Recent Progress on the Research of 3D Printing in Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Fundamentals of 3D Printing Technology
2.1. Overview of 3D Printing Technology
2.1.1. Direct Ink Writing (DIW)
2.1.2. Fused Filament Fabrication (FFF)
2.1.3. Stereolithography (SLA)
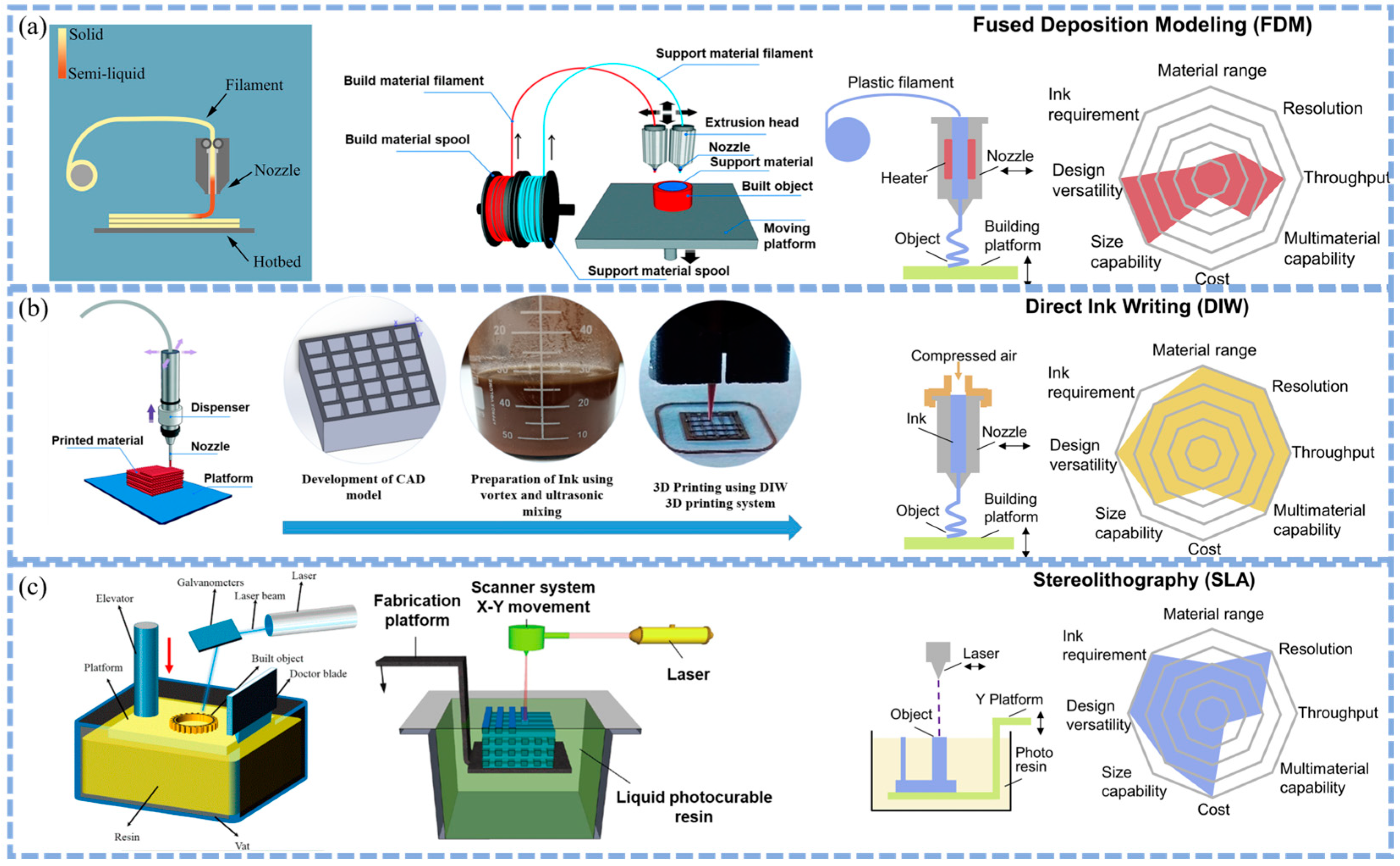
2.2. Comparison of the Advantages and Disadvantages of Different 3D Printing Technologies (DIW vs. FFF vs. SLA) for Battery Components
3. Application of 3D Printing Technology in AZIBs
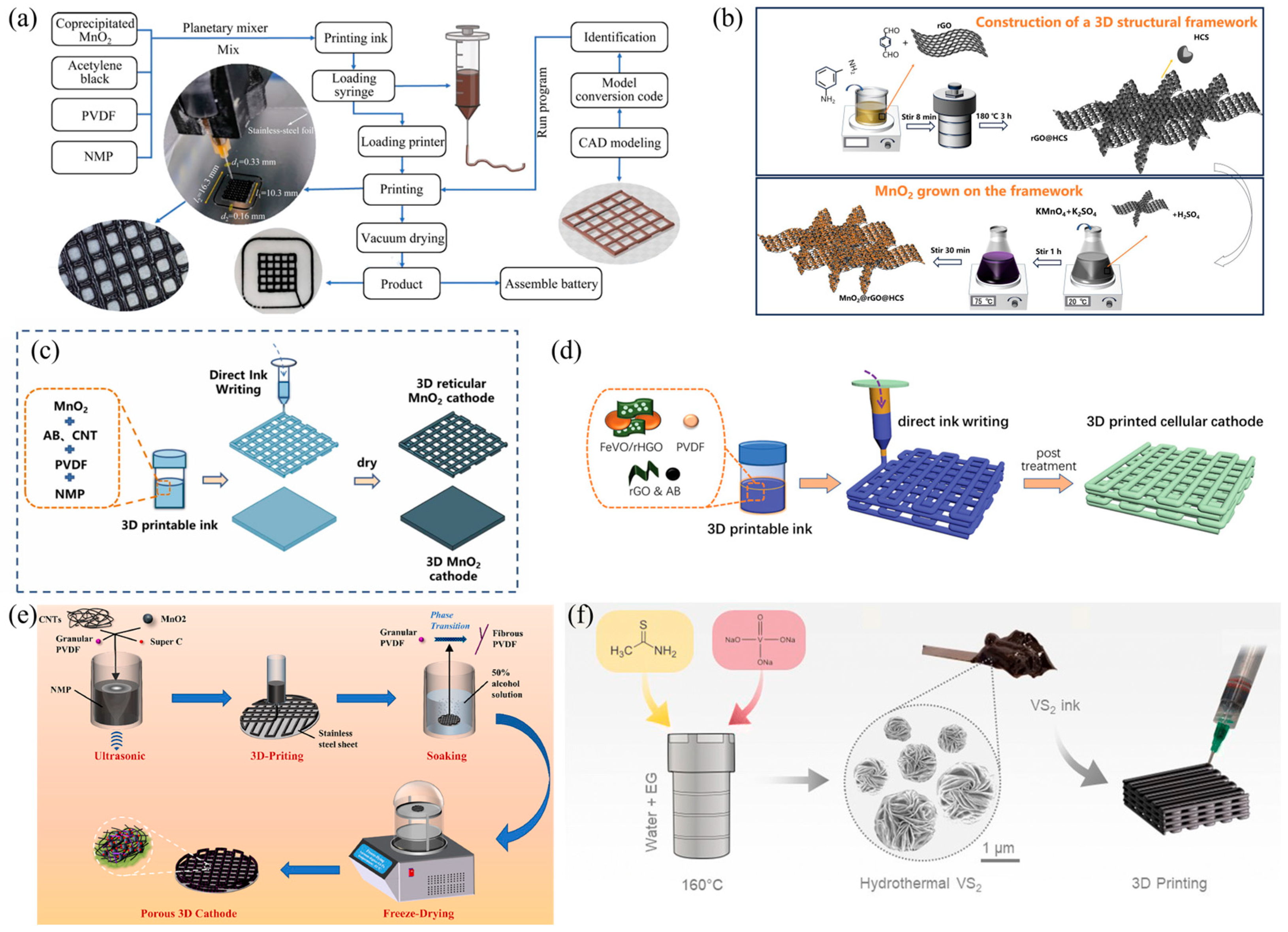
3.1. Cathode Design and Fabrication
3.1.1. Cathode Materials
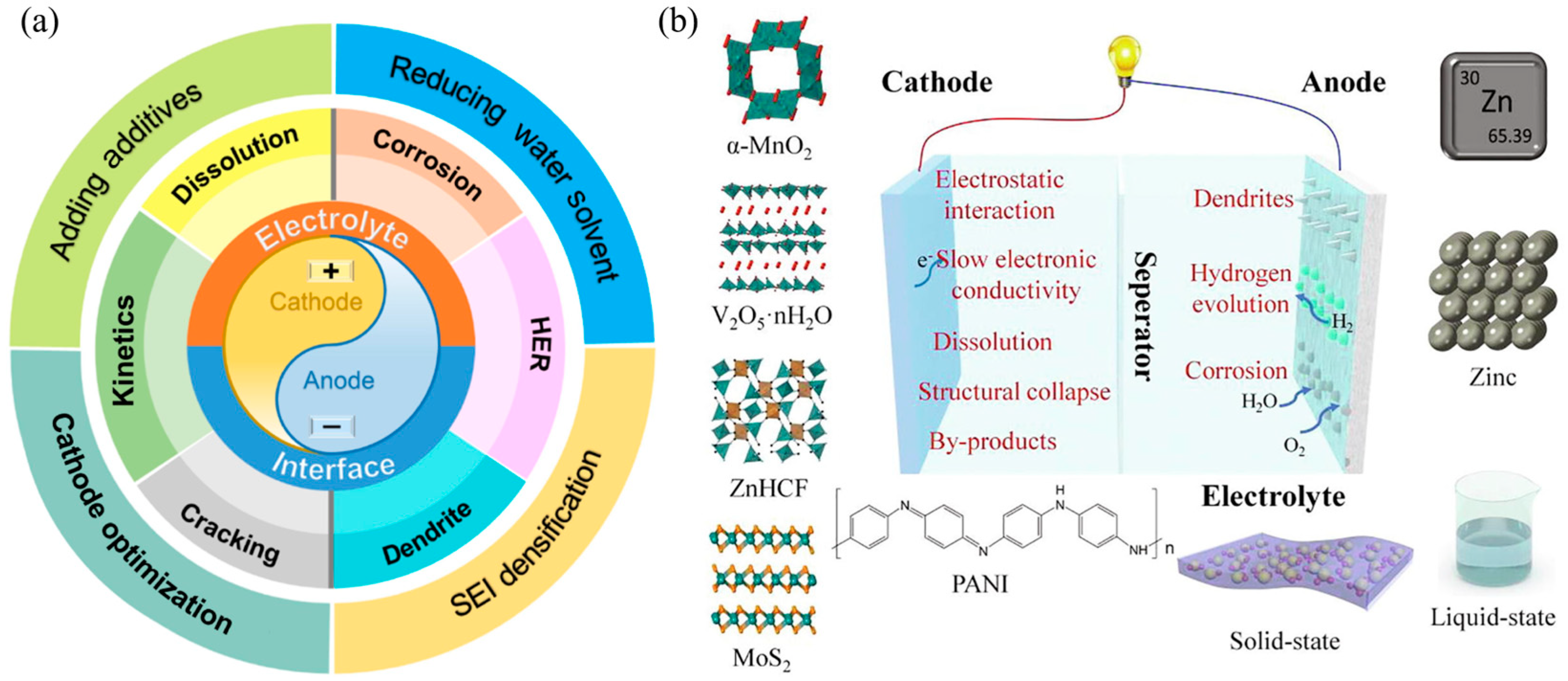
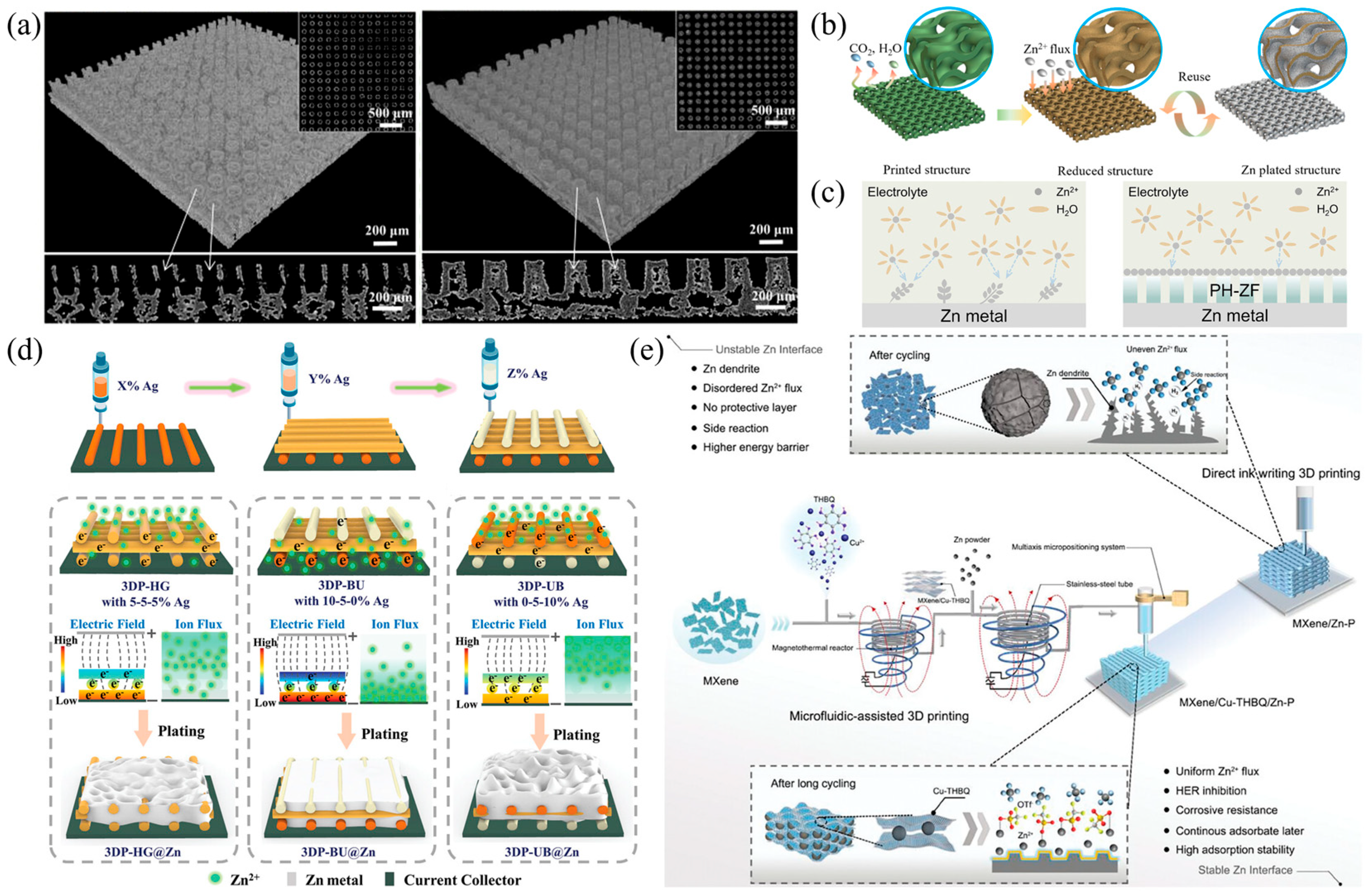
3.1.2. Anode Materials
3.2. Electrolyte and Separator
3.3. Full-Cell Packaging
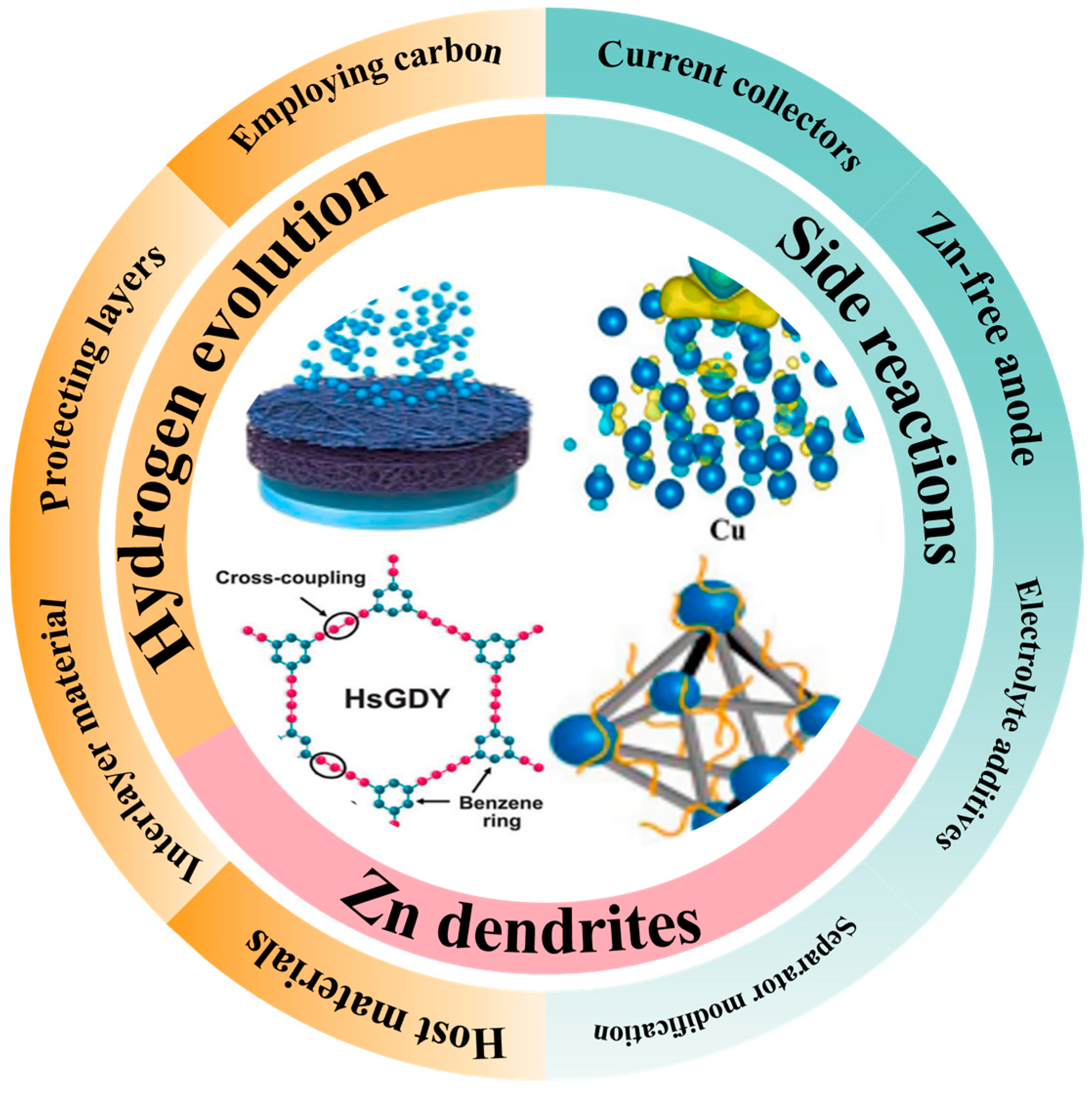
4. Challenges of 3D Printing Technology in AZIBs
4.1. Challenges in Terms of Materials
4.1.1. Development of Inks/Slurries
4.1.2. Inter-Material Compatibility
4.1.3. Material Adaptability and Modification
4.2. Challenges in Terms of Design
4.2.1. Electrode Structure Design
4.2.2. Battery Integration Design
4.2.3. Customized Design
4.3. Challenges in Terms of Applications
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, D.; Lin, Y.; Pan, R.; Li, J.; Zhu, A.; Zhang, T.; Liu, K.; Feng, D.; Liu, K.; Zhou, Y.; et al. Water-Restrained Hydrogel Electrolytes with Repulsion-Driven Cationic Express Pathways for Durable Zinc-Ion Batteries. Nano-Micro Lett. 2025, 17, 193. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Ma, H.; Yang, M.; Dai, Y.; Yu, H.; Hao, Y.; Wang, Z.; Wang, B.; Hu, M.; et al. An Ultra-Stable, High-Energy and Wide-Temperature-Range Aqueous Alkaline Sodium-Ion Battery with the Microporous C4N/rGO Anode. Nano-Micro Lett. 2025, 17, 158. [Google Scholar] [CrossRef]
- Gao, X.; Liu, K.; Su, C.; Zhang, W.; Dai, Y.; Parkin, I.P.; Carmalt, C.J.; He, G. From Bibliometric Analysis: 3D Printing Design Strategies and Battery Applications with a Focus on Zinc-Ion Batteries. SmartMat 2024, 5, e1197. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, L.; Yu, R.; Lu, R.; Li, J.; He, R.; Wu, Y.; Zhang, W.; Hong, X.; Chen, W.; et al. Ultrahigh Stable Methanol Oxidation Enabled by a High Hydroxyl Concentration on Pt Clusters/MXene Interfaces. J. Am. Chem. Soc. 2022, 144, 15529–15538. [Google Scholar] [CrossRef]
- Wang, F.; Tseng, J.; Liu, Z.; Zhang, P.; Wang, G.; Chen, G.; Wu, W.; Yu, M.; Wu, Y.; Feng, X. A Stimulus-Responsive Zinc–Iodine Battery with Smart Overcharge Self-Protection Function. Adv. Mater. 2020, 32, 2000287. [Google Scholar] [CrossRef]
