Design of Vitrimers with Simultaneous Degradable and Dynamic Crosslinkers: Mechanical and Thermal Behavior Based on Transesterification Reactions Between β-Amino Esters and Hydroxylated Acrylate/Methacrylate Monomers
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
2.3. Monomer Purification
2.4. Synthetic Strategy for the Preparation of the Crosslinkers
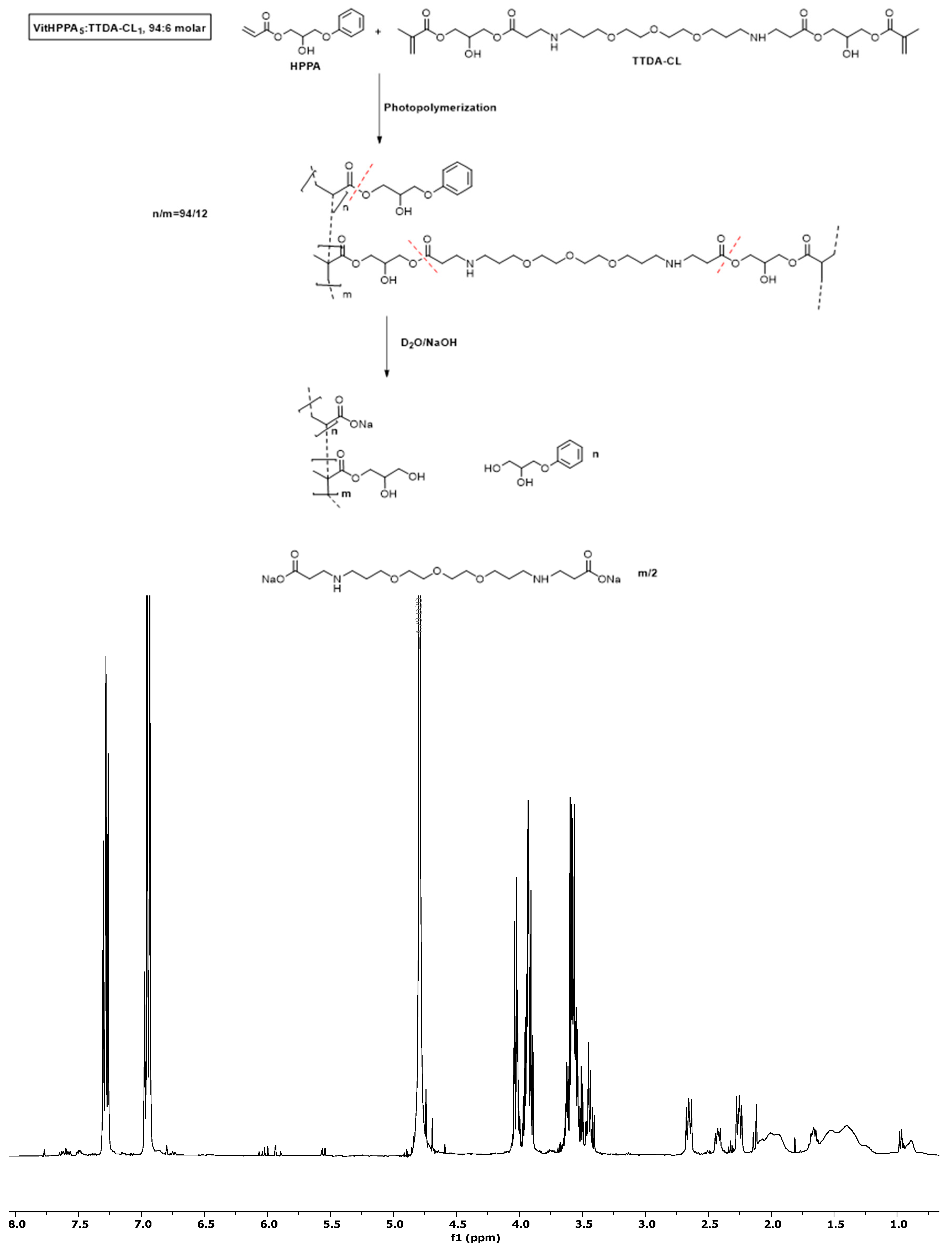
2.4.1. Preparation of Vitrimers by Photopolymerization
2.4.2. Post-Curing of the Photopolymerized Vitrimers
3. Results and Discussion
3.1. Design of the Vitrimer Formulation
3.2. Preparation of the Vitrimers by Photopolymerization
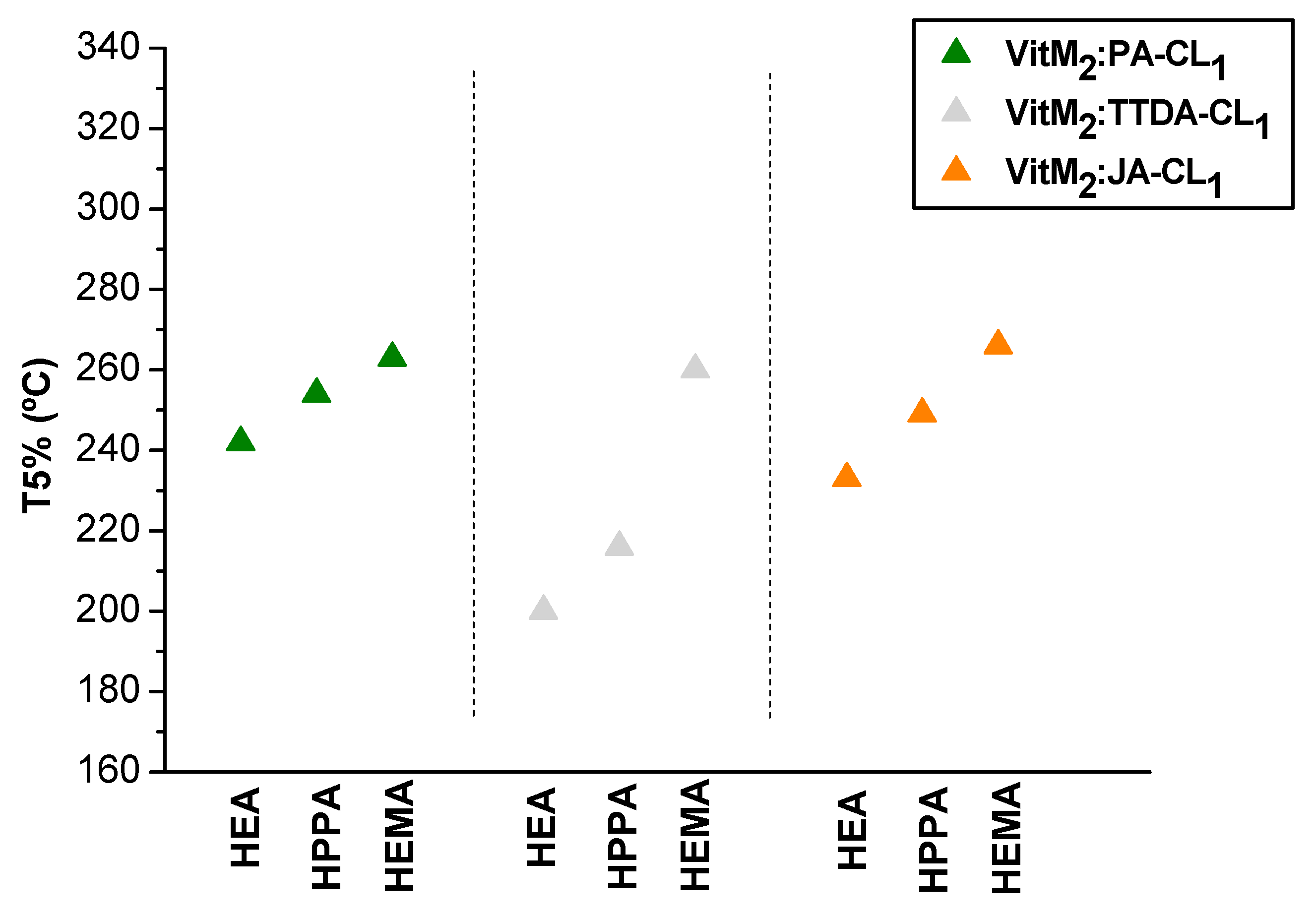
3.3. Thermal Behavior of the Vitrimers: Evaluation of the Tg and the Degradation Temperature
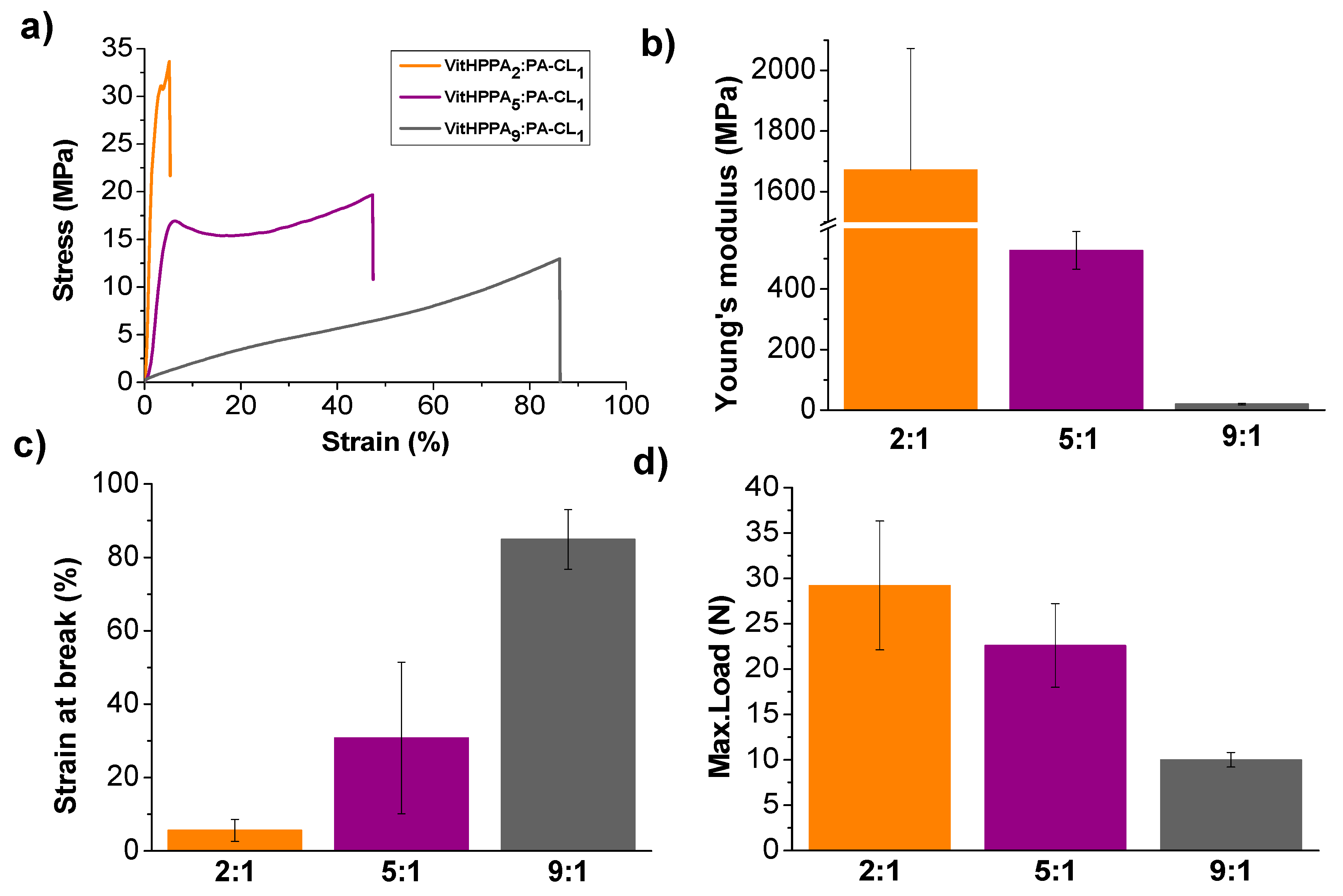
3.4. Mechanical Properties of the Vitrimers as a Function of the Chemical Composition
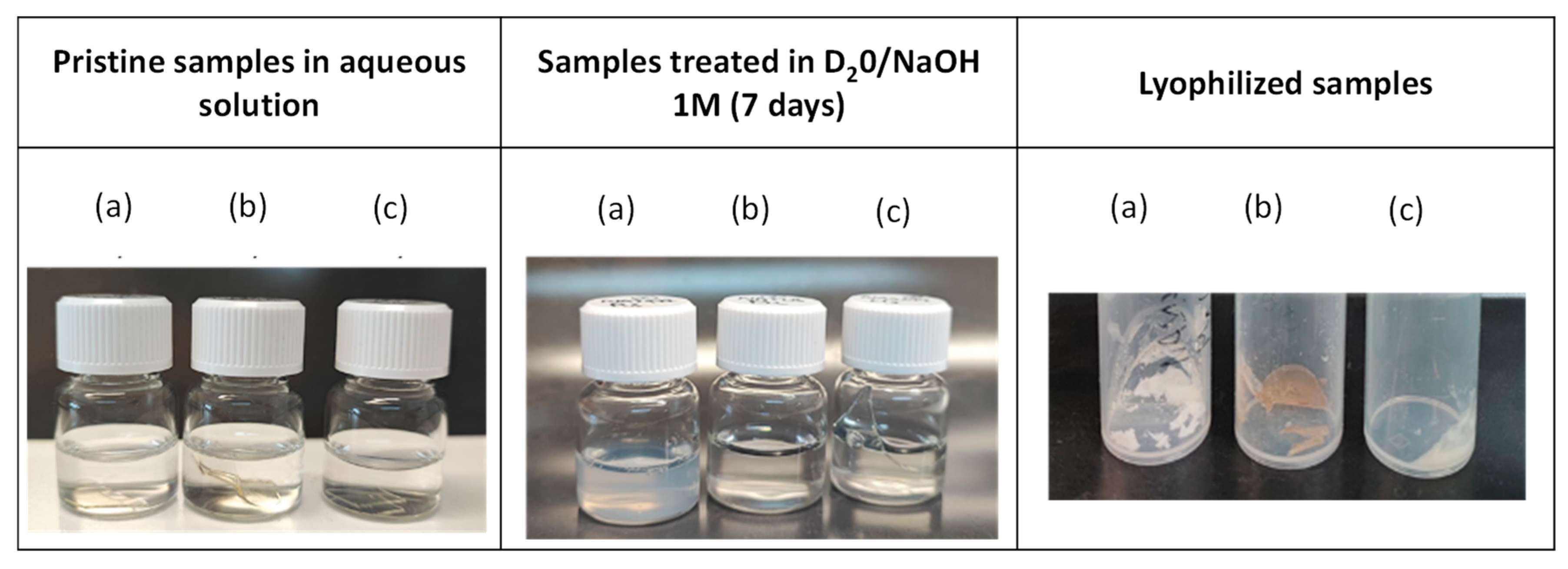
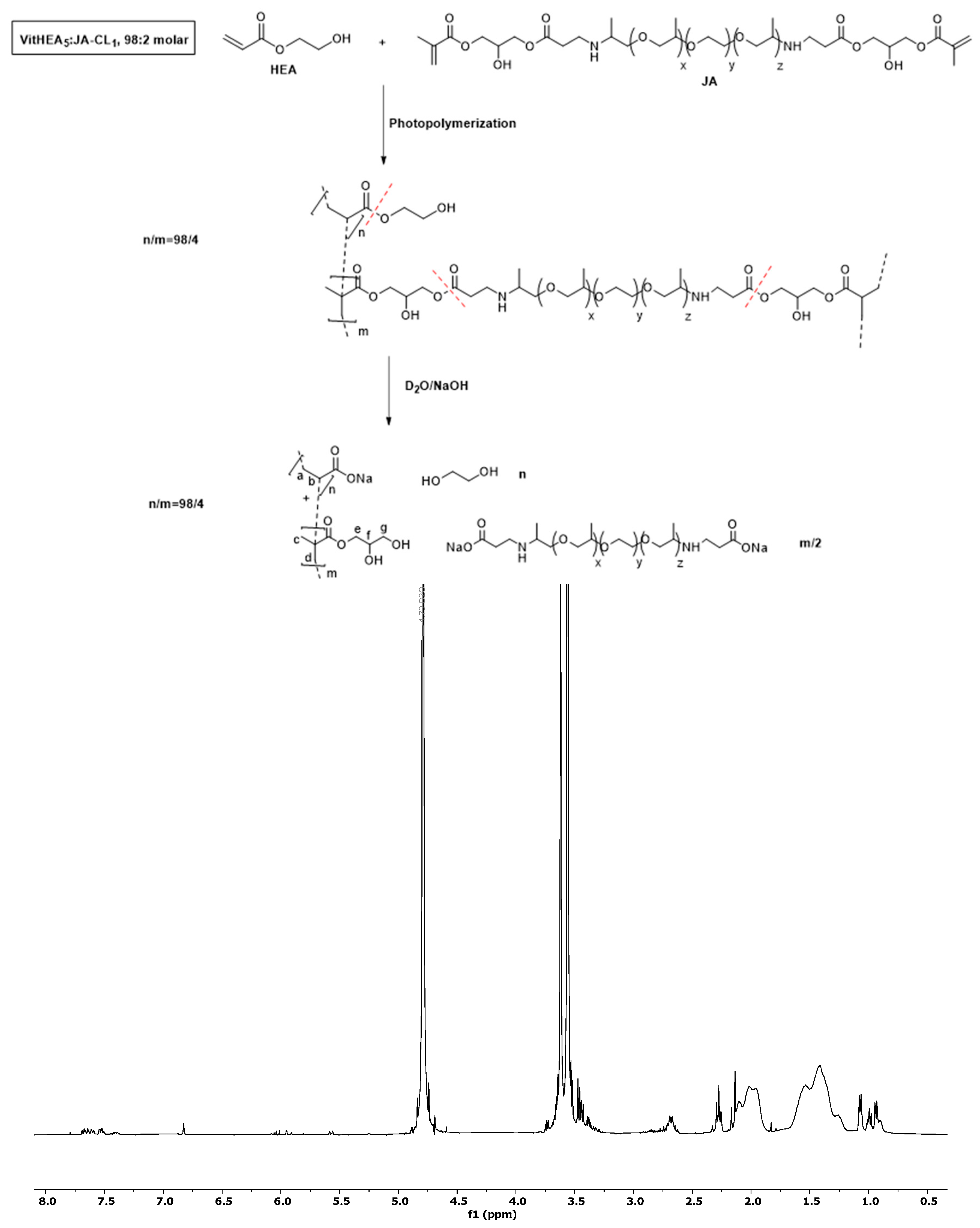
3.5. Evaluation of the Recyclability of the Vitrimers
- (a)
- Recyclability of the vitrimers by reprocessing
- (b)
- Hydrolysis of the vitrimers and formation of linear polymer chains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Huang, S.; Wang, H.; Ahmad, W.; Ahmad, A.; Vatin, N.I.; Mohamed, A.M.; Deifalla, A.F.; Mehmood, I. Plastic Waste Management Strategies and Their Environmental Aspects: A Scientometric Analysis and Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 4556. [Google Scholar] [CrossRef]
