The Use of Non-Degradable Polymer (Polyetheretherketone) in Personalized Orthopedics—Review Article
Abstract
1. Introduction
2. Characteristics of Implants in Orthopedics
2.1. Materials Used in the Production of Orthopedic Implants
2.2. Manufacturing Technologies Used in the Production of Orthopedic Implants
3. Polymers in Medicine
3.1. Classification of Polymers for Medical Applications
3.2. Characteristics of PEEK in Orthopedic Implants
3.2.1. Properties of Polyetheretherketone
3.2.2. Crystallinity of PEEK
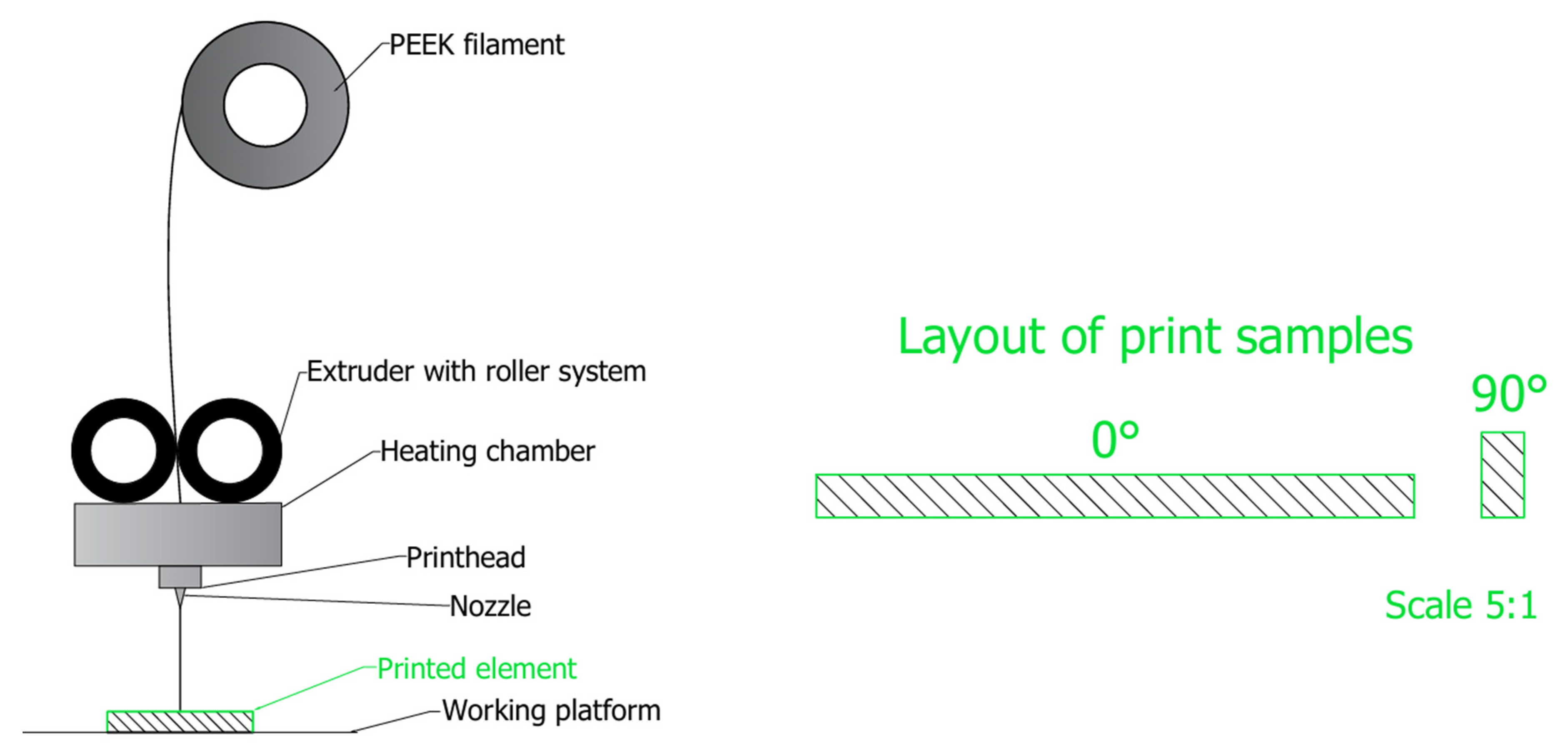
3.2.3. The Technological Process of Creating a Personalized PEEK Implant
3.2.4. Additive Technologies Using PEEK for Personalized Orthopedic Implants
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEEK | Polyetheretherketone |
| FFF | Fused Filament Fabrication |
| PBF | Powder Bed Fusion |
| SLM | Selective Laser Melting |
| DMLS | Direct Metal Laser Sintering |
| EBM | Electron Beam Melting |
| PMMA | Polymethylmethacrylate |
| PAEKs | Polyaryletherketones |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| DICOM | Digital Imaging and Communications in Medicine |
| CAD | Computer-Aided Design |
| STL | Standard Tessellation Language |
| MDR | Medical Device Regulation |
| VOCs | Volatile Organic Compounds |
| SEM | Scanning Electron Microscopy |
| IBE | Ion Beam Etching |
| CNTs | Carbon Nanotubes |
| DSC | Differential Scanning Calorimetry |
| BGs | Bioactive Glasses |
| PLGA | Poly(lactic-co-glycolic acid) |
| PEG | Polyethylene Glycol |
References
- Piszczatowski, S.; Pauk, J.; Oksiut, Z. Aktualne Problemy Inżynierii Biomedycznej; Oficyna Wydawnicza Politechniki Białostockiej: Bialystok, Poland, 2020; pp. 6–7. [Google Scholar]
- Trwanie Życia. Available online: https://stat.gov.pl/obszary-tematyczne/ludnosc/trwanie-zycia/ (accessed on 23 May 2025).
