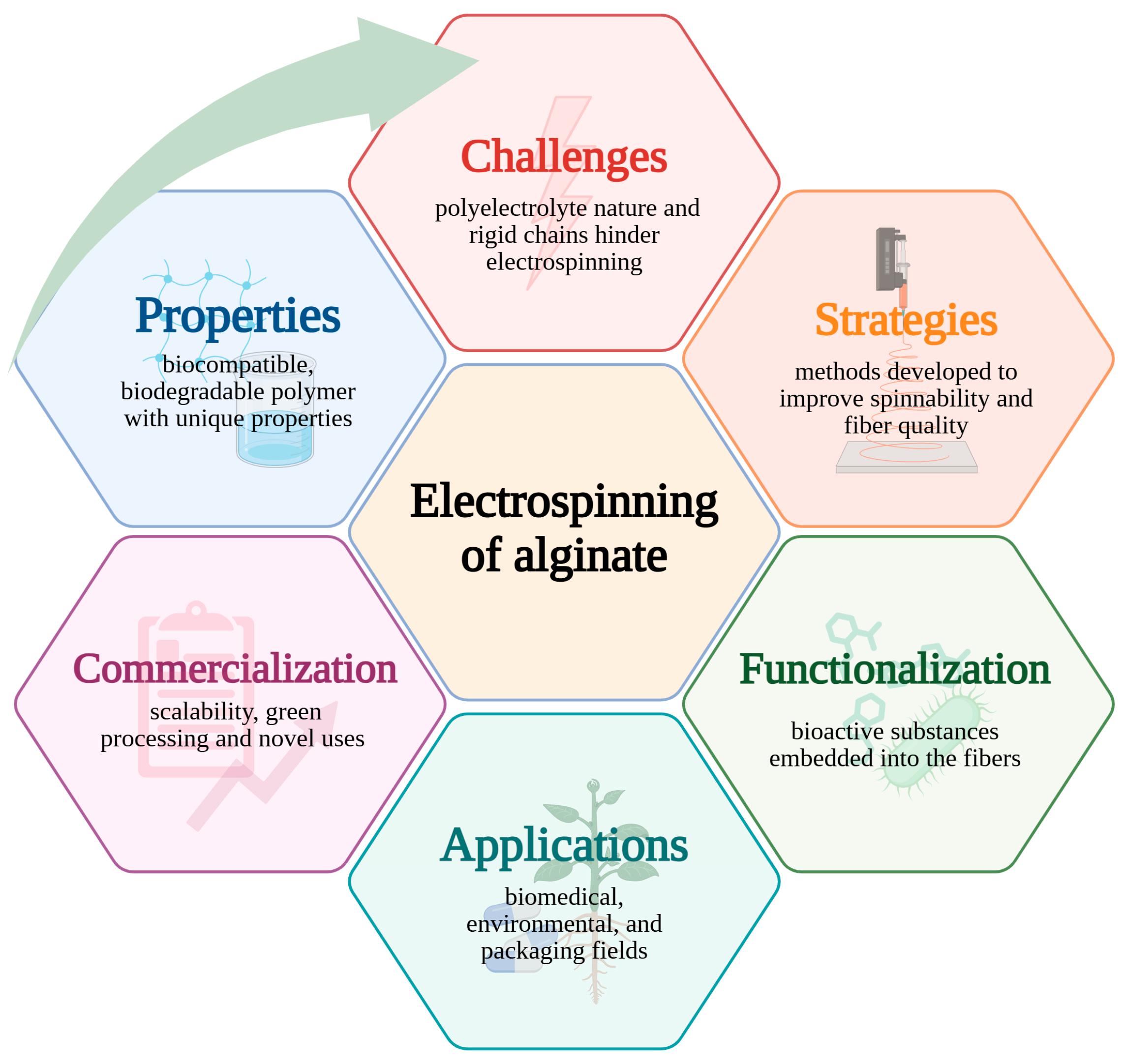
Towards Enhanced Electrospinning of Alginate—Can Recent Strategies Overcome Limitations? A Review
Abstract
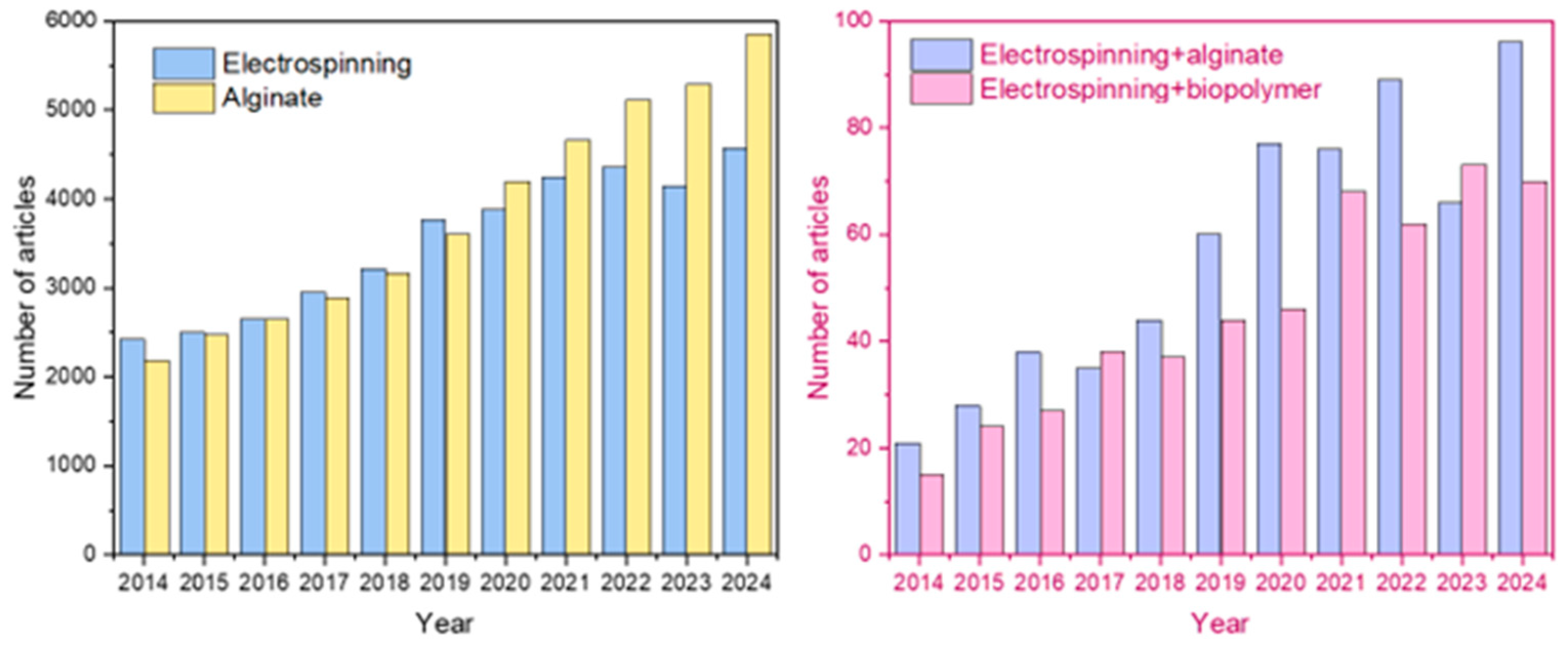
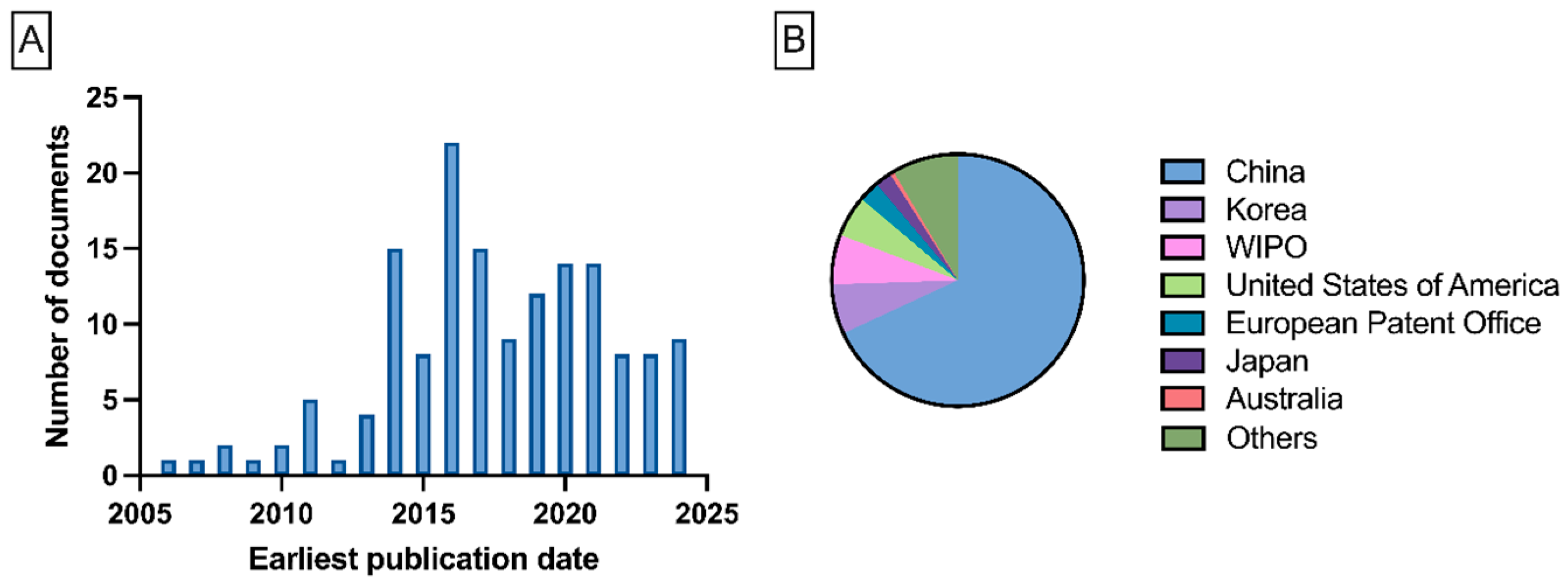
1. Introduction
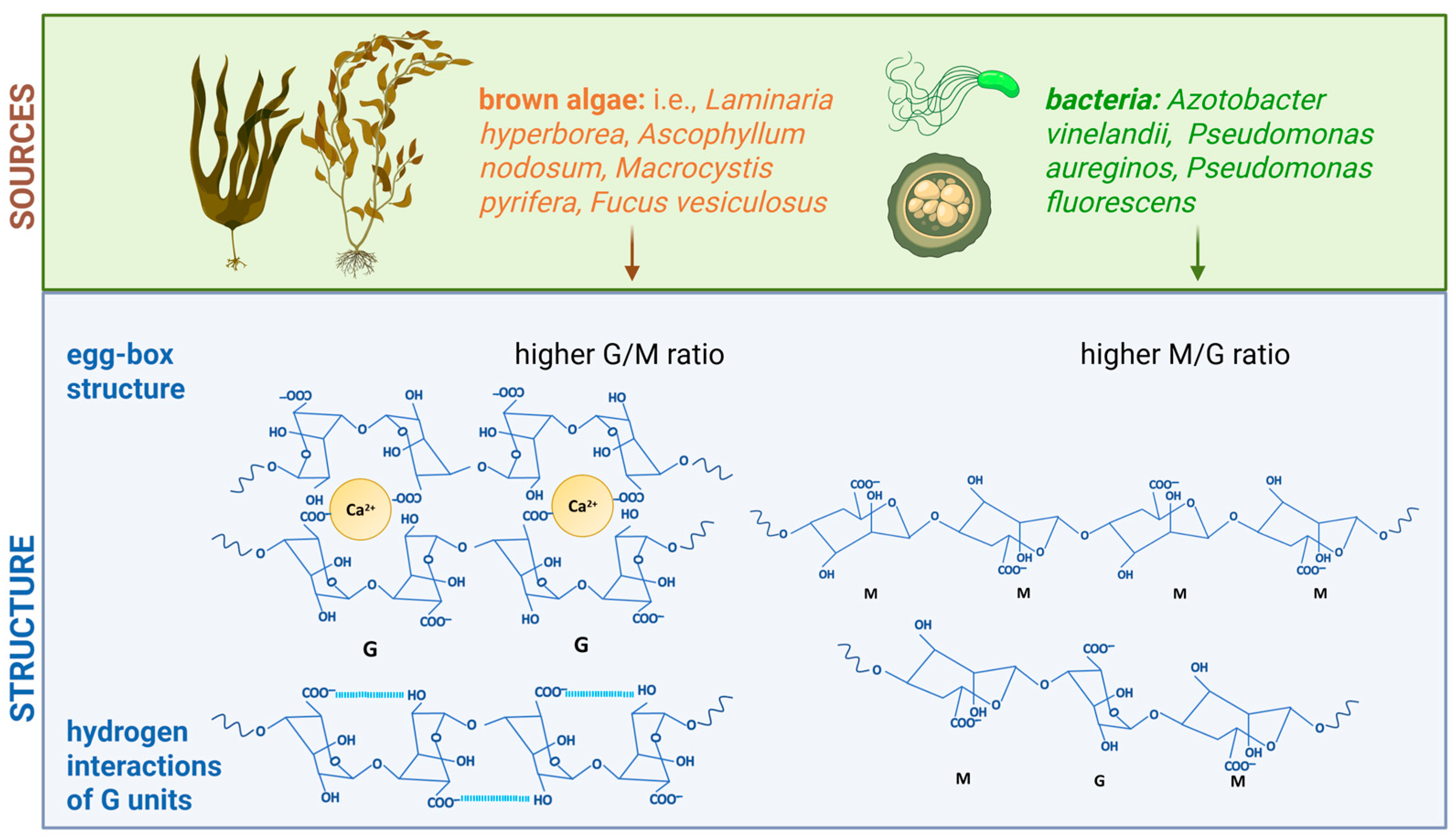
2. Alginate: Structure, Properties, and Potential in Electrospinning Applications
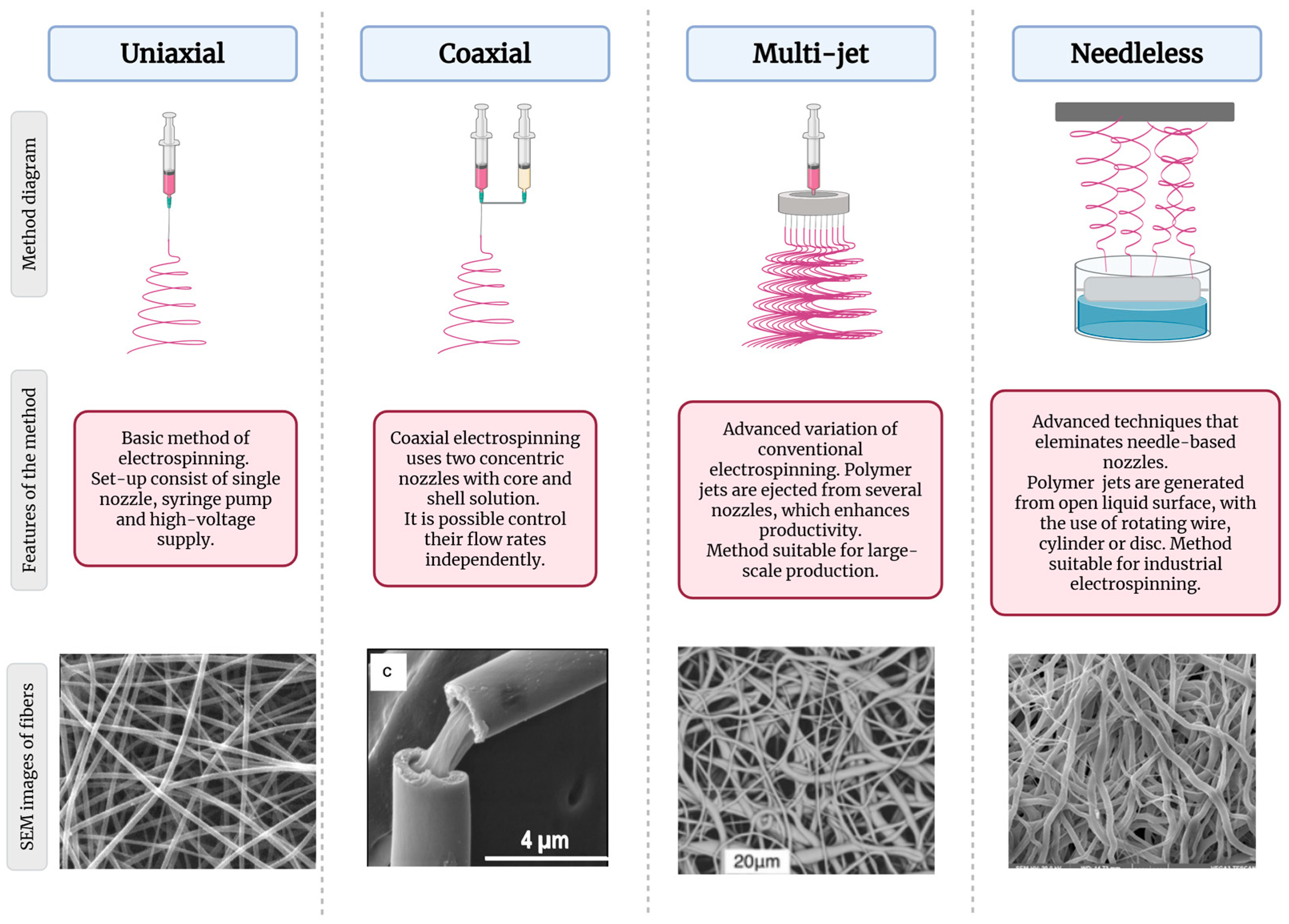
3. Electrospinning of Alginate-Based Fibers
3.1. Influence of Molecular Weight and M/G Ratio on Alginate Electrospinning
3.2. Improving Alginate Spinnability and Fiber Functionality
Blending Alginate with Synthetic Polymers
3.3. Improving Alginate Spinnability and Fiber Functionality
3.3.1. Blending Alginate with Synthetic Polymers
3.3.2. Blending Alginate with Biopolymers
3.3.3. Chemical Modification of Alginate
3.3.4. Surfactants as Additives
3.4. Optimization of Electrospinning Parameters
3.5. Crosslinking Methods for Enhanced Fiber Stability
3.6. Green Processing Approaches and Long-Term Stability
3.6.1. Green Processing Strategies for Alginate Electrospinning
3.6.2. Long-Term Stability and Degradation of Alginate Nanofibers
4. Applications of Electrospun Alginate Fibers
4.1. Microorganism-Loaded Alginate Fibers
| Polymers | Solvents | Surfactant | Microorganism | Electrospinning Conditions | Crosslinking | Application | Results/Conclusion/Problems/Perspectives | Reference |
|---|---|---|---|---|---|---|---|---|
| SA, PVA | water | - | Lactobacillus paracasei KS-199 | U = 22 kV | - | method of probiotic encapsulation; probiotic food | -Bacteria incorporation yielded beaded fibers (diameter increased from 305 nm to 842 nm) -Electrospinning did not remarkably influence L. paracasei’s stability or metabolism -Nanofiber mats exhibited high melting temperature, suggesting potential for heat-processed/baked foods. | [114] |
| Q = 1.2 mL/h | ||||||||
| L = 10 cm | ||||||||
| SA, corn starch | water | - | Lactobacillus acidophilus LA5, L. rhamnosus 23,527 LGG, Bifidobacterium bifidum, B. animalis | U = 24 kV | freeze-drying after the process | method of probiotic encapsulation; oral consumption of probiotics | -Free nanofibers had a diameter of 295 nm; probiotics-loaded nanofibers had a diameter of 797 nm -Enhanced viability was observed compared to non-immobilized bacteria -Nanocapsules protected probiotic cells from gastric acid and bile salt effects -Electrospinning significantly enhanced the acid tolerance and viability of the tested bacteria. -Optimized methods and biopolymer formulas are needed to improve the survival/viability of probiotic species/strains in digestive juices and expand their food/supplement application. | [130] |
| Q = 1.5 mL/h | ||||||||
| L = 12 cm | ||||||||
| T = 25 °C | ||||||||
| core: SA/bacteria shell: SA/PEO/CaCO3/PS80 | water | PS80 | Lactococcus lactis 11454 | U = 17.5 kV | CaCl2 in water; CaCl2 in 1:1 water/glycerol | delivery system of probiotics to the gut | -Flowing alginate in the core needle helped to reduce interfacial tension variance that can occur where both solutions meet at the coaxial needle tip -CaCO3 was mixed into the shell solution to improve the acid survivability of encapsulated bacteria. -Survivability after the electrospinning process was 108 (10 times smaller than the initial ratio) -Release from fiber in simulated gastrointestinal tract model was significantly higher with the addition of CaCO3 (no release w/o CaCO3) -The antacid enabled the survivability of encapsulated bacteria in the stomach | [15] |
| Q= Core: 0.35, Shell:0.70 mL/h | ||||||||
| L = 18 cm | ||||||||
| T = 23 °C | ||||||||
| RH = 20–30% | ||||||||
| SA, gelatin | water | - | Lactobacillus acidophilus, Limosilactobacillus reuteri, Lacticaseibacillus casei, Lacticaseibacillus rhamnosus | Q = 0.5 mL/h | - | food packaging | -Encapsulation of probiotics reduced mechanical strength but increased resistance against water -After 14 days at 4 °C, 25 °C, and 37 °C, probiotic survival in nanofibers ranks: L. acidophilus > L. reuteri > L. casei > L. rhamnosus (7.37–9.35 log CFU/g) -With a decrease in viscosity, the stabilization of the jet increases -The need to check the release of bacteria over time to determine the required dose to inhibit the growth of pathogens | [118] |
| U = 25 kV | ||||||||
| L = 15 cm | ||||||||
| T = 25 °C | ||||||||
| SA, PEO + inulin | water | - | Lactobacillus paragasseri K7 | U = 60 kV | - | delivery system of probiotics (drugs, hygiene products, urogenital infections treatment) | -Addition of inulin caused an increase in viscosity when spinning solutions and the nanofibers were potentially formed only when the solution did not contain inulin -One of the limitations presents the release study where only samples immediately after the performed electrospun process were taken for analysis. These results are not enough to determine the significance of the effect of ageing and storage conditions. | [125] |
| L = 21 cm | ||||||||
| SA, PVA + inulin | water | - | Lactobacillus fermentum | U= 20 kV | - | delivery system of probiotics | -The formation of beads was observed in the fibers -The addition of inulin influenced the survival of probiotic bacteria -Addition of inulin increased the surface tension of the solution | [126] |
| Q = 1.5 mL/h | ||||||||
| L = 10 cm | ||||||||
| T = 25 °C | ||||||||
| RH = 50% | ||||||||
| SA, PEO | water | PS80 | E. coli with GFP plasmid | U = 17.5 kV | - | medicine- probiotics delivery to the gut | -The addition of surfactant caused the formation of smooth fibers; -The change in conductivity and surface tension of the solution can be significant to produce smooth fibers, especially with the addition of microorganisms | [109] |
| Q = 2 mL/h | ||||||||
| L = 17 cm | ||||||||
| T = 23 °C | ||||||||
| RH = 20–30% | ||||||||
| SA, PEO | water | - | Bacillus strains | U = 15–17 kV | - | wound healing | -Nanofiber diameters in all prepared formulations ranged from 200 to 300 nm -60% (in case of PEO/SA 60/40 nanofibers) and 40% (in case of PEO/SA 20/80 nanofibers) of the viable spores were released from spore-loaded nanofibers in the first hour of the release experiment. -The presence of SA and the increase of its content in the electrospinning solution resulted in significantly smaller nanofiber diameters (p < 0.05), which can be attributed to the increased charge density in the polymer solution | [129] 1 |
| Q = 0.325 mL/h | ||||||||
| L = 15 cm |
4.2. Fibers for Drug Delivery Applications
4.3. Alginate Fibers Modified with Nanoparticles
4.4. Incorporation of Plant Extracts into Alginate Fibers
| Polymers | Solvents | Surfactant | Active Component | Electrospinning Conditions | Crosslinking | Application | Results/Conclusion/Problems/Perspectives | Reference |
|---|---|---|---|---|---|---|---|---|
| SA, PEO | water | - | curcumin | L = 15 cm U = 15–23 kV Q = 0.3–1 mL/h t = 3 h Room temperature RH = 40–50% | TFA | environmental pollution control, food packaging and tissue engineering | -TFA crosslinking and the addition of curcumin increased the mechanical properties of the fibers. -Curcumin content up to 10% increased mechanical strength; higher concentrations have a negative effect | [41] |
