Properties of Polybenzoxazine-Based Conducting Materials in Energy-Related Applications
Abstract
1. Introduction
1.1. Various Energy-Related Applications and Their Key Challenges
1.2. Materials Used in Combination with Polybenzoxazine
1.3. Role of Hetero Atom in PBz/PBz Derived Carbon
2. Incorporating Conductivity into PBz
3. Intriguing Properties of PBz and Their Composites
4. Tailored Porosity and Surface Area in PBz-Derived Carbons for Enhanced Electrochemical Activity
5. Toughening Strategies for PBz Composites
6. Conclusions, Key Findings, and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.; Du, C.; Yang, S. Synthesis of graphene oxide/polybenzoxazine-based nitrogen-containing porous carbon nanocomposite for enhanced supercapacitor properties. Electrochim. Acta 2017, 251, 12–24. [Google Scholar]
- Arza, C.R.; Ishida, H.; Maurer, F.H.J. Quantifying Dispersion in Graphene Oxide/Reactive Benzoxazine Monomer Nanocomposites. Macromolecules 2014, 47, 3685. [Google Scholar] [CrossRef]
- Alhassan, S.M.; Qutubuddin, S.; Schiraldi, D.A.; Agag, T.; Ishida, H. Preparation and Thermal Properties of Graphene Oxide/Main Chain Benzoxazine Polymer. Eur. Polym. J. 2013, 49, 3825. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Feng, C.; Sun, Y.; Li, K. Synthesis of Polybenzoxazine Based Nitrogen-Rich Porous Carbons for Carbon Dioxide Capture. Nanoscale 2015, 7, 6534. [Google Scholar] [CrossRef] [PubMed]
- Sawaryn, C.; Landfester, K.; Taden, A. Benzoxazine Miniemulsions Stabilized with Multifunctional Main-chain Benzoxazine Protective Colloids. Macromolecules 2011, 44, 5650. [Google Scholar] [CrossRef]
- Shen, L.; Ding, H.; Wang, W.; Guo, Q. Fabrication of Ketjen black-polybenzoxazine superhydrophobic conductive composite coatings. Appl. Surf. Sci. 2013, 268, 297–301. [Google Scholar] [CrossRef]
- Wang, K.W.; Hu, N.X.; Xu, G.; Qi, Y. Stable superhydrophobic composite coatings made from an aqueous dispersion of carbon nanotubes and a fluoropolymer. Carbon 2011, 49, 1769–1774. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhou, J.; Zhang, D.; Zhang, A. Dramatic toughness enhancement of benzoxazine/epoxy thermosets with a novel hyperbranched polymeric ionic liquid. Chem. Eng. J. 2018, 334, 1371–1382. [Google Scholar] [CrossRef]
- Froimowicz, P.; Zhang, K.; Ishida, H. Intramolecular hydrogen bonding in benzoxazines: When structural design becomes functional. Chem. Eur. J. 2016, 22, 2691–2707. [Google Scholar] [CrossRef] [PubMed]
- Puchot, L.; Verge, P.; Fouquet, T.; Vancaeyzeele, C.; Vidal, F.; Habibi, Y. Breaking the symmetry of dibenzoxazines: A paradigm to tailor the design of bio-based thermosets. Green Chem. 2016, 18, 3346–3353. [Google Scholar] [CrossRef]