- Węgrzyn, M. Wydłużanie Życia Ludności a Problem Zapewnienia Bezpieczeństwa Zdrowotnego. Stud. Ekon. 2014, 167, 77–86. [Google Scholar]
- Zuzda, J.G.; Latosiewicz, R.; Quintana, M.S. Innowacyjny Program Ćwiczeń Rotacyjnych Jako Profilaktyka i Leczenie Dysfunkcji Stawu Biodrowego; Politechnika Białostocka: Białystok, Poland, 2024; pp. 23–24. [Google Scholar]
- Klimiuk, P.A.; Kuryliszyn-Moskal, A. Choroba zwyrodnieniowa stawów. In Wielka Interna Reumatologia Wydanie II; Medical Tribune Polska: Warsaw, Poland, 2016; pp. 1–8. [Google Scholar]
- Wierzbicka, J.; Brukwicka, I.; Kopański, Z.; Rowiński, J.; Furmanik, F. Selected aspects of human ageing process—Review papers. J. Clin. Healthc. 2017, 82, 2–4. [Google Scholar]
- NIK. Realizacja Świadczeń Zdrowotnych w Zakresie Endoprotezoplastyki Stawu Biodrowego i Kolanowego; NIK: Warsaw, Poland, 2021. [Google Scholar]
- Koniec Stanu Zagrożenia Epidemicznego. Available online: https://www.gov.pl/web/rpp/koniec-stanu-zagrozenia-epidemicznego (accessed on 20 June 2025).
- Czy NFZ Refunduje Endoprotezy? Available online: https://www.kliniki.pl/wiedza/czy-nfz-refunduje-endoprotezy/ (accessed on 29 June 2025).
- Marciniak, J. Biomateriały; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2013. [Google Scholar]
- Żaneta, G. Three-dimensional model for assessing the pore volume of biomaterials intendend for implantation. In Computational Modelling of Biomechanics and Biotribology in the Musculoskeletal System; Politechnika Śląska: Zabrze, Poland, 2022; pp. 8–18. Available online: https://repolis.bg.polsl.pl/dlibra/publication/83977/edition/74877?language=en (accessed on 4 August 2025).
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; Ribeiro da Silva, L.R.; Lawrence, A.A. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Chevalier, J.; Pezzotti, G. Ceramics and ceramic coatings in orthopaedics. J. Eur. Ceram. Soc. 2025, 35, 4327–4339. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 1–3. [Google Scholar] [CrossRef]
- ISO 5832-1:2024; Implants for Surgery—Metallic Materials—Part 1: Wrought Stainless Steel. ISO: Geneva, Switzerland, 2024.
- Stal Nierdzewna 316LVM. Available online: https://pl.china-stainless-steels.com/stainless-steel-plate/stainless-steel-316lvm.html (accessed on 29 June 2025).
- Świeczko-Żurek, B. Biomateriały; Politechnika Gdańska: Gdańsk, Poland, 2009; pp. 81–90. [Google Scholar]
- ISO 5832-3:2021; Implants for Surgery—Metallic Materials, Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy. ISO: Geneva, Switzerland, 2021.
- ISO 5832-11:2024; Implants for Surgery—Metallic Materials Part 11: Wrought Titanium 6-Aluminium 7-Niobium Alloy. ISO: Geneva, Switzerland, 2024.
- ASTM F1713-08:2021; Standard Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications (UNS R58130). ASTM: West Conshohocken, PA, USA, 2021.
- Shaikh, M.; Kahwash, F.; Lu, Z.; Alkhreisat, M.; Mohammad, A.; Shyha, I. Revolutionising orthopaedic implants—A comprehensive review on metal 3D printing with materials, design strategies, manufacturing technologies, and post-process machining advancements. Int. J. Adv. Manuf. Technol. 2024, 134, 1043–1076. [Google Scholar] [CrossRef]
- Wojnarowska, W.; Sandecki, K. Polieteroeteroketon (PEEK) w zastosowaniach medycznych. In Badania i Rozwój Młodych Naukowców w Polsce Nauki techniczne i inżynieryjne Część IX MN; Młodzi Naukowcy: Poznań, Poland, 2018; pp. 120–121. [Google Scholar]
- Revision Total Hip Replacement. Available online: https://orthoinfo.aaos.org/en/treatment/revision-total-hip-replacement/ (accessed on 30 June 2025).