| SA, PVA | water | - | purple cabbage anthocyanins | L = 12 cm | GA | tissue engineering | -Uniform nanofibers were obtained with proper incorporation of the extract into the nanofibers and produced mats worked well for monitoring pH -Psychochemical properties of polymer solution strongly affect the morphology of fibers; addition of PVA increases the conductivity and disruption of the jet structure | [154] |
| Q = 0.7 mL/h | ||||||||
| U = 18 kV (without extract) | ||||||||
| U = 14 kV (with extract) | ||||||||
| SA, PVA | water | - | BWA | Q = 0.5 mL/h | - | intelligent packaging | -As anthocyanin concentration increased, fiber diameter and tensile strength decreased, while thickness, moisture content and antioxidant properties increased -High sensitivity to the pH environment- color changes -Formation of the beads and agglomerates due to increased viscosity of the solution, due to the addition of BWA, which affected the fiber structure and diameter | [157] |
| U = 20 ± 0.5 kV | ||||||||
| L = 10 cm | ||||||||
| T = 25 °C | ||||||||
| RH = 50% | ||||||||
| SA, PVA | water | - | Lawsonia inermis and Scrophularia striata extracts | U = 10 kV | GA | biomedical application—wound healing | -As the concentration of the extracts increased, the tensile strength decreased -Extracts caused faster wound healing -One limitation is the need for further investigation into the long-term biocompatibility and degradation | [158] |
| Q = 0.4 mL/h | ||||||||
| L = 12 cm | ||||||||
| SA, PCL | water, chloroform, methanol | - | Salvia abrotanoides essential oil | U = 25 kV L = 15 cm shell solution: Q = 0.2 mL/h core solution: Q = 1 mL/h T = 25 °C RH = 30% | - | tissue engineering—wound healing | -The average diameter of nanofibers: 187 ± 51 nm, improved tensile strength -Application of ZnO NPs and essential oils increased antimicrobial activity -The scaffold accelerated the healing time with total wound healing over 14 days in mouse models carrying full-thickness wounds compared to the nanofibrous scaffold without additives. -While the drug release profile of the membrane was satisfactory, close to 50% of the bioactive agents were not released in 5 days | [152] |
| SA, PVA | water | - | Malva Sylvestris extract | T = 25 °C | GA | tissue engineering—wound healing | -Diameter ranged from approximately 100–200 nm, without beads -At 21st day of treatment SA/PVA/extract dressing demonstrated a wound closure rate of 93% while the control (without an extract: 60%) | [159] |
| Q = 0.4 mL/h | ||||||||
| U = 12 kV | ||||||||
| L = 12 cm | ||||||||
| SA, PEO, poly(3-hydroxybutyric acid) | water, ethanol, hexafluoro-2- propanol, | - | embelin | Q = 2 mL/h electric potential: 1 kV/cm L = 15 cm | GA | tissue engineering—wound healing | -Coaxial mats showed antibacterial properties (because of the addition of embelin), improved physicochemical properties -Core-shell structure also ensures a sustained release profile -A limitation of the study is that the manufacturing techniques for nanofibrous mats may not be able to produce mats that are large enough or contain the structural integrity to treat deep wounds effectively | [156] |
| SA, bentonite (clay) | water | - | shrimp enzyme extract | Q = 10 mL/h | CaCl2 (collector solution) with or without chitosan in aqueous or acetic acid solutions | feeds for aquaculture | -The average sizes ranged from 360 to 790 μm (wet) and 260–516 μm (freeze-dried), with larger sizes for those including chitosan and bentonite -SA–chitosan–bentonite was the most suitable for immobilizing exogenous proteases -Clay sonication pre-treatment can prevent mineral agglomeration and achieve better dispersion within the composite matrix. This method not only enhances barrier properties but also helps preserve the enzymes | [160] |
| U = 15 kV | ||||||||
| L = 20 cm | ||||||||
| SA, PVA | water | - | cardamom extract | U = 15 kV | GA | tissue engineering—wound healing | -Optimum content of extract was 10 wt%, resulting in fibers with average diameter of 233 ± 33 nm. -Nanofibers showed no toxicity against fibroblast cells -Increasing the amount of PVA in the blend solution reduces the surface charge density of SA, leading to the formation of a thicker jet during the electrospinning process - When L is too short, beads form because the jet lacks sufficient time to stretch properly, and the diameter of fibers increases | [161] |
| Q = 1 mL/h | ||||||||
| L = 10 cm | ||||||||
| SA, PVA, Poly-(D, L,-O-lactide)-co-glycoside | SA, PVA: water Poly-(D, L,-O-lactide)-co-glycoside: chloroform, DMF | - | Capparis sepiaria plant extract | Q = 10 µL/min | - | tissue engineering—wound healing | -Exhibited smooth and porous surfaces, and showed high % cell viability of 90% and above, revealing non-toxicity and biocompatibility -Poly-(D, L,-O-lactide)-co-glycoside addition enhanced the surface charge density and conductivity of the polymer solution, increasing the jet’s stretching force and leading to nanofibers with a smaller diameter | [162] |
| U = 15 kV | ||||||||
| L = 15 cm | ||||||||
| SA, PEO | water | Pluronic F-127 | OEO | U= 26 kV; 30 kV Q= 0.8 mL/h L= 15 cm Room temperature RH = 40–60% | CaCl2 for 10 min; dH2O for 23 h | medical textiles | -Addition of OEO caused lower viscosity -The smallest beads were obtained with the applied voltage of 26 kV -Without soaking in water for 24 h—fibers were embedded in PEO film layers; the same sample, but with the additional step of soaking in water for 24 h, showed clear fibers, without PEO -Mats had antimicrobial activity against MRSA -The crosslinking process is necessary to prevent the SA nanofibers from dissolving in water for their end-use | [155] 1 |
5. Commercialization of the Electrospinning Process
5.1. Alginate Electrospinning Patents
| Name | Number | Short Description | Origin | Reference |
|---|---|---|---|---|
| Nano fibrous frame material with sodium alginate as matrix and its preparation method | CN100443126C | The patent provides a method for producing nanofibers based on SA/PVA or SA/PEO, with the addition of glycerin and a surfactant. The invention also describes a chemical crosslinking process. | China | [164] |
| Method for preparing pure sodium alginate nano fiber membrane material | CN101230150A | The patent describes an electrospinning process of pure SA dissolved in a water-ethanol solution, performed by fluid electrospinning. | China | [165] |
| Alginate-based nanofibers and related scaffolds | US2009087469A1 | The patent provides a method for producing nanofibers based on a SA–PEO solution with the addition of a surfactant and a co-solvent (DMSO) to improve the spinnability of alginate. | United States of America | [166] |
| Method for preparing sodium alginate-chitosan nano-grade medical dressing | CN104069536A | The patent provides a method of producing nanofibers based on a solution of SA–co-polymer (e.g., PVA, PEO). Process is performed by fluid electrospinning, where a receiving solution is chitosan with a crosslinking agent (e.g., CaCl2) | China | [163] |
| Method for preparing sodium alginate electro-spinning nanofibers by chemical crosslinking | CN106222798A | The patent describes a method for producing nanofibers using a SA solution, with epichlorohydrin added to achieve chemical crosslinking of the alginate. | China | [167] |
| Preparation method of sodium alginate/polyvinyl alcohol nanofiber | CN106283269A | The patent describes an electrospinning method based on a SA–PVA solution. | China | [168] |