- Raj, M.; Ghosh, A.; Ilame, T.; Kumar, S. Plastic Waste Management Strategies: Planning through Sustainable Lens and Way Forward towards Circular Economy. Discov. Sustain. 2025, 6, 361. [Google Scholar] [CrossRef]
- Nassani, A.A.; Hussain, H.; Condrea, E.; Grigorescu, A.; Yousaf, Z.; Haffar, M. Zero Waste Management: Investigation of Green Technology, the Green Supply Chain, and the Moderating Role of CSR Intentions. Sustainability 2023, 15, 4169. [Google Scholar] [CrossRef]
- Nishida, H. Development of Materials and Technologies for Control of Polymer Recycling. Polym. J. 2011, 43, 435–447. [Google Scholar] [CrossRef]
- Peterson, G.I.; Larsen, M.B.; Boydston, A.J. Controlled Depolymerization: Stimuli-Responsive Self-Immolative Polymers. Macromolecules 2012, 45, 7317–7328. [Google Scholar] [CrossRef]
- Gurrala, L.; Morais, A.R.C. Supercritical Fluids for Enhanced Chemical Transformation of Postconsumer Plastics: A Review. ChemCatChem 2025, 17, e202401725. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Towards Truly Sustainable Polymers: A Metal-Free Recyclable Polyester from Biorenewable Non-Strained γ-Butyrolactone. Angew. Chem. Int. Ed. 2016, 55, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Godwin, B.; Baran, M.J.; Nazir, R.; Wulff, J.E. A Cleavable Crosslinking Strategy for Commodity Polymer Functionalization and Generation of Reprocessable Thermosets. Angew. Chem. 2023, 135, e202304708. [Google Scholar] [CrossRef]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.-L.; Park, S.-J. Preparation and Characterization of Carbon Fiber-Reinforced Thermosetting Composites: A Review. Carbon Lett. 2015, 16, 67–77. [Google Scholar] [CrossRef]
- Caydamli, Y.; Heudorfer, K.; Take, J.; Podjaski, F.; Middendorf, P.; Buchmeiser, M.R. Transparent Fiber-Reinforced Composites Based on a Thermoset Resin Using Liquid Composite Molding (LCM) Techniques. Materials 2021, 14, 6087. [Google Scholar] [CrossRef]
- Gore, P.M.; Kandasubramanian, B. Functionalized Aramid Fibers and Composites for Protective Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 16537–16563. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently Crosslinked Polymers with Dynamic Network Topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Hoe, G.X.D.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef]