- Zasińska, K.; Seramak, T.; Łubiński, J.I. Comparison of the abrasion resistance of the selected biomaterials for friction components in orthopedic endoprostheses. Tribologia 2015, 264, 190–191. [Google Scholar]
- Tardelli, J.D.C.; Bolfarini, C.; Cândido Dos Reis, A. Comparative analysis of corrosion resistance between beta titanium and Ti-6Al-4V alloys: A systematic review. J. Trace Elem. Med. Biol. 2020, 62, 126618. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Moretti, B.; Pesce, V.; MacCagnano, G.; Vicenti, G.; Lovreglio, P.; Soleo, L.; Apostoli, P. Peripheral neuropathy after hip replacement failure: Is vanadium the culprit? Lancet 2012, 379, 1676. [Google Scholar] [CrossRef] [PubMed]
- ISO 5832-12:2019; Implants for Surgery—Metallic Materials—Part 12: Wrought Cobalt-Chromium Molybdenum Alloy. ISO: Geneva, Switzerland, 2019.
- Cnc Machining Cobalt Chrome Hardness Alloy Valve Parts Corrosion Resistance. Available online: https://polish.cobaltchromealloy.com/sale-9568965-cnc-machining-cobalt-chrome-hardness-alloy-valve-parts-corrosion-resistance.html (accessed on 20 June 2025).
- Tytan R56400. Available online: https://emetal.eu/tytan/tytan_grade_5_R56400_Ti-6Al-4V_3.7165/ (accessed on 20 June 2025).
- Rościszewska, M. Manufacturing and Modification of Porous Titanium Structures for Implants; Politechnika Gdańska: Gdańsk, Poland, 2023; pp. 28–30. [Google Scholar]
- Titane Ti 13Nb 13Zr FT 00—Version 0. Available online: https://acnis-titanium.com/wp-content/uploads/2020/04/UK-Ti-13Nb-13Zr_-FT00.pdf (accessed on 20 June 2025).
- Zasińska, K.; Piątkowska, A. The Evaluation of the abrasive wear of the Ti13Nb13Zr alloy implanted by nitrogen ions for friction components of the hip joint endoprostheses. Tribologia 2015, 264, 178–179. [Google Scholar]
- Zawadzki, P. A Method of Precise Shaping of Bone Surfaces; Politechnika Poznańska: Poznań, Poland, 2023; pp. 21–23. [Google Scholar]
- Pręty Tytanowe. Available online: https://wolften.pl/pl/prety-tytanowe/ (accessed on 24 May 2025).
- Tuli, N.T.; Khatun, S.; Rashid, A.B. Unlocking the future of precision manufacturing: A comprehensive exploration of 3D printing with fiber-reinforced composites in aerospace, automotive, medical, and consumer industries. Heliyon 2024, 10, e27328. [Google Scholar] [CrossRef]
- Singh, H.N.; Agrawal, S.; Kuthe, A.M. Design of customized implants and 3D printing of symmetric and asymmetric cranial cavities. J. Mech. Behav. Biomed. Mater. 2023, 146, 106061. [Google Scholar] [CrossRef]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.-H.; Ramesh, S.; Harun, W.S.W.; Kadirgama, K. A novel design, analysis and 3D printing of Ti-6Al-4V alloy bio-inspired porous femoral stem. J. Mater. Sci. Mater. Med. 2020, 31, 2–7. [Google Scholar] [CrossRef]
- Fashanu, F.F.; Marcellin-Little, D.J.; Linke, B.S. Review of surface finishing of additively manufactured metal implants. In Proceedings of the ASME 2020 15th International Manufacturing Science and Engineering Conference MSEC2020, Online, 3 September 2020; pp. 2–8. [Google Scholar] [CrossRef]
- Orłowska, A.; Szewczenko, J.; Kajzer, W.; Goldsztajn, K.; Basiaga, M. Study of the Effect of Anodic Oxidation on the Corrosion Properties of the Ti6Al4V Implant Produced from SLM. J. Funct. Biomater. 2023, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, A. Funkcjonalizacja Powierzchni Wysokoporowatego Implantu Międzykręgowego Wytworzonego Metodą Przyrostową; Politechnika Śląska: Politechnika Śląska, Poland, 2024; pp. 31–36. [Google Scholar]
- Kajzer, W.; Wielgus, G.; Kajzer, A. Mechanical and Physicochemical Properties of Ti6Al4V Alloy After Plastic Working and 3D Printing Intended for Orthopedics Implants. Appl. Sci. 2024, 14, 11181. [Google Scholar] [CrossRef]
- Kajzer, A.; Wielgus, G.; Drobina, K.; Żurawska, A.; Kajzer, W. The Influence of Heat and Surface Treatment on the Functional Properties of Ti6Al4V Alloy Samples Obtained by Additive Technology for Applications in Personalized Implantology. Appl. Sci. 2025, 15, 8311. [Google Scholar] [CrossRef]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- Nelson, K.; Kelly, C.N.; Gall, K. Effect of stress state on the mechanical behavior of 3D printed porous Ti6Al4V scaffolds produced by laser powder bed fusion. Mater. Sci. Eng. 2022, 286, 116013. [Google Scholar] [CrossRef]
- Balasubramainian, N.K.; Kothandaraman, L.; Sathish, T.; Giri, J.; Ammarullah, M.I. Optimization of process parameters to minimize circularity error and surface roughness in fused deposition modelling (FDM) using Taguchi method for biomedical implant fabrication. Adv. Manuf. Polym. Compos. Sci. 2024, 10, 2406156. [Google Scholar] [CrossRef]
- Karta Charakterystyki Materiału. Available online: https://zatorski.pl/wp-content/uploads/2018/02/PEEK.pdf (accessed on 22 June 2025).