| Antibacterial liquid-absorption type sodium alginate-based composite nanofiber medical dressing | CN108837179A | The invention provides an antibacterial composite nanofiber based on SA–PVA solution enriched with a cationic polymer. The patent also describes a thermal crosslinking method. | China | [169] |
| Chitosan/calcium alginate needleless electrospinning nanofiber membrane for medical dressings and preparation method thereof | CN109267240A | The patent provides a method for producing nanofibers based on SA–PVA or SA–PEO solutions with the addition of a chitosan–PEO composite solution, performed using needleless electrospinning. | China | [170] |
| Preparation method of sodium alginate and chitosan composite nanofiber | CN111020745A | The invention describes a method for producing nanofibers based on a SA–chitosan solution. | China | [171] |
| Porous sodium alginate nanofiber scaffold material and preparation method thereof | CN113577397A | The patent provides a method for producing nanofibers based on a SA–PVA solution. The invention describes a foaming and crosslinking process to obtain a porous material. | China | [172] |
| Chitosan-alginate composite nanofiber membrane, as well as the preparation method and application thereof | CN112760811A | The patent provides a method for producing nanofibers based on a SA–chitosan–gelatin solution, incorporating drying. | China | [172] |
| Alginate-based fibers and uses thereof | US2022033995A1 | The patent describes a method for producing nanofibers based on SA–cellulose with the addition of CaCl2. | United States of America | [173] |
| OMVs-loaded sodium alginate nanofiber membrane, as well as the preparation method and application thereof | CN118480968A | The patent describes a method for producing nanofibers based on a PVA–SA solution loaded with OMVs. | China | [174] |
5.2. Electrospinning Process on an Industrial Scale
| Company | Set-Up Scales Available | Reference |
|---|---|---|
| Elmarco (Liberec, Czech Republic) | laboratory and industrial | [182,183] |
| SKE (Research Equipment) (London, UK) | laboratory, pilot, industrial | [184,185] |
| Nano FiberLabs (Foshan, China) | laboratory, pilot, industrial | [186] |
| Electrospinning (Didcot, UK) | laboratory, pilot, industrial | [187,188] |
| Fnm Co. (Milan, Italy) | laboratory, pilot, industrial | [189,190] |
| INOVENSO (Cambridge, MA, USA) | laboratory, pilot, industrial | [191,192] |
| Nanoflux (Singapore) | laboratory, pilot, industrial | [193] |
| Fluidnatek by Bioinica (Valencia, Spain) | laboratory, industrial | [194,195] |
| Progene Link Sdn Bhd (Subang Jaya, Malaysia) | laboratory, pilot, industrial | [196] |
| NanoLab (Waltham, MA, USA) | laboratory, pilot, industrial | [196] |
6. Research Directions and Knowledge Gaps
- Systematic studies on the source, purity, and molecular characteristics of alginate need to be carried out. Many research papers neglect the critical role of raw material variability in the electrospinning process. Alginates from different suppliers, or even from different production batches, can vary significantly in their molecular weight, block composition (M/G ratio), and viscosity. These differences directly affect solution behavior, jet stability, and fiber formation. Comparative studies using alginates of various origins and properties, processed under identical conditions, are urgently needed. Developing standardized protocols for polymer characterization will help define guidelines for selecting alginates that are suitable for fiber production.
- The development of fully biodegradable systems without synthetic polymer carriers is a necessity. Currently, most alginate electrospinning methods require the use of synthetic polymers such as PEO or PVA to facilitate fiber formation. Although these materials improve processability, they reduce the biodegradability of the final product and pose obstacles for sustainable development and green manufacturing. Future research should focus on creating entirely natural systems using only biopolymers, such as pullulan, chitosan, or gelatin, combined with alginate. The goal is to develop universal and scalable electrospinning methods that eliminate synthetic additives while maintaining the quality of the fibers.
- Interaction of process parameters with modified alginate systems: While the spinning conditions (voltage, distance, temperature, humidity) are well described for standard blends, these blends may behave differently when novel, chemically modified, or biopolymer-only alginate systems are used. There is a need for integrated studies that couple formulation chemistry with systematic process optimization (i.e., response surface methodology) methodologies.
- Fiber structure-function correlations for targeted applications: Many studies focus on describing the morphology and mechanical properties of alginate-based fibers, but often overlook how these features impact their practical performance. To design materials suited for specific applications, it is essential to understand how the fiber’s structure, including its porosity, surface area, and crosslinking, relates to key functional outcomes, including drug release, microbial survival, and gas permeability. Future research should include standardized tests that directly measure these functions to help connect the structure of fibers with their real-world performance.
- Evaluation of long-term stability and degradation in real-use conditions: Current studies typically focus on short-term fiber stability, which is usually measured in hours or days. However, real-world applications require predictable behavior over weeks or months. This is critical for wound dressings, agricultural mats, packaging materials, and filters. Long-term studies should assess aging, moisture uptake, degradation kinetics, and storage stability. Furthermore, it is essential to understand how crosslinked alginate nanofibers behave in biological and environmental systems to ensure safe and environmentally friendly degradation.
- Investigation of interactions between the electrospinning process and living microorganisms: Encapsulating live microorganisms into nanofibers is an emerging and promising direction; however, there is limited knowledge about how this process affects cell viability and function. Research is needed to study how shear forces, electric fields, and osmotic pressure changes during electrospinning impact cell membranes, gene expression, and metabolic activity. Long-term functional studies should assess whether microbes retain their properties, including probiotic activity, biofilm formation, and enzymatic degradation abilities, after encapsulation.
- Development of greener, safer solvent systems for electrospinning: Although alginate is often electrospun from aqueous solutions, blending it with PEO or PVA typically requires additional solvents or post-processing steps that may involve organic compounds. There is a growing need to develop non-toxic, environmentally friendly solvent systems that are suitable for large-scale production. Ethanol–water mixtures, natural deep eutectic solvents, or enzymatic modification techniques could provide promising alternatives.