- Zhang, Z.P. Polymer Engineering Based on Reversible Covalent Chemistry: A Promising Innovative Pathway towards New Materials and New Functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic Covalent Chemistry in Polymer Networks: A Mechanistic Perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Zhu, G.; Houck, H.A.; Spiegel, C.A.; Selhuber-Unkel, C.; Hou, Y.; Blasco, E. Introducing Dynamic Bonds in Light-based 3D Printing. Adv. Funct. Mater. 2024, 34, 2300456. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent Development of Repairable, Malleable and Recyclable Thermosetting Polymers through Dynamic Transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef]
- Zhou, Y.; Goossens, J.G.P.; Sijbesma, R.P.; Heuts, J.P.A. Poly(Butylene Terephthalate)/Glycerol-Based Vitrimers via Solid-State Polymerization. Macromolecules 2017, 50, 6742–6751. [Google Scholar] [CrossRef]
- Ji, F.; Liu, X.; Lin, C.; Zhou, Y.; Dong, L.; Xu, S.; Sheng, D.; Yang, Y. Reprocessable and Recyclable Crosslinked Polyethylene with Triple Shape Memory Effect. Macromol. Mater. Eng. 2019, 304, 1800528. [Google Scholar] [CrossRef]
- Kumar, A.; Connal, L.A. Biobased Transesterification Vitrimers. Macromol. Rapid Commun. 2023, 44, e2200892. [Google Scholar] [CrossRef]
- Mu, E.; Mu, R. Bismethacrylate-Based Hybrid Monomers via Michael-Addition Reactions. Macromolecules 2001, 34, 5778–5785. [Google Scholar]
