Extraction and Conversion of Carboxymethyl Cellulose from Okara Soybean Residue via Soda AQ Pulping: Integration of Predictive Models and Process Control
Abstract
1. Introduction
2. Materials and Methods
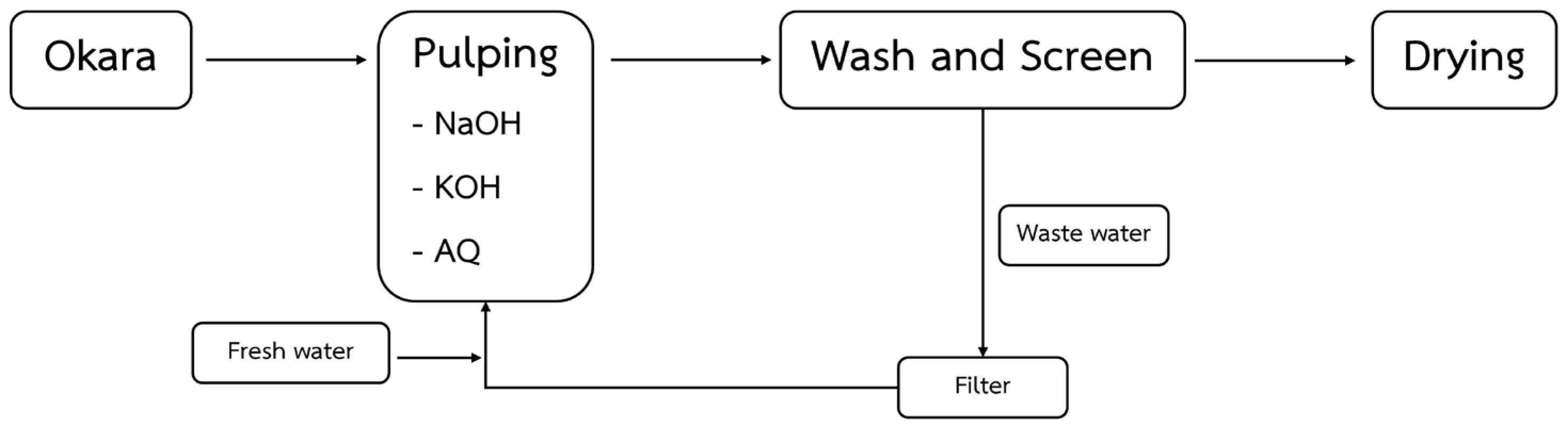
2.1. Soda AQ Pulping Process Description
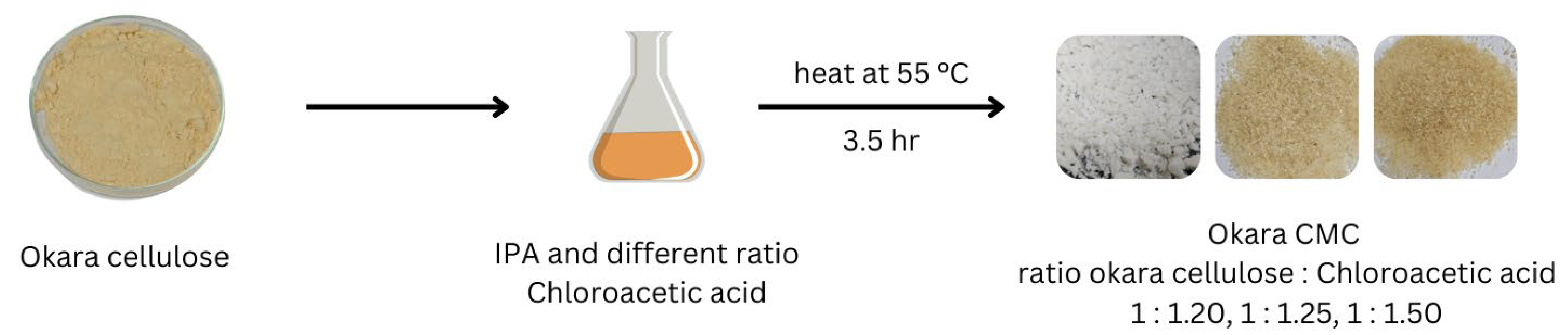
2.2. Preparation of Carboxymethyl Cellulose from Okara Soybean Pulp
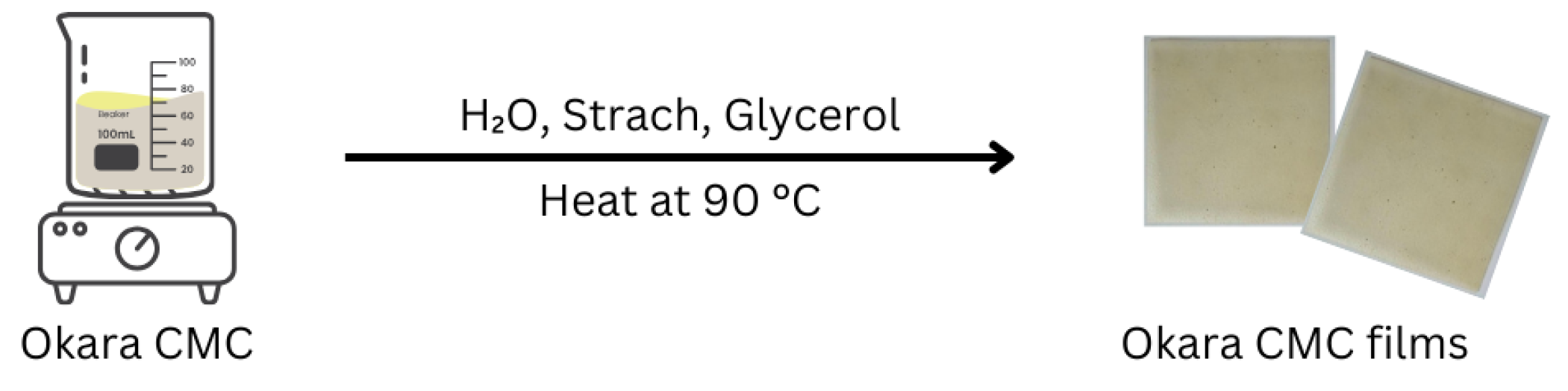
2.3. Preparation of CMC Film from Okara
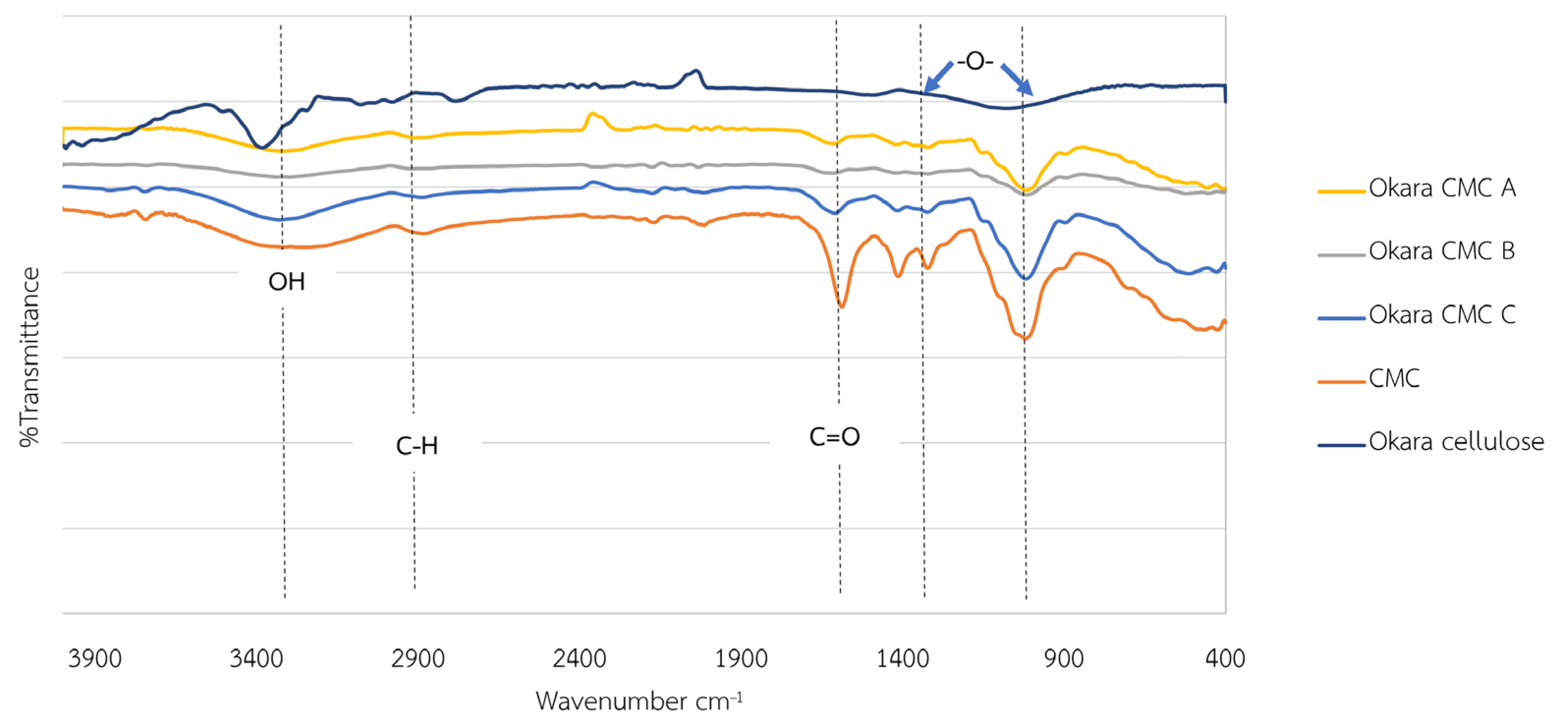
2.4. Characterizations
2.5. Case Study: Process Variables
- NaOH—the concentration of NaOH used in the pulping process. NaOH is commonly used as a cooking liquor to break down fat, protein, and lignin in the okara feedstock.
- KOH—the concentration of KOH used, if applicable. KOH is used in addition to or instead of NaOH for pulping.
- AQ—the concentration of AQ, which serves as a pulping additive to improve delignification efficiency.
- H2O—the amount of H2O used in the pulping process, which serves as a solvent for the chemicals and facilitates the pulping reaction.
2.6. Data Collection, Model Development, and Processing
2.7. Machine Learning Implementation
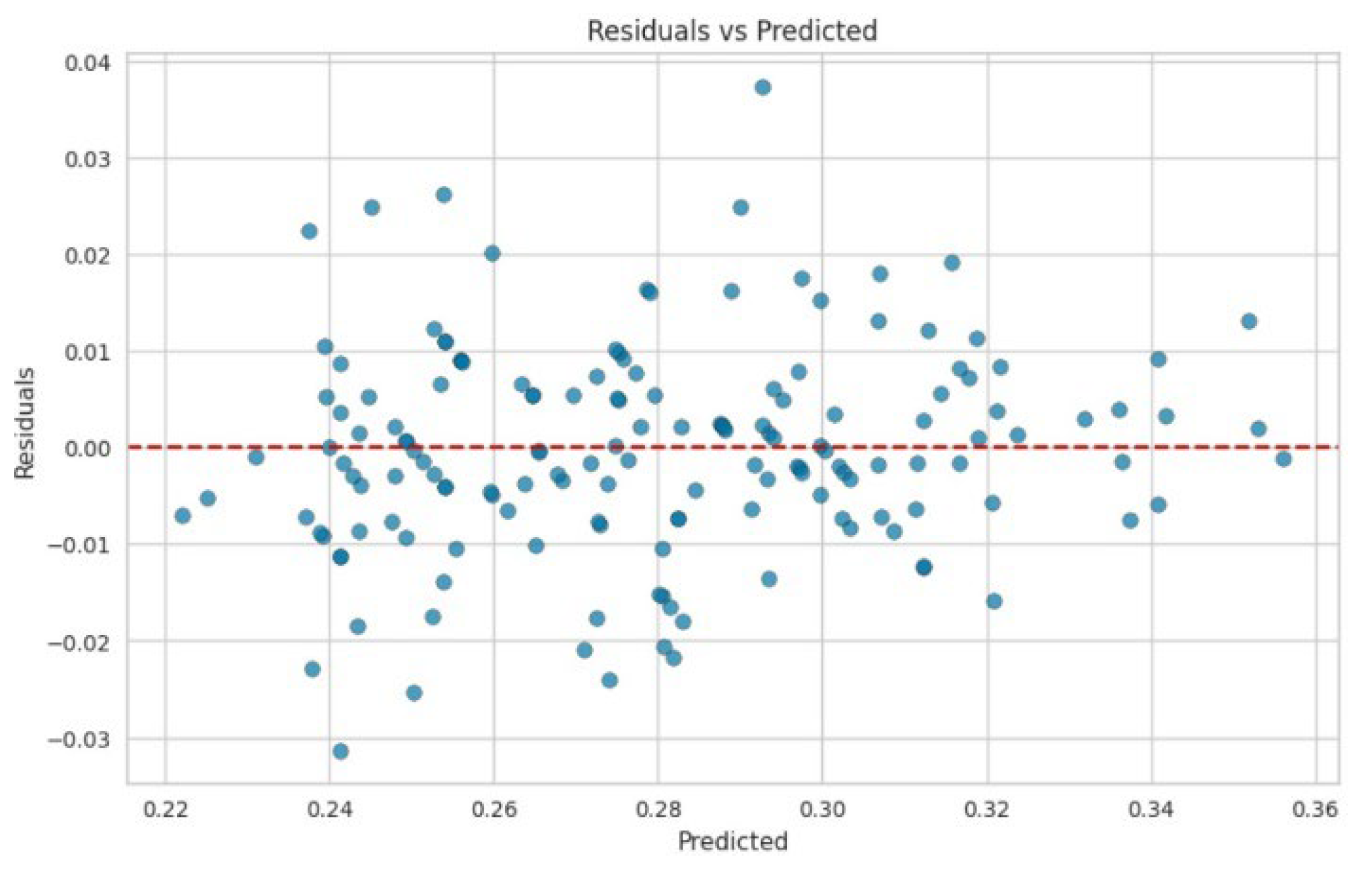
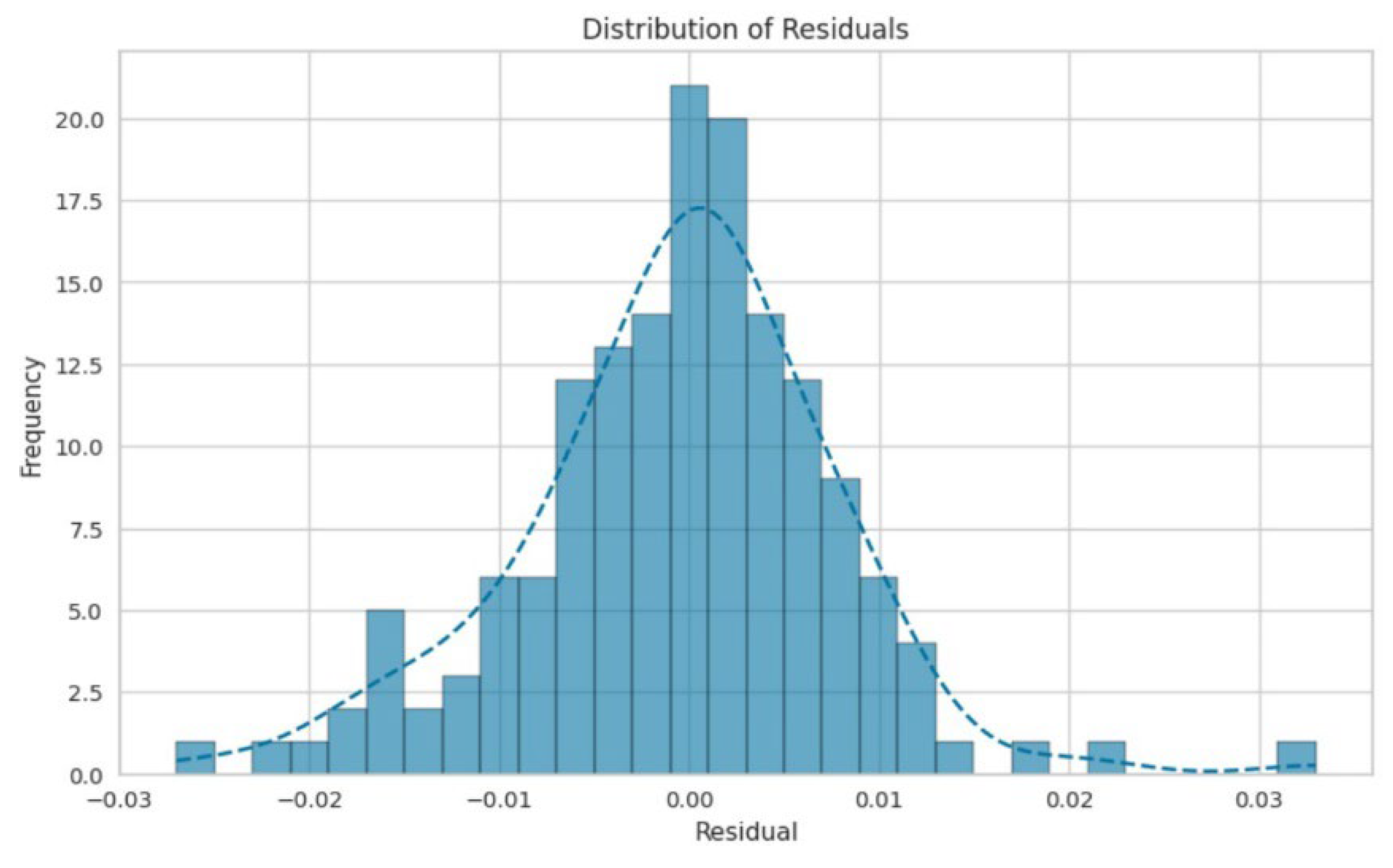
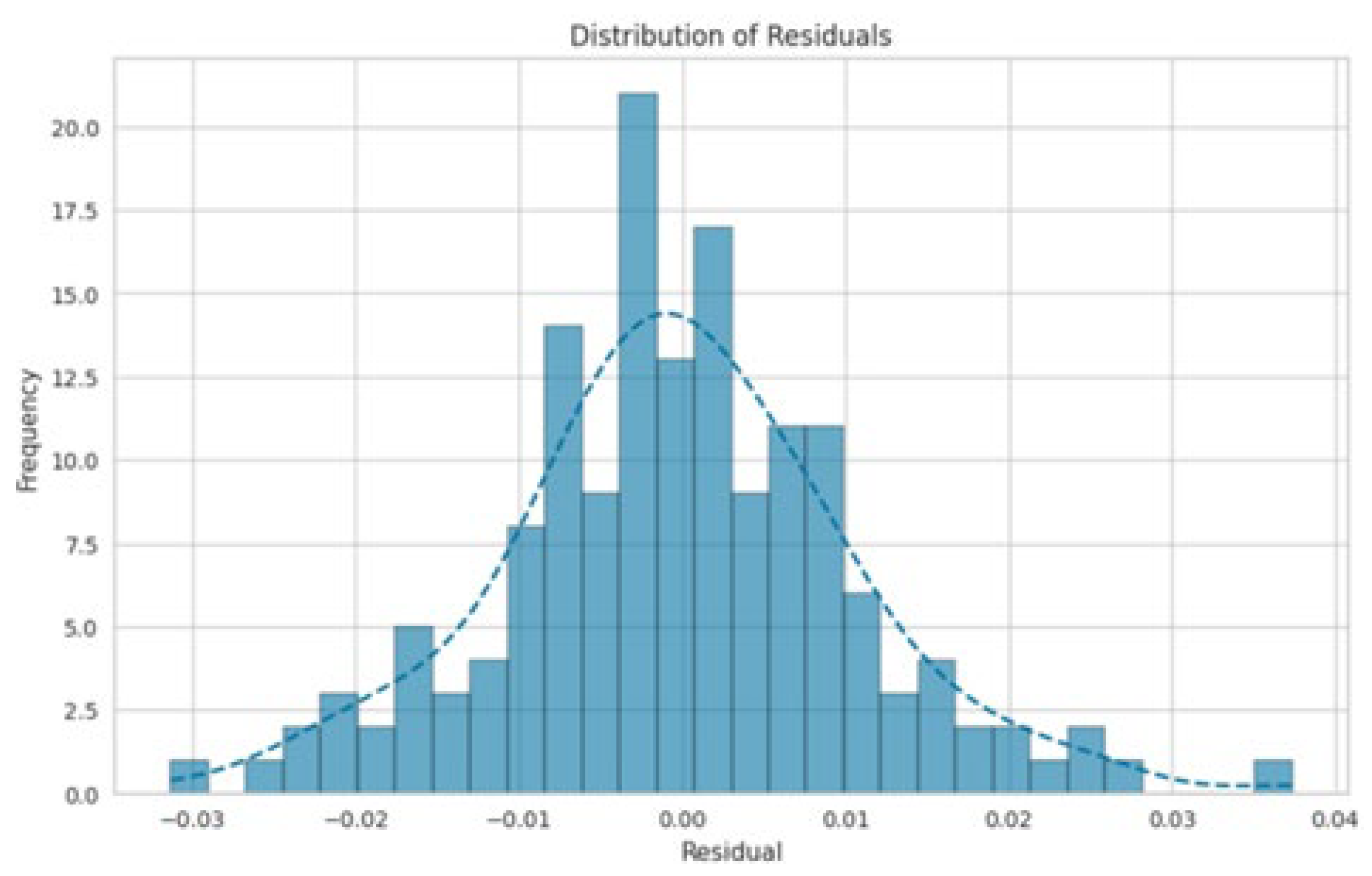
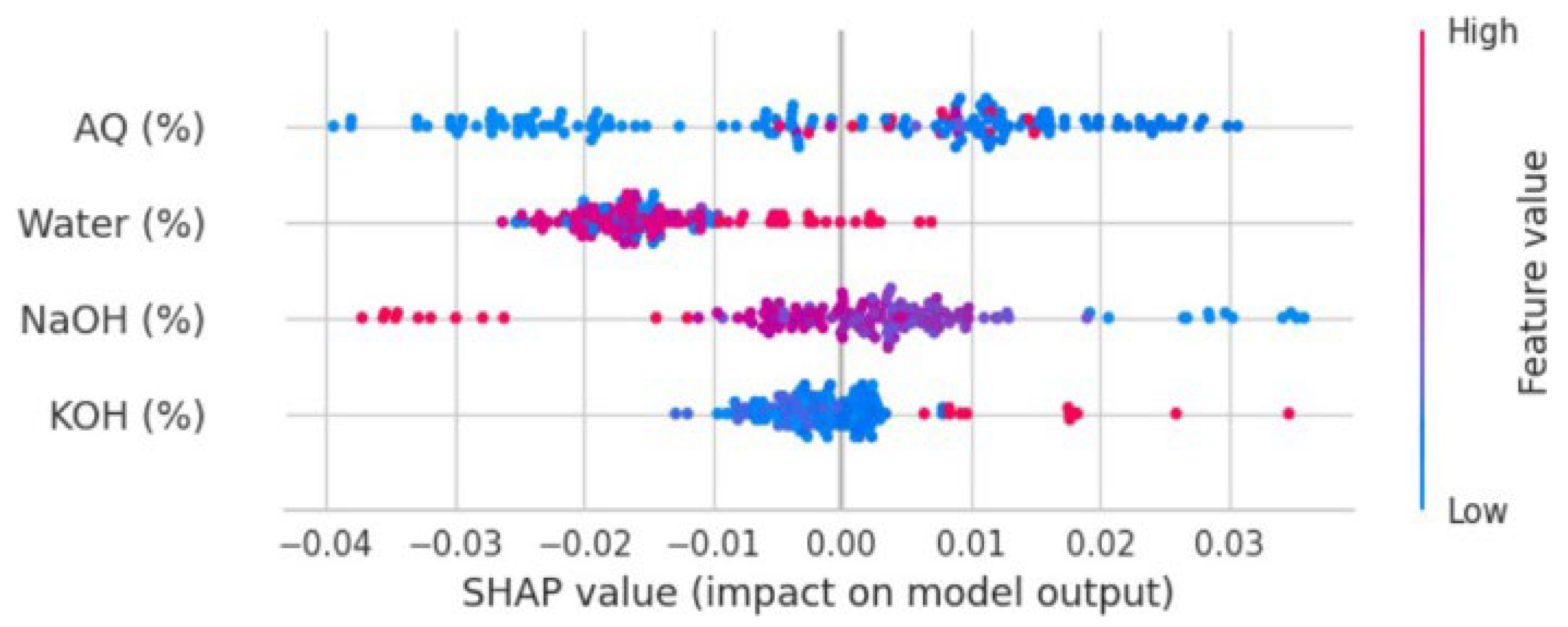
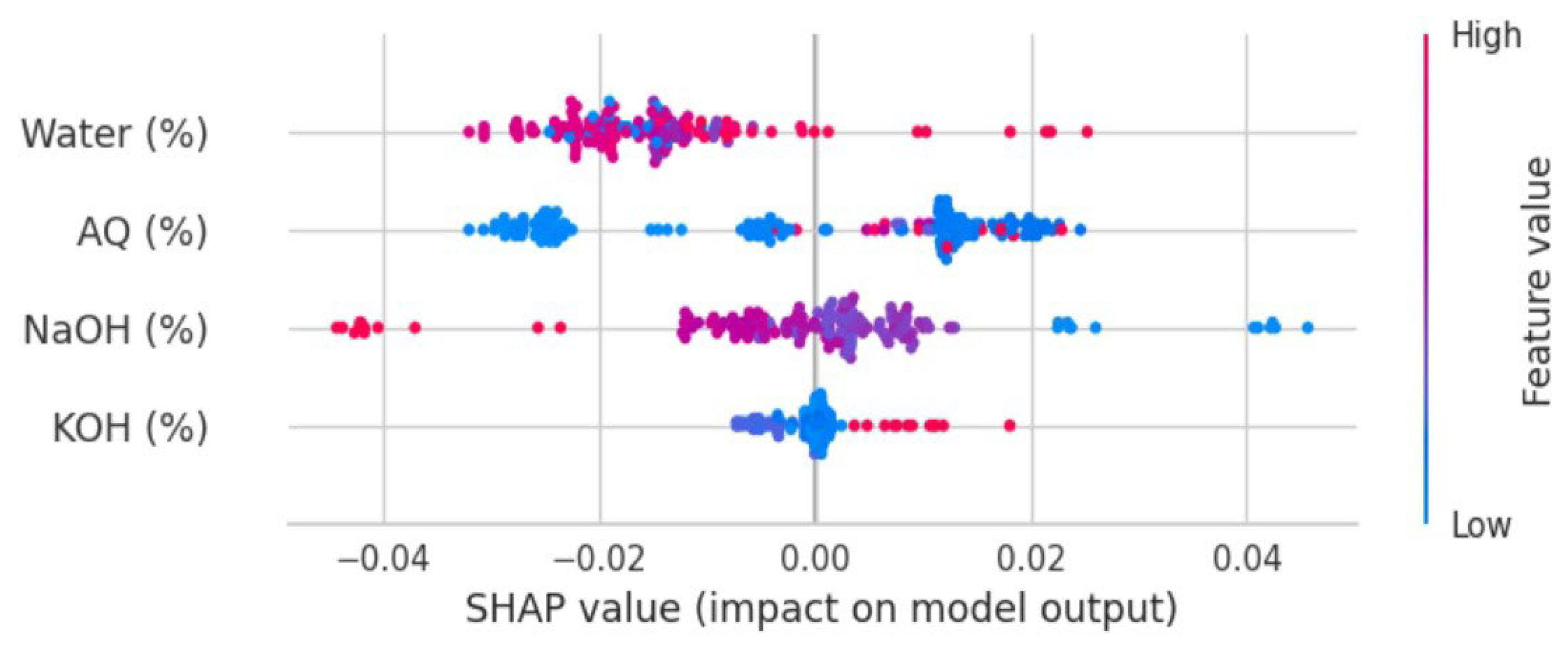
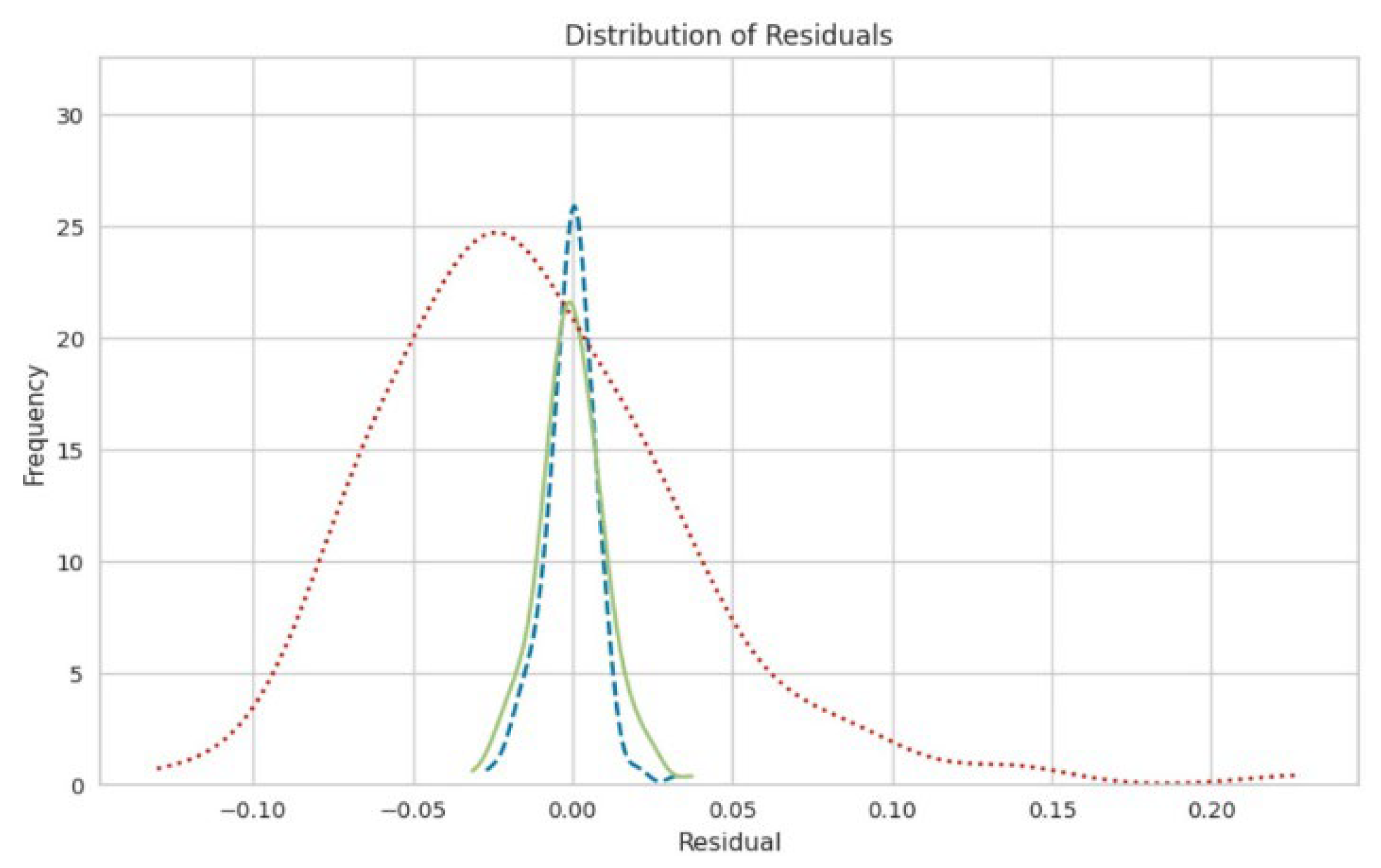
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Food Price Index. FAO. Available online: https://www.fao.org/worldfoodsituation/foodpricesindex/en/ (accessed on 10 February 2025).