- To enable industrial implementation, it is necessary to develop standardized protocols for the reproducible and scalable production of alginate nanofibers. This includes solving issues related to quality control, real-time process monitoring, and safe handling of materials. Collaboration with industry partners will be crucial to adapting laboratory methods for large-scale production while meeting regulatory, economic, and environmental requirements.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Amphiphilic alginate derivative |
| BWA | Black wolfberry anthocyanin |
| CPD | Carbon polymer dot |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| FAO | Food and Agriculture Organization |
| G | Guluronic acid |
| GA | Glutaraldehyde |
| GFP | Green fluorescent protein |
| GRAS | Generally recognized as safe |
| HAc | Acetic acid |
| HDF | Human dermal fibroblasts |
| L | Needle-to-collector distance |
| LAB | Lactic acid bacteria |
| M | Mannuronic acid |
| MRSA | Methicyllin-resistant Staphylococcus aureus |
| MTT | 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide |
| NHS | N-Hydroxysuccinimide |
| NPs | Nanoparticles |
| O/W | Oil-in-water |
| OEO | Oregano essential vulgare oil |
| PAA | Polyacrylic acid |
| PCL | Polycaprolactone |
| PDA | Polydopamine |
| PEO | Polyethylene oxide |
| PHB | 3-hydroxybutanoic acid |
| PLA | Polylactic acid |
| PS80 | Polysorbate 80 |
| PVA | Polyvinyl alcohol |
| Q | Feed rate |
| RH | Humidity |
| SA | Sodium alginate |
| SOD | Superoxide dismutase |
| T | Temperature |
| TFA | Trifluoroacetic acid |
| U | Voltage |
| WHO | World Health Organization |
References
- Kogje, M.; Satdive, A.; Mestry, S.; Mhaske, S.T. Biopolymers: A Comprehensive Review of Sustainability, Environmental Impact, and Lifecycle Analysis. Iran. Polym. J. (Engl. Ed.) 2025, n.a., 1–44. [Google Scholar] [CrossRef]
- Abdu, M.T.; Abuhasel, K.A.; Alquraish, M.; Nagy, S.; Khodir, S.; Ali, A.A. Selected Natural Fibers and Their Electrospinning. J. Polym. Res. 2023, 30, 1–35. [Google Scholar] [CrossRef]
- Lee, J.W.; Song, K.H. Fibrous Hydrogels by Electrospinning: Novel Platforms for Biomedical Applications. J. Tissue Eng. 2023, 14, 20417314231191881. [Google Scholar] [CrossRef]
- Lu, G.; Tian, T.; Wang, Y. Advanced Electrospinning Technology Applied to Polymer-Based Sensors in Energy and Environmental Applications. Polymers 2024, 16, 839. [Google Scholar] [CrossRef]
- Al-Attabi, A.; Ahmed, A.T.; Merza, M.S.; Shakier, H.G.; Zabibah, R.S.; Al-Hetty, H.R.A.K.; Baher, H. Electrospinning in Food Packaging: Current Trend and Future Direction. Packag. Technol. Sci. 2024, 37, 571–584. [Google Scholar] [CrossRef]
- Meraz-Dávila, S.; Pérez-García, C.E.; Feregrino-Perez, A.A. Challenges and Advantages of Electrospun Nanofibers in Agriculture: A Review. Mater. Res. Express 2021, 8, 042001. [Google Scholar] [CrossRef]
- Mirtič, J.; Balažic, H.; Zupančič, Š.; Kristl, J. Effect of Solution Composition Variables on Electrospun Alginate Nanofibers: Response Surface Analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Sodium Alginate Solutions: Correlation between Rheological Properties and Spinnability. J. Mater. Sci. 2019, 54, 8034–8046. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication Challenges and Trends in Biomedical Applications of Alginate Electrospun Nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Yu, D.G.; Bligh, S.W.A. Alginate-Based Electrospun Nanofibers and the Enabled Drug Controlled Release Profiles: A Review. Biomolecules 2024, 14, 789. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Env. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Akshay Kumar, K.P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Makvandi, P.; Černík, M.; Padil, V.V.T.; Varma, R.S. Electrospun Fibers Based on Botanical, Seaweed, Microbial, and Animal Sourced Biomacromolecules and Their Multidimensional Applications. Int. J. Biol. Macromol. 2021, 171, 130–149. [Google Scholar] [CrossRef]
- Diep, E.; Schiffman, J.D. Targeted Release of Live Probiotics from Alginate-Based Nanofibers in a Simulated Gastrointestinal Tract. RSC Appl. Polym. 2024, 2, 719–725. [Google Scholar] [CrossRef]
- Colín-Orozco, J.; Colín-Orozco, E.; Valdivia-Barrientos, R. Production of Nanofibers by Electrospinning as Carriers of Agrochemical. Fibers 2024, 12, 64. [Google Scholar] [CrossRef]
- Klapstova, A.; Honzikova, P.; Dasek, M.; Ackermann, M.; Gergelitsova, K.; Erben, J.; Samkova, A.; Jirkovec, R.; Chvojka, J.; Horakova, J. Effective Needleless Electrospinning for the Production of Tubular Scaffolds. ACS Appl. Eng. Mater. 2024, 2, 492–500. [Google Scholar] [CrossRef]
- Kumar, N.; Sridharan, D.; Palaniappan, A.; Dougherty, J.A.; Czirok, A.; Isai, D.G.; Mergaye, M.; Angelos, M.G.; Powell, H.M.; Khan, M. Scalable Biomimetic Coaxial Aligned Nanofiber Cardiac Patch: A Potential Model for “Clinical Trials in a Dish”. Front. Bioeng. Biotechnol. 2020, 8, 567842. [Google Scholar] [CrossRef] [PubMed]
- Wojasiński, M.; Goławski, J.; Ciach, T. Blow-assisted multi-jet electrospinning of poly-L-lactic acid nanofibers. J. Polym. 2017, 24, 76. [Google Scholar] [CrossRef]
- Rashtchian, M.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Simorgh, S. Fabricating Alginate/Poly(Caprolactone) Nanofibers with Enhanced Bio-Mechanical Properties via Cellulose Nanocrystal Incorporation. Carbohydr. Polym. 2020, 233, 115873. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Me-chanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, N.; Kumar, P. A Review on Sources, Modification Techniques, Properties and Potential Applications of Alginate-Based Modified Polymers. Eur. Polym. J. 2024, 213, 113078. [Google Scholar] [CrossRef]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Gao, C.; Avérous, L. Alginate-Based Materials: Enhancing Properties through Multiphase Formula-tion Design and Processing Innovation. Mater. Sci. Eng. R Rep. 2024, 159, 100799. [Google Scholar] [CrossRef]
- Khatun, M.R.; Bhattacharyya, A.; Gunbayar, M.; Jo, Y.O.; Noh, I. Gelatin-Alginate Hydrogel for near-Field Electrospinning Assisted 3D and 4-Axis Bioprinting. Carbohydr. Polym. 2025, 348, 122853. [Google Scholar] [CrossRef] [PubMed]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical Properties of Alginate-Based Films: Effect of Ionic Crosslinking and Mannuronic and Guluronic Acid Ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Sheng, S.Y.; Lu, C.G.; Zhang, Y.Q.; Zou, J.Y.; Lei, Y.Y.; Gu, Y.; Hong, H. Effects of Glycogen Synthase Kinase-3β Inhibitor TWS119 on Proliferation and Cytokine Production of TILs From Human Lung Cancer. J. Immunother. 2018, 41, 319–328. [Google Scholar] [CrossRef]
- Wen, P.; Feng, K.; Yang, H.; Huang, X.; Zong, M.H.; Lou, W.Y.; Li, N.; Wu, H. Electrospun Core-Shell Struc-tured Nanofilm as a Novel Colon-Specific Delivery System for Protein. Carbohydr. Polym. 2017, 169, 157–166. [Google Scholar] [CrossRef]
- Ju, J.; Yang, J.; Zhang, W.; Wei, Y.; Yuan, H.; Tan, Y. Seaweed Polysaccharide Fibers: Solution Properties, Pro-cessing and Applications. J. Mater. Sci. Technol. 2023, 140, 1–18. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl Chitosan/Sodium Al-ginate-Based Micron-Fibers Fabricated by Emulsion Electrospinning for Periosteal Tissue Engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.M.; Wu, R.Q.; Wei, Y.S.; Zong, M.H.; Linhardt, R.J.; Wu, H. A Novel Route for Double-Layered Encapsulation of Probiotics with Improved Viability under Adverse Conditions. Food Chem 2020, 310, 125977. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A Novel Alginate/Gelatin Sponge Combined with Curcumin-Loaded Electrospun Fibers for Postoperative Rapid Hemostasis and Prevention of Tumor Recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef]
- Zhou, T.; NajafiKhoshnoo, S.; Esfandyarpour, R.; Kulinsky, L. Dissolvable Calcium Alginate Microfibers Pro-duced via Immersed Microfluidic Spinning. Micromachines 2023, 14, 318. [Google Scholar] [CrossRef]
- Holmberg, S.; Garza-Flores, N.A.; Almajhadi, M.A.; Chávez-Madero, C.; Lujambio-Angeles, A.; Jind, B.; Bau-tista-Flores, C.; Mendoza-Buenrostro, C.; Pérez-Carrillo, E.; Wickramasinghe, H.K.; et al. Fabrication of Mul-tilayered Composite Nanofibers Using Continuous Chaotic Printing and Electrospinning: Chaotic Electro-spinning. ACS Appl. Mater. Interfaces 2021, 13, 37455–37465. [Google Scholar] [CrossRef]
- Najafiasl, M.; Osfouri, S.; Azin, R.; Zaeri, S. Alginate-Based Electrospun Core/Shell Nanofibers Containing Dexpanthenol: A Good Candidate for Wound Dressing. J. Drug Deliv. Sci. Technol. 2020, 57, 101708. [Google Scholar] [CrossRef]
- Nie, H.; He, A.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effects of Chain Conformation and Entanglement on the Electrospinning of Pure Alginate. Biomacromolecules 2008, 9, 1362–1365. [Google Scholar] [CrossRef]
- Fang, D.; Liu, Y.; Jiang, S.; Nie, J.; Ma, G. Effect of Intermolecular Interaction on Electrospinning of Sodium Alginate. Carbohydr. Polym. 2011, 85, 276–279. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of Food Proteins and Polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.; Garcia-Cela, E.; Magan, N.; Rahatekar, S.S. Electrospinning Alginate/Polyethylene Oxide and Curcumin Composite Nanofibers. Mater. Lett. 2020, 270, 127662. [Google Scholar] [CrossRef]
- Li, C.; Shang, W.; Huang, Y.; Ge, J.; Ye, J.; Qu, X.; Guo, Q.; Wang, C.; Hu, P.; Liu, Y. Sodium Alginate/Chitosan Composite Scaffold Reinforced with Biodegradable Polyesters/Gelatin Nanofibers for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2025, 285, 138054. [Google Scholar] [CrossRef]
- Hajiabbas, M.; Alemzadeh, I.; Vossoughi, M. A Porous Hydrogel-Electrospun Composite Scaffold Made of Oxidized Alginate/Gelatin/Silk Fibroin for Tissue Engineering Application. Carbohydr. Polym. 2020, 245, 116465. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Kim, G.H. Nano/Microscale Topographically Designed Alginate/PCL Scaffolds for Inducing Myoblast Alignment and Myogenic Differentiation. Carbohydr. Polym. 2019, 223, 115041. [Google Scholar] [CrossRef]
- Penton, K.E.; Kinler, Z.; Davis, A.; Spiva, J.A.; Hamilton, S.K. Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog. Polymers 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated Electrospun Alginate-Containing Fibers as Novel Delivery Systems for Regenerative Purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef]
- Azarsa, S.; Pezeshki-Modaress, M.; Yazdian, F.; Bagher, Z.; Chahsetareh, H.; Simorgh, S.; Heidari, M.K.; Davachi, S.M. Nanofiber/Hydrogel Composite Scaffolds Based on Alginate Sulfate and Extracellular Matrix for Cartilage Tissue Engineering Applications. Process Biochem. 2024, 136, 60–71. [Google Scholar] [CrossRef]
- Petrova, V.A.; Golovkin, A.S.; Mishanin, A.I.; Romanov, D.P.; Chernyakov, D.D.; Poshina, D.N.; Skorik, Y.A. Cytocompatibility of Bilayer Scaffolds Electrospun from Chitosan/Alginate-Chitin Nanowhiskers. Biomedicines 2020, 8, 305. [Google Scholar] [CrossRef]
- Moridi, H.; Gh, A.B. Sodium Alginate/Polyvinyl Pyrrolidone/Zinc Oxide @silica Schiff-Base Nanofiber Membrane for Single and Binary Removal of Copper and Nickel Cations from Aqueous Medium. Res. Chem. Intermed. 2022, 48, 4643–4670. [Google Scholar] [CrossRef]
- Dodero, A.; Donati, I.; Scarfì, S.; Mirata, S.; Alberti, S.; Lova, P.; Comoretto, D.; Alloisio, M.; Vicini, S.; Castellano, M. Effect of Sodium Alginate Molecular Structure on Electrospun Membrane Cell Adhesion. Mater. Sci. Eng. C 2021, 124, 112067. [Google Scholar] [CrossRef]
- Castellano, M.; Alloisio, M.; Darawish, R.; Dodero, A.; Vicini, S. Electrospun Composite Mats of Alginate with Embedded Silver Nanoparticles: Synthesis and Characterization. J. Therm. Anal. Calorim. 2019, 137, 767–778. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Z.; Sun, J.; Geng, C.; Bu, Q.; Wu, D.; Xia, Y. Electrospinning Highly Concentrated Sodium Alginate Nanofibres without Surfactants by Adding Fluorescent Carbon Dots. Nanomaterials 2020, 10, 565. [Google Scholar] [CrossRef]
- Wang, S.; Ju, J.; Wu, S.; Lin, M.; Sui, K.; Xia, Y.; Tan, Y. Electrospinning of Biocompatible Alginate-Based Nanofiber Membranes via Tailoring Chain Flexibility. Carbohydr. Polym. 2020, 230, 115665. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Wang, X.; Chang, Z.; Jiang, Y.-C.; Han, S.; Cui, Z.; Han, X.; Li, Q. Loading and Sustained Release of Sodium Alginate Membranes on Pyridirubicin Chloride. Mater. Res. Express 2021, 8, 065402. [Google Scholar] [CrossRef]
- Daemi, H.; Mashayekhi, M.; Pezeshki Modaress, M. Facile Fabrication of Sulfated Alginate Electrospun Nanofibers. Carbohydr. Polym. 2018, 198, 481–485. [Google Scholar] [CrossRef]
- Xiao, Q.; Lim, L.T. Pullulan-Alginate Fibers Produced Using Free Surface Electrospinning. Int. J. Biol. Macromol. 2018, 112, 809–817. [Google Scholar] [CrossRef]
- Asadi-Korayem, M.; Akbari-Taemeh, M.; Mohammadian-Sabet, F.; Shayesteh, A.; Daemi, H. How Does Counter-Cation Substitution Influence Inter- and Intramolecular Hydrogen Bonding and Electrospinnability of Alginates. Int. J. Biol. Macromol. 2021, 171, 234–241. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled Synthesis of Sodium Alginate Electrospun Nanofiber Membranes for Multi-Occasion Adsorption and Separation of Methylene Blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.-Q.; Zhang, W.-Y.; Chang, C.; Han, J.-L.; Tian, F.; Hao, X.-D. Calcium Alginate Production through Forward Osmosis with Reverse Solute Diffusion and Mechanism Analysis. Membranes 2023, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Hasan Aneem, T.; Firdous, S.O.; Anjum, A.; Wong, S.Y.; Li, X.; Arafat, M.T. Enhanced Wound Healing of Ciprofloxacin Incorporated PVA/Alginate/PAA Electrospun Nanofibers with Antibacterial Effects and Controlled Drug Release. Mater. Today Commun. 2024, 38, 107950. [Google Scholar] [CrossRef]