- Kolanadiyil, S.N.; Minami, M.; Endo, T. Synthesis and thermal properties of difunctional benzoxazines with attached oxazine ring at the para-, meta-, and ortho-position. Macromolecules 2017, 50, 3476–3488. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, Q.; Deng, Y.Y.; Zhu, R.Q.; Gu, Y. A novel benzoxazine/epoxy blend with multiphase structure. RSC Adv. 2014, 4, 238–242. [Google Scholar] [CrossRef]
- Xi, Y.Q.; Yang, P.; Miao, Y.; Zhang, C.L.; Gu, Y. Blends of sulfonated polysulfone/polysulfone/4,4′-diaminodiphenyl methane-based benzoxazine: Multiphase structures and properties. Polym. Int. 2015, 64, 118–125. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Lin, Y.F.; Ran, Q.C.; Zhu, R.Q.; Gu, Y. Modification of benzoxazine with arylether-ether-ketone diphenol: Preparation and characterization. RSC Adv. 2017, 7, 1617–1625. [Google Scholar] [CrossRef]
- Barwe, S.; Andronescu, C.; Engels, R.; Conzuelo, F.; Seisel, S.; Wilde, P.; Chen, Y.-T.; Masa, J.; Schuhmann, W. Cobalt metalloid and polybenzoxazine derived composites for bifunctional oxygen electrocatalysis. Electrochim. Acta 2019, 297, 1042–1051. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Kiskan, B.; Ghosh, N.N.; Yagci, Y. Polybenzoxazine-based composites as high-performance materials. Polym. Int. 2011, 60, 167–177. [Google Scholar] [CrossRef]
- Low, H.Y.; Ishida, H. Structural effects of phenols on the thermal and thermo-oxidative degradation of polybenzoxazines. Polymer 1999, 40, 4365–4376. [Google Scholar] [CrossRef]
- Lin, C.H.; Cai, S.X.; Leu, T.S.; Hwang, T.Y.; Lee, H.H. Synthesis and properties of flame-retardant benzoxazines by three approaches. J. Polym. Sci. A Polym. Chem. 2006, 44, 3454–3468. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, W.; Zhu, R.; Ran, Q.; Gu, Y. Nitrogen configuration of polybenzoxazine carbide. High Temp. Mater. Process. 2015, 34, 245–250. [Google Scholar] [CrossRef]
- Hemvichian, K.; Ishida, H. Thermal decomposition processes in aromatic amine-based polybenzoxazines investigated by TGA and GCeMS. Polymer 2002, 43, 4391–4402. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.B.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.F.; Abdelnaser, S.; EL-Mahdy, A.F.M.; Kuo, S.-W. One-pot synthesis of heteroatom-rich anthraquinone-based benzoxazine-linked porous organic polymers for high performance supercapacitors. Electrochim. Acta 2025, 511, 145397. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, C.; Liang, J.; Wu, W. Electrode materials and device architecture strategies for flexible supercapacitors in wearable energy storage. J. Mater. Chem. A 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Xu, J.; He, Y.; Bi, S.; Wang, M.; Yang, P.; Wu, D.; Wang, J.; Zhang, F. An olefin-linked covalent organic framework as a flexible thin-film electrode for a high-performance micro-supercapacitor. Angew. Chem. Int. Ed. 2019, 58, 12065–12069. [Google Scholar] [CrossRef]