- Chao, D.; Qiao, S.-Z. Toward High-Voltage Aqueous Batteries: Super- or Low-Concentrated Electrolyte? Joule 2020, 4, 1846–1851. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable Nickel–3D Zinc Batteries: An Energy-Dense, Safer Alternative to Lithium-Ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mahandra, H.; Marthi, R.; Ji, X.; Zhao, W.; Chae, S.; Traversy, M.; Li, W.; Yu, F.; Li, L.; et al. An Overview on the Life Cycle of Lithium Iron Phosphate: Synthesis, Modification, Application, and Recycling. Chem. Eng. J. 2024, 485, 149923. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Zong, W.; Yang, C.; Mo, L.; Ouyang, Y.; Guo, H.; Ge, L.; Miao, Y.-E.; Rao, D.; Zhang, J.; Lai, F.; et al. Elucidating Dual-Defect Mechanism in Rhenium Disulfide Nanosheets with Multi-Dimensional Ion Transport Channels for Ultrafast Sodium Storage. Nano Energy 2020, 77, 105189. [Google Scholar] [CrossRef]
- Zanoletti, A.; Carena, E.; Ferrara, C.; Bontempi, E. A Review of Lithium-Ion Battery Recycling: Technologies, Sustainability, and Open Issues. Batteries 2024, 10, 38. [Google Scholar] [CrossRef]
- Lu, X.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.M.; Hinds, G.; Kendrick, E.; et al. 3D Microstructure Design of Lithium-Ion Battery Electrodes Assisted by X-Ray Nano-Computed Tomography and Modelling. Nat. Commun. 2020, 11, 2079. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-Extinguishing Organic Electrolytes for Safe Batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, Q.; Yang, H.; Zhang, J.; Wang, W. The Enormous Potential of Sodium/Potassium-Ion Batteries as the Mainstream Energy Storage Technology for Large-Scale Commercial Applications. Adv. Mater. 2024, 36, 2405989. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Zong, W.; Zhu, X.; Mo, L.; Chao, G.; Fan, W.; Lai, F.; Miao, Y.-E.; Liu, T.; Yu, Y. A Universal Spinning-Coordinating Strategy to Construct Continuous Metal–Nitrogen–Carbon Heterointerface with Boosted Lithium Polysulfides Immobilization for 3D-Printed Li—S Batteries. Adv. Sci. 2022, 9, 2203181. [Google Scholar] [CrossRef] [PubMed]
- Kaliyappan, K.; Or, T.; Deng, Y.-P.; Hu, Y.; Bai, Z.; Chen, Z. Constructing Safe and Durable High-Voltage P2 Layered Cathodes for Sodium Ion Batteries Enabled by Molecular Layer Deposition of Alucone. Adv. Funct. Mater. 2020, 30, 1910251. [Google Scholar] [CrossRef]
- Lv, T.; Peng, Y.; Zhang, G.; Jiang, S.; Yang, Z.; Yang, S.; Pang, H. How About Vanadium-Based Compounds as Cathode Materials for Aqueous Zinc Ion Batteries? Adv. Sci. 2023, 10, 2206907. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Q.; Liu, L.; Li, D.; Xu, Y.; Wang, Y.; Liu, J.; Huang, C.; Xiong, F.; Wang, B.; et al. Stable Zinc Metal Anode with an Ultrathin Carbon Coating for Zinc-Ion Batteries. J. Electroanal. Chem. 2023, 936, 117357. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Muhammed Shafi, P.; Karthik, R.; Dhakal, G.; Kim, S.-H.; Kim, M.; Shim, J.-J. Safe and Extended Operating Voltage Zinc-Ion Battery Engineered by a Gel-Polymer/Ionic-Liquid Electrolyte and Water Molecules Pre-Intercalated V2O5 Cathode. J. Mol. Liq. 2022, 367, 120399. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible Epitaxial Electrodeposition of Metals in Battery Anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Fu, L.; Wu, S.; Tang, Y.; Ji, X.; Wang, H. The Three-Dimensional Dendrite-Free Zinc Anode on a Copper Mesh with a Zinc-Oriented Polyacrylamide Electrolyte Additive. Angew. Chem. Int. Ed. 2019, 58, 15841–15847. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent Progress and Perspectives on Aqueous Zn-Based Rechargeable Batteries with Mild Aqueous Electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Nie, C.; Wang, G.; Wang, D.; Wang, M.; Gao, X.; Bai, Z.; Wang, N.; Yang, J.; Xing, Z.; Dou, S. Recent Progress on Zn Anodes for Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300606. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, H.; Lu, J.; Hou, Y.; Liu, P.; Dong, S.; Pang, H.; Zhang, Y. 3D Printing of MXene-Enhanced Ferroelectric Polymer for Ultrastable Zinc Anodes. Adv. Funct. Mater. 2024, 34, 2305550. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Xie, X.; Li, Z.; Wang, P.; Lu, B.; Liang, S.; Tang, Y.; Zhou, J. A Functionalized Separator Enables Dendrite-Free Zn Anode via Metal-Polydopamine Coordination Chemistry. InfoMat 2023, 5, e12374. [Google Scholar] [CrossRef]
- Tian, H.; Feng, G.; Wang, Q.; Li, Z.; Zhang, W.; Lucero, M.; Feng, Z.; Wang, Z.-L.; Zhang, Y.; Zhen, C.; et al. Three-Dimensional Zn-Based Alloys for Dendrite-Free Aqueous Zn Battery in Dual-Cation Electrolytes. Nat. Commun. 2022, 13, 7922. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, T.; He, Z.; Lu, B.; Liang, S.; Zhou, J. Interfacial Engineering Strategy for High-Performance Zn Metal Anodes. Nano-Micro Lett. 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.-G.; You, Z.; Wei, C.; Kang, F.; Wei, B. Rechargeable Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Future Perspectives. Mater. Today 2021, 42, 73–98. [Google Scholar] [CrossRef]
- Chen, M.; Yang, M.; Han, X.; Chen, J.; Zhang, P.; Wong, C.-P. Suppressing Rampant and Vertical Deposition of Cathode Intermediate Product via PH Regulation Toward Large-Capacity and High-Durability Zn//MnO2 Batteries. Adv. Mater. 2024, 36, 2304997. [Google Scholar] [CrossRef]
- Ye, J.-J.; Li, P.-H.; Hou, Z.; Zhang, W.; Zhu, W.; Jin, S.; Ji, H. Se-Dopant Modulated Selective Co-Insertion of H+ and Zn2+ in MnO2 for High-Capacity and Durable Aqueous Zn-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202410900. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Jiao, Q.; Zhao, Y.; Li, H.; Feng, C. Tailoring Porous Three-Dimensional (Co,Mn)(Co,Mn)2O4/PPy Architecture towards High-Performance Cathode for Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2023, 465, 142897. [Google Scholar] [CrossRef]