- ISO 23153-1; Plastics—Polyetheretherketone (PEEK) Moulding and Extrusion Materials, Part 1: Designation System and Basis for Specifications. ISO: Geneva, Switzerland, 2020.
- Sharma, N.; Honigmann, P.; Cao, S.; Thieringer, F.M. Dimensional characteristics of FDM 3D printed PEEK implant for craniofacial reconstruction. Trans. Addit. Manuf. Meets Med. 2020, 2, 1–2. [Google Scholar]
- Definicja 1: Polimery. Available online: https://epodreczniki.open.agh.edu.pl/handbook/29/module/635/reader (accessed on 27 May 2025).
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Ariffin, M.K.A.M.; Kim, C.L.S.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Technologia FDM/FFF. Available online: https://drukarki3d.pl/technologie/technologia-fdm-fff/ (accessed on 22 June 2025).
- Available online: https://www.sitech3d.pl/artykul/218/tworzywo-sztuczne-polimer-peek-wlasciwosci (accessed on 22 June 2025).
- Ostapczuk, M. Polieteroeteroketon-PEEK; Politechnika Warszawska: Warsaw, Poland, 2024; pp. 1–3. [Google Scholar]
- Polyether Ether Ketone (PEEK Plastic): Properties & Material Guide. Available online: https://www.specialchem.com/plastics/guide/polyetheretherketone-peek-thermoplastic (accessed on 27 May 2025).
- Ortega-Martínez, J.; Farré-Lladós, M.; Cano-Batalla, J.; Cabratosa-Termes, J. Polyetheretherketone (PEEK) as a medical and dental material. A literature review. Med. Res. Arch. 2017, 5, 3–6. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Sun, C.; Li, D. Transfer film effects induced by 3D-printed polyether-ether-ketone with excellent tribological properties for joint prosthesis. Bio-Des. Manuf. 2024, 7, 44–49. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K.; Sinai, M. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Polieteroeteroketon Peek. Available online: https://plastics.pl/produkty-katalogi/polprodukty-aluminium-tworzywa/polieteroeteroketon-peek (accessed on 27 May 2025).
- Moharil, S.; Reche, A.; Durge, K. Polyetheretherketone (PEEK) as a Biomaterial: An Overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef]
- Zhen, H.; Zhao, B.; Quan, L.; Fu, J. Effect of 3D Printing Process Parameters and Heat Treatment Conditions on the Mechanical Properties and Microstructure of PEEK Parts. Polymers 2023, 15, 2209. [Google Scholar] [CrossRef]
- Post-Processing of 3D Prints. Available online: https://3dgence.com/3dnews/post-processing-of-3d-prints (accessed on 31 July 2025).
- European Parliament. Regulation (EU) 2017/745 of the European Parliament and of the Council, on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. Off. J. Eur. Union 2017, 117, 1–175. [Google Scholar]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef]
- Szymaniec, O.; Kowalski, G.; Hurny, B.; Kwaczyński, K.; Półtorak, Ł. 3D printing—An electrochemist point of view. Chem. News 2024, 78, 5–8. [Google Scholar] [CrossRef]
- Adarsh, S.H.; Nagamadhu, M. Effect of Printing Parameters on Mechanical Properties and Warpage of 3D-Printed PEEK/CF-PEEK Composites Using Multi-Objective Optimization Techniqu. J. Compos. Sci. 2025, 9, 208. [Google Scholar] [CrossRef]
- Jak Zagwarantować Precyzję Wymiarową Drukując PEEK? Available online: https://vshaper.com/pl/blog-pl/jak-zagwarantowac-precyzje-wymiarowa-drukujac-peek (accessed on 30 May 2025).