- Saber, A.F.; Sharma, S.U.; Lee, J.T.; EL-Mahdy, A.F.M.; Kuo, S.-W. Carbazole-conjugated microporous polymers from Suzuki–Miyaura coupling for supercapacitors. Polymer 2022, 254, 125070. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.J.; Feng, L.; Du, C.; Jian, S.J.; Yang, W.S.; Wu, Y.M.A.; Jiang, S.H.; He, S.J.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, C.; Zhang, S.Y.; Yu, S.T.; Huang, L. Self-assembly of biomass-based hybrid hydrogel electrode for an additive-free flexible supercapacitor. J. Mater. Chem. 2022, 10, 16853–16865. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Balasubramanian, R.; Parveen, A.S.; Kim, S.-C. Direct synthesis of nitrogen-rich carbon sheets via polybenzoxazine as highly active electrocatalyst for water splitting. Int. J. Hydrogen Energy 2018, 43, 13266–13275. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Ullah, K.; Ye, S.; Zhu, L.; Jo, S.B.; Jang, W.K.; Cho, K.Y.; Oh, W.C. Noble metal doped graphene nanocomposites and its study of photocatalytic hydrogen evolution. Solid State Sci. 2014, 31, 91–98. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Sethuraman, M.G.; Lee, Y.R. Ultrasonic synthesis, characterization and energy applications of Ni–B alloy nanorods. J. Taiwan Inst. Chem. Eng. 2017, 80, 901–907. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shen, H.; Li, Z.; Zhang, S.; Zhao, Y.; Bi, X.; Wang, Y.; Cui, H.; Zhuo, S. Porous carbon materials with dual N, S-doping and uniform ultramicroporosity for high performance supercapacitors. Electrochim. Acta 2016, 209, 557–564. [Google Scholar] [CrossRef]
- Zhang, Y.; Zang, J.; Han, C.; Jia, S.; Tian, P.; Gao, H.; Wang, Y. Molybdenum oxide and molybdenum carbide coated carbon black as an electrocatalyst for hydrogen evolution reaction in acidic media. Int. J. Hydrogen Energy 2017, 42, 26985–26994. [Google Scholar] [CrossRef]
- Witpathomwong, S.; Okhawilai, M.; Jubsilp, C.; Karagiannidis, P.; Rimdusit, S. Highly filled graphite/graphene/carbon nanotube in polybenzoxazine composites for bipolar plate in PEMFC. Int. J. Hydrogen Energy 2020, 45, 30898–30910. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Xu, S.; He, J.; Jin, S.; Tan, B. Heteroatom-rich porous organic polymers constructed by benzoxazine linkage with high carbon dioxide adsorption affinity. J. Colloid Interface Sci. 2018, 509, 457–462. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347. [Google Scholar] [CrossRef]
