Mechanistic Insights into the Non-Monotonic Flame Retardancy of CPVC/ABS Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CPVC/ABS Composites
2.3. Characterization
3. Results and Discussion
3.1. Flame Retardancy of CPVC and CPVC/ABS Composites
3.1.1. Limiting Oxygen Index and UL-94 Tests
3.1.2. Cone Calorimeter Test
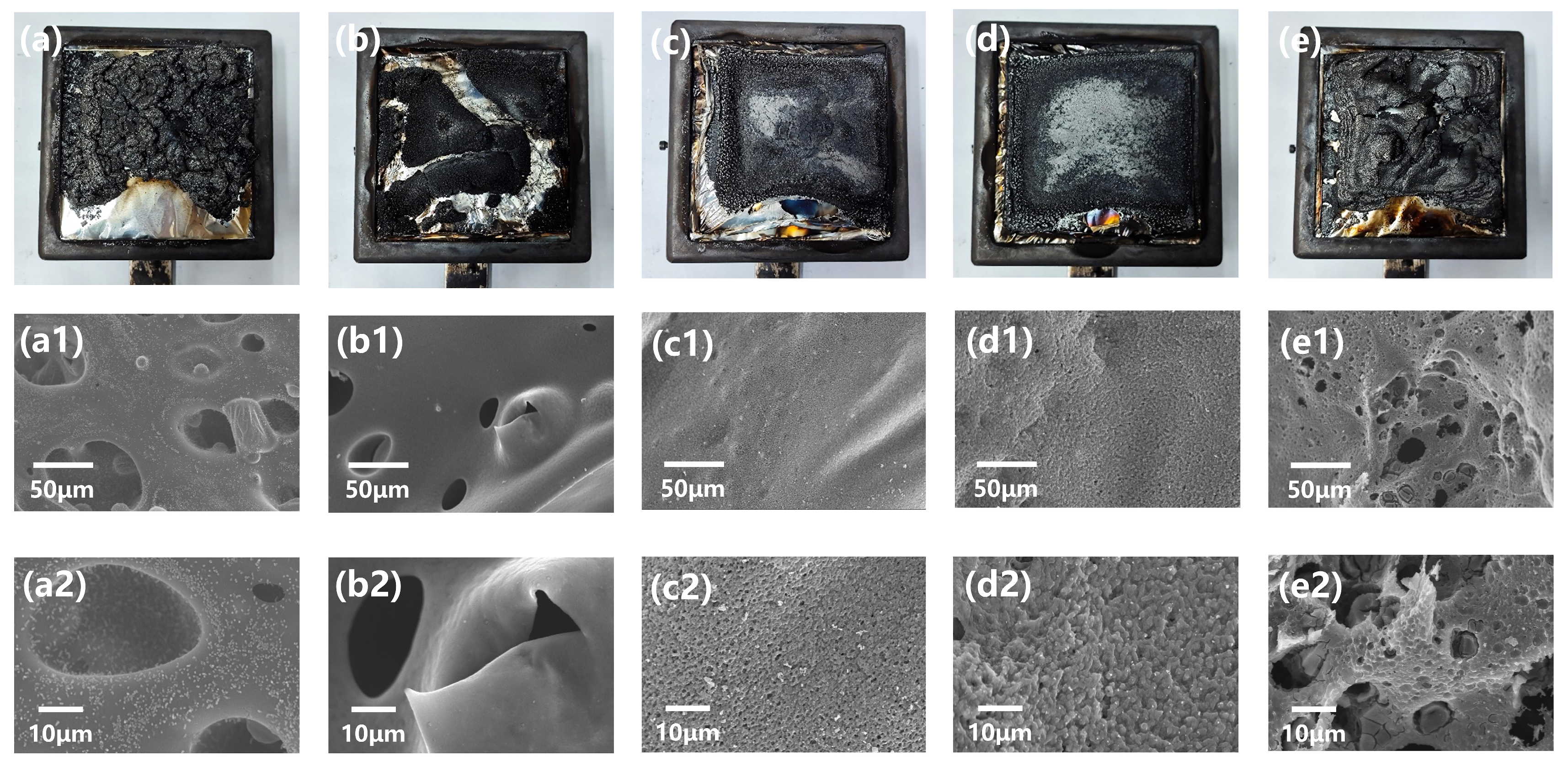
3.2. Analysis of the Char of CPVC and CPVC/ABS Composites
3.2.1. The Morphology of Residual Char
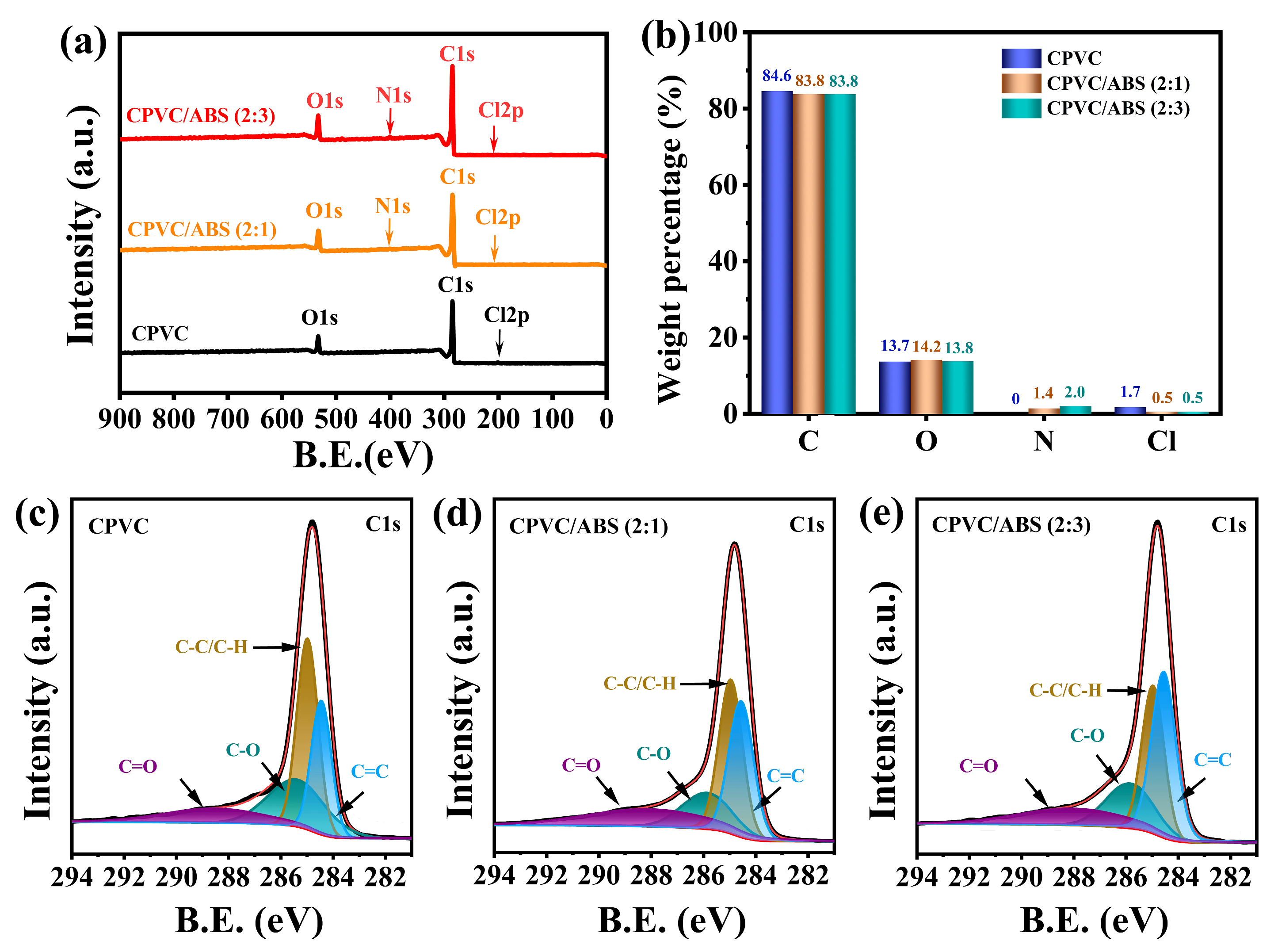
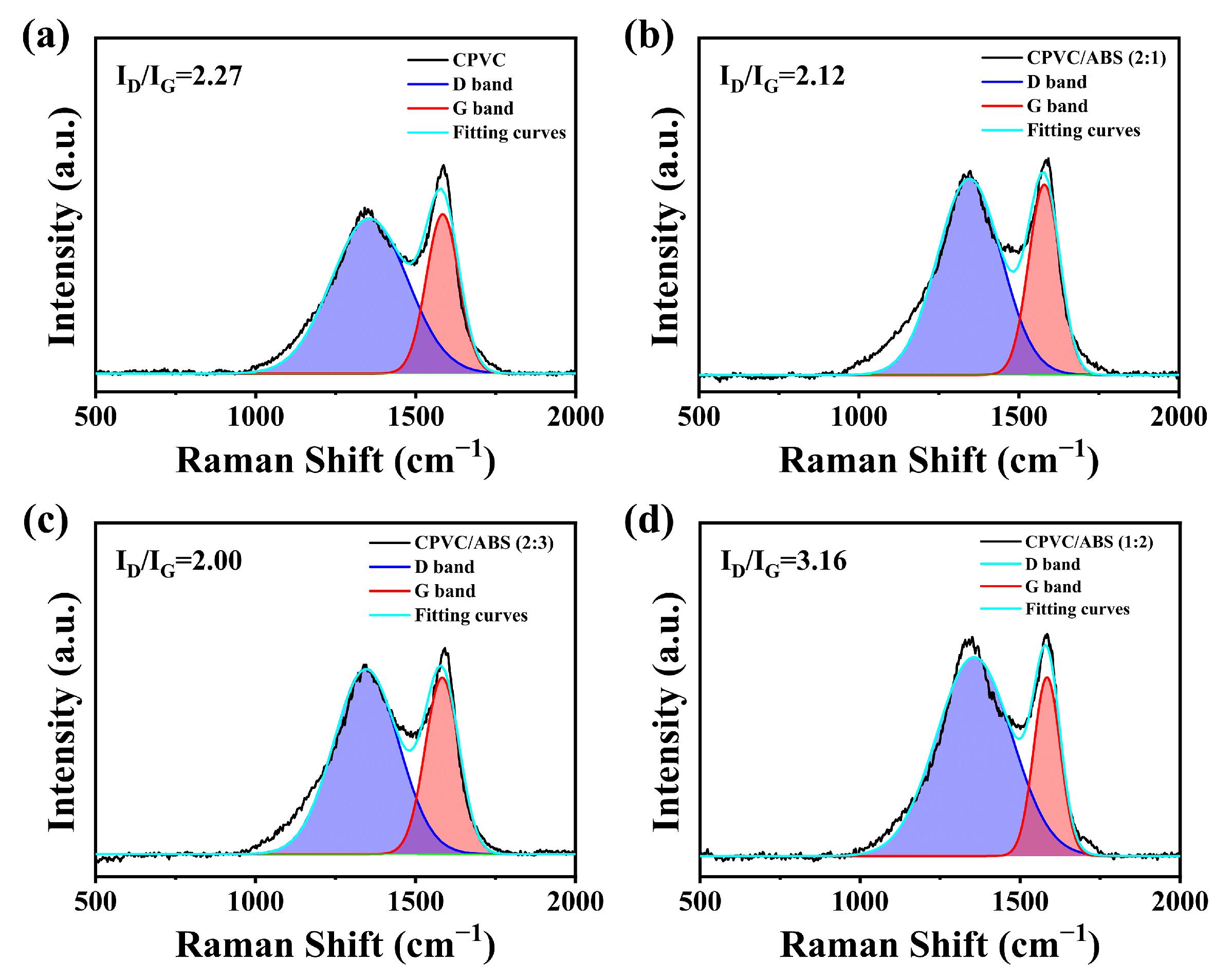
3.2.2. The Composition and Structure of Residual Char
3.3. Degradation Behavior of CPVC and CPVC/ABS Composites
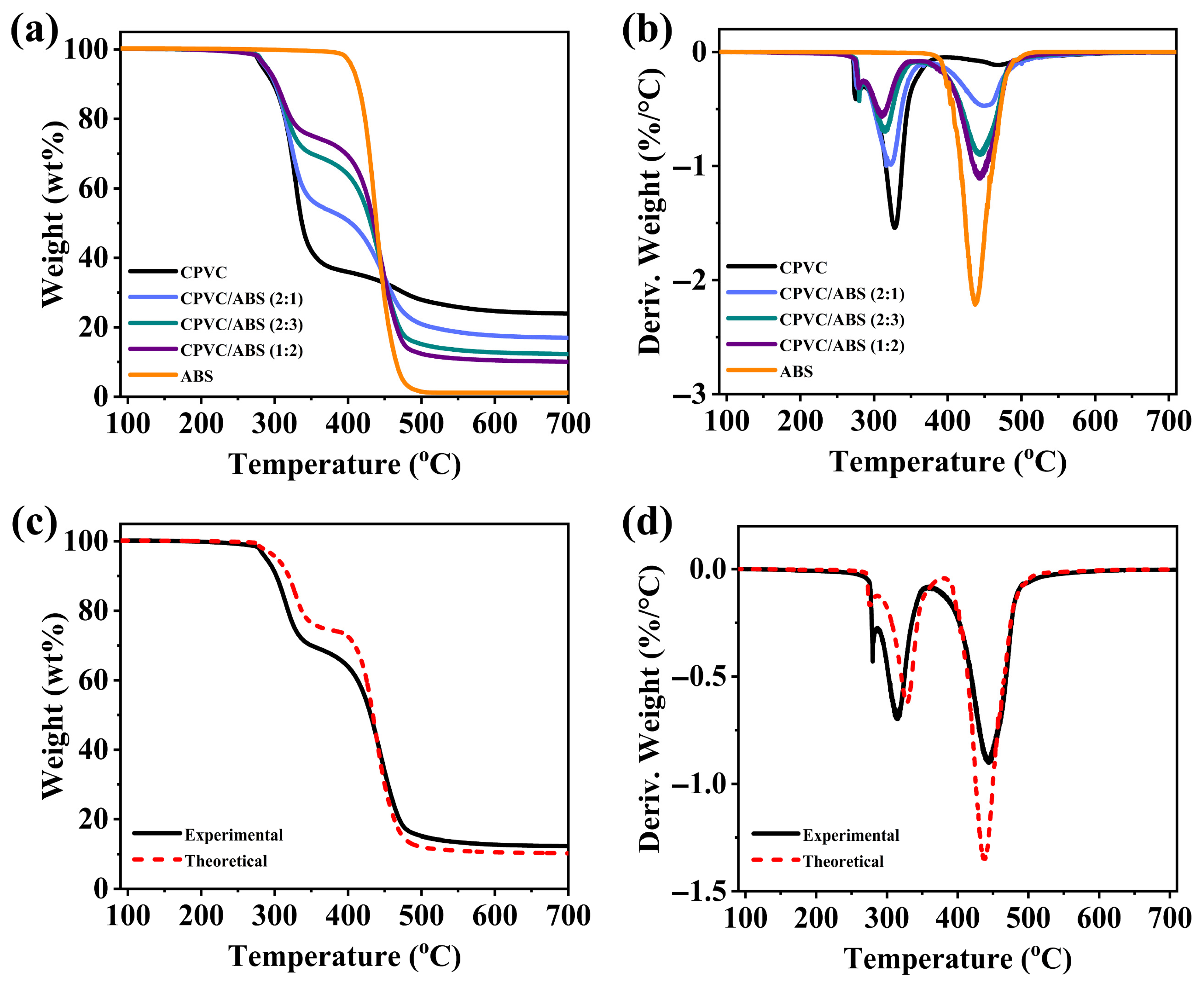
3.3.1. Thermal Stability of CPVC and CPVC/ABS Composites
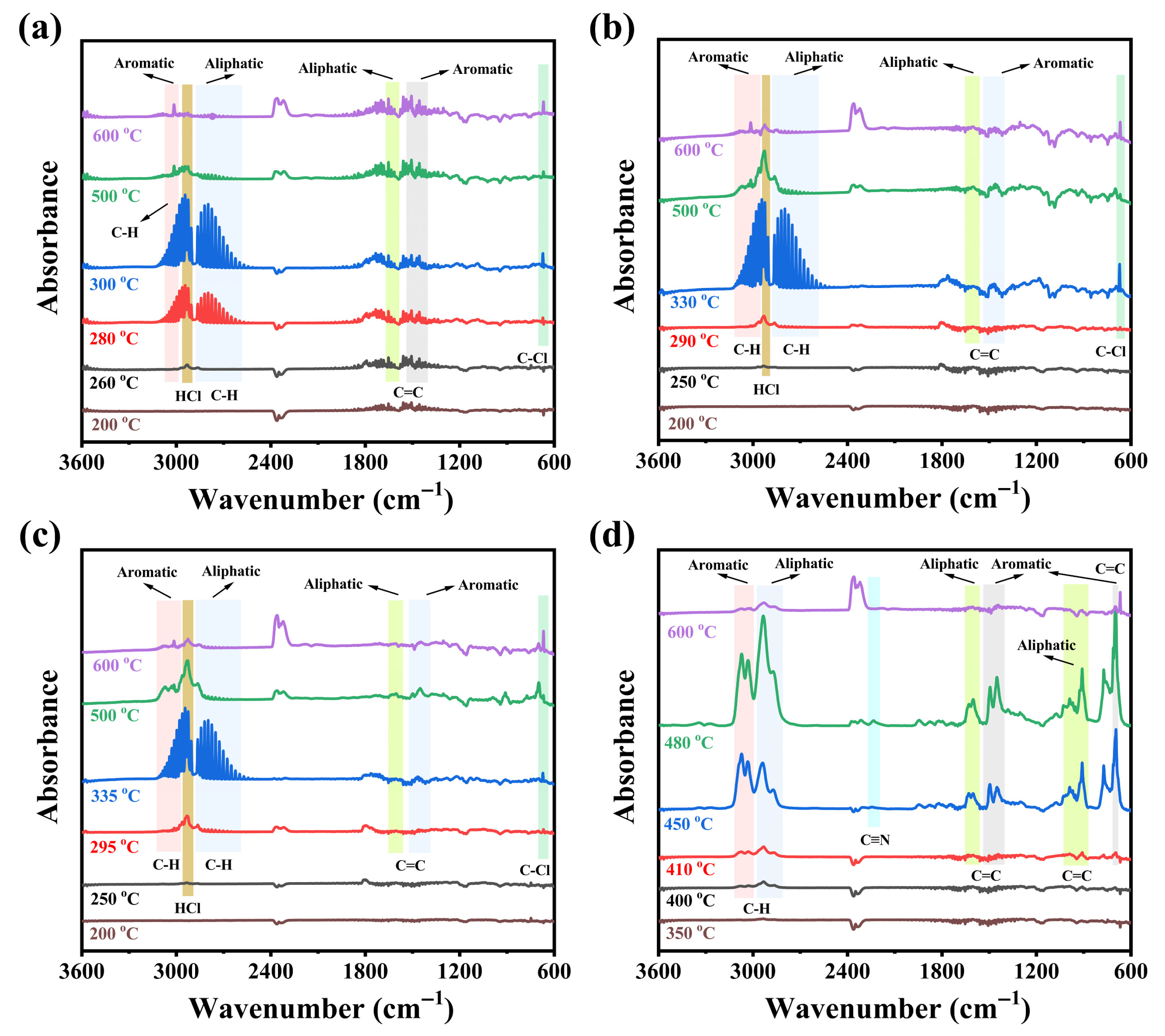
3.3.2. Analysis of the Gas Phase Pyrolysis Products
3.4. Flame-Retardant Mechanism of CPVC/ABS Composites
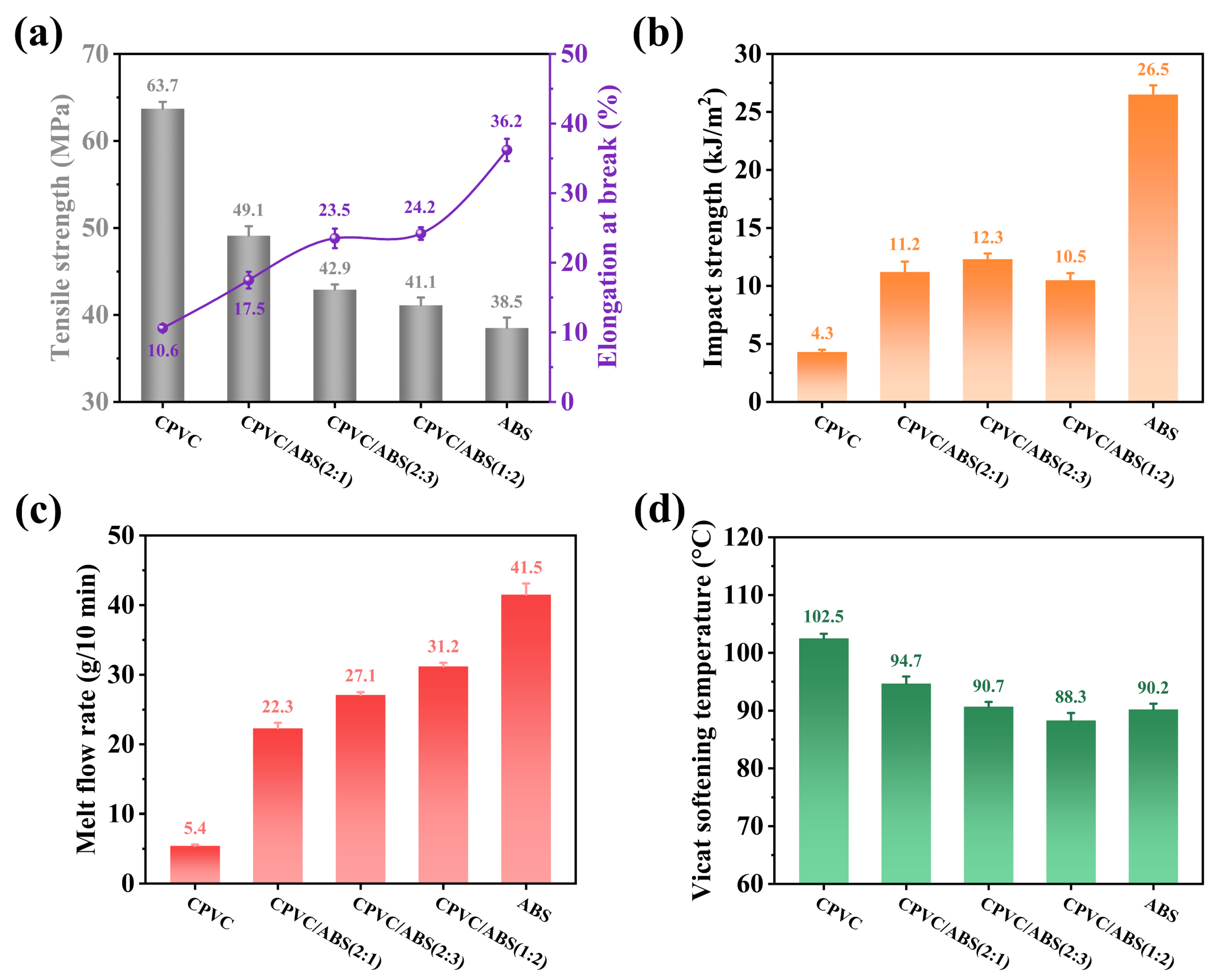
3.5. Comprehensive Performance of CPVC/ABS Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshmukh, D.; Kulkarni, H.; Srivats, D.S.; Bhanushali, S.; More, A.P. Recycling of acrylonitrile butadiene styrene (ABS): A review. Polym. Bull. 2024, 81, 1–38. [Google Scholar] [CrossRef]
- Roma, P.; Luda, M.P.; Camino, G. Synergistic action of fluorine-containing additives in bromine/antimony fire retardant ABS. Polym. Degrad. Stab. 1999, 64, 497–500. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, X.; Luo, X.; Mai, B. Leaching of brominated flame retardants (BFRs) from BFRs-incorporated plastics in digestive fluids and the influence of bird diets. J. Hazard. Mater. 2020, 393, 122397. [Google Scholar] [CrossRef]