- Liu, M.; Hu, A.; Yan, Z.; Chen, J.; He, M.; Zhou, B.; Pan, Y.; Fan, Y.; Gao, D.; Long, J. Enhancing Zn2+ Diffusion for Dendrite-Free Zinc Anodes via a Robust Zincophilic Clay Mineral Coating. Chem. Eng. J. 2024, 479, 147410. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, F.; Bao, D.; Chen, Y.; Hu, L.; Zhu, J. Two-Dimensional V2O3 Nanosheets Embedded in a Carbon Matrix with Enhanced Performance for Aqueous Zn-Ion Battery. Mater. Lett. 2024, 357, 135706. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Q.; Zou, X.; Zhang, B.; An, L. High-Energy-Density Aqueous Zinc-Ion Batteries: Recent Progress, Design Strategies, Challenges, and Perspectives. Adv. Mater. 2025, 2501361. [Google Scholar] [CrossRef]
- Ma, H.; Tian, X.; Wang, T.; Tang, K.; Liu, Z.; Hou, S.; Jin, H.; Cao, G. Tailoring Pore Structures of 3D Printed Cellular High-Loading Cathodes for Advanced Rechargeable Zinc-Ion Batteries. Small 2021, 17, 2100746. [Google Scholar] [CrossRef]
- Zhou, W.; Wan, Z.; Feng, J.; Hao, Z. Interface Engineering for Manipulating the Zinc Deposition and Achieving Stable Zinc Ion Batteries. Nano Today 2024, 58, 102461. [Google Scholar] [CrossRef]
- Yu, Z.; Shan, H.; Zhong, Y.; Zhang, X.; Hong, G. Leveraging Advanced X-Ray Imaging for Sustainable Battery Design. ACS Energy Lett. 2022, 7, 3151–3176. [Google Scholar] [CrossRef]
- Li, X.; Xiang, J.; Qiu, L.; Chen, X.; Zhao, Y.; Wang, Y.; Yue, Q.; Gao, T.; Liu, W.; Xiao, D.; et al. Unlocking the Stable Interface in Aqueous Zinc-Ion Battery with Multifunctional Xylose-Based Electrolyte Additives. J. Energy Chem. 2025, 100, 770–778. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, H.; Guo, Y.; Xu, C.; Deng, Y.; Deng, Z.; Zhang, Y.; Wu, H.; Cai, W.; Zhang, Y. Chemical and Electrochemical Synergistic Weaving Stable Interface Enabling Longevous Zinc Plating/Stripping Process. Chem. Eng. J. 2023, 457, 141305. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Zhu, M.; You, F.; Lin, W.; Chan, D.; Lin, W.; Li, P.; Tang, Y.; Zhang, Y. Revitalizing Zinc-Ion Batteries with Advanced Zinc Anode Design. Nanoscale Horiz. 2023, 8, 29–54. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Fang, G.; Sun, H.; Cao, X.; Zhou, J.; Pan, A.; Liang, S. Suppressing By-Product via Stratified Adsorption Effect to Assist Highly Reversible Zinc Anode in Aqueous Electrolyte. J. Energy Chem. 2021, 55, 549–556. [Google Scholar] [CrossRef]
- Xue, M.; Bai, J.; Wu, M.; He, Q.; Zhang, Q.; Chen, L. Carbon-Assisted Anodes and Cathodes for Zinc Ion Batteries: From Basic Science to Specific Applications, Opportunities and Challenges. Energy Storage Mater. 2023, 62, 102940. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Xin, Y.; Ge, Y.; Xie, H.; Cai, S.; Zhao, C.; Zhang, H.; Tian, H. Quaternary Alloy Interfaces for Stable Zinc Anodes for High-Performance Aqueous Zinc-Ion Batteries With Long-Term Cycling Stability. Small 2025, 21, 2502569. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Q.; Xia, Q.; Lei, Y.; Long Huang, X.; Tebyetekerwa, M.; Song Zhao, X. Interface Challenges and Optimization Strategies for Aqueous Zinc-Ion Batteries. J. Energy Chem. 2023, 77, 642–659. [Google Scholar] [CrossRef]