- Feng, J.Y.; Wang, R.; Thakur, K.; Ni, Z.J.; Zhu, Y.Y.; Hu, F.; Zhang, J.G.; Wei, Z.J. Evolution of okara from waste to value-added food ingredient: An account of its bio-valorization for improved nutritional and functional effects. Trends Food Sci. Technol. 2021, 116, 8. [Google Scholar] [CrossRef]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Ohno, A.; Ano, T.; Shoda, M. Production of the antifungal peptide antibiotic, iturin by Bacillus subtilis NB22 in solid state fermentation. J. Ferment. Bioeng. 1993, 75, 23–27. [Google Scholar] [CrossRef]
- Khare, S.K.; Jha, K.; Sinha, L.K. Preparation and nutritional evaluation of okara fortified biscuits. J. Dairy. Foods Home Sci. 1995, 14, 91–94. [Google Scholar]
- Li, B.; Zhang, Y.; Yang, H.; Li, R. Effect of drying methods on functional properties of bean curd dregs. J. Henan Inst. Sci. Technol. 2008, 36, 64–66. [Google Scholar]
- Ahn, S.H.; Oh, S.C.; Choi, I.; Han, G.; Jeong, H.; Kim, K.; Yoon, Y.; Yang, I. Environmentally friendly wood preservatives formulated with enzymatic-hydrolyzed okara, copper and/or boron salts. J. Hazard. Mater. 2010, 178, 604–611. [Google Scholar] [CrossRef]
- Liu, K. Food use of whole soybeans. In Soybeans; Elsevier: Amsterdam, The Netherlands, 2008; pp. 441–481. [Google Scholar]
- Xiao, C. 22-Functional soy products. In Functional Foods, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2011; pp. 534–556. [Google Scholar]