- Wu, N.; Xiu, Z.; Du, J. Preparation of microencapsulated aluminum hypophosphite and flame retardancy and mechanical properties of flame-retardant ABS composites. J. Appl. Polym. Sci. 2017, 134, 45008. [Google Scholar] [CrossRef]
- Realinho, V.; Arencón, D.; Antunes, M.; Velasco, J.I. Effects of a Phosphorus Flame Retardant System on the Mechanical and Fire Behavior of Microcellular ABS. Polymers 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Tang, W.; Xi, W.; Qian, L.; Wang, J.; Qiu, Y.; Chen, Y. Improving the fracture toughness, flame retardancy and smoke suppression of ABS by core-shell elastic flame retardant particles with P/Si synergistic effect. Polym. Degrad. Stab. 2024, 228, 110893. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Lin, Y.; Li, R.; Zhang, F. Layered double hydroxides used as flame retardant for engineering plastic acrylonitrile–butadiene–styrene (ABS). J. Phys. Chem. Solids 2012, 73, 1514–1517. [Google Scholar] [CrossRef]
- Wang, B.; Wu, B.; Zhang, G.; Yang, B. In-situ growth of layered double hydroxide on montmorillonite nanosheets to improve the flame retardant performance of ABS resin. J. Vinyl Addit. Technol. 2023, 29, 435–447. [Google Scholar] [CrossRef]
- Zhu, S.E.; Wang, F.D.; Liu, J.J.; Wang, L.L.; Wang, C.; Yuen, A.C.Y.; Chen, T.B.Y.; Kabir, I.I.; Yeoh, G.H.; Lu, H.D.; et al. BODIPY coated on MXene nanosheets for improving mechanical and fire safety properties of ABS resin. Compos. Part B Eng. 2021, 223, 109130. [Google Scholar] [CrossRef]