- Navarro, R.; Monterde, C.; Molina, S.; Pérez-Perrino, M.; Reviriego, F.; Del Prado, A.; Gallardo, A.; Reinecke, H. Understanding the Regioselectivity of Michael Addition Reactions to Asymmetric Divinylic Compounds. RSC Adv. 2017, 7, 56157–56165. [Google Scholar] [CrossRef]
- Liz-Basteiro, P.; Sanz-Horta, R.; Reviriego, F.; Martínez-Campos, E.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A. High Resolution Molds, Sacrificial in Aqueous Media, Obtained by Vat Photopolymerization 3D Printing. Addit. Manuf. 2023, 75, 103758. [Google Scholar] [CrossRef]
- Lynn, D.M.; Langer, R. Degradable Poly(β-Amino Esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Al Thaher, Y.; Latanza, S.; Perni, S.; Prokopovich, P. Role of Poly-Beta-Amino-Esters Hydrolysis and Electrostatic Attraction in Gentamicin Release from Layer-by-Layer Coatings. J. Colloid Interface Sci. 2018, 526, 35–42. [Google Scholar] [CrossRef]
- Zhang, B.; Kowsari, K.; Serjouei, A.; Dunn, M.L.; Ge, Q. Reprocessable Thermosets for Sustainable Three-Dimensional Printing. Nat. Commun. 2018, 9, 1831. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Chen, K.; Dunn, C.K.; Wu, J.; Li, V.C.F.; Qi, H.J. 3D Printing of Highly Stretchable, Shape-Memory, and Self-Healing Elastomer toward Novel 4D Printing. ACS Appl. Mater. Interfaces 2018, 10, 7381–7388. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific—Materials & Structural Analysis. In Curing an Acrylate with FTIR Spectroscopy; AzoM: Manchester, UK, 2021.