- Wang, Y.; Müller, W.-D.; Rumjahn, A.; Schmidt, F.; Schwitalla, A.D. Mechanical properties of fused filament fabricated PEEK for biomedical applications depending on additive manufacturing parameters, Journal of the Mechanical Behavior of Biomedical. Materials 2021, 115, 2–4. [Google Scholar] [CrossRef]
- Sun, C.; Kang, J.; Yang, C.; Polg, J.; Su, Y.; Dong, E.; Liu, Y.; Yao, S.; Shi, C.; Pang, H.; et al. Additive manufactured polyether-ether-ketone implants for orthopaedic applications: A narrative review. Biomater Transl. 2022, 3, 116–133. [Google Scholar] [PubMed]
- Palmara, G.; Frascella, F.; Chiad`o, A.; Roppolo, I.; Chiappone, A. Functional 3D printing: Approaches and bioapplications. Biosens. Bioelectron. 2021, 112849, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Golbang, A.; Jindal, S.; Dixon, D.; McIlhagger, A.; Harkin-Jones, A.; Crawford, D.; Mancuso, E. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J. Mech. Behav. Biomed. Mater. 2021, 121, 104601. [Google Scholar] [CrossRef]
- Kang, J.; Zheng, J.; Hui, Y.; Li, D. Mechanical Properties of 3D-Printed PEEK/HA Composite Filaments. Polymers 2022, 4293, 4293. [Google Scholar] [CrossRef]
- Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials 2015, 8, 3815–3830. [Google Scholar] [CrossRef]
- Khallaf, R.M.; Emam, A.E.; Mostafa, A.A.; Nassif, M.S.; Hussein, T.S. Strength and bioactivity of PEEK composites containing multiwalled carbon nanotubes and bioactive glass. J. Mech. Behav. Biomed. Mater. 2023, 144, 105964. [Google Scholar] [CrossRef]
- Senra, M.R.; Marques, M.F.V.; Monteiro, S.N. Poly (Ether-Ether-Ketone) for Biomedical Applications: From Enhancing Bioactivity to Reinforced-Bioactive Composites—An Overview. Polymers 2023, 15, 373. [Google Scholar] [CrossRef]
- Primc, G. Strategies for Improved Wettability of Polyetheretherketone (PEEK) Polymers by Non-Equilibrium Plasma Treatment. Polymers 2022, 14, 5319. [Google Scholar] [CrossRef] [PubMed]
- Małek, A.; Konefał-Góral, J.; Kluska, S.; Tkacz-Śmiech, K.; Jonas, S.; Wierzchoń, T. Synthesis and properties of silicon carbonitride layers on polyetheretherketone. Eng. Biomater. 2013, 119, 1–5. [Google Scholar]
- Zrozumienie Technologii Suchego Trawienia w Przemyśle Półprzewodników. Available online: https://pl.semicorex.com/news-show-5219.html (accessed on 29 May 2025).
- Lisoń, J.; Taratura, A.; Paszenda, Z.; Szindler, M.; Basiaga, M. Perspectives in Prevention of Biofilm for Medical Applications. Coatings 2022, 12, 197. [Google Scholar] [CrossRef]
- Tekin, S.; Değer, Y.; Demirci, F. Evaluation of the Use of PEEK Material in Implant-Supported Fixed Restorations by Finite Element Analysis. Niger. J. Clin. Pract. 2019, 22, 1252–1258. [Google Scholar] [CrossRef]
- Mbogoria, M.; Vaisha, A.; Vaishyaa, R.; Haleemb, A.; Javaid, M. Poly-Ether-Ether-Ketone (PEEK) in orthopaedic practice- A current concept review. J. Orthop. Rep. 2022, 1, 2–9. [Google Scholar] [CrossRef]
- Qi, D.; Wang, N.; Cheng, Y.; Zhao, Y.; Meng, L.; Yue, X.; She, P.; Gao, H. Application of Porous Polyetheretherketone Scaffold/Vancomycin-Loaded Thermosensitive Hydrogel Composites for Antibacterial Therapy in Bone Repair. Macromol. Biosci. 2022, 22, e2200114. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.; Zhu, W.; Weng, X. Polyetheretherketone development in bone tissue engineering and orthopedic surgery. Front Bioeng. Biotechnol. 2023, 11, 1207277. [Google Scholar] [CrossRef] [PubMed]
- Titanium Ti-6Al-4V (Grade 5), Annealed. Available online: https://asm.matweb.com/search/specificmaterial.asp?bassnum=mtp641 (accessed on 31 July 2025).
- PEEK—Polyetheretherketone. Available online: https://www.biesterfeld.com/pl/pl/product/ketaspire-peek (accessed on 31 July 2025).
- Accelerated Ageing and Characterisation of UHMWPE Used in Orthopaedic Implants. Available online: https://www.azom.com/properties.aspx?ArticleID=909 (accessed on 31 July 2025).