- Aadil, K.R.; Nathani, A.; Sharma, C.S.; Lenka, N.; Gupta, P. Fabrication of Biocompatible Alginate-Poly(Vinyl Alcohol) Nanofibers Scaffolds for Tissue Engineering Applications. Mater. Technol. 2018, 33, 507–512. [Google Scholar] [CrossRef]
- Suratman, A.; Astuti, D.N.; Jonathan, R.; Kuncaka, A.; Yusuf, Y. Fabrication of Alginate-Based Electrospun Nanofibers for Carbon Dioxide Removal. Indones. J. Chem. 2022, 22, 317–330. [Google Scholar] [CrossRef]
- Vicini, S.; Mauri, M.; Vita, S.; Castellano, M. Alginate and Alginate/Hyaluronic Acid Membranes Generated by Electrospinning in Wet Conditions: Relationship between Solution Viscosity and Spinnability. J. Appl. Polym. Sci. 2018, 135, 46390. [Google Scholar] [CrossRef]
- Majidi, S.S.; Slemming-Adamsen, P.; Hanif, M.; Zhang, Z.; Wang, Z.; Chen, M. Wet Electrospun Alginate/Gelatin Hydrogel Nanofibers for 3D Cell Culture. Int. J. Biol. Macromol. 2018, 118, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of Composite Alginate-Based Electrospun Membranes Loaded with ZnO Nanoparticles. Carbohydr. Polym. 2020, 227. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Wang, Q.; Yu, N.; Zhang, X.; Su, S. Electrospinning Novel Sodium Alginate/MXene Nanofiber Membranes for Effective Adsorption of Methylene Blue. Polymers 2023, 15, 2110. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey Loaded Alginate/PVA Nanofibrous Membrane as Potential Bioactive Wound Dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, P.; Ju, J.; Wang, Q.; Hao, L.; Wang, R.; Sui, K.; Tan, Y. Gelatin/Alginate Composite Nanofiber Membranes for Effective and Even Adsorption of Cationic Dyes. Compos. B Eng. 2019, 162, 671–677. [Google Scholar] [CrossRef]
- Haghbin, M.; Sadeghi-Avalshahr, A.; Hassanzadeh, H.; Moloodi, A.; Harati, Z. Preparation of Porous Alginate-Based Smart Dressings Used in Real-Time Monitoring of PH in Chronic Wounds by Evaluating Two Fabrication Routes: Freeze-Drying vs. Electrospinning. J. Porous Mater. 2023, 30, 1953–1963. [Google Scholar] [CrossRef]
- Mauri, M.; Vicini, S.; Castellano, M. Gelling Process of Sodium Alginate with Bivalent Ions Rich Microsphere: Nature of Bivalent Ions. AIP Conf. Proc. 2016, 1736, 020007. [Google Scholar] [CrossRef]
- Eskandani, M.; Derakhshankhah, H.; Jahanban-Esfahlan, R.; Jaymand, M. Biomimetic Alginate-Based Electroconductive Nanofibrous Scaffolds for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2023, 249, 125991. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.C.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun Alginate Fibers: Mixing of Two Different Poly(Ethylene Oxide) Grades to Improve Fiber Functional Properties. Nanomaterials 2018, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Diep, E.; Schiffman, J.D. Ethanol-Free Cross-Linking of Alginate Nanofibers Enables Controlled Release into a Simulated Gastrointestinal Tract Model. Biomacromolecules 2023, 24, 2908–2917. [Google Scholar] [CrossRef]
- Anyfantis, G.C.; Hajiali, H.; Mele, E.; Marras, S.; Carzino, R.; Marini, L.; Papadopoulou, E.L.; Athanassiou, A. Investigation of the Electro-Spinnability of Alginate Solutions Containing Gold Precursor HAuCl4. J. Colloid. Interface Sci. 2016, 483, 60–66. [Google Scholar] [CrossRef]
- Jadbabaei, S.; Kolahdoozan, M.; Naeimi, F.; Ebadi-Dehaghani, H. Preparation and Characterization of Sodium Alginate-PVA Polymeric Scaffolds by Electrospinning Method for Skin Tissue Engineering Applications. RSC Adv. 2021, 11, 30674–30688. [Google Scholar] [CrossRef]
- Xu, W.; Shen, R.; Yan, Y.; Gao, J. Preparation and Characterization of Electrospun Alginate/PLA Nanofibers as Tissue Engineering Material by Emulsion Eletrospinning. J. Mech. Behav. Biomed. Mater. 2017, 65, 428–438. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Simultaneous Adsorption for Cationic and Anionic Dyes Using Chitosan/Electrospun Sodium Alginate Nanofiber Composite Sponges. Carbohydr. Polym. 2022, 276, 118728. [Google Scholar] [CrossRef]
- Negahdari, N.; Alizadeh, S.; Majidi, J.; Saeed, M.; Ghadimi, T.; Tahermanesh, K.; Arabsorkhi-Mishabi, A.; Pezeshki-Modaress, M. Heat-Treated Alginate-Polycaprolactone Core-Shell Nanofibers by Emulsion Electrospinning Process for Biomedical Applications. Int. J. Biol. Macromol. 2024, 275, 133709. [Google Scholar] [CrossRef]
- Nista, S.V.G.; Bettini, J.; Mei, L.H.I. Coaxial Nanofibers of Chitosan-Alginate-PEO Polycomplex Obtained by Electrospinning. Carbohydr. Polym. 2015, 127, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Fuh, Y.K.; Wu, Y.C.; He, Z.Y.; Huang, Z.M.; Hu, W.W. The Control of Cell Orientation Using Biodegradable Alginate Fibers Fabricated by Near-Field Electrospinning. Mater. Sci. Eng. C 2016, 62, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.S.; Parivar, K.; Hayati Roodbari, N.; Mashayekhan, S.; Amini, N. Fabrication and Characterization of Biaxially Electrospun Collagen/Alginate Nanofibers, Improved with Rhodotorula Mucilaginosa Sp. GUMS16 Produced Exopolysaccharides for Wound Healing Applications. Int. J. Biol. Macromol. 2022, 196, 194–203. [Google Scholar] [CrossRef]
- Safi, S.; Morshed, M.; Hosseini Ravandi, S.A.; Ghiaci, M. Study of Electrospinning of Sodium Alginate, Blended Solutions of Sodium Alginate/Poly(Viny1 Alcoho1) and Sodium Alginate/Poly(Ethylene Oxide). J. Appl. Polym. Sci. 2007, 104, 3245–3255. [Google Scholar] [CrossRef]
- Ahmed Al-moalemi, H.; Izwan Abd Razak, S.; Pauliena Mohd Bohari, S. Electrospun Sodium Alginate/Poly(Ethylene Oxide) Nanofibers for Wound Healing Applications: Challenges and Future Directions. Cellul. Chem. Technol. 2022, 56, 251–270. [Google Scholar] [CrossRef]
- Modesto-López, L.B.; Pérez-Arjona, A.; Ganán-Calvo, A.M. Flow Blurring-Enabled Production of Polymer Filaments from Poly(Ethylene Oxide) Solutions. ACS Omega 2019, 4, 2693–2701. [Google Scholar] [CrossRef]
- Aloma, K.K.; Sukaryo, S.; Fahlawati, N.I.; Dahlan, K.; Oemar, S. Synthesis of Nanofibers from Alginate-Polyvinyl Alcohol Using Electrospinning Methods. Macromol. Symp. 2020, 391, 1900199. [Google Scholar] [CrossRef]
- Monne, M.A.; Howlader, C.Q.; Mishra, B.; Chen, M.Y. Synthesis of Printable Polyvinyl Alcohol for Aerosol Jet and Inkjet Printing Technology. Micromachines 2021, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Mwiiri, F.K.; Daniels, R. Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing. Molecules 2020, 25, 4799. [Google Scholar] [CrossRef] [PubMed]
- Kirbas, Z.; Altay, F. Uniaxial Electrospinning Encapsulation of Bioactive Peptides into Green Nanofibers Containing Pullulan-Alginate-CaCl2. Int. J. Pept. Res. Ther. 2025, 31, 19. [Google Scholar] [CrossRef]
- Abdulkadhim, M.K.; Habeeb, S.A. The Possibility of Producing Uniform Nanofibers from Blends of Natural Biopolymers. Mater. Perform. Charact. 2022, 11, 313–323. [Google Scholar] [CrossRef]
- Ahmetoglu, U.; Gungor, M.; Kilic, A. Alginate/Gelatin Blend Fibers for Functional High-Performance Air Filtration Applications. Int. J. Biol. Macromol. 2025, 294, 139389. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, H.; Sun, W.; Feng, Y.; Li, J.; Lin, Q.; Shi, Z.; Wang, X. Synthesis of Amphiphilic Alginate Derivatives and Electrospinning Blend Nanofibers: A Novel Hydrophobic Drug Carrier. Polym. Bull. 2015, 72, 3097–3117. [Google Scholar] [CrossRef]
- Kyzioł, A.; Michna, J.; Moreno, I.; Gamez, E.; Irusta, S. Preparation and Characterization of Electrospun Alginate Nanofibers Loaded with Ciprofloxacin Hydrochloride. Eur. Polym. J. 2017, 96, 350–360. [Google Scholar] [CrossRef]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning Alginate-Based Nanofibers: From Blends to Crosslinked Low Molecular Weight Alginate-Only Systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, Q.; Wang, L.; Long, B.; Salleh, K.M.; Anuar, N.I.S.; Zakaria, S. Diameter Optimization of Polyvinyl Alcohol/Sodium Alginate Fiber Membranes Using Response Surface Methodology. Mater. Chem. Phys. 2021, 271, 124969. [Google Scholar] [CrossRef]
- Hossain, M.F.; Rahman, M. Preparation and Characterization of the Electrospun Alginate Nanofibers. J. Text. Sci. Technol. 2021, 07, 91–100. [Google Scholar] [CrossRef]
- Murthe, S.S.; Mohamed Saheed, M.S.; Perumal, V.; Mohamed Saheed, M.S.; Mohamed, N.M. Electrospun Nanofibers for Biosensing Applications. In Nanobiosensors for Biomolecular Targeting; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–267. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Draczynski, Z. Preparation and Characterization of the Advanced Alginate-Based Nanofibrous Nonwoven Using EDC/NHS Coupling Agent by Electrospinning. J. Text. Inst. 2022, 113, 1908–1916. [Google Scholar] [CrossRef]
- Hajiali, H.; Heredia-Guerrero, J.A.; Liakos, I.; Athanassiou, A.; Mele, E. Alginate Nanofibrous Mats with Adjustable Degradation Rate for Regenerative Medicine. Biomacromolecules 2015, 16, 936–943. [Google Scholar] [CrossRef]
- Weerasinghe, U.A.; Wu, T.; Chee, P.L.; Yew, P.Y.M.; Lee, H.K.; Loh, X.J.; Dan, K. Deep Eutectic Solvents towards Green Polymeric Materials. Green. Chem. 2024, 26, 8497–8527. [Google Scholar] [CrossRef]
- Vannuchi, N.; Ramos, S.d.P.; Mazzo, T.M.; Longo, E.; Bonsanto, F.P.; Braga, A.R.C.; de Rosso, V.V. Natural Deep Eutectic Solvents (NADES)-Extracted Anthocyanins: Bioaccessibility in Electrospun PEO Microfibers. Food Res. Int. 2024, 177, 113898. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Han, S.; Yang, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers 2020, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Dulnik, J.; Sajkiewicz, P. Crosslinking of Gelatin in Bicomponent Electrospun Fibers. Materials 2021, 14, 3391. [Google Scholar] [CrossRef]
- Phang, Y.N.; Chee, S.Y.; Lee, C.O.; Teh, Y.L. Thermal and Microbial Degradation of Alginate-Based Superabsorbent Polymer. Polym. Degrad. Stab. 2011, 96, 1653–1661. [Google Scholar] [CrossRef]
- Diep, E.; Schiffman, J.D. Electrospinning Living Bacteria: A Review of Applications from Agriculture to Health Care. ACS Appl. Bio Mater. 2023, 6, 951–964. [Google Scholar] [CrossRef]
- Sun, Q.; Yin, S.; He, Y.; Cao, Y.; Jiang, C. Biomaterials and Encapsulation Techniques for Probiotics: Current Status and Future Prospects in Biomedical Applications. Nanomaterials 2023, 13, 2185. [Google Scholar] [CrossRef]
- Fareed, F.; Saeed, F.; Afzaal, M.; Imran, A.; Ahmad, A.; Mahmood, K.; Shah, Y.A.; Hussain, M.; Ateeq, H. Fabrication of Electrospun Gum Arabic–Polyvinyl Alcohol Blend Nanofibers for Improved Viability of the Probiotic. J. Food Sci. Technol. 2022, 59, 4812–4821. [Google Scholar] [CrossRef]
- Serrano-Delgado, A.; Quintanilla-Carvajal, M.X. Electrospinning Microencapsulation of Lactobacillus Fermentum K73 Using Gelatin as the Main Component of a Food-Grade Matrix. Microorganisms 2023, 11, 2682. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, H. Recent Advances in Probiotics Encapsulation by Electrospinning. ES Food Agrofor. 2020, 2, 3–12. [Google Scholar] [CrossRef]
- Diep, E.; Schiffman, J.D. Encapsulating Bacteria in Alginate-Based Electrospun Nanofibers. Biomater. Sci. 2021, 9, 4364–4373. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Grzywaczyk, A.; Zdarta, A.; Jankowska, K.; Biadasz, A.; Zdarta, J.; Jesionowski, T.; Kaczorek, E.; Smułek, W. New Biocomposite Electrospun Fiber/Alginate Hydrogel for Probiotic Bacteria Immobilization. Materials 2021, 14, 3861. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Buahom, J.; Siripornadulsil, S.; Sukon, P.; Sooksawat, T.; Siripornadulsil, W. Survivability of Freeze- and Spray-Dried Probiotics and Their Effects on the Growth and Health Performance of Broilers. Vet. World 2023, 16, 1849–1865. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An Alternative Way to Encapsulate Probiotics within Electrospun Alginate Nanofibers as Monitored under Simulated Gastrointestinal Conditions and in Kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef]
- Li, X.-M.; Che, L.-H.; Wu, Y.; Li, C.; Xu, B.-C. An Effective Strategy for Improving the Freeze-Drying Survival Rate of Lactobacillus Curvatus and Its Potential Protective Mechanism. Food Biosci. 2024, 58, 103794. [Google Scholar] [CrossRef]
- Hirsch, E.; Pantea, E.; Vass, P.; Domján, J.; Molnár, M.; Suhajda, Á.; Andersen, S.K.; Vigh, T.; Verreck, G.; Marosi, G.J.; et al. Probiotic Bacteria Stabilized in Orally Dissolving Nanofibers Prepared by High-Speed Electrospinning. Food Bioprod. Process. 2021, 128, 84–94. [Google Scholar] [CrossRef]
- Gul, S.; Durante-Mangoni, E. Unraveling the Puzzle: Health Benefits of Probiotics—A Comprehensive Review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef]
- Ghalehjooghi, H.D.; Tajik, H.; Shahbazi, Y. Development and Characterization of Active Packaging Nanofiber Mats Based on Gelatin-sodium Alginate Containing Probiotic Microorganisms to Improve the Shelf-Life and Safety Quality of Silver Carp Fillets. Int. J. Food Microbiol. 2023, 384, 109984. [Google Scholar] [CrossRef]
- Zupani, Š.; Škrlec, K.; Kocbek, P.; Kristl, J.; Berlec, A. Effects of Electrospinning on the Viability of Ten Species of Lactic Acid Bacteria in Poly(Ethylene Oxide) Nanofibers. Pharmaceutics 2019, 11, 483. [Google Scholar] [CrossRef]
- Singh, T.P.; Malik, R.K.; Kaur, G. Cell Surface Proteins Play an Important Role in Probiotic Activities of Lactobacillus Reuteri. Nutrire 2016, 41, 5. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yang, Y.-R.; Cheng, G.; Yang, Z.-Q. Lactic Acid Bacteria Biofilms and Their Antimicrobial Potential against Pathogenic Microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef]