- Schieber, A.; Stintzing, F.; Carle, R. By-products of plant food processing as a source of functional compounds recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, A. Soluble soybean polysaccharide. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G., Williams, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2009; pp. 693–709. [Google Scholar]
- Wang, L.; Cui, Q.; Pan, S.; Li, Y.; Jin, Y.; Yang, H.; Li, T.; Zhang, Q. Facile isolation of cellulose nanofibers from soybean residue. Carbohydr. Polym. Technol. Appl. 2021, 2, 100172. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper-based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Chauhan, S.; Meena, B.L. Introduction to pulp and paper industry: Global scenario. Phys. Sci. Rev. 2021, 6, 81–109. [Google Scholar]
- Li, P.; Xu, Y.; Yin, L.; Liang, X.; Wang, R.; Liu, K. Development of raw materials and technology for pulping—A brief review. Polymers 2023, 15, 4465. [Google Scholar] [CrossRef] [PubMed]
- Alén, R. Chapter 3a—Pulp mills and wood-based biorefineries. In Industrial Biorefineries and White Biotechnology; Academic Press: Cambridge, MA, USA, 2015; pp. 91–125. [Google Scholar]
- Kalyoncu, E.E. Eco-friendly pulping of banana pseudo-stem wastes with potassium-based processes. Cellul. Chem. Technol. 2022, 56, 131–140. [Google Scholar] [CrossRef]
- Chandra, M.R.G.S.; Madakka, M. Comparative biochemistry and kinetics of microbial lignocellulolytic enzymes. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 147–159. [Google Scholar]