- Bose, S.; Akdogan, E.K.; Balla, V.K.; Ciliveri, S.; Colombo, P.; Franchin, G.; Ku, N.; Kushram, P.; Niu, F.; Pelz, J.; et al. 3D Printing of Ceramics: Advantages, Challenges, Applications, and Perspectives. J. Am. Ceram. Soc. 2024, 107, 7879–7920. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Mu, Y.; Guo, B.; Zeng, G.; Xiang, Y.; Zeng, L.; Bai, J.; Yang, J. Towards Reusable 3D-Printed Graphite Framework for Zinc Anode in Aqueous Zinc Battery. Energy Storage Mater. 2024, 70, 103454. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.H.; Koh, J.J.; Li, Y.; Ma, Y.; Ding, J.; Wang, J.; Hu, Z.; Wang, J.; Chen, W.; et al. Design and Manufacture of 3D-Printed Batteries. Joule 2021, 5, 89–114. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G.; Lu, J.; Hou, Y.; Bao, Y.; Liu, P.; Zhang, Y. 3D Printing-Induced Phase Transition in Ferroelectric Porous Polymer Coatings for Stable Zinc Anodes. Small 2025, 21, 2408848. [Google Scholar] [CrossRef]
- Ji, G.; Sun, M.; Li, M.; Zheng, J. Sulfonate-Modified Covalent Organic Framework Integrated Hydrogel Electrolyte: Enhancing AZIBs Performances by Tailoring Microstructures and Functional Groups. Adv. Funct. Mater. 2025, 2500110. [Google Scholar] [CrossRef]
- Zhang, Y. Optimization of Layering Algorithm and Print Order in 3D Printing. Highlights Sci. Eng. Technol. 2024, 120, 122–128. [Google Scholar] [CrossRef]
- Shen, F.; Tang, C.; Sun, X.; Song, Y.; Cai, J. Recent Advances in 3D Printing Technologies for Lithium-Sulfur Batteries. Small 2025, 21, 2412182. [Google Scholar] [CrossRef]
- Sani, A.R.; Zolfagharian, A.; Kouzani, A.Z. Artificial Intelligence-Augmented Additive Manufacturing: Insights on Closed-Loop 3D Printing. Adv. Intell. Syst. 2024, 6, 2400102. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z. Interfacial Regulation for 3D Printing Based on Slice-Based Photopolymerization. Adv. Mater. 2023, 35, 2300903. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, X.; Hao, Z.; Li, Q.; Wu, F.; Wang, K.; Li, Y.; Li, W.; Gao, H. Advances in Polysaccharide-Based Microneedle Systems for the Treatment of Ocular Diseases. Nano-Micro Lett. 2024, 16, 268. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, D.; Wang, J. Challenges and Opportunities in Preserving Key Structural Features of 3D-Printed Metal/Covalent Organic Framework. Nano-Micro Lett. 2024, 16, 157. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, H.-Y.; Chae, C.; Lee, S.Y.; Kim, S.-H. Advanced Additive Manufacturing of Structurally-Colored Architectures. Adv. Mater. 2024, 36, 2307917. [Google Scholar] [CrossRef]
