. 2021, 121, 104873. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Wei, Y.; Li, X.; Li, J.; Shi, S.Q.; Li, J. Bioinspired hyperbranched protein adhesive based on boronic acid-functionalized cellulose nanofibril and water-soluble polyester. Comp. B 2021, 219, 108943. [Google Scholar] [CrossRef]
- Stubelius, A.; Lee, S.; Almutairi, A. The chemistry of boronic acids in nanomaterials for drug delivery. Acc. Chem. Res. 2019, 52, 3108–3119. [Google Scholar] [CrossRef]
- Van Duin, M.; Peters, J.A.; Kieboom, A.P.G.; Van Bekkum, H. Studies on borate esters: The pH dependence of the stability of esters of boric acid and borate in aqueous medium as studied by 11B NMR. Tetrahedron 1984, 40, 2901–2911. [Google Scholar] [CrossRef]
- Hall, D.G. (Ed.) Structure, properties, and preparation of boronic acid derivatives. Overview of their reactions and applications. In Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine; Wiley-VCH Verlag: Weinheim, Germany, 2005; pp. 1–99. [Google Scholar]
- Rietjens, M.; Steenbergen, P.A. Crosslinking mechanism of boric acid with diols revisited. EurJIC 2005, 6, 1162–1174. [Google Scholar] [CrossRef]
- Ishihara, K.; Mouri, Y.; Funahashi, S.; Tanaka, M. Mechanistic study of the complex formation of boric acid. Inorg. Chem. 1991, 30, 2356–2360. [Google Scholar] [CrossRef]
- Raval, P.; Thomas, N.; Hamdouna, L.; Delevoye, L.; Lafon, O.; Reddy, G.N. Boron adsorption kinetics of microcrystalline cellulose and polymer resin. Langmuir 2023, 39, 5384–5395. [Google Scholar] [CrossRef]
- Shen, H.; Zheng, X.; Dong, L.; Huang, D. Bioinspired high-strength borate cross-linked microfibrillated cellulose composite laminate with self-extinguishing flame retardance and superhydrophobicity for self-cleaning. ACS Omega 2023, 8, 41458–41468. [Google Scholar] [CrossRef]
- Yildirim, M.; Candan, Z.; Gonultas, O. Chemical performance analysis of nanocellulose/boron-compound-reinforced hybrid UF resin. Green Mater. 2022, 10, 90–96. [Google Scholar] [CrossRef]
- Yildirim, M.; Candan, Z. Performance properties of particleboard panels modified with nanocellulose/boric acid. BioResources 2021, 16, 1875–1890. [Google Scholar] [CrossRef]
- Moreno, P.; Villamizar, N.; Perez, J.; Bayona, A.; Roman, J.; Moreno, N.; Cardozo, N.S.M. Fire-resistant cellulose boards from waste newspaper, boric acid salts, and protein binders. Clean Technol. Environ. 2021, 23, 1537–1546. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, D.; Carosio, F.; Camino, G.; Bergström, L. Tuning the nanocellulose-borate interaction to achieve highly flame retardant hybrid materials. Chem. Mater. 2016, 28, 1985–1989. [Google Scholar] [CrossRef]
- Fu, D.; Yang, R.; Wang, R.; Wang, Y.; Li, Y.; Bian, H. A triple-crosslinked, self-healing, polyvinyl alcohol/nanocellulose hydrogel for versatile sensing application. Carbohydr. Polym. 2024, 334, 122060. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, C.; Su, J.; Zhang, W.; Wei, S.; Tian, W.; Liu, X.; Cheng, W.; Li, X.; Li, X.; et al. A dual-crosslinked self-healing and antibacterial nanocellulose hydrogel for monitoring of human motions. Mater. Des. 2022, 215, 110464. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppala, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Vineeth, S.K.; Mahanwar, P.A.; Gadekar, P.T. Effect of addition of boric acid on thermo-mechanical properties of microcrystalline cellulose/polyvinyl alcohol blend and applicability as wood adhesive. J. Adhes. Sci. Technol. 2021, 35, 1072–1086. [Google Scholar] [CrossRef]
- Rouhi, M.; Garavand, F.; Heydari, M.; Mohammadi, R.; Sarlak, Z.; Cacciotti, I.; Razavi, S.H.; Mousavi, M.; Parandi, E. Fabrication of novel antimicrobial nanocomposite films based on polyvinyl alcohol, bacterial cellulose nanocrystals, and boric acid for food packaging. J. Food Meas. Char. 2024, 18, 2146–2161. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Ago, M.; Rojas, O.J. Hybrid films of chitosan, cellulose nanofibrils and boric acid: Flame retardancy, optical and thermo-mechanical properties. Carbohydr. Polym. 2017, 177, 13–21. [Google Scholar] [CrossRef]
- Yaylaci, E.U. Antibacterial Effects of Boric Acid Against Aquatic Pathogens. J. Anat. Eviron. Anim. Sci. 2021, 6, 240–244. [Google Scholar]
- Durmaz, E.; Ates, S. Characterisation of surfaces coated with different nanocellulose-based suspensions. Int. J. Surf. Sci. Eng. 2023, 17, 141–164. [Google Scholar] [CrossRef]
- Feinberg, H.; Hanks, T.W. Polydopamine: A bioinspired adhesive and surface modification platform. Polym. Internat. 2021, 71, 578–582. [Google Scholar] [CrossRef]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-independent surface chemistry beyond polydopamine coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.K.; Zhao, B. Adhesion properties of self-polymerized dopamine thin film. Open Surf. Sci. J. 2011, 3, 115–122. [Google Scholar] [CrossRef]
- Samyn, P. Polydopamine and cellulose: Two biomaterials with excellent compatibility and applicability. Polym. Rev. 2021, 61, 814–865. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F.; Jonsson, M. The reactivity of hydroxyl radicals toward boric acid as a function of pH. J. Phys. Chem. A 2024, 128, 7593–7600. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 5849. [Google Scholar] [CrossRef]
- Hong, S.H.; Hong, S.; Ryou, M.H.; Choi, J.W.; Kang, S.M.; Lee, H. Sprayable ultrafast polydopamine surface modifications. Adv. Mater. Interfac. 2016, 3, 1500857. [Google Scholar] [CrossRef]
- Graveson, I.; English, R. Low Energy Method for the Preparation of Non-Derivatized Nanocellulose. U.S. Patent US20150158955A1, 11 June 2015. [Google Scholar]
- ISO 1518-1; Paints and Varnishes—Determination of Scratch Resistance Part 1: Constant-Loading Method. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 2409; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 25178-2; Geometrical Product Specifications (GPS)—Surface Texture: Areal, Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 19403-2; Paints and Varnishes—Wettability, Part 2: Determination of the Surface Free Energy of Solid Surfaces by Measuring the Contact Angle. International Organization for Standardization: Geneva, Switzerland, 2024.