- Stutz, H.; Illers, K.-H.; Mertes, J. A Generalized Theory for the Glass Transition Temperature of Crosslinked and Uncrosslinked Polymers. J. Polym. Sci. B Polym. Phys. 1990, 28, 1483–1498. [Google Scholar] [CrossRef]
- Chen, M.; Si, H.; Zhang, H.; Zhou, L.; Wu, Y.; Song, L.; Kang, M.; Zhao, X.-L. The Crucial Role in Controlling the Dynamic Properties of Polyester-Based Epoxy Vitrimers: The Density of Exchangeable Ester Bonds (υ). Macromolecules 2021, 54, 10110–10117. [Google Scholar] [CrossRef]
- Lessard, J.J.; Garcia, L.F.; Easterling, C.P.; Sims, M.B.; Bentz, K.C.; Arencibia, S.; Savin, D.A.; Sumerlin, B.S. Catalyst-Free Vitrimers from Vinyl Polymers. Macromolecules 2019, 52, 2105–2111. [Google Scholar] [CrossRef]


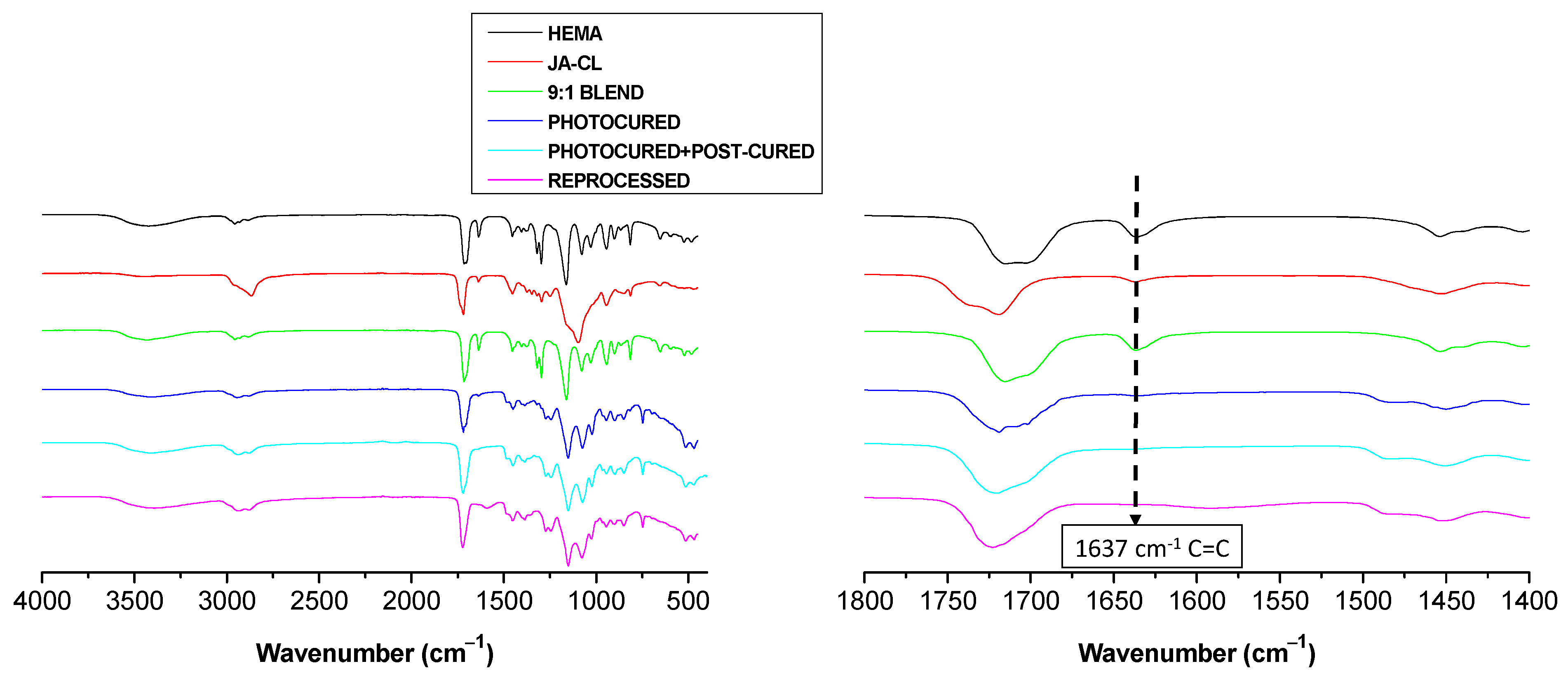
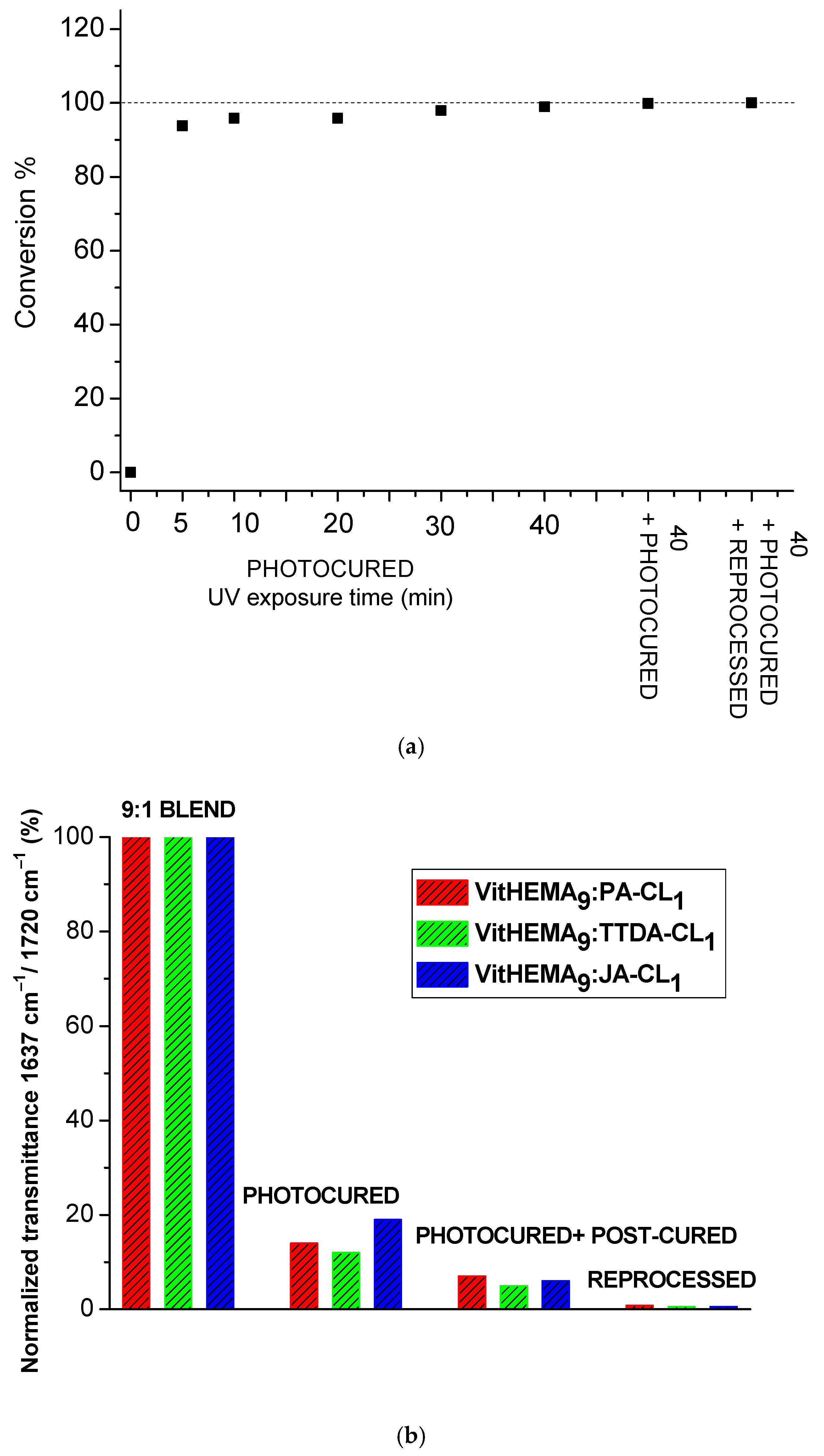
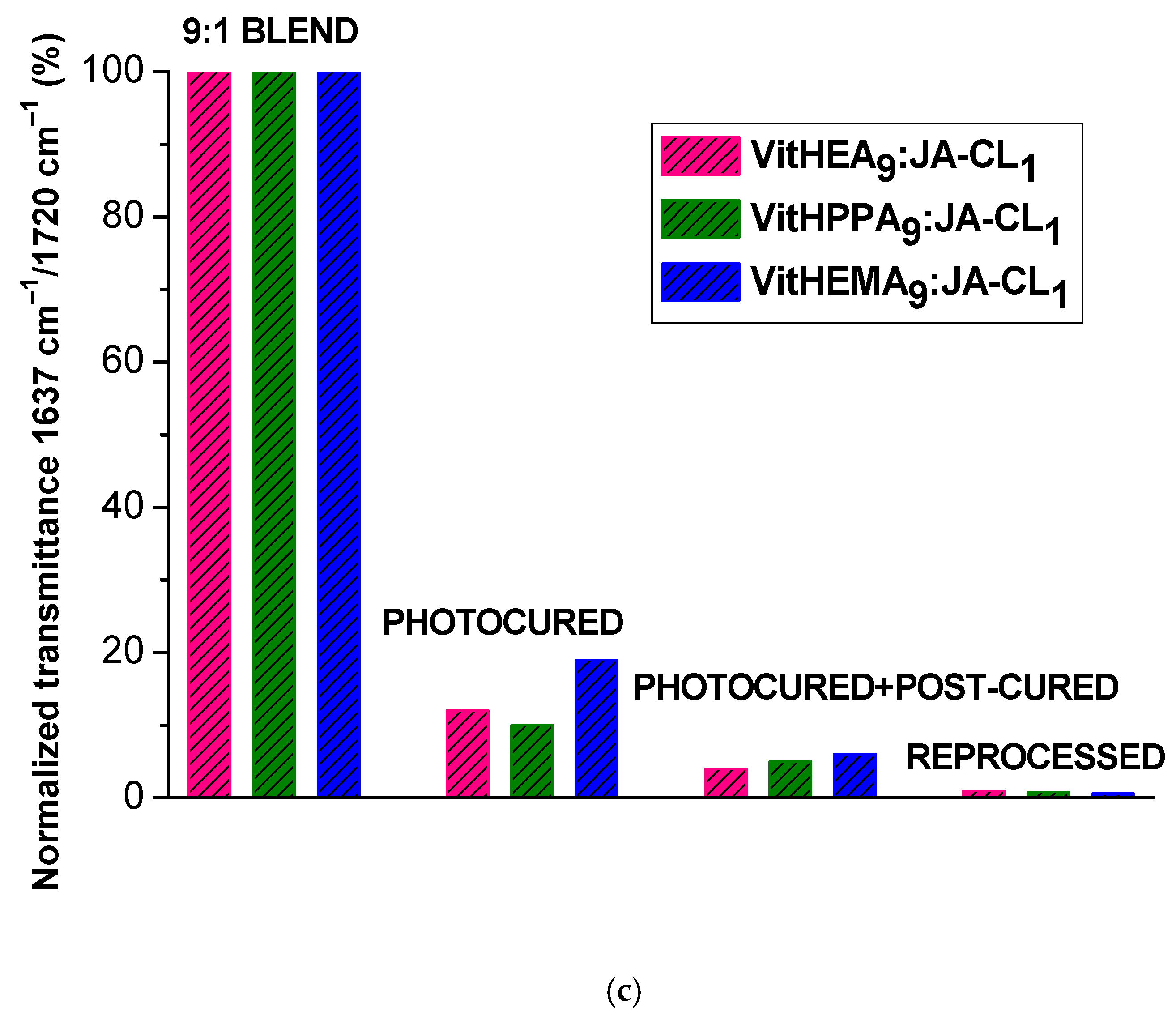

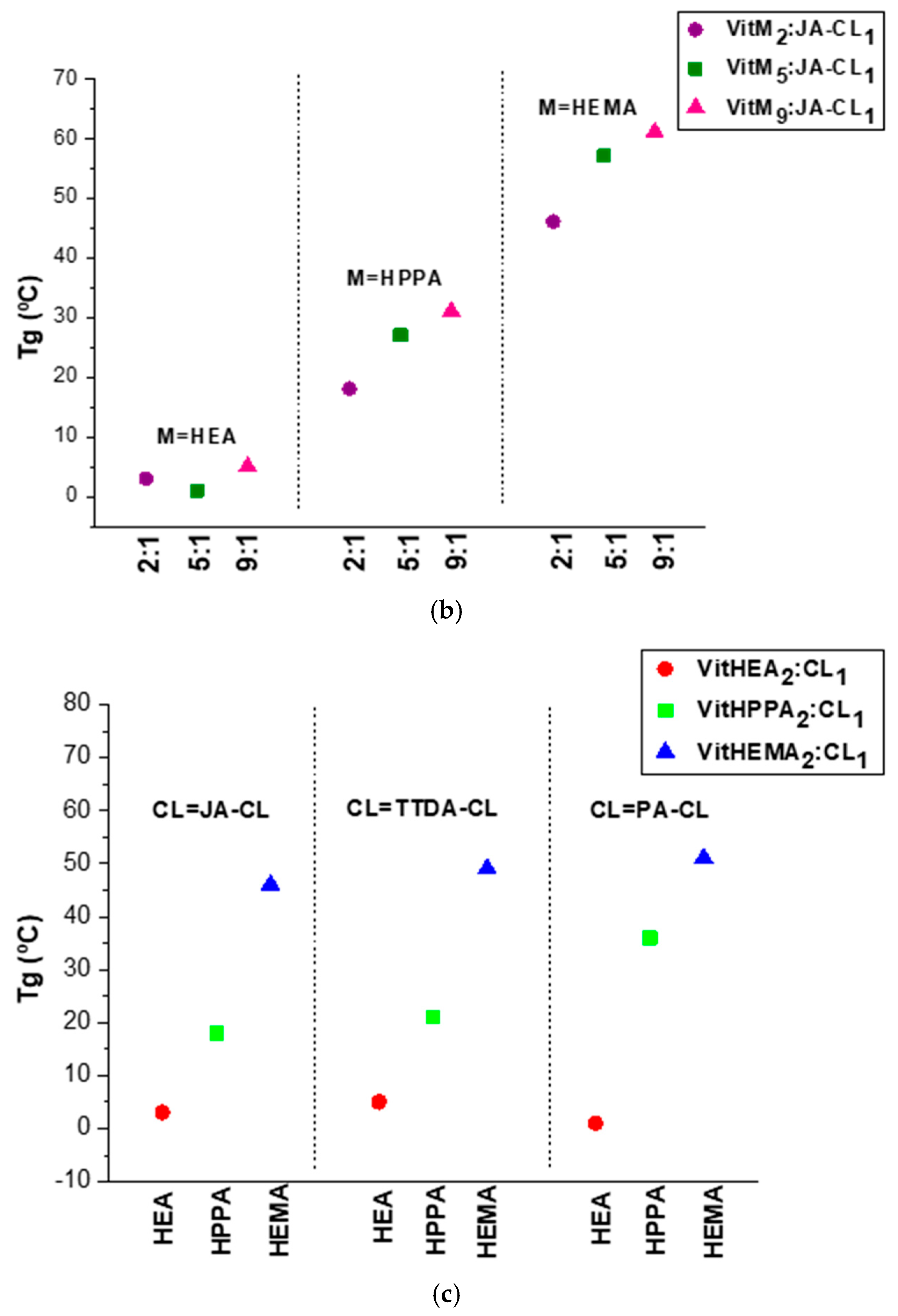




| Sample Code | Monomer (wt%) | Monomer (Mol%) | Crosslinker (wt%) | Crosslinker (Mol%) | Photoinitiator (wt%) |
|---|---|---|---|---|---|
| VitHEA2:JA-CL1 | 67 | 95 | 33 | 5 | 2 |
| VitHEMA2:JA-CL1 | 67 | 94 | 33 | 6 | 2 |
| VitHPPA2:JA-CL1 | 67 | 90 | 33 | 10 | 2 |
| VitHEMA9:JA-CL1 | 90 | 99 | 10 | 1 | 2 |
| VitHEMA9:TTDA-CL1 | 90 | 98 | 10 | 2 | 2 |