- Waqas, M.; Keirouz, A.; Sanira Putri, M.K.; Fazal, F.; Diaz Sanchez, F.J.; Ray, D.; Koutsos, V.; Radacsi, N. Design and Development of a Nozzle-Free Electrospinning Device for the High-Throughput Production of Biomaterial Nanofibers. Med. Eng. Phys. 2021, 92, 80–87. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Uhljar, L.É.; Alshweiat, A.; Katona, G.; Chung, M.; Radacsi, N.; Kókai, D.; Burián, K.; Ambrus, R. Comparison of Nozzle-Based and Nozzle-Free Electrospinning for Preparation of Fast-Dissolving Nanofibers Loaded with Ciprofloxacin. Pharmaceutics 2022, 14, 1559. [Google Scholar] [CrossRef] [PubMed]
- Simonič, M.; Slapničar, Š.; Trček, J.; Matijašić, B.B.; Lorbeg, P.M.; Vesel, A.; Zemljič, L.F.; Peršin Fratnik, Z. Probiotic Lactobacillus Paragasseri K7 Nanofiber Encapsulation Using Nozzle-Free Electrospinning. Appl. Biochem. Biotechnol. 2023, 195, 6768–6789. [Google Scholar] [CrossRef] [PubMed]
- Duman, D.; Karadag, A. Inulin Added Electrospun Composite Nanofibres by Electrospinning for the Encapsulation of Probiotics: Characterisation and Assessment of Viability during Storage and Simulated Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2021, 56, 927–935. [Google Scholar] [CrossRef]
- Adamczyk, P.A.; Reed, J.L. Escherichia Coli as a Model Organism for Systems Metabolic Engineering. Curr. Opin. Syst. Biol. 2017, 6, 80–88. [Google Scholar] [CrossRef]
- Mckenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus Subtilis Endospore: Assembly and Functions of the Multilayered Coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Grilc, N.K.; Zidar, A.; Kocbek, P.; Rijavec, T.; Colja, T.; Lapanje, A.; Jeras, M.; Gobec, M.; Mlinarič-Raščan, I.; Gašperlin, M.; et al. Nanofibers with Genotyped Bacillus Strains Exhibiting Antibacterial and Immunomodulatory Activity. J. Control. Release 2023, 355, 371–384. [Google Scholar] [CrossRef]
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. [Google Scholar] [CrossRef]
- Dragar, Č.; Roškar, R.; Kocbek, P. The Incorporated Drug Affects the Properties of Hydrophilic Nanofibers. Nanomaterials 2024, 14, 949. [Google Scholar] [CrossRef]
- Jiffrin, R.; Razak, S.I.A.; Jamaludin, M.I.; Hamzah, A.S.A.; Mazian, M.A.; Jaya, M.A.T.; Nasrullah, M.Z.; Majrashi, M.; Theyab, A.; Aldarmahi, A.A.; et al. Electrospun Nanofiber Composites for Drug Delivery: A Review on Current Progresses. Polymers 2022, 14, 3725. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Sharma, C.S.; Majumdar, S. Electrospun Nanofibres in Drug Delivery: Advances in Controlled Release Strategies. RSC Adv. 2023, 13, 7312–7328. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.D.; Voicu, M.E.; Prodana, M.; Demetrescu, I.; Anuta, V.; Draganescu, D. Electrospun PCL Wires Loaded with Vancomycin on Zirconium Substrate. Materials 2023, 16, 7237. [Google Scholar] [CrossRef]
- Jenvoraphot, T.; Thapsukhon, B.; Daranarong, D.; Molloy, R.; Supanchart, C.; Krisanaprakornkit, S.; Topham, P.D.; Tighe, B.; Mahomed, A.; Punyodom, W. Tetracycline-Loaded Electrospun Poly(l -Lactide- Co-ϵ-Caprolactone) Membranes for One-Step Periodontal Treatment. ACS Appl. Polym. Mater. 2022, 4, 2459–2469. [Google Scholar] [CrossRef]
- Akduman, C.; Özgüney, I.; Kumbasar, E.P.A. Preparation and Characterization of Naproxen-Loaded Electrospun Thermoplastic Polyurethane Nanofibers as a Drug Delivery System. Mater. Sci. Eng. C 2016, 64, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, J.; Wuertz-Kozak, K.; Ferguson, S.J.; Rottmar, M.; Avaro, J.; Elbs-Glatz, Y.; Chung, M.; Rossi, R.M. Ibuprofen-Loaded Electrospun Poly(Ethylene-Co-Vinyl Alcohol) Nanofibers for Wound Dressing Applications. Nanoscale Adv. 2023, 5, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast Dissolving Paracetamol/Caffeine Nanofibers Prepared by Electrospinning. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef]
- Abid, S.; Wang, L.; Haider, M.K.; Mayakrishnan, G.; Lakshminarayanan, R.; Kim, K.O.; Ullah, A.; Kim, I.S. Investigating Alginate and Chitosan Electrospun Nanofibers as a Potential Wound Dressing: An in Vitro Study. Nanocomposites 2024, 10, 254–267. [Google Scholar] [CrossRef]
- Wongkanya, R.; Chuysinuan, P.; Pengsuk, C.; Techasakul, S.; Lirdprapamongkol, K.; Svasti, J.; Nooeaid, P. Electrospinning of Alginate/Soy Protein Isolated Nanofibers and Their Release Characteristics for Biomedical Applications. J. Sci. Adv. Mater. Devices 2017, 2, 309–316. [Google Scholar] [CrossRef]
- Çerçi, A.; Demir, E.S.; Karaca, E.; Güzel, Ç.B.; Osman, B. Preparation and Characterization of Amoxicillin-Loaded Polyvinyl Alcohol/Sodium Alginate Nanofibrous Mat: Drug Release Properties, Antibacterial Activity, and Cytotoxicity. Arab. J. Sci. Eng. 2024, 50, 77–91. [Google Scholar] [CrossRef]
- Sadeghi-Aghbash, M.; Rahimnejad, M.; Adeli, H.; Feizi, F. Catecholamines Polymerization Crosslinking for Alginate-Based Burn Wound Dressings Developed with Ciprofloxacin and Zinc Oxide Interactions. Int. J. Biol. Macromol. 2024, 260, 129400. [Google Scholar] [CrossRef]
- Ju, T.; Gaisford, S.; Williams, G.R. Ciprofloxacin-Loaded Electrospun Nanofibres for Antibacterial Wound Dressings. J. Drug Deliv. Sci. Technol. 2024, 91, 105264. [Google Scholar] [CrossRef]
- Nematpour, N.; Moradipour, P.; Zangeneh, M.M.; Arkan, E.; Abdoli, M.; Behbood, L. The Application of Nanomaterial Science in the Formulation a Novel Antibiotic: Assessment of the Antifungal Properties of Mucoadhesive Clotrimazole Loaded Nanofiber versus Vaginal Films. Mater. Sci. Eng. C 2020, 110, 110635. [Google Scholar] [CrossRef]
- Yi, N.; Wang, M.; Song, L.; Feng, F.; Li, J.; Xie, R.; Zhao, Z.; Chen, W. Highly Hygroscopicity and Antioxidant Nanofibrous Dressing Base on Alginate for Accelerating Wound Healing. Colloids Surf. B Biointerfaces 2023, 225, 113240. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Y.; Song, Y.; Chen, J.; Wu, Y.; Guo, R.; He, Y.; Shen, L.; Wang, B.; Huang, S.; et al. Alginate/Silk Fibroin/Zn2+ Composite Microspheres for Site-Specific Delivery for Enhanced Ulcerative Colitis Therapy. Chem. Eng. J. 2024, 495, 153441. [Google Scholar] [CrossRef]
- Casula, L.; Zidar, A.; Kristl, J.; Jeras, M.; Kralj, S.; Fadda, A.M.; Zupančič, Š. Development of Nanofibers with Embedded Liposomes Containing an Immunomodulatory Drug Using Green Electrospinning. Pharmaceutics 2023, 15, 1245. [Google Scholar] [CrossRef]
- Esentürk, İ.; Balkan, T.; Güngör, S.; Saraç, S.; Erdal, M.S. Preparation and Characterization of Naftifine-Loaded Poly(Vinyl Alcohol)/Sodium Alginate Electrospun Nanofibers. Braz. J. Pharm. Sci. 2020, 56, e18440. [Google Scholar] [CrossRef]
- Wang, W.; Liu, M.Y.; Shafiq, M.; Li, H.Y.; Hashim, R.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Mo, X. Synthesis of Oxidized Sodium Alginate and Its Electrospun Bio-Hybrids with Zinc Oxide Nanoparticles to Promote Wound Healing. Int. J. Biol. Macromol. 2023, 232, 123480. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Li, L.; Jia, L. Fabrication of Alginate-Based Nanofibers Loaded with ZnO Nanoparticles for Adsorption of Tetracyclines from Environmental Waters. Mater. Today Commun. 2023, 34, 105214. [Google Scholar] [CrossRef]
- Sasan, S.; Molavi, A.M.; Moqadam, K.H.; Farrokhi, N.; Oroojalian, F. Enhanced Wound Healing Properties of Biodegradable PCL/Alginate Core-Shell Nanofibers Containing Salvia Abrotanoides Essential Oil and ZnO Nanoparticles. Int. J. Biol. Macromol. 2024, 279, 135152. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Chen, J.; Li, J.; Chen, J. In Situ Assembly of Silver Nanoparticles throughout Electrospun Oriented Alginate Nanofibers for Hazardous Rust Trace Detection on Bronze. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 308, 123739. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Osman, B.; Göktalay, G.; Özer, E.T.; Şahan, Y.; Becerir, B.; Karaca, E. Design and in Vivo Evaluation of Alginate-Based PH-Sensing Electrospun Wound Dressing Containing Anthocyanins. J. Polym. Res. 2021, 28, 50. [Google Scholar] [CrossRef]
- Lu, H.; Butler, J.A.; Britten, N.S.; Venkatraman, P.D.; Rahatekar, S.S. Natural Antimicrobial Nano Composite Fibres Manufactured from a Combination of Alginate and Oregano Essential Oil. Nanomaterials 2021, 11, 2062. [Google Scholar] [CrossRef]
- Singaravelu, S.; Madhan, B.; Abrahamse, H.; Dhilip Kumar, S.S. Multifunctional Embelin- Poly (3-Hydroxybutyric Acid) and Sodium Alginate-Based Core-Shell Electrospun Nanofibrous Mat for Wound Healing Applications. Int. J. Biol. Macromol. 2024, 265, 131128. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Y.; Cui, J. Rapid-Response Nanofiber Films against Ammonia Based on Black Wolfberry Anthocyanins, Polyvinyl Alcohol and Sodium Alginate for Intelligent Packaging. Int. J. Biol. Macromol. 2024, 279, 135390. [Google Scholar] [CrossRef]
- Akbarpour, A.; Rahimnejad, M.; Sadeghi-Aghbash, M.; Feizi, F. Bioactive Nanofibrous Mats Constructs: Sep-arate Efficacy of Lawsonia Inermis and Scrophularia Striata Extracts in PVA/Alginate Matrices for En-hanced Wound Healing. Int. J. Biol. Macromol. 2024, 277, 134545. [Google Scholar] [CrossRef]
- Akbarpour, A.; Rahimnejad, M.; Sadeghi-Aghbash, M.; Feizi, F. Poly(Vinyl Alcohol)/Alginate Nanofibrous Mats Containing Malva Sylvestris Extract: Synthesis, Characterization, in Vitro and in Vivo Assessments for Burn Wound Applications. Int. J. Pharm. 2024, 654, 123928. [Google Scholar] [CrossRef]
- Rodriguez, Y.E.; Laitano, M.V.; Zanazzi, A.N.; Fernádez-Gimenez, A.V.; de los Ángeles Pereira, N.; Rivero, G. Turning Fishery Waste into Aquafeed Additives: Enhancing Shrimp Enzymes Immobilization in Alginate-Based Particles Using Electrohydrodynamic Atomization. Aquaculture 2024, 587, 740846. [Google Scholar] [CrossRef]
- Najafi, S.; Gholipour-Kanani, A.; Eslahi, N.; Bahrami, S.H. Study on Release of Cardamom Extract as an Antibacterial Agent from Electrospun Scaffold Based on Sodium Alginate. J. Text. Inst. 2021, 112, 1482–1490. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Motaung, K.S.C.M.; Adeyemi, S.A.; Ubanako, P.; Ngema, L.; Fonkui, T.Y.; Ndinteh, D.T.; Kumar, P.; Choonara, Y.E.; Aderibigbe, B.A. Sodium Alginate-Based Nanofibers Loaded with Capparis Sepiaria Plant Extract for Wound Healing. J. Biomater. Sci. Polym. Ed. 2024, 35, 2380–2401. [Google Scholar] [CrossRef]
- Sun, K. Method for Preparing Sodium Alginate-Chitosan Nano-Grade Medical Dressing. CN Patent 104069536A, 1 October 2014. [Google Scholar]
- Zhongze, G.; Zhangqi, F.; Ting, W.; Nongyue, H. Nano Fibrous Frame Material with Sodium Alginate as Matrix and Its Preparing Method. CN Patent 100443126C, 17 December 2008. [Google Scholar]
- Aihua, H.; Zhichao, H.; Huarong, N. Method for Preparing Pure Sodium Alginate Nano Fiber Membrane Material. CN Patent 101230150A, 30 July 2008. [Google Scholar]
- Zhang, M.; Bhattarai, N. Alginate-Based Nanofibers and Related Scaffolds. U.S. Patent 2009087469A1, 2 April 2009. [Google Scholar]
- Tan, Y.; Sui, K.; Yu, X.; Xia, Y.; Yu, M.; Wu, S.; Wang, L. Method for Preparing Sodium Alginate Electro-Spinning Nanofiber by Chemical Crosslinking. CN Patent 106222798A, 14 December 2016. [Google Scholar]
- Cao, J. Preparation Method of Sodium Alginate/Polyvinyl Alcohol Nanofiber. CN Patent 106283269A, 4 January 2017. [Google Scholar]
- Sun, F.; Zhang, P.; Shu, Q. Antibacterial Liquid-Absorption Type Sodium Alginate Based Composite Nanofiber Medical Dressing. CN Patent 108837179A, 20 November 2018. [Google Scholar]
- Wang, Y.; Lei, H.; Ding, Z. Chitosan/Calcium Alginate Needleless Electrospinning Nanofiber Membrane for Medical Dressings and Preparation Method Thereof. CN Patent 109267240A, 25 January 2019. [Google Scholar]
- Chu, J.; Chu, X.; Chen, D. Preparation Method of Sodium Alginate and Chitosan Composite Nanofiber. CN Patent 111020745A, 17 April 2020. [Google Scholar]
- Liu, Y.; Xiao, J.; Wan, W.; Li, L.; Gao, X.; Zhang, D.; Zhong, N.; Liu, H.; Ma, X.; Liu, F.; et al. Porous Sodium Alginate Nanofiber Scaffold Material and Preparation Method Thereof. CN Patent 113577397A, 2 November 2021. [Google Scholar]
- Schiros, T.; Skocir, A.; Nesser, A.; Callaghan, T.; Gosiewski, A.; Russell, S.T.; An, D.; Antrobus, R.; Mosher, C.; Lu, H. Alginate-Based Fibers and Their Uses Thereof. U.S. Patent 2022033995A1, 3 February 2022. [Google Scholar]
- Zhang, H.; Tan, Y.; Wei, J.; Liu, S. OMVs-Loaded Sodium Alginate Nanofiber Membrane as Well as Preparation Method and Application Thereof. CN Patent 118480968A, 13 August 2024. [Google Scholar]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable Manufacturing and Applications of Nanofibers. Mater. Today 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Hwang, M.; Karenson, M.O.; Elabd, Y.A. High Production Rate of High Purity, High Fidelity Nafion Nanofibers via Needleless Electrospinning. ACS Appl. Polym. Mater. 2019, 1, 2731–2740. [Google Scholar] [CrossRef]
- Malara, A. Environmental Concerns on the Use of the Electrospinning Technique for the Production of Polymeric Micro/Nanofibers. Sci. Rep. 2024, 14, 8293. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Grothe, T.; Großerhode, C.; Hauser, T.; Kern, P.; Stute, K.; Ehrmann, A. Needleless Electrospinning of PEO Nanofiber Mats. In Proceedings of the Second International Conference on Mechanics, Materials and Structural Engineering (ICMMSE 2017), Beijing, China, 14–16 April 2017. [Google Scholar]
- Nanofibers Are the Future of Your Products|Elmarco. Available online: https://www.elmarco.com/ (accessed on 28 March 2025).