- Bajpai, P. Pulping calculations. In Biermann’s Handbook of Pulp and Paper; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 353–373. [Google Scholar]
- Iakovleva, M.; van Heiningen, A. Kinetics of fractionation by SO2–ethanol–water (SEW) treatment: Understanding the deconstruction of spruce wood chips. RSC Adv. 2012, 2, 3057–3068. [Google Scholar] [CrossRef]
- Holtzapple, M. Lignin. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3535–3542. [Google Scholar]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose-based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Almonti, D.; Baiocco, G.; Ucciardello, N. Pulp and paper characterization by means of artificial neural networks for effluent solid waste minimization—A case study. J. Process Control 2021, 105, 283–291. [Google Scholar] [CrossRef]
- El-Sayed, E.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Non-wood fibers as raw material for pulp and paper industry. Nord. Pulp Pap. Res. J. 2020, 35, 215–230. [Google Scholar] [CrossRef]
- Bajpai, P. Green Chemistry and Sustainability in Pulp and Paper Industry; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bozaci, E.; Tagaç, A.A. Extraction and Characterization of New Cellulosic Fiber from Catalpa bignonioides Fruits for Potential Use in Sustainable Products. Polymers 2023, 15, 201. [Google Scholar] [CrossRef]
- Eyupoglu, S.; Merdan, N. Physicochemical Properties of New Plant Based Fiber from Lavender Stem. J. Nat. Fibers 2022, 19, 9248–9258. [Google Scholar] [CrossRef]