- Thubsuang, U.; Manmuanpom, N.; Chokaksornsan, N.; Sommut, C.; Singhawat, K.; Payaka, A.; Wongkasemjit, S.; Chaisuwan, T. Efficient CO2 adsorption on porous carbon with nitrogen functionalities based on polybenzoxazine: High-pressure adsorption characteristics. Appl. Surf. Sci. 2023, 607, 155120. [Google Scholar] [CrossRef]
- Bai, B.C.; Kim, E.A.; Lee, C.W.; Lee, Y.-S.; Im, J.S. Effects of surface chemical properties of activated carbon fibers modified by liquid oxidation for CO2 adsorption. Appl. Surf. Sci. 2015, 353, 158–164. [Google Scholar] [CrossRef]
- Liu, J.; Sun, N.; Sun, C.; Liu, H.; Snape, C.; Li, K.; Wei, W.; Sun, Y. Spherical potassium intercalated activated carbon beads for pulverised fuel CO2 post-combustion capture. Carbon 2015, 94, 243–255. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene: Graphene oxide and other graphenoids. Electrochem. Commun. 2013, 36, 14. [Google Scholar] [CrossRef]
- McGrail, B.T.; Rodier, B.J.; Pentzer, E. Rapid functionalization of graphene oxide in water. Chem. Mater. 2014, 26, 5806. [Google Scholar] [CrossRef]
- Schuler, F.; Kerscher, B.; Beckert, F.; Thomann, R.; Mulhaupt, R. Hyperbranched polymeric ionic liquids with onion-like topology as transporters and compartmentalized systems. Angew. Chem. Int. Ed. 2013, 52, 455–458. [Google Scholar] [CrossRef]
- Fan, Y.J.; Zhang, D.P.; Wang, J.; Jin, H.B.; Zhou, Y.F.; Yan, D.Y. Preparation of anion exchangeable polymer vesicles through the self-assembly of hyperbranched polymeric ionic liquids. Chem. Commun. 2015, 51, 7234–7237. [Google Scholar] [CrossRef]
- Murugan, E.; Munusamy, K.; Babu, A.V. Development of aryl ether-free cross-linked polymer membranes for sustainable electrochemical energy conversion and storage applications. Chem. Eng. J. 2024, 501, 157473. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y. Experimental and computational investigation of Cu–N coordination bond strengthened polyaniline for stable energy storage. J. Mater. Sci. 2021, 56, 10135–10153. [Google Scholar] [CrossRef]
- Yi-Chiang, H.; Hsu-Feng, L.; Yu-Chao, T.; Chun-Che, L.; Mei-Ying, C.; Wen-Yao, H. Synthesis of novel sulfonated poly(arylene ether)s containing a tetra-trifluoromethyl side chain and multi-phenyl for proton exchange membrane fuel cell application. RSC Adv. 2017, 7, 33068–33077. [Google Scholar]
- Mohamed, M.G.; Su, B.-X.; Kuo, S.-W. Robust Nitrogen-Doped Microporous Carbon via Crown Ether-Functionalized Benzoxazine-Linked Porous Organic Polymers for Enhanced CO2 Adsorption and Supercapacitor Applications. ACS Appl. Mater. Interfaces 2024, 16, 40858–40872. [Google Scholar] [CrossRef] [PubMed]