- Samyn, P.; Cosemans, P.; Chandroth, A.M.; Takahashi, K.; Everaerts, J. Mechanical properties of spray-coated nanocellulose coatings. Surf. Coat. Technol. 2025, 496, 131601. [Google Scholar] [CrossRef]
- Schneider, A.; Hemmerlé, J.; Allais, M.; Didierjean, J.; Michel, M.; d’Ischia, M.; Ball, V. Boric acid as an efficient agent for the control of polydopamine self-assembly and surface properties. ACS Appl. Mater. Interf. 2018, 20, 7574–7580. [Google Scholar] [CrossRef]
- Alavia, W.; Seidel-Morgenstern, A.; Hermsdorf, D.; Lorenz, H.; Graber, T.A. Real-time crystal growth monitoring of boric acid from sodium or lithium sulfate containing aqueous solutions by atomic force microscopy. ACS Omega 2023, 8, 10822–10835. [Google Scholar] [CrossRef]
- Gao, Y.; Radaniello, W.; Toksoy, M.F.; Munhollon, T.; Haber, R. Improvement of crystallization and particle size distribution of boric acid in the processing of a boron carbide precursor. RCS Adv. 2015, 25, 19067–19073. [Google Scholar] [CrossRef]
- Harabor, A.; Rotaru, P.; Scorei, R.I.; Harabor, N.A. Non-conventional hexagonal structure for boric acid. J. Therm. Anal. Calor. 2014, 118, 1375–1384. [Google Scholar] [CrossRef]
- Jean, J.H. Crystallization of boric acid on low-k glass+ ceramic green tapes. Jpn. J. Appl. Phys. 1996, 35, L429. [Google Scholar] [CrossRef]
- Pan, R.; Cheng, Y.; Pei, Y.; Liu, J.; Tian, W.; Jiang, Y.; Tang, K.; Zhang, J.; Zheng, X. Cellulose materials with high light transmittance and high haze: A review. Cellulose 2023, 30, 4813–4826. [Google Scholar] [CrossRef]
- Ansari, F.; Ding, Y.; Berglund, L.A.; Dauskardt, R.H. Toward sustainable multifunctional coatings containing nanocellulose in a hybrid glass matrix. ACS Nano 2018, 12, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yue, Y.; Wu, Q.; Huang, C.; Pan, H.; Zhan, X.; Mei, C.; Xu, X. Effects of nanocellulose on the structure and properties of poly(vinyl alcohol)-borax hybrid foams. Cellulose 2017, 24, 4433–4448. [Google Scholar] [CrossRef]
- Zhang, J.; Koubaa, A.; Xing, D.; Liu, W.; Wang, Q.; Wang, X.M.; Wang, H. Improving lignocellulose thermal stability by chemical modification with boric acid for incorporating into polyamide. Mater. Des. 2020, 191, 108589. [Google Scholar] [CrossRef]
- Durmaz, E.; Ates, S. Effect of nanocellulose type and matrix material on production of nanocomposites. Cell. Chem. Technol. 2023, 57, 625–635. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samyn, P.; Cosemans, P.; Van der Eycken, E.V.; Coppola, G.A. Enhanced Mechanical Robustness of Sprayed Cellulose Nanofibril Coatings Through Internal Crosslinking with Boric Acid. Polymers 2025, 17, 2451. https://doi.org/10.3390/polym17182451
Samyn P, Cosemans P, Van der Eycken EV, Coppola GA. Enhanced Mechanical Robustness of Sprayed Cellulose Nanofibril Coatings Through Internal Crosslinking with Boric Acid. Polymers. 2025; 17(18):2451. https://doi.org/10.3390/polym17182451
Chicago/Turabian StyleSamyn, Pieter, Patrick Cosemans, Erik V. Van der Eycken, and Guglielmo A. Coppola. 2025. "Enhanced Mechanical Robustness of Sprayed Cellulose Nanofibril Coatings Through Internal Crosslinking with Boric Acid" Polymers 17, no. 18: 2451. https://doi.org/10.3390/polym17182451
APA StyleSamyn, P., Cosemans, P., Van der Eycken, E. V., & Coppola, G. A. (2025). Enhanced Mechanical Robustness of Sprayed Cellulose Nanofibril Coatings Through Internal Crosslinking with Boric Acid. Polymers, 17(18), 2451. https://doi.org/10.3390/polym17182451