- Baskaran, P.G.; Kathiresan, M.; Senthamaraikannan, P.; Saravanakumar, S.S. Characterization of New Natural Cellulosic Fiber from the Bark of Dichrostachys Cinerea. J. Nat. Fibers 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 17, 528–535. [Google Scholar] [CrossRef]
- Kono, H.; Oshima, K.; Hashimoto, H.; Shimizu, Y.; Tajima, K. NMR characterization of sodium carboxymethyl cellulose: Substituent distribution and mole fraction of monomers in the polymer chains. Carbohydr. Polym. 2016, 146, 1–9. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Nishinari, K.; Nagano, T. Wet Grinder-Treated Okara Improved Both Mechanical Properties and Intermolecular Forces of Soybean Protein Isolate Gels. Gels 2022, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Cheremisinoff, N.P.; Rosenfeld, P.E. Chapter 6—Sources of air emissions from pulp and paper mills. In Handbook of Pollution Prevention and Cleaner Production; Cheremisinoff, N.P., Rosenfeld, P.E., Eds.; William Andrew Publishing: Oxford, UK, 2010; pp. 179–259. [Google Scholar]
- Popy, R.S.; Nayeem, J.; Arafat, K.M.Y.; Rahman, M.M.; Jahan, M.S. Mild potassium hydroxide pulping of straw. Curr. Res. Green Sustain. Chem. 2020, 3, 100015. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Yin, L.; Wang, R.; Li, P.; Wang, J.; Liu, K. Sustainable Utilization of Pulp and Paper Wastewater. Water 2023, 15, 4135. [Google Scholar] [CrossRef]
- Huang, G.-L.; Shi, J.X.; Langrish, T.A.G. Environmentally friendly bagasse pulping with NH4OH–KOH–AQ. J. Clean. Prod. 2008, 16, 1287–1293. [Google Scholar] [CrossRef]