- Subhadip, R.; Swagata, P.; Priyadarsi, D. Recent progress on polymeric probes for formaldehyde sensing: A comprehensive review. Sci. Technol. Adv. Mater. 2024, 25, 2423597. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zeng, C.; Qu, D.; Tang, H.; Li, Y.; Su, B.-L.; Qu, D. Highly efficient synthesis of ordered nitrogen-doped mesoporous carbons with tunable properties and its application in high performance supercapacitors. J. Power Sources 2016, 321, 143–154. [Google Scholar] [CrossRef]
- Thubsuang, U.; Ishida, H.; Wongkasemjit, S.; Chaisuwan, T. Improvement in the pore structure of polybenzoxazine-based carbon xerogels through a silica templating method. J. Porous Mater. 2014, 21, 401–411. [Google Scholar] [CrossRef]
- Woo, S.W.; Dokko, K.; Nakano, H.; Kanamura, K. Preparation of three-dimensionally ordered macroporous carbon with mesoporous walls for electric double-layer capacitors. J. Mater. Chem. 2008, 18, 1674–1680. [Google Scholar] [CrossRef]
- Sayari, A.; Yang, Y. SBA-15 templated mesoporous carbon: New insights into the SBA-15 pore structure. Chem. Mater. 2005, 17, 6108–6113. [Google Scholar] [CrossRef]
- Lin, C.F.; Zhang, X.; Lin, H.; Wang, N.; Li, J.B.; Yang, X.Z. Synthesis of ordered mesoporous carbon using MCM-41 mesoporous silica as template. Adv. Mater. Res. 2006, 11–12, 543–546. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.; Fang, Z.; E, Y.; Wu, T.; Chen, F. Superhydrophobic and conductive properties of carbon nanotubes/polybenzoxazine nanocomposites coated ramie fabric prepared by solution-immersion process. Appl. Surf. Sci. 2014, 309, 218–224. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Wang, C.-F.; Su, Y.-C.; Kuo, S.-W.; Huang, C.-F.; Sheen, Y.-C.; Chang, F.-C. Low-surface-free-energy materials based on polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef]
- Zhang, D.H.; Liang, E.B.; Li, T.C.; Chen, S.F.; Zhang, J.H.; Cheng, X.J.; Zhou, J.L.; Zhang, A.Q. The effect of molecular weight of hyperbranched epoxy resins with a silicone skeleton on performance. RSC Adv. 2013, 3, 9522–9529. [Google Scholar] [CrossRef]
- Zhang, D.H.; Liang, E.B.; Li, T.C.; Chen, S.F.; Zhang, J.H.; Cheng, X.J.; Zhou, J.L.; Zhang, A.Q. Environment-friendly synthesis and performance of a novel hyperbranched epoxy resin with a silicone skeleton. RSC Adv. 2013, 3, 3095–3102. [Google Scholar] [CrossRef]
- Che, J.; Wu, K.; Lin, Y.; Wang, K.; Fu, Q. Largely improved thermal conductivity of HDPE/expanded graphite/carbon nanotubes ternary composites via filler network-network synergy. Compos. Part A 2017, 99, 32–40. [Google Scholar] [CrossRef]
- Kara, S.; Arda, E.; Dolastir, F.; Pekcan, O. Electrical and optical percolations of polystyrene latex-multiwalled carbon nanotube composites. J. Colloid Interface Sci. 2010, 344, 395–401. [Google Scholar] [CrossRef]