- Ho, M.; Ramirez, A.B.; Akbarnia, N.; Croiset, E.; Prince, E.; Fuller, G.G.; Kamkar, M. Direct Ink Writing of Conductive Hydrogels. Adv. Funct. Mater. 2025, 35, 2415507. [Google Scholar] [CrossRef]
- Zheng, Q.; Xie, B.; Xu, Z.; Wu, H. A Systematic Printability Study of Direct Ink Writing towards High-Resolution Rapid Manufacturing. Int. J. Extrem. Manuf. 2023, 5, 035002. [Google Scholar] [CrossRef]
- Guo, Z.; Fei, F.; Song, X.; Zhou, C. Analytical Study and Experimental Verification of Shear-Thinning Ink Flow in Direct Ink Writing Process. J. Manuf. Sci. Eng. 2023, 145, 071001. [Google Scholar] [CrossRef]
- Guo, Z.; Fei, F.; Song, X.; Zhou, C. Analytical Study of Shear-Thinning Fluid Flow in Direct Ink Writing Process. In Proceedings of the ASME 2022 17th International Manufacturing Science and Engineering Conference, West Lafayette, IN, USA, 27 June–1 July 2022. [Google Scholar]
- Barth, L.; Jung, M.; Seemann, R.; Lienkamp, K. 3D Printable Magnetic Soft Actuators–Ink Formulation, Rheological Characterization, and Hydrogel Actuator Prototypes. Macromol. Mater. Eng. 2025, 310, 2400431. [Google Scholar] [CrossRef]
- Li, C.; Feng, C.; Zhang, L.; Zhang, L.; Wang, L. Direct Ink Writing of Polymer-Based Materials—A Review. Polym. Eng. Sci. 2025, 65, 431–454. [Google Scholar] [CrossRef]
- Baniasadi, H.; Abidnejad, R.; Fazeli, M.; Lipponen, J.; Niskanen, J.; Kontturi, E.; Seppälä, J.; Rojas, O.J. Innovations in Hydrogel-Based Manufacturing: A Comprehensive Review of Direct Ink Writing Technique for Biomedical Applications. Adv. Colloid Interface Sci. 2024, 324, 103095. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.A.; Pan, Y. Direct Ink Writing on a Rotating Mandrel—Additive Lathe Micro-Manufacturing. J. Micro Nano-Manuf. 2024, 11, 021002. [Google Scholar] [CrossRef]
- Alam, A.; Saeed, G.; Kim, K.; Kim, J.K.; Park, H.S.; Lim, S. Direct Ink Writing (DIW) Printed High-Performance Asymmetric Supercapacitor Based on 0D@2D Silver-nanoparticles@MXene as Anode and 0D@2D MnO2-nanoparticles@MXene as Cathode Materials. J. Energy Storage 2023, 72, 108227. [Google Scholar] [CrossRef]
- Izumi, A.; Sanada, M.; Furuichi, K.; Teraki, K.; Matsuda, T.; Hiramatsu, K.; Munakata, H.; Kanamura, K. Development of High Capacity Lithium-Ion Battery Applying Three-Dimensionally Patterned Electrode. Electrochim. Acta 2012, 79, 218–222. [Google Scholar] [CrossRef]
- Nath, S.S.; Sai, A.; Sundriyal, P. (Invited) Direct Ink Writing of Supercapacitors for Flexible and Wearable Applications—IOPscience. In Proceedings of the 244th ECS Meeting, Gothenburg, Sweden, 8–12 October 2023. [Google Scholar] [CrossRef]
- Agócs, C.; Hanon, M.M.; Zsidai, L. A Comprehensive Review of Fused Deposition Modeling (FDM) Method Using PLA, ABS, and PET-G Polymers. Gradus 2024, 11, 1–12. [Google Scholar] [CrossRef]