- Pan, W.C.; Liu, Y.M.; Shiau, S.Y. Effect of Okara and Vital Gluten on Physico-Chemical Properties of Noodle. Czech J. Food Sci. 2018, 4, 36. [Google Scholar]
- Aussanasuwannakul, A.; Boonbumrung, S.; Pantoa, T. Valorization of Soybean Residue (Okara) by Supercritical Carbon Dioxide Extraction: Compositional, Physicochemical, and Functional Properties of Oil and Defatted Powder. Foods 2023, 12, 2698. [Google Scholar] [CrossRef]
- Hoenig, S. Basic Chemical Concepts and Tables; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Parikh, D.; Sachinvala, N.; Calamari, T.; Negulescu, I. Carboxymethylated Cotton for Moist Wound Healing. AATCC Rev. 2003, 3, 15. [Google Scholar]
- Casaburi, A.; Montoya Rojo, Ú.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Arthur, J.C., Jr. Chemical Modification of Cellulose and Its Derivatives; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Water content quantification by FTIR in carboxymethyl cellulose food additive. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Materzok, T.; Müller-Plathe, F. Molecular Structure and Dynamics in Wet Gecko β-Keratin. ACS Biomater. Sci. Eng. 2023, 9, 257–268. [Google Scholar] [CrossRef]

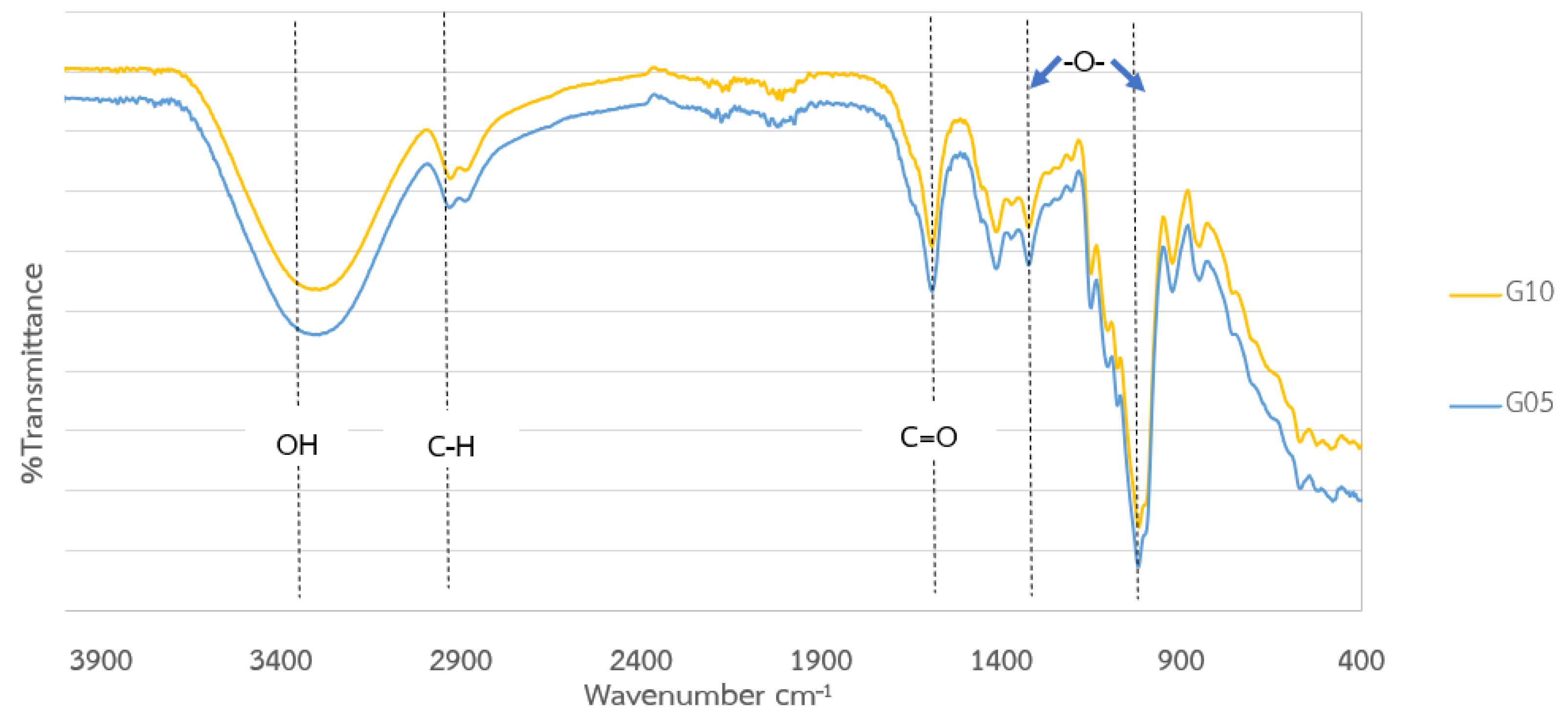
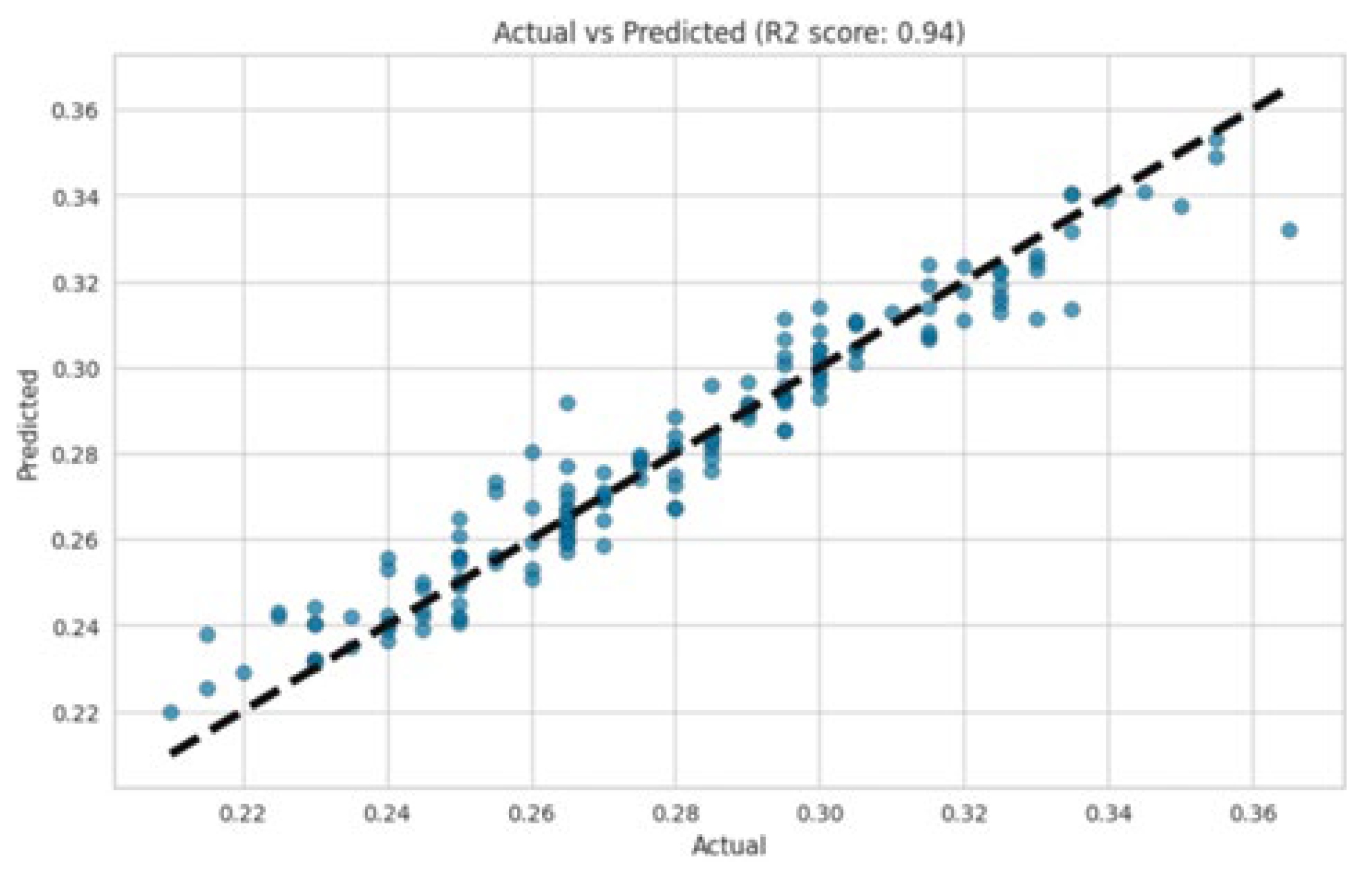
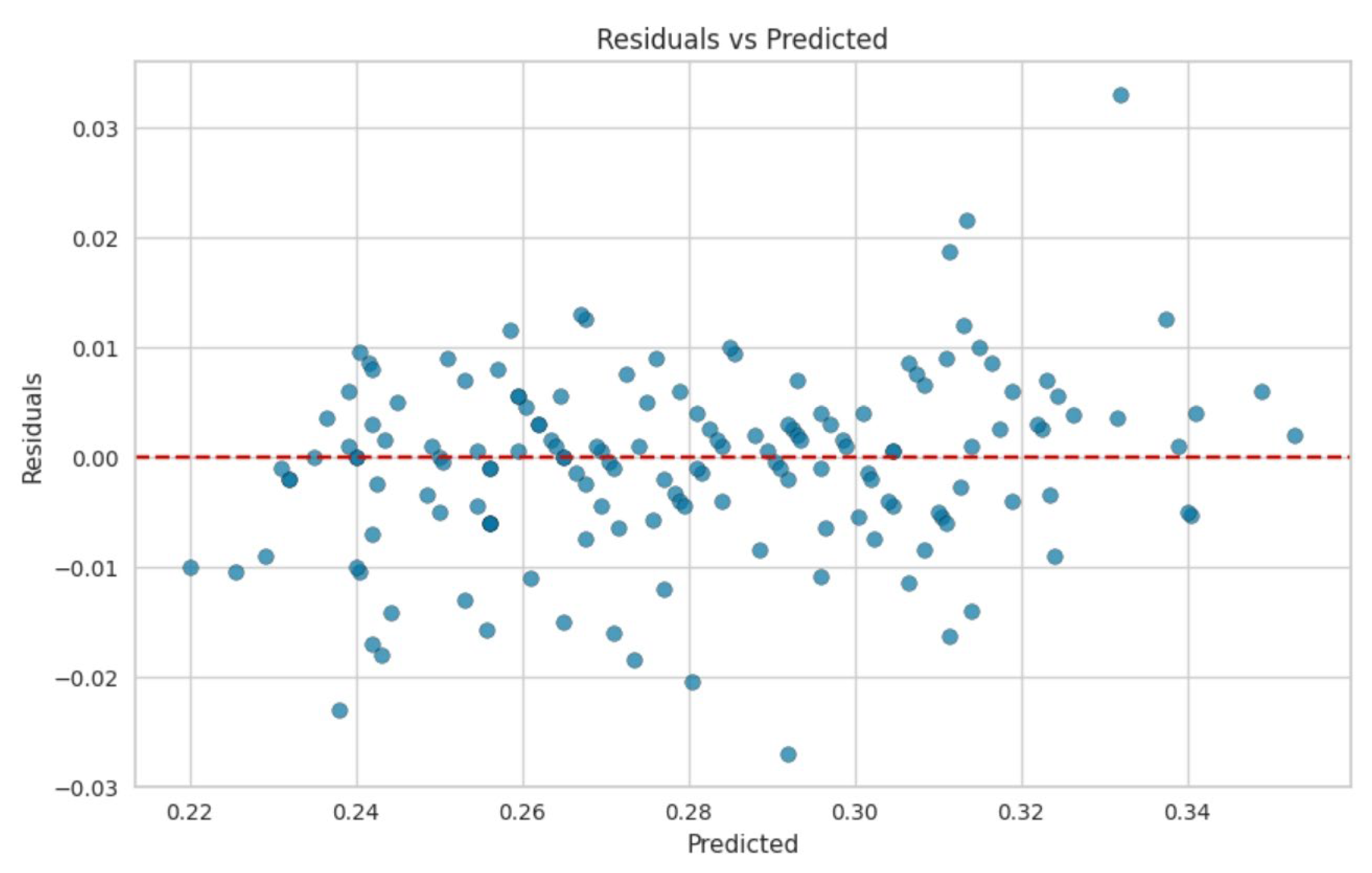
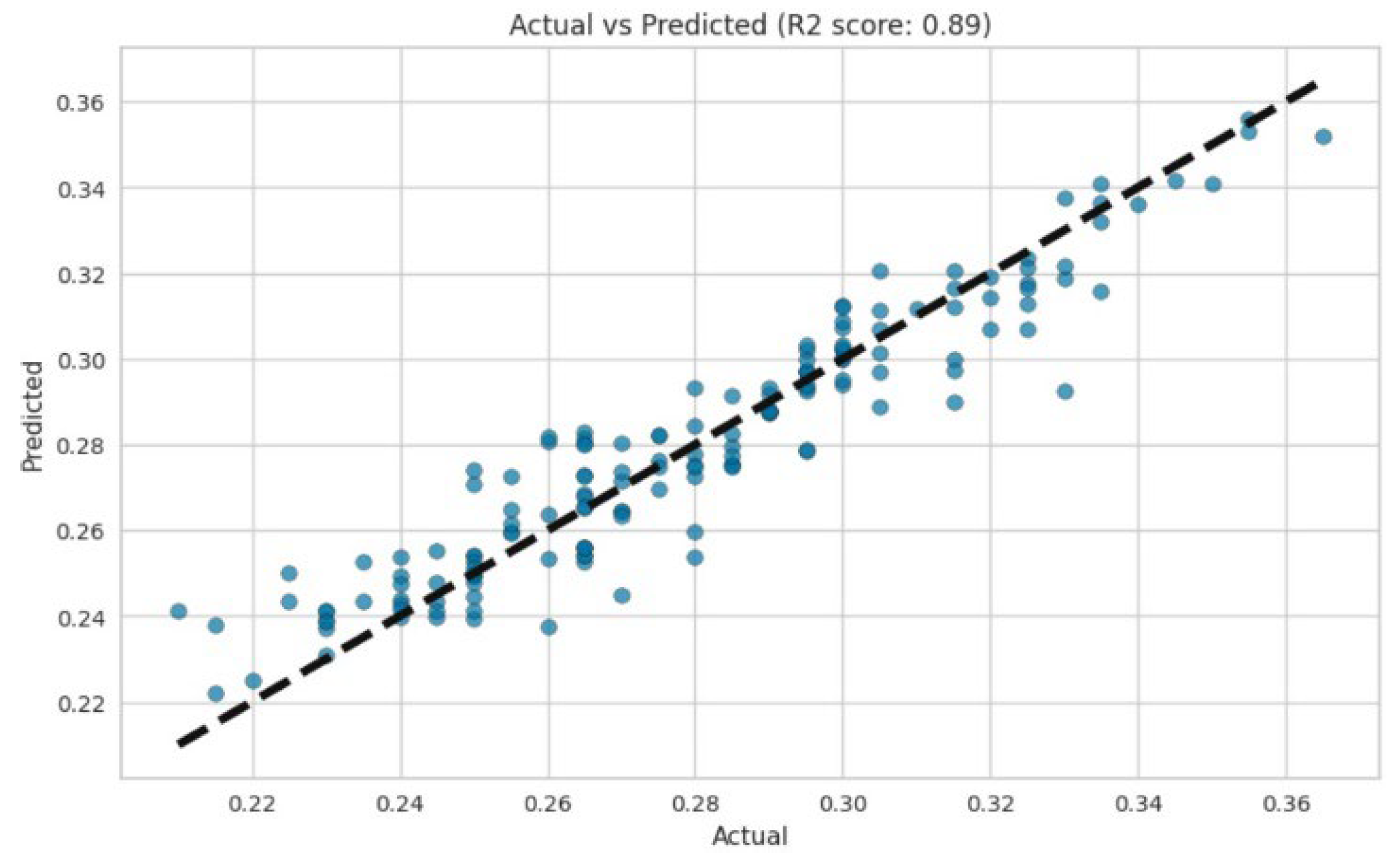
| Layer | Variable | Range (%) |
|---|---|---|
| Input | NaOH: base chemical | 0–50 |
| KOH: additional chemical | 0–50 | |
| AQ: catalyst | 0–5 | |
| H2O: intermediate | 60–100 | |