- Huang, Q.; Tong, Y.; Hu, B.; Huang, J.; Cao, X.; Yang, Z.; He, G. High-performance polybenzoxazine based composites PEMFC bipolar plates with a multi-layer structure for surface enrichment of conductive phase. Int. J. Hydrogen Energy 2023, 48, 32540–32552. [Google Scholar] [CrossRef]
- Liao, W.-N.; Jiang, F.-J.; Zhang, Y.; Zhou, X.-J.; He, Z.-Q. Highly conductive composite bipolar plate based on ternary carbon materials and its performance in redox flow batteries. Renew. Energy 2020, 152, 1310–1316. [Google Scholar] [CrossRef]
- Lv, B.; Shao, Z.; He, L.; Gou, Y.; Sun, S. A novel graphite/phenolic resin bipolar plate modified by doping carbon fibers for the application of proton exchange membrane fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 876–881. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Zakeri, M.; Nasef, M.M.; Miyake, M.; Mozarmnia, P.; Bazilah, N.A. Highly durable polybenzimidazole composite membranes with phosphonated graphene oxide for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2019, 412, 238–245. [Google Scholar] [CrossRef]
- Mohanty, P.; Kull, L.D.; Landskron, K. Porous covalent electron-rich organonitridic frameworks as highly selective sorbents for methane and carbon dioxide. Nat. Commun. 2011, 2, 401. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Islamoglu, T.; El-Kaderi, H.M. Benzothiazole- and benzoxazole-linked porous polymers for carbon dioxide storage and separation. J. Mater. Chem. A 2017, 5, 258–265. [Google Scholar] [CrossRef]
- Brousse, T.; Belanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Tian, L.; Wang, M.; Liao, G.; Sun, Y.; Chen, Y.; Hu, Y.; Lu, Z. Highly sulfonated polybenzoxazine proton exchange membrane with dimensional stability and low hydrogen permeability for water electrolysis. Chem. Eng. J. 2025, 517, 164596. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, R.; Zhang, J.; Luo, H.; Liu, Y.; Zou, Y.; Ren, X. Microwave-assisted rapid synthesis of nanoscale MOF-303 for hydrogel composites with superior proton conduction at ambient-humidity conditions. ACS Appl. Energy Mater. 2021, 4, 14681–14688. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Wang, S.; Li, S. Investigation of sulfonation degree and temperature on structure, thermal and membrane’s properties of sulfonated poly(ether ether ketone). Int. J. Hydrogen Energy 2023, 48, 13791–13803. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, C.; Chen, G.; Liu, Z.; Ma, M.; Xie, Q.; Zheng, N.; Yao, S. Synthesis of ultrathin nitrogen-doped graphitic carbon nanocages as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2013, 5, 2241. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Patil, U.M.; Shackery, I.; Sohn, J.S.; Lee, S.; Park, B.; Jun, S. High-Performance Supercapacitor Electrode Based on a Polyaniline Nanofibers/3D Graphene Framework as an Efficient Charge Transporter. J. Mater. Chem. A 2014, 2, 4989. [Google Scholar] [CrossRef]