- SKE Research Equipment. Available online: https://www.ske.it/ (accessed on 28 March 2025).
- Sedláčková, E.; Klusoňová, N.; Bursa, R.; Procházka, V.; Dvořáková, P.; Jílková, K.; Jankovský, O.; Macháček, J.; Havlík Míka, M. Characterization of Silicon-Based Fibers Prepared by Electrospinning for Potential Li-Ion Battery Anodes. Mater. Lett. 2024, 377, 137352. [Google Scholar] [CrossRef]
- Nanofiberlabs. Available online: https://www.nanofiberlabs.com/ (accessed on 28 March 2025).
- TONG LI TECH. Available online: https://www.electrospinningstore.com/ (accessed on 28 March 2025).
- Ali, A.; Islam, S.M.; Mohebbullah, M.; Uddin, M.N.; Hossain, M.T.; Saha, S.K.; Jamal, M.S.I. Antibacterial Electrospun Nanomat from Nigella/PVA System Embedded with Silver. J. Text. Inst. 2021, 112, 561–567. [Google Scholar] [CrossRef]
- Ahmadvand, E.; Salami, S.R.; Soofi, J.B.; Tabatabaeian, S.H. Catch-up Process in Nanotechnology Start-Ups: The Case of an Iranian Electrospinning Firm. Technol. Soc. 2018, 55, 1–8. [Google Scholar] [CrossRef]
- Fnm Co. Available online: https://fnm.ir/Default (accessed on 28 March 2025).
- INOVESO. Available online: https://www.inovenso.com/ (accessed on 28 March 2025).
- Okutan, N.; Terzi, P.; Altay, F. Affecting Parameters on Electrospinning Process and Characterization of Electrospun Gelatin Nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- NANOFLUX. Available online: https://www.nanoflux.com.sg/en/ (accessed on 28 March 2025).
- Fluidnatek. Available online: https://fluidnatek.com/electrospinning-machines/fluidnatek-ht-industrial-electrospinning-machine/?_gl=1*1v1tepr*_up*MQ..*_gs*MQ..&gclid=Cj0KCQjwkZm_BhDrARIsAAEbX1EYJBD-AaOmy1bL3sQSzjjJ_8iIcLq3L926yj2m8p6Y3yVfCzeLQIQaAl9-EALw_wcB (accessed on 28 March 2025).
- Al-Dhahebi, A.M.; Ling, J.; Krishnan, S.G.; Yousefzadeh, M.; Elumalai, N.K.; Saheed, M.S.M.; Ramakrishna, S.; Jose, R. Electrospinning Research and Products: The Road and the Way Forward. Appl. Phys. Rev. 2022, 9, 011319. [Google Scholar] [CrossRef]
- Progene Link. Available online: https://www.progenelink.com/ (accessed on 28 March 2025).
- Nanolab. Available online: https://nanolab-i.com/ (accessed on 28 March 2025).
| Molecular Weight kDa | Reference | M/G Ratio | Reference |
|---|---|---|---|
| <40 | [41] | 0.42 | [42] |
| 66 | [43] | 0.42 | [44] |
| 80–120 | [45] | 0.43 | [46] |
| 115 | [47] | 0.43 | [34] |
| 130 | [48] | 0.54 | [49] |
| 138 | [50] | 0.64 | [51] |
| 140 | [47] | 1.05 | [52] |
| 144–148 | [50] | 1.25 | [53] |
| 158 | [43] | 1.25 | [54] |
| 120–200 | [55] | 1.25 | [56] |
| 120–200 | [57] | 1.25 | [58] |
| 120–190 | [59] | 1.30 | [60] |
| 150–180 | [61] | 1.42 | [62] |
| 200 | [63] | 1.49 | [64] |
| 200 | [51] | 1.56 | [25] |
| 220 | [52] | 1.56 | [65] |
| 220 | [66] | 1.56 | [8] |
| 323 | [58] | 1.56 | [67] |
| 323 | [68] | 1.56 | [69] |
| 324 | [53] | 1.56 | [70] |
| 396 | [64] | 1.56 | [71] |
| 486 | [50] | 1.61 | [43] |
| 500 | [25] | 2.33 | [72] |
| 500 | [65] | 2.65 | [73] |
| Polymers | Solvents | Surfactant | Electrospinning Conditions | Crosslinking | Application | Results/Conclusion/Problems/Perspectives | Reference |
|---|---|---|---|---|---|---|---|
| SA, PVA | water | - | U = (1;15.50;30 kV) | GA | skin tissue engineering | -Optimal solution: SA:PVA 1:6,5 nanofibers with regular size and narrow diameter (<170 nm) were obtained at 15–30 kV, 0.55–1.00 mL/h and 12.5–20.0 cm -Pure PVA was slightly toxic and irritant for surrounding tissues; its biocompatibility can be improved with the addition of natural polymers | [75] |
| L = (5;12.50;20 cm) | |||||||
| Q = (0.1;0.55;1 mL/h) | |||||||
| SA, PLA | water, chloroform | Span 80 | Q = 0.5 mL/h | CaCl2 | tissue engineering | -Emulsion electrospinning is a good method to produce fibers with good mechanical strength (only with crosslinking) | [76] |
| L = 15 cm | |||||||
| U = 15 kV | |||||||
| SA, PEO | water, ethanol | Triton X-100 | L = 20 cm | CaCl2 | wastewater treatment | -Solution of electrospun SA/PEO fibers in water mixed with chitosan. Produced composite sponges have potential in an adsorption | [77] |
| U = 15 kV | |||||||
| T = 25 °C | |||||||
| RH = 30% | |||||||
| SA, PEO | water, ethanol | Triton X-100 | Q = 0.30 mL/h | CaCl2/TFA/GA | water treatment | -Different crosslinkers could adjust the efficient adsorption at varied environmental conditions -CaCl2-crosslinked membranes showed the best tensile strength, and TFA-crosslinked membranes had the highest specific surface area. | [58] |
| L = 15–20 cm | |||||||
| U = 20–28 kV | |||||||
| T = 30–35 °C | |||||||
| RH = 30–35% | |||||||
| SA, PEO | water | PS80 | Q = 2 mL/h | CaCl2 in different environments | biomedical application | -In each case, crosslinked fibers did not dissolve in water as uncrosslinked fibers did and retained their morphology -fibers are more stable in the acidic gastric modeling environment -Ethanol is not ideal for the encapsulation/delivery of biologics, as it can cause cell death and promote oxidative stress that damages proteins and DNA -Presence of glycerol in calcium alginate microbeads (presence in solvent mixture) improved their stability and extended the release time -Fibers crosslinked in neutral and basic solutions were not able to maintain their fibrous structure after submersion in PBS for 2 h -The stability of the fibers in acidic environments and their swelling in higher pH environments can be beneficial for the targeted release of active ingredients | [73] |
| U = 17.5 kV | |||||||
| L = 18 cm | |||||||
| T = 25 °C | |||||||
| RH = 20–30% | |||||||
| SA, PEO, methacrylated gelatin | water | F127 | Q = 0.1 mL/h | CaCl2, UV | tissue engineering | -In addition to Ca2+, fibers were also crosslinked by UV, which further stabilized the gelatin -The fibers promoted the adhesion and proliferation of mesenchymal stem cells for 5 weeks -The solvent/polymer interactions provide the surface tension, conductivity and viscosity of the solution as the primary factors that influence electrospinning -The low surface tension of the nonsolvent ethanol used in the bath prevented fiber from dense packing, thus allowing the generation of a 3D macroporous structure which favors cell motility | [64] |
| U = 7 kV | |||||||
| L = 7 cm | |||||||
| SA, PEO, PCL | water, chloroform, DMF | Tween 80 | U = 29 kV | CaCl2 | tissue engineering and biomedical applications | -Core-shell fibers showed better mechanical strength than SA-PCL fibers -SA was modified with heat treatment to decrease the molecular weight; (better than degrading the molecule) | [78] |
| L = 13 cm | |||||||
| Q = 1 mL/min | |||||||
| SA, pollulan | water | - | free-surface electrospinning RH = 30–33% T = 23 °C U = 30 kV V of carriage contains polymer solution: 80 mm/s Distance from grounded electrode to wire electrode: 18 cm | CaCl2 was added to the solution, not after electrospinning | food, nutraceutical, and pharmaceutical applications | -Addition of CaCl2 (up to 0.045%, w/w) resulted in smooth fibers that were smaller in diameter and more thermally stable than those without the addition of CaCl2 -The branched fibers are caused by the electric field induced by the excess charge carried on the primary jet -Promising usage as an active food packaging material (w/o ethanol) | [56] |
| SA, PEO, chitosan | water, HAc, ethanol | - | U = 27 kV | - | pharmaceutical and biomedical aaplications | -Core-shell fiber diameter: 154 ± 35 nm -The fiber structure was preserved even after 24 h in water -When the distance between the needle tip and the collector decreases, a greater number of defects in the membrane appear | [79] |
| L = 10 cm | |||||||
| Q = 0.4 mL/h | |||||||
| PVA+SA/KA/LiA | water | - | U = 20 kV L = 15 cm Q = 0.2 mL/h Room temperature RH = 25% | - | without a specific application | -Too much alginate causes bead formation -Substitution of Li+ as a hard acid, instead of Na+, causes a stronger interaction with the hard base of the carboxylate anion in the alginate backbone and weakens the intramolecular hydrogen bonds and electrospinnability of alginate -Li cation improves electrospinnability and the problem is not in the viscosity (because Li salts have the highest viscosity of the alginate salts) -The results have indicated that the effect of conductivity on the nanofiber diameter is more dominant than solution viscosity | [57] |
| SA, PEO polypyrrole film (not in the solution) | SA/PEO: water Polypyrrole: ammonium persulfate | - | NEAR-FIELD ELECTROSPINNING L = 1 mm U = 0.8 kV Room temperature | CaCl2 | tissue engineering | -Polypyrrole is a suitable substrate for cell growth, and alginate is a material unfavorable for cell adhesion, so the alternate arrangement of these materials (contrasting pattern) allows precise cell positioning -By adjusting the level of ionic crosslinking, the stability of alginate fibers can be adjusted, allowing the fibers to initially guide cell positioning and then gradually degrade, creating more space for cell growth | [80] |
| SA, PCL, carboxymethyl chitosan | water, 1,1,1,3,3,3-hexafluoro-2-propanol, | Span 80 | Q = 0.6 mL/h | CaCl2 | periosteal tissue engineering | -Micron-fibers showed an average diameter of 2.381 ± 1.068 μm with excellent tensile strength and biocompatibility -The addition of the hydrophilic carboxymethyl chitosan and SA could form highly entangled polymer molecular chains and affect the evaporation rate of the solvent. Thus, the surface of the fibers becomes relatively smooth after the addition of carboxymethyl chitosan and SA -The addition of the emulsifier affected the diameter of the fibers | [32] |
| U = 16 kV | |||||||
| L = 15 cm | |||||||
| T = 25 °C | |||||||
| RH = 55% | |||||||
| SA, PEO | water, NaCl | Triton X-100 | Q = 0.75 mL/h | SrCl2 | tissue engineering | -Three alginates (i.e., M.pyr, L.hyp, A.nod) were evaluated -Fibroblasts and keratinocytes did not show any preference for the three tested mats, but the former presented a much greater adhesion -Alginate with an evident polyelectrolyte nature, are found to promote cell viability better -Domination of G blocks and high Mw increase charge density, enhancing polyelectrolyte behavior and forcing chains into a rod-like conformation, which reduces viscosity-concentration scaling factors | [50] |
| L = 15 cm | |||||||
| U = 12.5 kV | |||||||
| T = 25 °C | |||||||
| RH = 50% | |||||||
| camphorsulfonic acid-doped alginate-poly(aniline), PVA | water | - | n.a. | GA, CaCl2 (double crosslinking) | tissue engineering | -The contact angles of the developed scaffolds with water drop were obtained as 51 ± 2.4° and 58 ± 2.7°, which confirm their excellent wettabilities for tissue engineering applications -The scaffolds have acceptable cytocompatibilities and could improve the MG-63 cells’ adhesion and proliferation -The porous morphologies of both samples may originate from the freeze-drying process | [71] |
| SA, PEO, collagen, exopolysaccharide | water, HAc | - | L = 15 cm 100 rpm Collagen: Q = 0.5 mL/h U = 17 kV SA/PEO: Q = 0.5 mL/h U = 27 kV collagen/exopolysaccharide: Q = 1 mL/h U = 15 kV | GA | tissue engineering | -Among the nanofibrous scaffolds, Collagen-SA/PEO + exopolysaccharide 2% nanofiber showed better mechanical properties and cellular behavior than others -The high porosity of nanofibers can negatively impact their mechanical properties | [81] 1 |
| Polymers | Solvents | Surfactant | Drug | Electrospinning Conditions | Crosslinking | Application | Results/Conclusion/Problems/Perspectives | Reference |