- Elsonbaty, A.; MRashad, A.; Abass, O.Y.; Abdelghany, T.Y.; MAlfauiomy, A. A Survey of Fused Deposition Modeling (FDM) Technology in 3D Printing. J. Eng. Res. Rep. 2024, 26, 304–312. [Google Scholar] [CrossRef]
- Bembenek, M.; Kowalski, Ł.; Kosoń-Schab, A. Research on the Influence of Processing Parameters on the Specific Tensile Strength of FDM Additive Manufactured PET-G and PLA Materials. Polymers 2022, 14, 2446. [Google Scholar] [CrossRef]
- Safaei, B.; Memarzadeh, A.; Asmael, M.; Sahmani, S.; Zeeshan, Q.; Jen, T.-C.; Qin, Z. Challenges and Advancements in Additive Manufacturing of Nylon and Nylon Composite Materials: A Comprehensive Analysis of Mechanical Properties, Morphology, and Recent Progress. J. Mater. Eng. Perform. 2024, 33, 6261–6305. [Google Scholar] [CrossRef]
- Sousa, H.C.; Ruben, R.B.; Viana, J.C. On the Fused Deposition Modelling of Personalised Bio-Scaffolds: Materials, Design, and Manufacturing Aspects. Bioengineering 2024, 11, 769. [Google Scholar] [CrossRef]
- Hiremath, S.; Sahu, A.R.; Majumder, H. Modeling and Analysis of Fused Deposition Modeling (FDM) Process Parameters on the Mechanical Properties of Thermoplastic Parts. AIP Conf. Proc. 2023, 2427, 020063. [Google Scholar] [CrossRef]
- Wang, A.; Tang, X.; Zeng, Y.; Zou, L.; Bai, F.; Chen, C. Carbon Fiber-Reinforced PLA Composite for Fused Deposition Modeling 3D Printing. Polymers 2024, 16, 2135. [Google Scholar] [CrossRef]
- Sandanamsamy, L.; Mogan, J.; Rajan, K.; Harun, W.S.W.; Ishak, I.; Romlay, F.R.M.; Samykano, M.; Kadirgama, K. Effect of Process Parameter on Tensile Properties of FDM Printed PLA. Mater. Today: Proc. 2024, 109, 1–6. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Y. Optimization of Parameters for FDM Process with Functional Input Based on LS-SVR. AIP Adv. 2022, 12, 025108. [Google Scholar] [CrossRef]
- Kananathan, J.; Samykano, M.; Kadirgama, K.; Ramasamy, D.; Rahman, M.M. Comprehensive Investigation and Prediction Model for Mechanical Properties of Coconut Wood–Polylactic Acid Composites Filaments for FDM 3D Printing. Eur. J. Wood Wood Prod. 2021, 80, 75–100. [Google Scholar] [CrossRef]
- Pérez, M.; Medina-Sánchez, G.; García-Collado, A.; Gupta, M.; Carou, D. Surface Quality Enhancement of Fused Deposition Modeling (FDM) Printed Samples Based on the Selection of Critical Printing Parameters. Materials 2018, 11, 1382. [Google Scholar] [CrossRef]
- Patel, R.; Desai, C.; Kushwah, S.; Mangrola, M.H. A Review Article on FDM Process Parameters in 3D Printing for Composite Materials. Mater. Today Proc. 2022, 60, 2162–2166. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, J.; Zhang, Y.; Liu, G.; Yi, X.; Liu, X. FDM 3D Printed MXene/Recycled Carbon Fibre Reinforced Polylactic Acid Composites: Interface Optimization, Toughening and Enhanced Electromagnetic Shielding Performance. Compos. Commun. 2024, 48, 101953. [Google Scholar] [CrossRef]