| VitHEMA9:PA-CL1 | 90 | 97 | 10 | 3 | 2 |
| VitHPPA2:PA-CL1 | 67 | 82 | 33 | 18 | 2 |
| VitHPPA5:PA-CL1 | 83 | 92 | 17 | 8 | 2 |
| VitHPPA9:PA-CL1 | 90 | 95 | 10 | 5 | 2 |
| Sample Code | Tg (°C) | Degradation Onset Temperature (T5%) | Young’s Modulus (MPa) |
|---|---|---|---|
| VitHEA2:JA-CL1 | 3 | 233 | 2.3 ± 0.2 |
| VitHEA5:JA-CL1 | 1 | 234 | 2.2 ± 0.3 |
| VitHEA9:JA-CL1 | 5 | 256 | 1.3 ± 0.1 |
| VitHPPA2:JA-CL1 | 18 | 249 | 4.5 ± 0.1 |
| VitHPPA5:JA-CL1 | 27 | 259 | 4.9 ± 0.7 |
| VitHPPA9:JA-CL1 | 31 | 266 | 5.1 ± 0.4 |
| VitHEMA2:JA-CL1 | 46 | 266 | 470 ± 51 |
| VitHEMA5:JA-CL1 | 57 | 277 | 1072 ± 33 |
| VitHEMA9:JA-CL1 | 61 | 272 | 1362 ± 156 |
| VitHEA2:TTDA-CL1 | 5 | 200 | 7.8 ± 0.5 |
| VitHPPA2:TTDA-CL1 | 21 | 216 | 9.2 ± 0.3 |
| VitHEMA2:TTDA-CL1 | 49 | 260 | n.d. |
| VitHEA2:PA-CL1 | 1 | 242 | 14.1 ± 1.3 |
| VitHPPA2:PA-CL1 | 36 | 254 | 1672 ± 401 |
| VitHEMA2:PA-CL1 | 51 | 263 | n.d. |
| VitHPPA5:TTDA-CL1 | 25 | 213 | 5.7 ± 0.5 |
| VitHPPA5:PA-CL1 | 40 | 259 | 527 ± 62 |
| VitHPPA9:PA-CL1 | 34 | 192 | 20 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayensa, N.; Reviriego, F.; Reinecke, H.; Gallardo, A.; Elvira, C.; Rodríguez-Hernández, J. Design of Vitrimers with Simultaneous Degradable and Dynamic Crosslinkers: Mechanical and Thermal Behavior Based on Transesterification Reactions Between β-Amino Esters and Hydroxylated Acrylate/Methacrylate Monomers. Polymers 2025, 17, 2448. https://doi.org/10.3390/polym17182448