- Zinola, C.F.; Castro Luna, A.M.; Triaca, W.E.; Arvia, A.J. Kinetics and mechanism of the electrochemical reduction of molecular oxygen on platinum in KOH. J. Appl. Electrochem. 1994, 24, 531–541. [Google Scholar] [CrossRef]
- Gorlin, Y.; Chung, C.-J.; Nordlund, D.; Clemens, B.M.; Jaramillo, T.F. Mn3O4 supported on glassy carbon. ACS Catal. 2012, 2, 2687–2694. [Google Scholar] [CrossRef]
- Medina, D.; Barwe, S.; Masa, J.; Seisel, S.; Schuhmann, W.; Andronescu, C. Optimizing the synthesis of Co/Co–Fe nanoparticles/N-doped carbon composite materials as bifunctional oxygen electrocatalysts. Electrochim. Acta 2019, 318, 281–289. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Aparicio, S.; Canlier, A.; Yavuz, C.T. Insights of CO2 adsorption performance of amine impregnated mesoporous silica (SBA-15) at wide range pressure and temperature conditions. Int. J. Greenh. Gas Control 2015, 43, 22–32. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Adsorption equilibrium and kinetics of CO2, CH4, N2O, and NH3 on ordered mesoporous carbon. J. Colloid Interface Sci. 2010, 345, 402–409. [Google Scholar] [CrossRef]
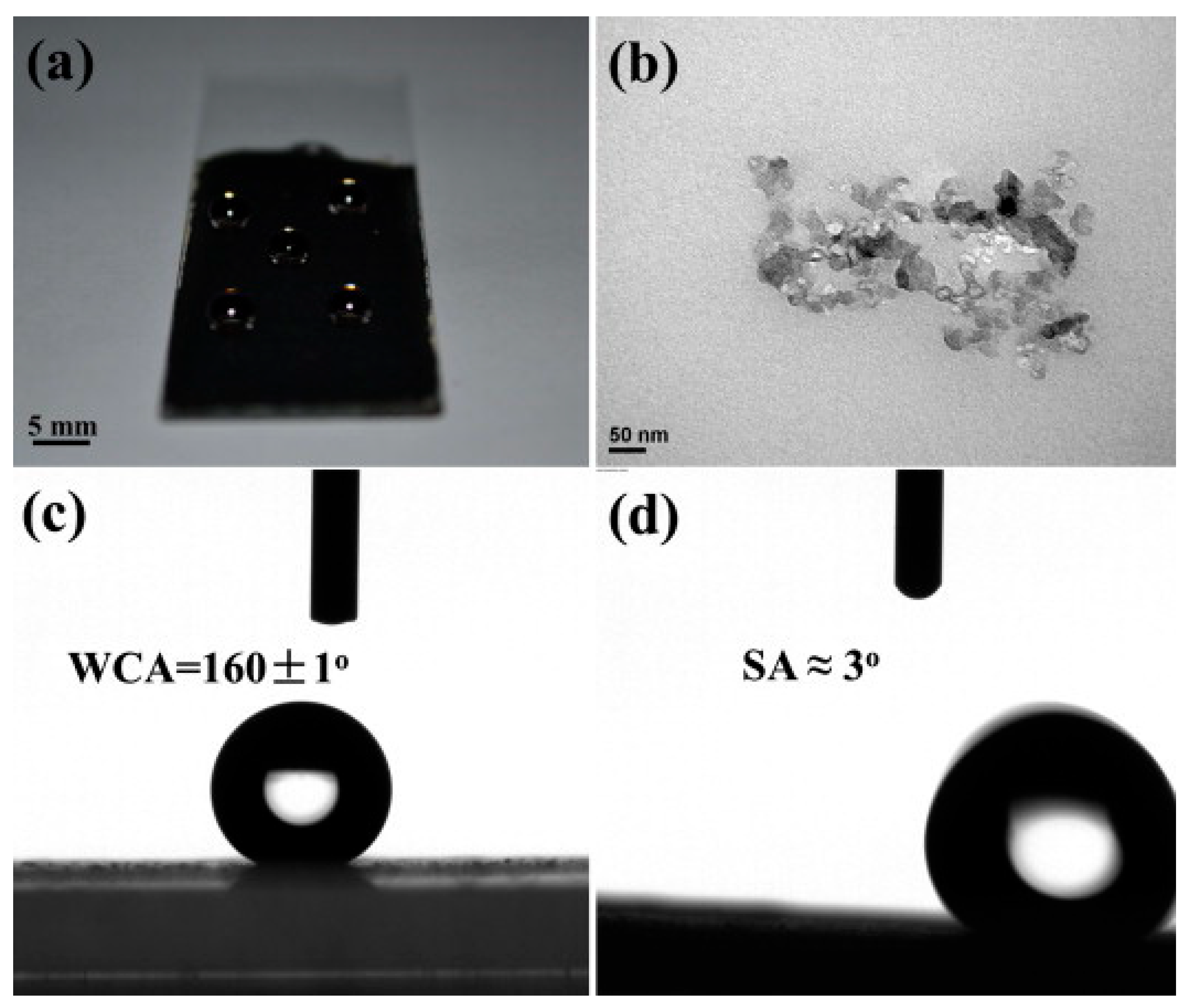
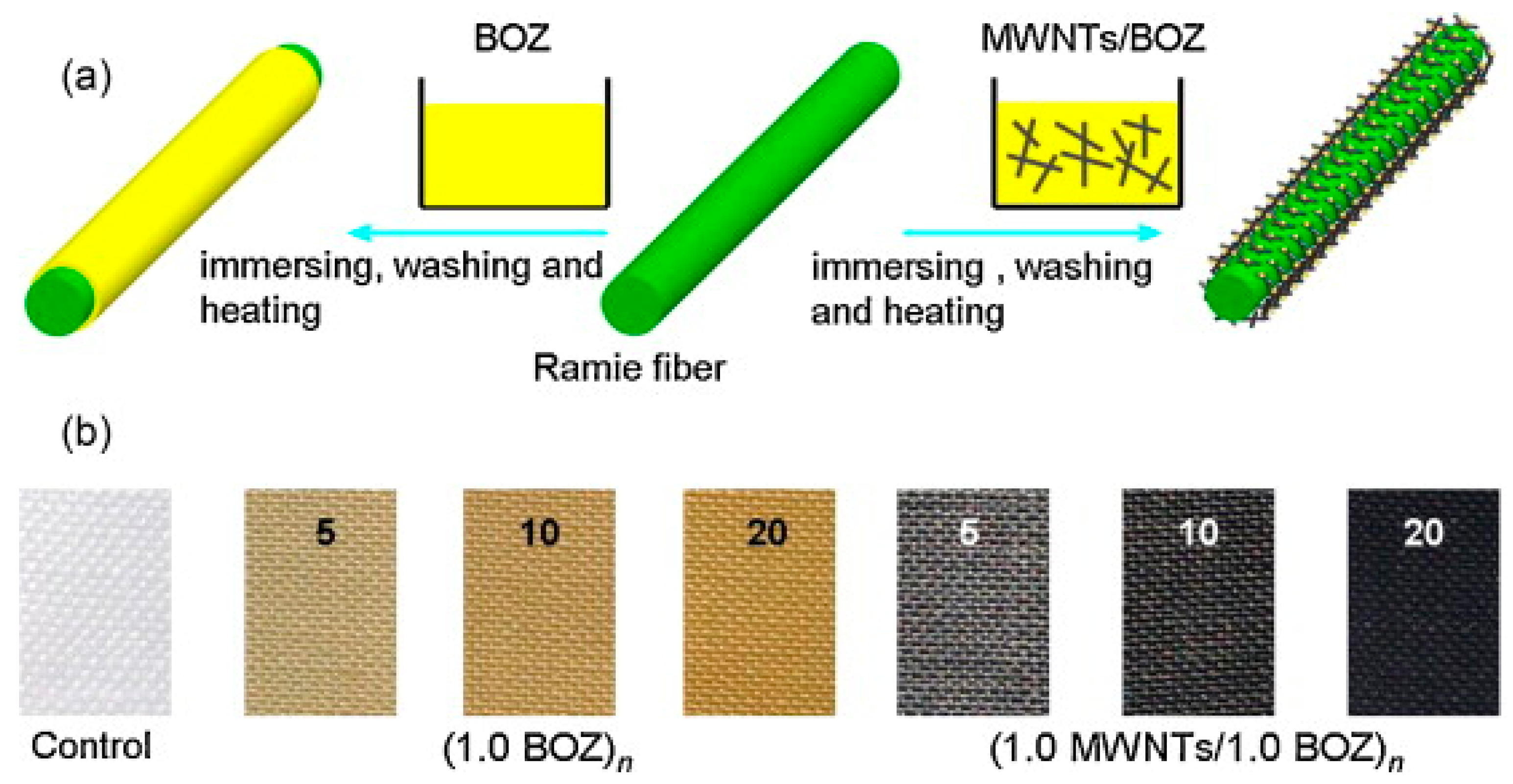
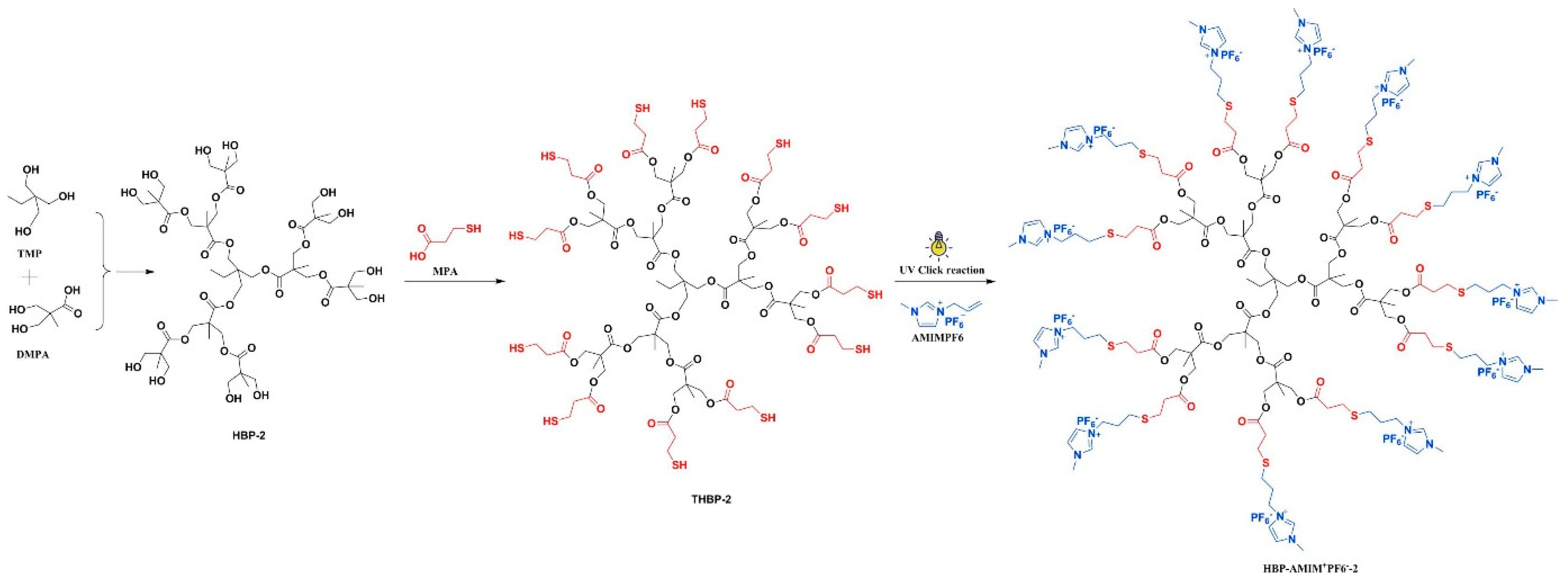
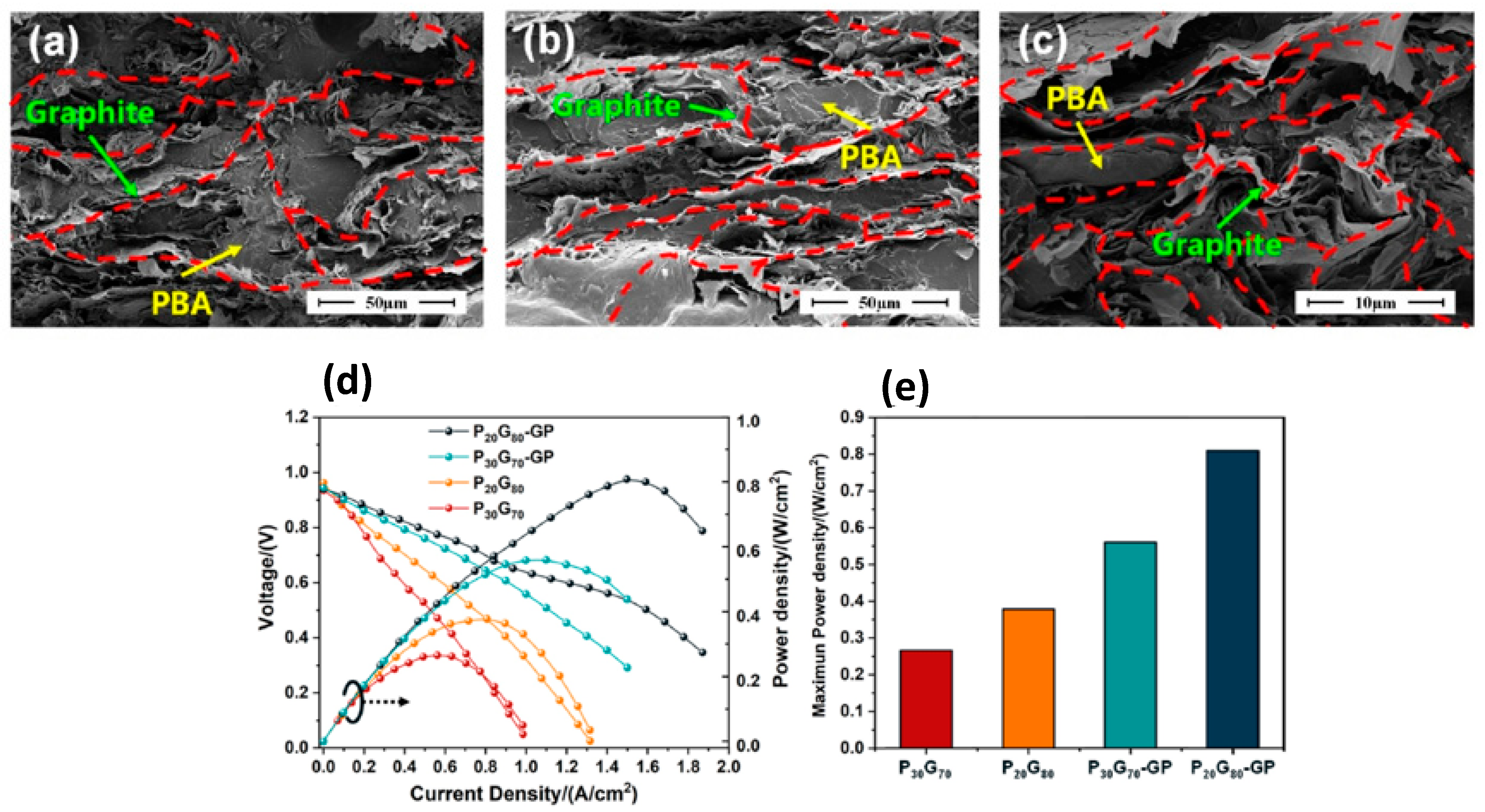

| Material | Composition | Performance | Application |
|---|---|---|---|
| KB-PBZ Composite [6] (Bisphenol-A based Bzo) | Ketjen Black + PBz | WCA ≈ 160°, SA ≈ 3°, Conductive surface | Supercapacitor/ Superhydrophobic Coating |
| MWNTs/PBZ [61] (Bzo with carbonitrile group) | MWNTs in BOZ matrix, 20 immersion cycles | WCA up to 152°, SA ≈ 3°, Sheet resistance: 3.41 × 103 Ω/sq | Superhydrophobic and Conductive Textile |
| HBP-AMIM+PF6−/BA-ECC [8] (Bisphenol-A based Bzo) | Hyperbranched Ionic Liquid + Benzoxazine/Epoxy | Tensile strength ↑ 76.6%, Impact strength ↑ 80.4%, Tg ↑, T5% ↑ 30 °C | Structural Composite |
| Graphene/Graphite/CNT-PBZ [41] (Bisphenol-A based Bzo) | 7.5% graphene + variable CNT/graphite | Conductivity: 364 S/cm, Thermal conductivity: 21.3 W/m·K, Flexural strength: 41.5 MPa | PEMFC Bipolar Plate |
| PBA/EG Multilayer BP [71] (Bisphenol-A based Bzo) | Graphite-PBA-Graphite layers | In-plane conductivity: 278.85 S/cm, Flexural strength: 75.75 MPa, WCA ≈ 99°–102°, ASR: 9.70 mΩ·cm2 | PEMFC |
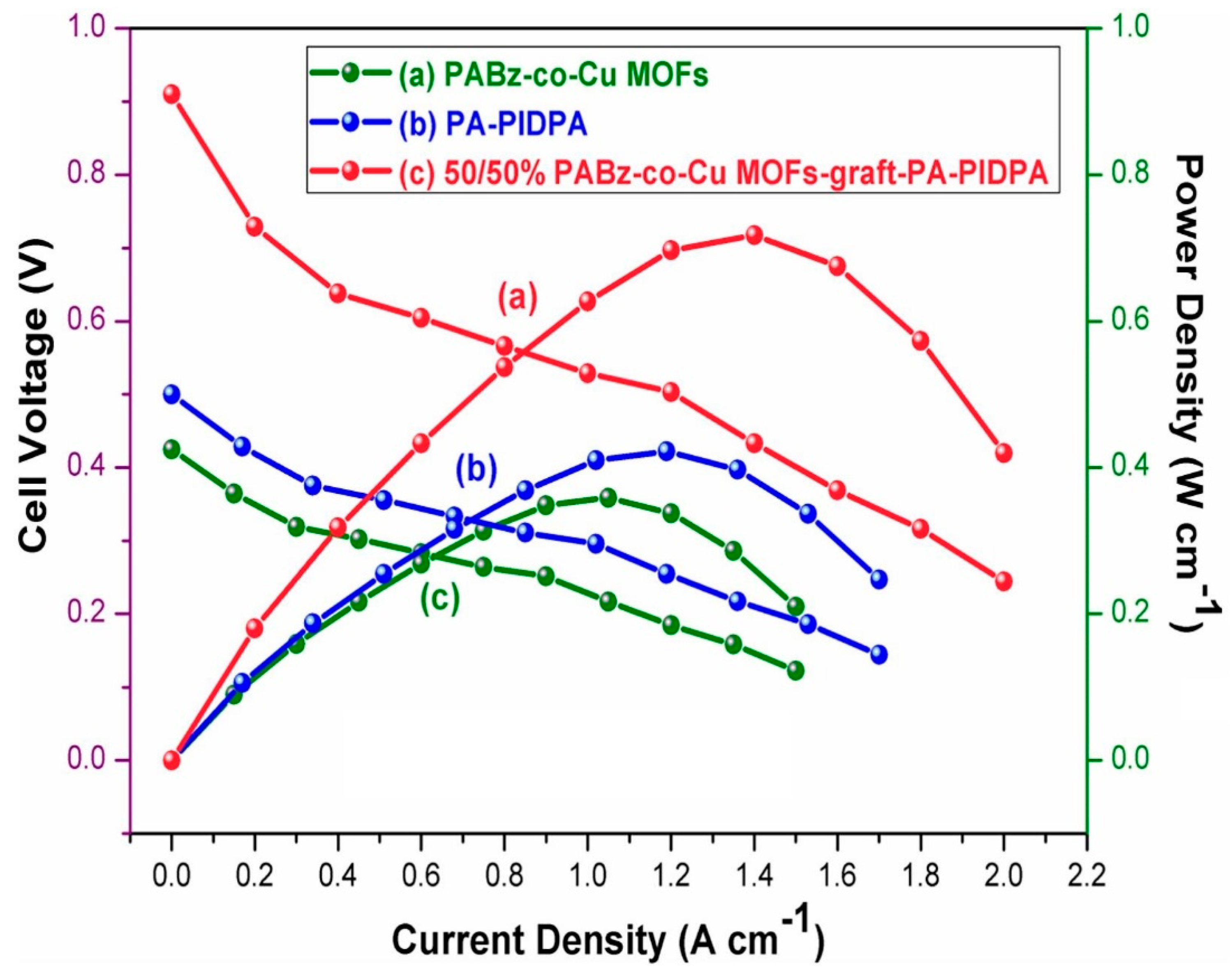
| PABz-co-Cu MOFs-graft-PIDPA [53] (Bzo from ethylene diamine and 5-sulphosalicylic acid) | MOF-linked benzoxazine membrane | Proton conductivity: 0.0757 S/cm, Capacitance: 387 F/g, Tensile strength: 3.87 MPa | HT-PEMFC & Supercapacitor |