- Ghonjizade-Samani, F.; Haurie, L.; Malet, R.; Realinho, V. Study of using cork powder as an adjuvant bio-flame retardant in acrylonitrile-butadiene-styrene flame retardant formulations. Polym. Degrad. Stab. 2024, 225, 110825. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, W.; Li, S.; Zhang, S.; Chen, X.; Wang, B.; Du, L. Constructing surface oxygen vacancies defect-La-MOF@MXene for enhanced fire safety and smoke suppression properties in robust ABS nanocomposites. Polym. Degrad. Stab. 2025, 234, 111251. [Google Scholar] [CrossRef]
- Kong, Q.; Tang, Y.; Hu, Y.; Song, L.; Liu, H.; Li, L. Thermal stability and flame retardance properties of acrylonitrile-butadiene-styrene/polyvinyl chloride/organophilic Fe-montmorillonite nanocomposites. J. Polym. Res. 2011, 19, 9751. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, Z.; Zhang, J. Study of the phase morphology and toughness in poly (vinyl chloride)/acrylonitrile–styrene-acrylic/styrene–butadiene–styrene ternary blends influenced by interfacial/surface tension. J. Appl. Polym. Sci. 2019, 136, 47721. [Google Scholar] [CrossRef]
- Li, Y.; Lv, L.; Wang, W.; Zhang, J.; Lin, J.; Zhou, J.; Dong, M.; Gan, Y.; Seok, I.; Guo, Z. Effects of chlorinated polyethylene and antimony trioxide on recycled polyvinyl chloride/acryl-butadiene-styrene blends: Flame retardancy and mechanical properties. Polymer 2020, 190, 122198. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, W.; Zhang, Z.; Xu, B.; Liang, J.; Cao, X.; Zhao, S.; Cui, J.; Gao, A.; Zhang, G.; et al. Facile preparation of high-performance and multifunctional PVC-based nanocomposites with segregated structure achieved by volume repulsion and toughening effects of ABS. Eur. Polym. J. 2021, 161, 110867. [Google Scholar] [CrossRef]
- Yildiz, C.; Seki, Y.; Kizilkan, E.; Sarikanat, M.; Altay, L. Development of Halogen-Free Flame Retardant Acrylonitrile Butadiene Styrene (ABS) Based Composite Materials. Chem. Select 2023, 8, e202300989. [Google Scholar] [CrossRef]
- Carty, P.; White, S. Anomalous flammability behaviour of CPVC (chlorinated poly vinylchloride) in blends with ABS (acrylonitrile-butadiene-styrene) containing flame-retarding/smoke-suppressing compounds. Polymer 1997, 38, 1111–1119. [Google Scholar] [CrossRef]
- Mi, H.; Huang, X.; Jar, P.-Y.B. Influence of Acetone and Primer on Strength and Ductility of Chlorinated Poly(vinyl chloride). Polymers 2023, 15, 489. [Google Scholar] [CrossRef] [PubMed]
- Elakesh, E.O.; Richard Hull, T.; Price, D.; Carty, P. Thermal decomposition of chlorinated poly(vinyl chloride) (CPVC). J. Vinyl Addit. Technol. 2003, 9, 116–126. [Google Scholar] [CrossRef]
- Li, A.; Huang, B.; Zhang, W.; Ding, Y.; Zhou, R. Experimental study on pyrolysis gas products of chlorinated polyvinyl chloride and its smoke properties during combustion. J. Therm. Anal. Calorim. 2022, 147, 8213–8224. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C.A. The thermal degradation of acrylonitrile-butadiene-styrene terpolymei as studied by TGA/FTIR. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Brebu, M.; Vasile, C. Thermal behaviour of binary and ternary copolymers containing acrylonitrile. Polym. Degrad. Stab. 2013, 98, 1889–1897. [Google Scholar] [CrossRef]