Ayensa N, Reviriego F, Reinecke H, Gallardo A, Elvira C, Rodríguez-Hernández J. Design of Vitrimers with Simultaneous Degradable and Dynamic Crosslinkers: Mechanical and Thermal Behavior Based on Transesterification Reactions Between β-Amino Esters and Hydroxylated Acrylate/Methacrylate Monomers. Polymers. 2025; 17(18):2448. https://doi.org/10.3390/polym17182448
Chicago/Turabian StyleAyensa, Naroa, Felipe Reviriego, Helmut Reinecke, Alberto Gallardo, Carlos Elvira, and Juan Rodríguez-Hernández. 2025. "Design of Vitrimers with Simultaneous Degradable and Dynamic Crosslinkers: Mechanical and Thermal Behavior Based on Transesterification Reactions Between β-Amino Esters and Hydroxylated Acrylate/Methacrylate Monomers" Polymers 17, no. 18: 2448. https://doi.org/10.3390/polym17182448
APA StyleAyensa, N., Reviriego, F., Reinecke, H., Gallardo, A., Elvira, C., & Rodríguez-Hernández, J. (2025). Design of Vitrimers with Simultaneous Degradable and Dynamic Crosslinkers: Mechanical and Thermal Behavior Based on Transesterification Reactions Between β-Amino Esters and Hydroxylated Acrylate/Methacrylate Monomers. Polymers, 17(18), 2448. https://doi.org/10.3390/polym17182448