| Output | Yield | 20–99 |
| Parameter | Description |
|---|---|
| n_estimators | Number of decision trees in the forest. Set to 100 to balance accuracy and computational efficiency. |
| max_depth | Maximum depth of each tree. Limited to 8 to prevent overfitting. |
| min_samples_split | Minimum number of samples required to split an internal node. Set to 5 to ensure robustness against noise. |
| min_samples_leaf | Minimum number of samples required to be at a leaf node. Set to 2 to avoid overly specific splits. |
| max_features | Number of features to consider when looking for the best split. Set to “auto” to utilize all available features for optimal split decisions. |
| bootstrap | Whether bootstrap samples are used when building trees. Enabled to increase tree diversity. |
| criterion | Function to measure the quality of a split. Used “mse” (mean squared error), suitable for regression tasks. |
| Entry | NaOH (%) | KOH (%) | AQ (%) | H2O (%) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 50 | 0 | - | 50 | 85 | 26.5 |
| 2 | 40 | 10 | - | 50 | 85 | 24.0 |
| 3 | 30 | 10 | 0.1 | 59.9 | 85 | 24.0 |
| 4 | 30 | 0 | 0.1 | 69.9 | 85 | 23.0 |
| 5 | 20 | 10 | 0.1 | 69.9 | 85 | 24.5 |
| 6 | 20 | 0 | 0.1 | 79.9 | 85 | 26.5 |
| 7 | 10 | 40 | - | 50 | 85 | 31.0 |
| 8 | 0 | 50 | - | 50 | 85 | 34.5 |
| Entry | Okara Pulp: Chloroacetic Acid (Ratio) | Okara Pulp: IPA (Ratio) | Yield (wt%) | DS |
|---|---|---|---|---|
| A | 1:1.20 | 1:20 | 23.85 | 0.266 |
| B | 1:1.25 | 1:20 | 23.64 | 0.281 |