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
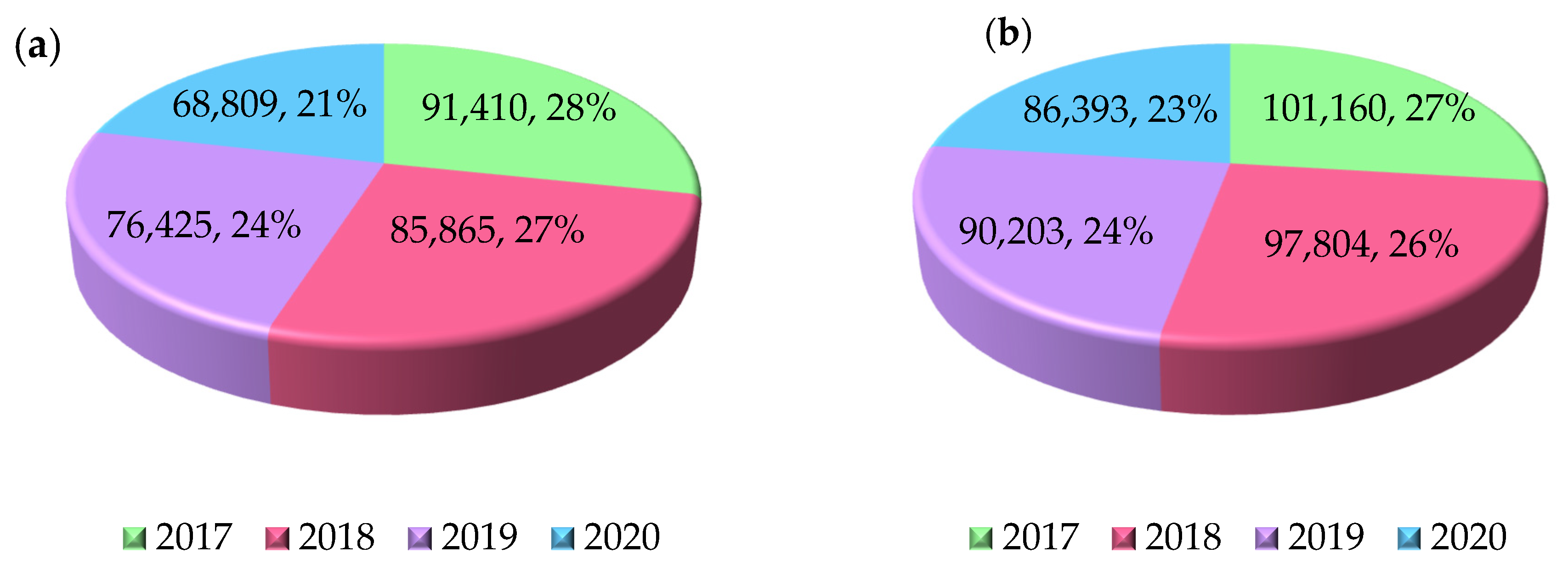
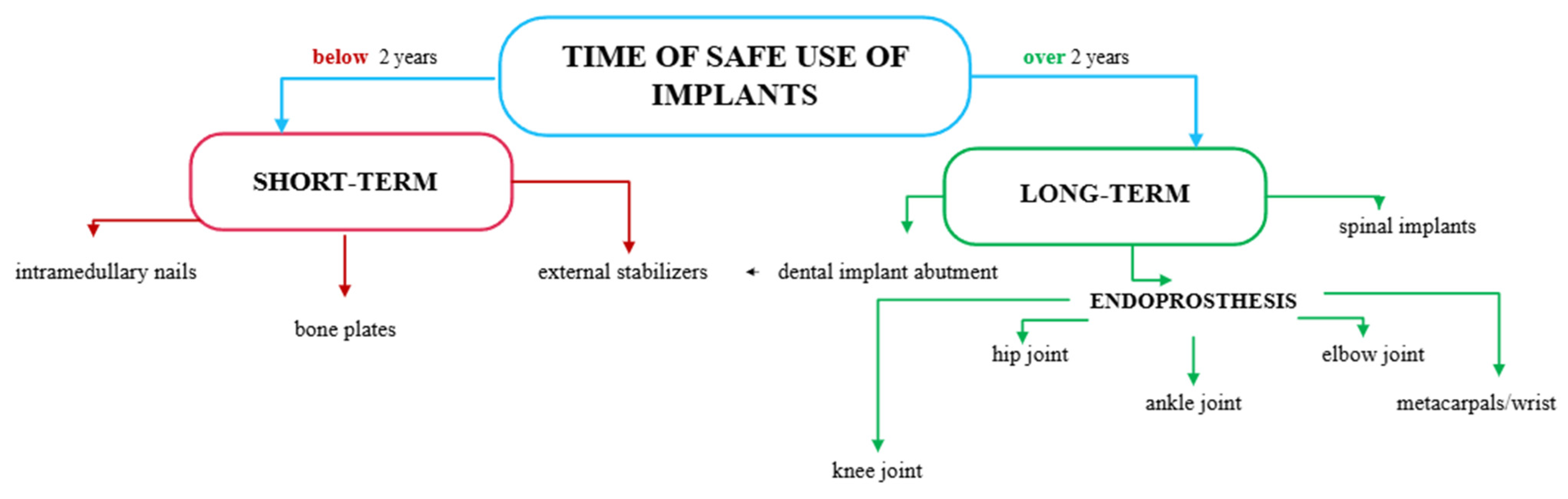
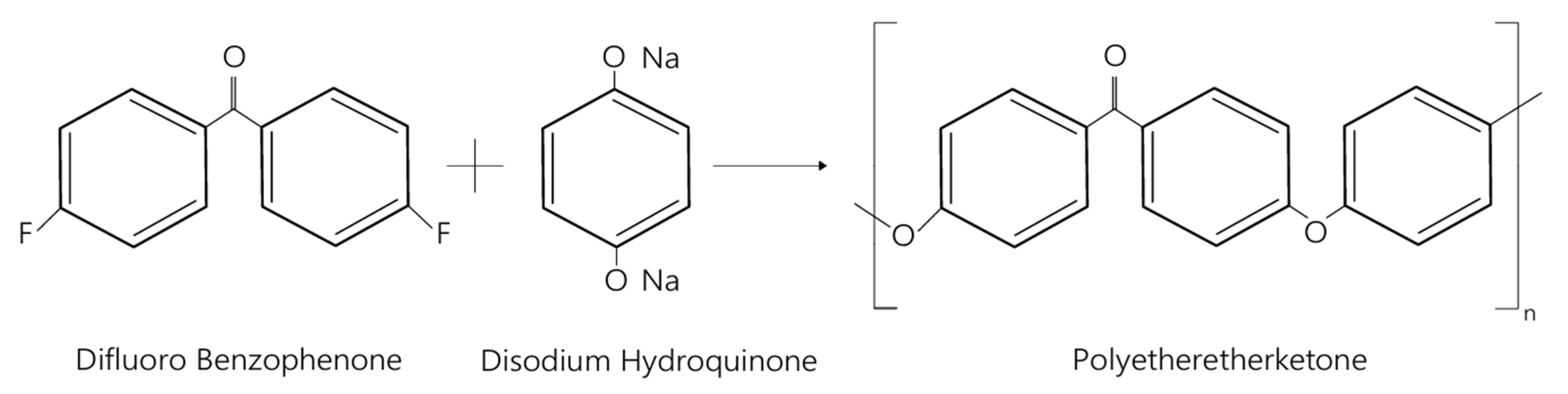
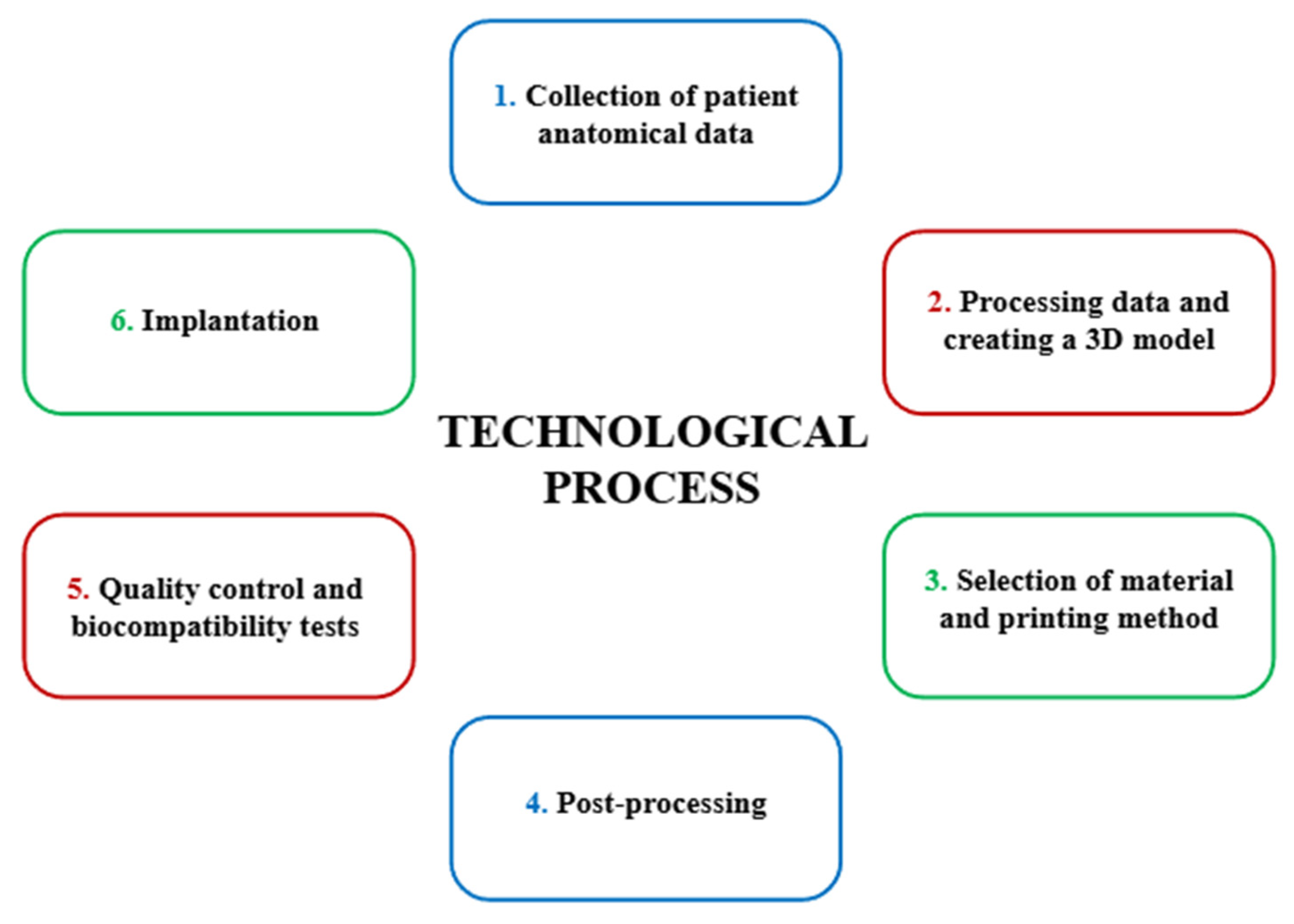
| Material | Rm [MPa] | Rp0,2 [MPa] | A [%] | E [MPa] | ρ [g/cm3] |
|---|---|---|---|---|---|
| Cr-Ni-Mo | 860 | 690 | 10 | 193,000 | 8.0 |
| CoCrMo | 1000 | 700 | 12 | 227,000 | 8.4 |
| Ti6Al4V | 860 | 795 | 10 | 112,000 | 4.4 |
| Ti6Al7Nb | 900 | 800 | 10 | 110,000 | 4.5 |
| Ti13Nb13Zr | 973 | 836 | 10 | 79,000 | 4.9 |
| Cortical bone | 200 | * | 1.4 | 17,700 | 1.6–2.0 |
| Rm [MPa] | Rp0,2 [MPa] | A [%] | E [MPa] | ρ [g/cm3] |
|---|---|---|---|---|
| 116 | 116 | 15 | 4044 | 1.3 |
| Strength Tests | Tensile [MPa] | Bending [MPa] | |
|---|---|---|---|
| Orientation of Test Samples | 0° | 67.14 | 122.53 |
| 90° | 66.97 | 156.84 | |
| Angle Fills | ±10° | 69.35 | 144.16 |