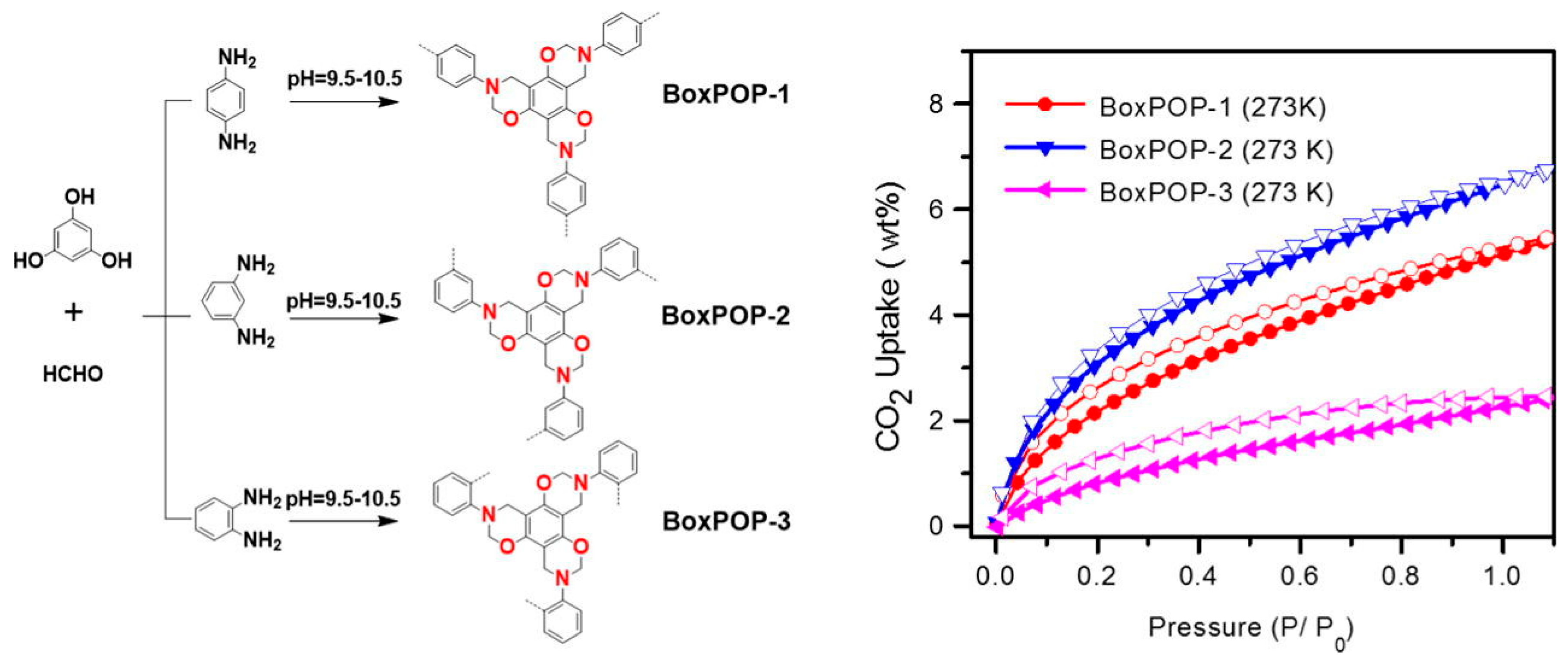
| BoxPOP-1/2 [44] (Bzo from diaminobenzenes and phloroglucinol) | Benzoxazine-linked porous polymers | CO2 uptake: 5.5–6.8 wt.%, Surface area: 225–231 m2/g, Qst: 27.8–29.8 kJ/mol | CO2 Capture |
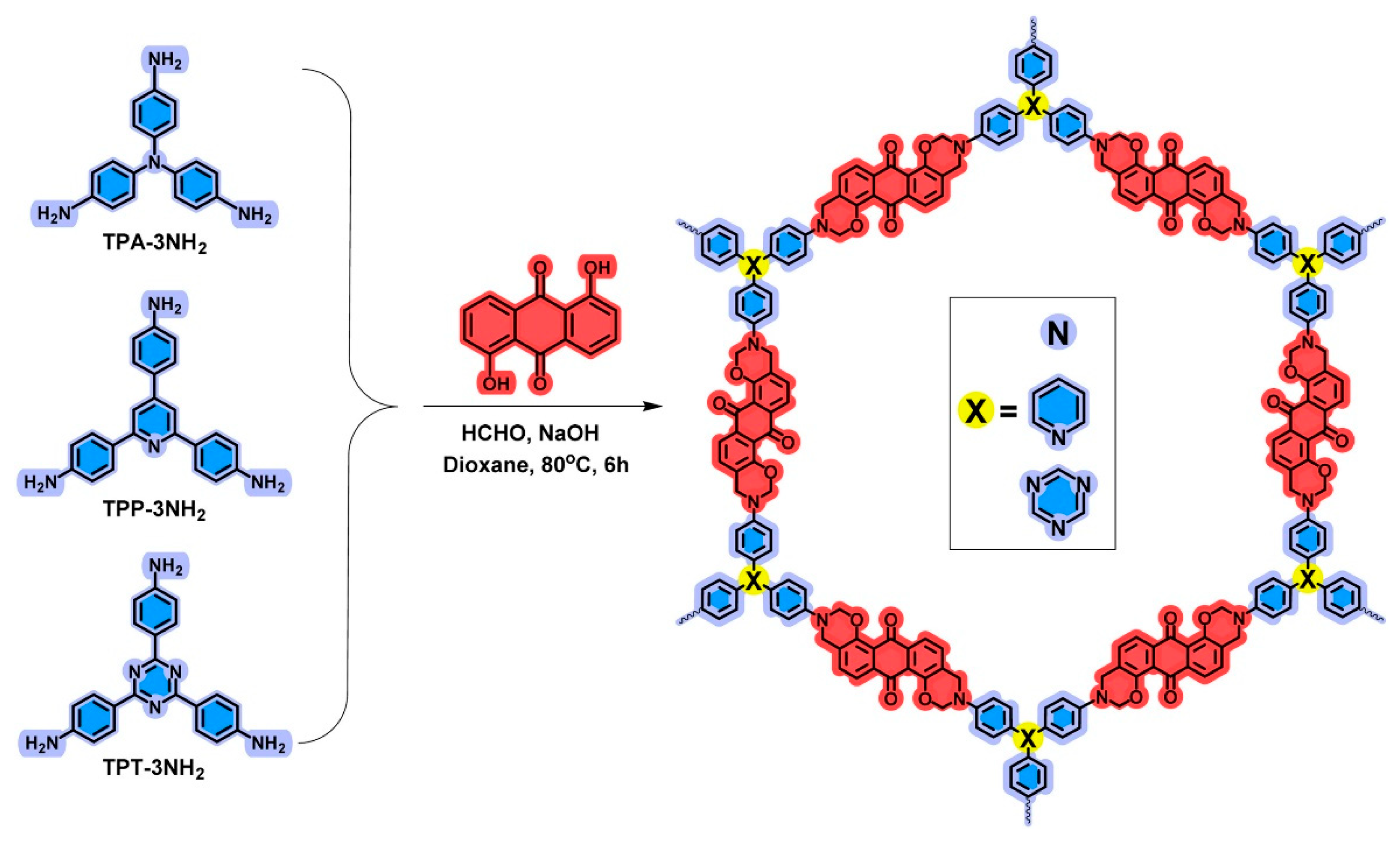
| An-TPA POP [27] (Bzo from 1,5-dihydroxyanthraquinone and triamines) | Benzoxazine with tris(4-aminophenyl) amine | Surface area: 26.51 m2/g, Capacitance: 117.7 F/g | Supercapacitor |
| PUBZ Membrane (40%) [79] (Bzo from sodium 4-hydroxybenzenesulfonate and 2-(2-aminoethoxy)ethanol) | Sulfonated PBZ + HDI | Proton conductivity: 128 mS/cm @ 80 °C, IEC: 1.42 mmol/g, Peak current density: 1.29 A/cm2 @ 1.95 V | PEM Water Electrolysis |
| GO/NC [1] (Bzo from phenolphthalein and urea) | Graphene oxide + PBz-derived N-carbon | Surface area: 1493 m2/g, Conductivity: 11.73 S/cm, Capacitance: 405.6 F/g | Supercapacitor |
| N-CSs from EM-Bz [33] (Bzo from eugenol and melamine) | Eugenol-melamine derived N-carbon sheets | Onset potential: −10 mV (vs. RHE), Tafel slope: 45 mV/dec | HER Electrocatalyst |
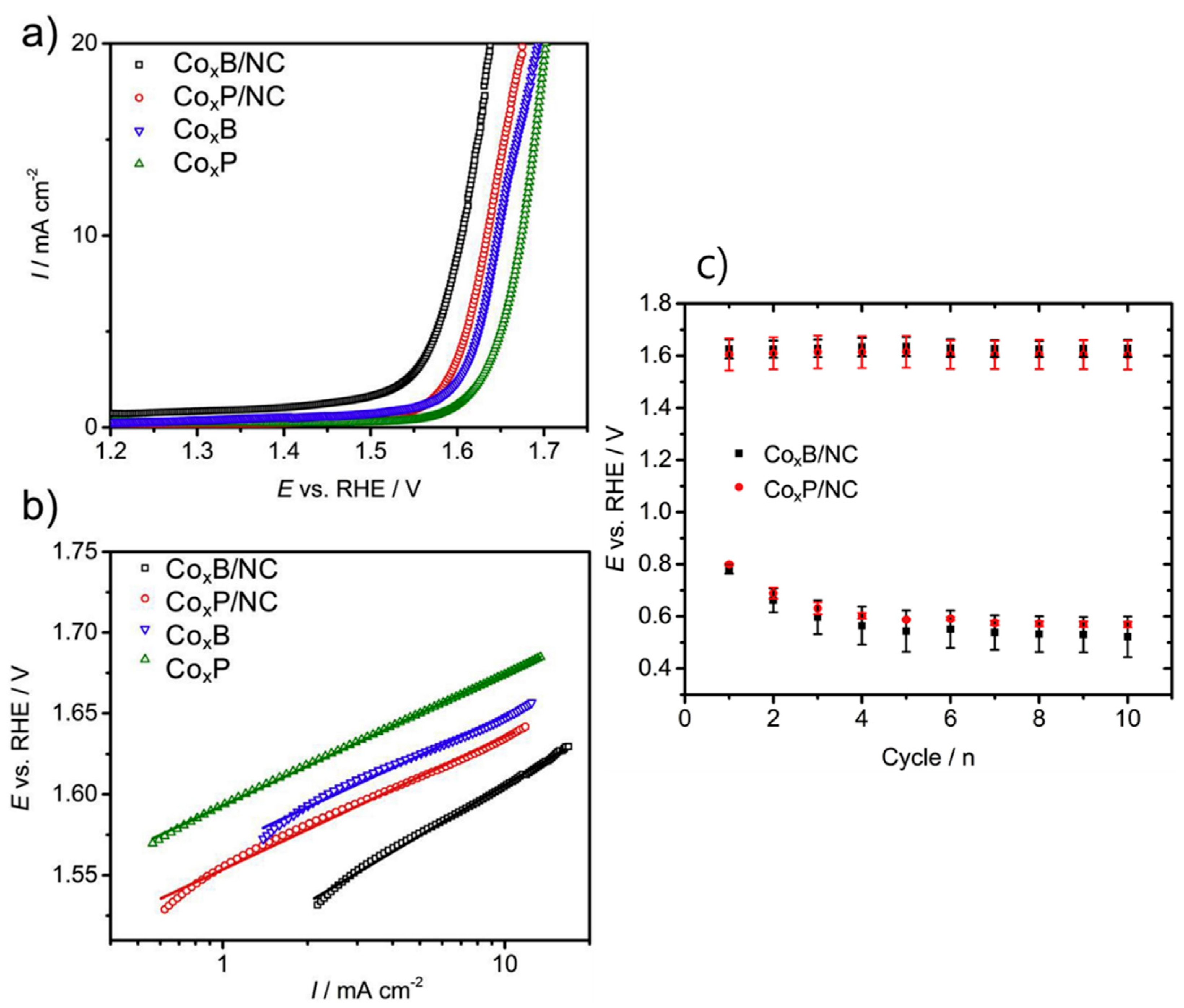
| CoₓB/NC, CoₓP/NC [15] (Bzo from bisphenol A and tetraethylenepentaamine) | Co-metalloids in PBz-derived N-doped carbon | Round-trip voltage: 0.81 V, Good bifunctional stability | ORR/OER Electrocatalyst |
| Co/CoxFey/NC-NH3 [88] (Bzo from bisphenol A and tetraethylenepentaamine) | CoFe LDH + BA-tepa PBz | Onset potential (ORR): 0.82 V, OER: 1.59 V, Round-trip: 0.77 V | ORR/OER Catalyst |
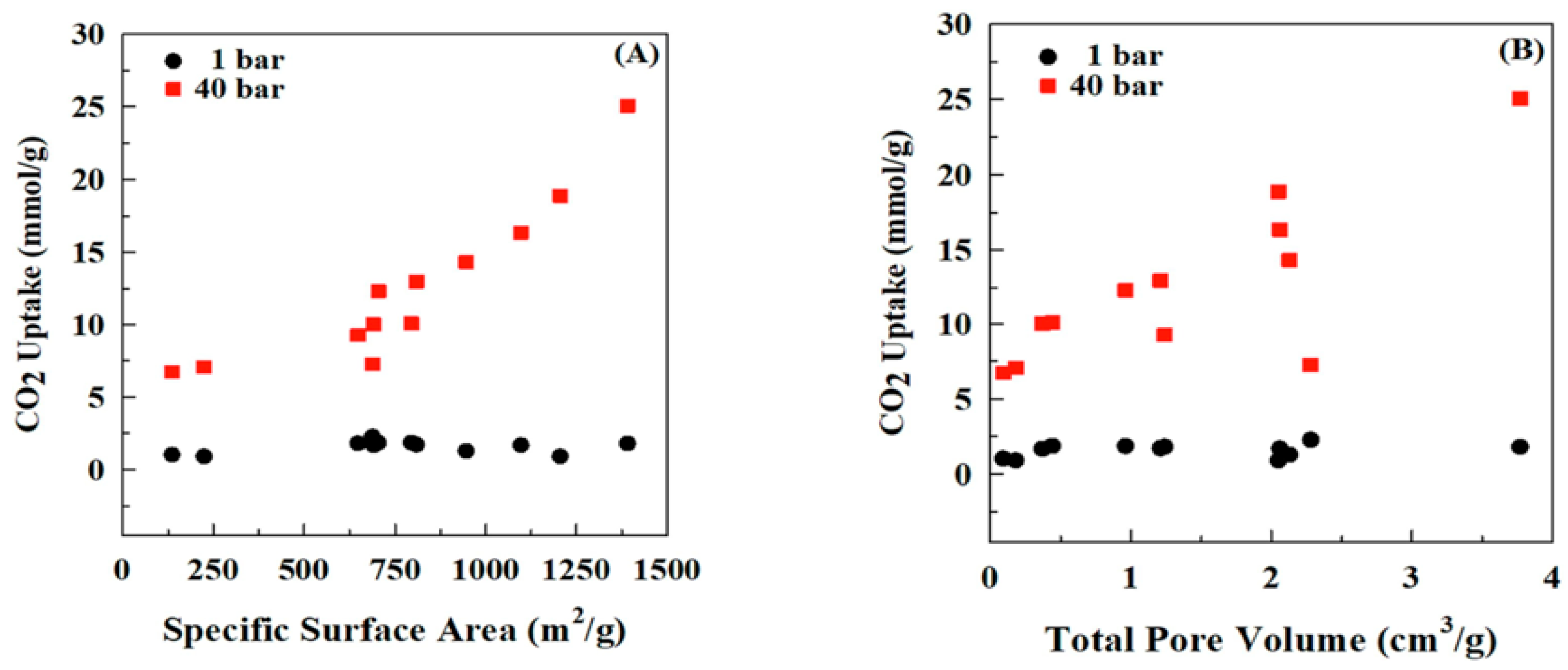
| AC40%Si-800 [46] (Bzo from bisphenol A and Tetraethylenepentaamine) | PBz-derived porous carbon with silica template | CO2 uptake: 25.07 mmol/g @ 40 bar, 30 °C | CO2 Capture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrafali, S.P.; Periyasamy, T.; Bari, G.A.K.M.R.; Lee, J. Properties of Polybenzoxazine-Based Conducting Materials in Energy-Related Applications. Polymers 2025, 17, 2194. https://doi.org/10.3390/polym17162194
Asrafali SP, Periyasamy T, Bari GAKMR, Lee J. Properties of Polybenzoxazine-Based Conducting Materials in Energy-Related Applications. Polymers. 2025; 17(16):2194. https://doi.org/10.3390/polym17162194
Chicago/Turabian StyleAsrafali, Shakila Parveen, Thirukumaran Periyasamy, Gazi A. K. M. Rafiqul Bari, and Jaewoong Lee. 2025. "Properties of Polybenzoxazine-Based Conducting Materials in Energy-Related Applications" Polymers 17, no. 16: 2194. https://doi.org/10.3390/polym17162194
APA StyleAsrafali, S. P., Periyasamy, T., Bari, G. A. K. M. R., & Lee, J. (2025). Properties of Polybenzoxazine-Based Conducting Materials in Energy-Related Applications. Polymers, 17(16), 2194. https://doi.org/10.3390/polym17162194