|---|---|---|---|---|---|---|---|---|
| SA, PEO, isolated soy protein | water, NaOH (adjusting pH) | - | vancomycin antibiotic | U = 15 kV | CaCl2 | biomedical applications | -Smooth and homogeneous SA/PEO/protein fibers with an average diameter of 200 nm were formed -The biocompatibility and non-toxicity of the fibers were confirmed by a cytotoxicity test using human fibroblasts -Increased alginate content showed a negative impact on spinnability and obtained fiber morphology (due to low spinnability of alginate) | [141] |
| L = 15 cm | ||||||||
| Q = 0.5 mL/h | ||||||||
| PVA, SA, PAA | HAc | - | ciprofloxacin (antibiotic) | U = 25 kV | - | tissue engineering—wound healing | -Ciprofloxacin release at neutral pH was approximately 40% and remained constant over 24 h -Fiber diameter of 141 ± 53 nm -PVA–SA–PAA–ciprofloxacin could release drugs for a prolonged period due to their lowest swelling ratio -Controlled release of ciprofloxacin enabled growth inhibition of bacteria for at least 7 days | [60] |
| L and Q not specified | ||||||||
| SA, PEO | water, DMSO | Triton X-100 | forsythin, CPDs | L = 17 cm | CaCl2 | tissue engineering—wound healing | -Membranes showed antibacterial and antioxidant properties -Addition of CPDs improved the mechanical strength of membranes -CPDs increased the conductivity of solutions, which allowed the production of nanofibers with a high SA amount (up to 92%) | [146] |
| U = 16–20 kV | ||||||||
| Q = 0.5 mL/h | ||||||||
| SA, silk fibroin | water | - | bornyl acetate (in ethanol) | Q = 0.8 mL/h | ZnCl2 | medicine- enhanced ulcerative colitis therapy | -Microsphere systems can protect bornyl acetate (the anti-inflammatory drug) from gastric degradation, ensuring its delivery to the colon -Introduction of protein polymers, such as silk fibroin, increases the bioavailability of the matrix—more protein binding sites | [147] |
| U = 16 kV | ||||||||
| SA, PVA | water | - | ciprofloxacin, ZnO NPs, dopamine | U = 10 kV | SA/PVA: GA SA/PVA/dopamine: ammonia solution | tissue engineering—wound healing | -The drug release and antibacterial assays demonstrated pH-responsive behavior, with increased drug release under higher pH conditions (simulating bacterial invasion) and strong antibacterial activity, achieving up to 99% inhibition -The burn wound model in rats, nanofibrous mats displayed excellent wound-healing ability in wound closure and tissue regeneration. -With the increase of time exposure to dopamine, the porosity of mats reduces, because of the hydrophilic properties of SA and PVA—that is why authors proposed to add the dopamine into the electrospinning solution -Crosslinked mats degrade more slowly in PBS than in water, with degradation significantly decreasing as crosslinking time increases. | [143] |
| Q = 0.4 mL/h | ||||||||
| L = 12 cm | ||||||||
| SA, PVA, agarose | water | - | ciproflaxin | U = 30 kV | drug release; biomedical application | -The optimal composition of the solution contained 3:7 2.5% (w/v) SA and 10% (w/v) PVA, produced smooth, uniform fibers with an average diameter of 185.1 nm. However, thin fibers were formed using a 1:2:1 ratio of 2.5% SA:10% PVA:0.5% agarose (M = 122.5 nm). These SA-PVA-Ag fibers still showed beading and thus were not drug–loaded -The addition of agarose made electrospinning difficult due to the low solubility of agarose in water -Charges on polymers can affect the release of drugs from carriers -Combining alginate with PVA or agarose via electrospinning should capitalize on the properties of both biocompatible materials in each fiber type to create a unique drug release profile for each scaffold | [45] | |
| Q = 7.5 μL/min | ||||||||
| L = 10 cm | ||||||||
| SA, PEO | water | Triton X-100 | levoflaxin | U = 15 kV | - | biomedical application | -SA-based nanofibers were 235 ± 43 nm in diameter -Nanofibers demonstrated effective antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa -A smaller fiber diameter can be achieved by using a different solvent, such as HAc, which evaporates more rapidly, resulting in thinner fibers -As the size of the nanofibers decreases, their surface area increases | [140] |
| Q = 0.4 mL/h | ||||||||
| L = 15 cm | ||||||||
| SA, PVA, dextran | water | clotrimazole | U = 15–17 kV | drug delivery—healing patches | -Fiber loaded with clotrimazole showed higher antifungal properties -Mats inhibited the growth of Candida albicans and Candida dubliniensis -Bioadhesion of the electrospun mat was two-fold higher than of the film due to the higher surface area. Lack of plasticizer in the formulation of nanofiber makes it more favorable from the perspective of safety and industrial production | [145] | ||
| Q = 0.7 mL/h | ||||||||
| L = 12 cm | ||||||||
| SA, PVA | water | amoxicillin | U = 25 kV | GA | tissue engineering—wound healing | -Diameter of fibers was 201.7 ± 30.9 nm -Average diameters of the nanofibers increased after GA crosslinking due to the flattening of the nanofibers -The nanofibrous mat has no cytotoxic effect on human normal keratinocyte cells -The lower the GA concentration, the higher the number of fusions at junction points of the nanofibers -Due to its absorption properties, the use of SA is important in the treatment of wounds and the absorption of exudate | [142] | |
| Q = 0.5 mL/h | ||||||||
| L = 15 cm | ||||||||
| SA, PEO | water | simvastatin | U = 22 kV (−5 kV) | tissue engineering—wound healing | -It was possible to obtain SA fibers with liposomes containing simvastatin and BHA (antioxidant) –Nanofibers were thicker—more than 300 nm in diameter -Considering poor solubility in water of simvastatin and the aim to avoid using organic solvents, green electrospinning was achieved by incorporating polymers into liposomal dispersions -Nanofibers with liposomes and antioxidants showed faster release of simvastatin (important in wound healing) | [148] | ||
| Q = 600 uL/h | ||||||||
| L = 15 cm | ||||||||
| T = 37 °C | ||||||||
| RH = 15% | ||||||||
| SA, PVA | water | Triton X-100 | ciproflaxin | Q = 0.3 mL/h | - | tissue engineering—wound healing | -70% of encapsulated drug was released during the first 10 h; the drug was fully released after 48 h -The drug release rate in a polymer matrix system can be dissolution and diffusion-controlled -Ciproflaxin encapsulated in PVA/SA fibers fully inhibited the growth of P. aeruginosa and S. aureus -Viproflaxin at the concentrations of 0.5 μg/mL and 1 μg/mL incorporated into PVA/SA fibers demonstrated antimicrobial activity -Addition of SA to the electrospinning solution leads to a smaller fiber diameter -Electrospun fibers had a high water uptake capacity | [144] |
| L = 14 cm | ||||||||
| T = 20–25 °C | ||||||||
| RH = 35–50% | ||||||||
| SA, PVA | water | - | naftifine | U = 15 kV Q = 1 mL/h L = 15 cm | 25% GTA vapor | tissue engineering—wound healing | -Optimum ratio of PVA:SA mixture was 8:2 -Average fiber diameter was 457.71 nm -19% of the drug was released within the first 30 min -Addition of the drug caused an increase in conductivity -Crosslinking process improved the hydrophilicity and mechanical properties of fibers -The solubility of the drug in the polymer solution affects the drug entrapment efficiency in nanofibers | [149] 1 |
| Polymers | Solvents | Surfactant | Nanoparticle | Electrospinning Conditions | Crosslinking | Application | Results/Conclusion/Problems/Perspectives | Reference |
|---|---|---|---|---|---|---|---|---|
| SA, PEO | water, DMSO | Triton X-100 | Ag NPs (by immersion in silver nitrate) | U = 12 kV | after electrospinning nanofiber membrane was immersed in silver nitrate solution for exchange of silver and sodium ions | surface corrosion analysis of bronze artifacts | -Ag–SA nanofiber membranes can successfully detect bronze patina trace | [153] |
| Q = 0.4 mL/h | ||||||||
| L = 12 cm | ||||||||
| SA, PCL | water, chloroform, methanol | - | ZnO NPs | U = 25 kV L = 15 cm Shell solution: Q = 0.2 mL/h Core solution: Q = 1 mL/h T = 25 °C RH = 30% | - | tissue engineering—wound healing | -The average diameter of nanofibers: 187 ± 51 nm, improved tensile strength -Application of ZnO NPs and essential oils increased antimicrobial activity -The scaffold accelerated the healing time with total wound healing over 14 days in mouse models carrying full-thickness wounds compared to the nanofibrous scaffold without additives -While the drug release profile of the membrane was satisfactory, close to 50% of the bioactive agents were not released in 5 days | [152] |
| SA, PEO | water | - | ZnO NPs | Q = 1.0 μL/s | CaCl2 | water treatment—removing residual tetracyclines | -Fabricated nanofibers showed great adsorption capacity and rapid adsorption for tetracyclines -Not only does PEO improve the electrospinability of SA but also ZnO NPs, forming a hydrogen bond with SA | [151] |
| U = 21 kV | ||||||||
| L = 15 cm | ||||||||
| oxidized SA, PEO | water, ethanol | Triton X-100 | ZnO NPs | Q = 0.8 mL/h U = 18 kV Collector speed: 120 rpm L = 15 cm T = 25 °C RH = 50% | adipic acid dihydrazide | tissue engineering—wound healing | -Membranes with ZnO NPs addition showed exceptional epithelialization, neovascularization, and anti-inflammatory response compared to membranes without ZnO NPs -The biodegradability of membranes can be adjusted by different concentrations of the crosslinking agent-the fibers became morphologically stable with the concentration increase -Oxidizing SA offers several advantages, such as generating multiple functional aldehyde groups, reducing Mw and viscosity, and enhancing biodegradability | [150] |
| SA, PEO | water | Triton X-100 | Ag NPs (by immersion in silver nitrate) | U = 11 kV (without Ag NPs) | BaCl2 | biomedical and food industrial fields | -Ag NPs slightly increased dimensions and were well distributed in the produced mats -The water–ethanol BaCl2 solution was chosen because ethanol acts as a non-solvent for alginate. The 60:40 ratio was optimal, as it allows dissolving of barium salt and not dissolving the alginate | [51] |
| U = 15 kV (with Ag NPs) | ||||||||
| L = 15 cm | ||||||||
| Q = 0.5 mL/h | ||||||||
| T = 25 °C | ||||||||
| SA, PEO | water | Triton X-100 | ZnO NPs | L = 15 cm | SrCl3 | without a specified application | -fibers diameter: 70–150 nm -PEO was eliminated by washing fibers in hot ethanol -SA with a low Mw and high M/G ratio or SA with a medium Mw and low M/G ratio should be preferred for combination with ZnO NPs | [65] 1 |
| U = 10–12 kV | ||||||||
| Q = 0.4–0.75 mL/h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, P.; Zwolińska, J.; Szopa, D.; Witek-Krowiak, A. Towards Enhanced Electrospinning of Alginate—Can Recent Strategies Overcome Limitations? A Review. Polymers 2025, 17, 2255. https://doi.org/10.3390/polym17162255
Wróbel P, Zwolińska J, Szopa D, Witek-Krowiak A. Towards Enhanced Electrospinning of Alginate—Can Recent Strategies Overcome Limitations? A Review. Polymers. 2025; 17(16):2255. https://doi.org/10.3390/polym17162255
Chicago/Turabian StyleWróbel, Paulina, Julia Zwolińska, Daniel Szopa, and Anna Witek-Krowiak. 2025. "Towards Enhanced Electrospinning of Alginate—Can Recent Strategies Overcome Limitations? A Review" Polymers 17, no. 16: 2255. https://doi.org/10.3390/polym17162255
APA StyleWróbel, P., Zwolińska, J., Szopa, D., & Witek-Krowiak, A. (2025). Towards Enhanced Electrospinning of Alginate—Can Recent Strategies Overcome Limitations? A Review. Polymers, 17(16), 2255. https://doi.org/10.3390/polym17162255