| ±30° lub ±20° | 56.65 | 122.53 | |
| Extrusion Speed | 0.8× | 31.78 | 56.48 |
| 1× 1.2× | 69.35 | 160.88 | |
| Strength Tests | Tensile [MPa] | Bending [MPa] | |
|---|---|---|---|
| Temperature | 150 °C | 70.84 | 157.37 |
| 300° | 74.24 | 167.13 | |
| Time | 30 min | 74.24 | 167.13 |
| 2 h | 77.26 | 172.98 | |
| Parameters | ||
|---|---|---|
| Temperature [°C] | Time [h] | Crystallinity [%] |
| 150 | 0.5 | 18.03 |
| 1 | 22.98 | |
| 2 | 23.77 | |
| Material | Young’s Modulus [MPa] | Fatigue Resistance [MPa] | Osseointegration | Clinical Performance |
|---|---|---|---|---|
| Ti6Al4V | 112,000 | 510 | very good | orthopedic implants |
| PEEK | 4044 | 121 | requires surface modification to improve osseointegration | hip and knee implants |
| UHMWPE | 790 | * | hip and knee implants | |
| Ceramics (ZrO2) | 200,000 | * | it has anticatarrhal properties and supports the remodeling process | hip joint socket |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielgus, G.; Kajzer, W.; Kajzer, A. The Use of Non-Degradable Polymer (Polyetheretherketone) in Personalized Orthopedics—Review Article. Polymers 2025, 17, 2158. https://doi.org/10.3390/polym17152158
Wielgus G, Kajzer W, Kajzer A. The Use of Non-Degradable Polymer (Polyetheretherketone) in Personalized Orthopedics—Review Article. Polymers. 2025; 17(15):2158. https://doi.org/10.3390/polym17152158
Chicago/Turabian StyleWielgus, Gabriela, Wojciech Kajzer, and Anita Kajzer. 2025. "The Use of Non-Degradable Polymer (Polyetheretherketone) in Personalized Orthopedics—Review Article" Polymers 17, no. 15: 2158. https://doi.org/10.3390/polym17152158
APA StyleWielgus, G., Kajzer, W., & Kajzer, A. (2025). The Use of Non-Degradable Polymer (Polyetheretherketone) in Personalized Orthopedics—Review Article. Polymers, 17(15), 2158. https://doi.org/10.3390/polym17152158