- Lu, L.F.; Price, D.; Milnes, G.J.; Carty, P.; White, S. GC/MS studies of ABS/CPVC blends. Polym. Degrad. Stab. 1999, 64, 601–603. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Li, D.F.; Fu, T.; He, L.; Wang, X.L.; Wang, Y.Z. Biomimetic construction peanut-leaf structure on ammonium polyphosphate surface: Improving its compatibility with poly(lactic acid) and flame-retardant efficiency simultaneously. Chem. Eng. J. 2021, 412, 128737. [Google Scholar] [CrossRef]
- Lou, S.; Wang, S.; Zhang, L.; Ma, L.; Liu, J.; Tang, T. Influence of phosphomolybdate modified with quaternary ammonium salts on enhancing the flame retardancy and antibacterial characteristics of epoxy resin/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2024, 228, 110914. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Wang, X.; Cheng, Z.; Yang, T.; Hu, Y. Utilizing the oxidation defects of black phosphorus to reduce and stabilize copper(I) molybdate to achieve flame retardancy and smoke suppression in polycarbonate composites. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108313. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame Retardancy Index for Thermoplastic Composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame Retardant Epoxy Composites on the Road of Innovation: An Analysis with Flame Retardancy Index for Future Development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Y.; Cheng, Y.; Huang, T.; Yu, B.; Zhu, M.; Yu, H. Synthesis of a charring agent containing triazine and benzene groups and its intumescent flame retardant performance for polypropylene. Polym. Degrad. Stab. 2022, 204, 110107. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Gengembre, L. XPS study of an intumescent coating: II. Application to the ammonium polyphosphate/pentaerythritol/ethylenic terpolymer fire retardant system with and without synergistic agent. Appl. Surf. Sci. 1997, 120, 15–29. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, T.; Ma, C.; Fang, Z.; Wang, D. Dependence of flame retardancy and smoke suppression properties of chloroprene rubber on zinc borate and antimony trioxide loadings. Mater. Today Chem. 2024, 36, 101966. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Yan, H.; Zhang, Y.; Wang, H.; Song, P.; Fang, Z. Transparent, highly thermostable and flame retardant polycarbonate enabled by rod-like phosphorous-containing metal complex aggregates. Chem. Eng. J. 2021, 409, 128223. [Google Scholar] [CrossRef]
- Feng, J.X.; Zhang, X.M.; Ma, S.Q.; Xiong, Z.; Zhang, C.Z.; Jiang, Y.H.; Zhu, J. Syntheses of Metallic Cyclodextrins and Their Use as Synergists in a Poly(Vinyl Alcohol)/Intumescent Flame Retardant System. Ind. Eng. Chem. Res. 2013, 52, 2784–2792. [Google Scholar] [CrossRef]
- Hu, Y.D.; Xu, P.; Gui, H.G.; Wang, X.X.; Ding, Y.S. Effect of imidazolium phosphate and multiwalled carbon nanotubes on thermal stability and flame retardancy of polylactide. Compos. Part A Appl. Sci. Manuf. 2015, 77, 147–153. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Yu, J. Comparison of the pyrolysis behavior of PC, ABS and PC/ABS. J. Anal. Appl. Pyrolysis 2024, 183, 106774. [Google Scholar] [CrossRef]