| C | 1:1.50 | 1:20 | 23.03 | 0.348 |
| D | Commercial CMC | 0.324 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichola, P.; Kitrungrotsakul, T.; Witthayolankowit, K.; Sampoompuang, C.; Lobyaem, K.; Khamphakun, P.; Tumthong, R. Extraction and Conversion of Carboxymethyl Cellulose from Okara Soybean Residue via Soda AQ Pulping: Integration of Predictive Models and Process Control. Polymers 2025, 17, 777. https://doi.org/10.3390/polym17060777
Srichola P, Kitrungrotsakul T, Witthayolankowit K, Sampoompuang C, Lobyaem K, Khamphakun P, Tumthong R. Extraction and Conversion of Carboxymethyl Cellulose from Okara Soybean Residue via Soda AQ Pulping: Integration of Predictive Models and Process Control. Polymers. 2025; 17(6):777. https://doi.org/10.3390/polym17060777
Chicago/Turabian StyleSrichola, Preeyanuch, Titinunt Kitrungrotsakul, Kuntawit Witthayolankowit, Chaiyaporn Sampoompuang, Keowpetch Lobyaem, Prapakorn Khamphakun, and Rawiwan Tumthong. 2025. "Extraction and Conversion of Carboxymethyl Cellulose from Okara Soybean Residue via Soda AQ Pulping: Integration of Predictive Models and Process Control" Polymers 17, no. 6: 777. https://doi.org/10.3390/polym17060777
APA StyleSrichola, P., Kitrungrotsakul, T., Witthayolankowit, K., Sampoompuang, C., Lobyaem, K., Khamphakun, P., & Tumthong, R. (2025). Extraction and Conversion of Carboxymethyl Cellulose from Okara Soybean Residue via Soda AQ Pulping: Integration of Predictive Models and Process Control. Polymers, 17(6), 777. https://doi.org/10.3390/polym17060777