- Pan, Y.T.; Yuan, Y.; Wang, D.Y.; Yang, R. An Overview of the Flame Retardants for Poly(vinyl chloride): Recent States and Perspective. Chin. J. Chem. 2020, 38, 1870–1896. [Google Scholar] [CrossRef]
- Suo, T.T.; Zhao, Z.Y.; Wang, Y.Z.; Deng, C. Chemical bridging of ammonium octamolybdate and silica for highly efficient smoke suppression of polyvinyl chloride-based composites. Compos. Part A Appl. Sci. Manuf. 2024, 190, 108650. [Google Scholar] [CrossRef]
- Goller, S.M.; Schartel, B.; Krüger, S. Phosphorus features halogen –calcium hypophosphite replaces antimony trioxide, reduces smoke, and improves flame retardancy. Thermochim. Acta 2024, 737, 179764. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, H.; Liu, Y.; Wang, Q. Synergy between piperazine pyrophosphate and aluminum diethylphosphinate in flame retarded acrylonitrile-butadiene-styrene copolymer. Polym. Degrad. Stab. 2021, 190, 109639. [Google Scholar] [CrossRef]



| Sample | ABS | CPVC/ABS (1:2) | CPVC/ABS (2:3) | CPVC/ABS (1:1) | CPVC/ABS (2:1) | CPVC |
|---|---|---|---|---|---|---|
| TTI (s) | 31 | 98 | 151 | 196 | 63 | 631 |
| PHRR (kW m−2) | 1077 | 243 | 131 | 210 | 126 | 65 |
| THR (MJ m−2) | 113.8 | 79.4 | 24.6 | 50.5 | 41.7 | 46.7 |
| PSPR (m2·s−1) | 0.211 | 0.130 | 0.084 | 0.101 | 0.055 | 0.012 |
| TSP (m2) | 26.7 | 20.5 | 18.9 | 25.8 | 9.8 | 7.3 |
| Residue (wt.%) | 0.8 | 11.2 | 18.1 | 14.7 | 10.3 | 15.9 |
| Sample | Cox/Ca | C=C/C-C(C-H) |
|---|---|---|
| CPVC | 0.62 | 0.66 |
| CPVC/ABS (2:1) | 0.55 | 0.93 |
| CPVC/ABS (2:3) | 0.53 | 1.29 |
| Sample | Td,5% (°C) | Tmax1 (°C) | MLRmax1 (%/°C) | Tmax2 (°C) | MLRmax2 (%/°C) | Residue (wt.%) | |
|---|---|---|---|---|---|---|---|
| Theoretical | Experimental | ||||||
| CPVC | 283 | 328 | 1.54 | 470 | 0.12 | / | 23.9 |
| CPVC/ABS (2:1) | 287 | 317 | 1.00 | 450 | 0.47 | 16.4 | 17.0 |
| CPVC/ABS (2:3) | 289 | 314 | 0.69 | 444 | 0.90 | 10.2 | 12.3 |
| CPVC/ABS (1:2) | 288 | 311 | 0.57 | 442 | 1.10 | 8.6 | 10.1 |
| ABS | 405 | 437 | 2.21 | / | / | / | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, L.; Zou, S.; Qin, P.; Zhu, Z.; Guo, S.; Ke, Q. Mechanistic Insights into the Non-Monotonic Flame Retardancy of CPVC/ABS Composite. Polymers 2025, 17, 2415. https://doi.org/10.3390/polym17172415
Zhang L, Liu L, Zou S, Qin P, Zhu Z, Guo S, Ke Q. Mechanistic Insights into the Non-Monotonic Flame Retardancy of CPVC/ABS Composite. Polymers. 2025; 17(17):2415. https://doi.org/10.3390/polym17172415
Chicago/Turabian StyleZhang, Long, Lewen Liu, Shengwen Zou, Peng Qin, Zhengzhu Zhu, Shaoyun Guo, and Qining Ke. 2025. "Mechanistic Insights into the Non-Monotonic Flame Retardancy of CPVC/ABS Composite" Polymers 17, no. 17: 2415. https://doi.org/10.3390/polym17172415
APA StyleZhang, L., Liu, L., Zou, S., Qin, P., Zhu, Z., Guo, S., & Ke, Q. (2025). Mechanistic Insights into the Non-Monotonic Flame Retardancy of CPVC/ABS Composite. Polymers, 17(17), 2415. https://doi.org/10.3390/polym17172415