- Yilmaz, S.; Gul, O.; Eyri, B.; Gamze Karsli Yilmaz, N.; Yilmaz, T. Comprehensive Characterization of 3D-Printed Bamboo/Poly(Lactic Acid) Bio Composites. Polym. Eng. Sci. 2023, 63, 2958–2972. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Wang, X.; Wang, Y.; Lu, X.; Wei, Z.; Mo, Q.; Sheng, Y.; Zhang, S.; Huang, C.; et al. Blending and Functionalisation Modification of 3D Printed Polylactic Acid for Fused Deposition Modeling. Rev. Adv. Mater. Sci. 2023, 62, 20230140. [Google Scholar] [CrossRef]
- Fafenrot, S.; Grimmelsmann, N.; Wortmann, M.; Ehrmann, A. Three-Dimensional (3D) Printing of Polymer-Metal Hybrid Materials by Fused Deposition Modeling. Materials 2017, 10, 1199. [Google Scholar] [CrossRef]
- Pavlovskii, A.A.; Pushnitsa, K.; Kosenko, A.; Novikov, P.; Popovich, A.A. 3D-Printed Lithium-Ion Battery Electrodes: A Brief Review of Three Key Fabrication Techniques. Materials 2024, 17, 5904. [Google Scholar] [CrossRef] [PubMed]
- Maurel, A.; Grugeon, S.; Fleutot, B.; Courty, M.; Prashantha, K.; Tortajada, H.; Armand, M.; Panier, S.; Dupont, L. Three-Dimensional Printing of a LiFePO4/Graphite Battery Cell via Fused Deposition Modeling. Sci. Rep. 2019, 9, 18031. [Google Scholar] [CrossRef]
- Gupta, P.; Bhat, M.; Khamkar, V.; Tandel, G.; Salunkhe, G. Designing of Cost Effective Resin 3D Printer Using UV LED. In Proceedings of the 2020 International Conference on Convergence to Digital World—Quo Vadis (ICCDW), Mumbai, India, 18–20 February 2020; pp. 1–4. [Google Scholar]
- Yadav, P.; Singh, K.; Bhaskar, J. Design and Development of Ultra Violet Curing Based 3-D Printer. Int. J. Adv. Prod. Ind. Eng. 2020, 5, 16–22. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Chua, G.S.; Tan, C.H. Abrasive Jet Deburring of Jewellery Models Built by Stereolithography Apparatus (SLA). J. Mater. Process. Technol. 1998, 83, 36–47. [Google Scholar] [CrossRef]
- Kong, Y. Application of 3D Printing Technology in Jewelry Design in the Era of Artificial Intelligence. In The International Conference on Cyber Security Intelligence and Analytics; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Caussin, E.; Moussally, C.; Le Goff, S.; Fasham, T.; Troizier-Cheyne, M.; Tapie, L.; Dursun, E.; Attal, J.-P.; François, P. Vat Photopolymerization 3D Printing in Dentistry: A Comprehensive Review of Actual Popular Technologies. Materials 2024, 17, 950. [Google Scholar] [CrossRef]
- Afridi, A.; Al Rashid, A.; Koç, M. Recent Advances in the Development of Stereolithography-Based Additive Manufacturing Processes: A Review of Applications and Challenges. Bioprinting 2024, 43, e00360. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef]
- Cingesar, I.K.; Marković, M.-P.; Vrsaljko, D. Effect of Post-Processing Conditions on Polyacrylate Materials Used in Stereolithography. Addit. Manuf. 2022, 55, 102813. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Byun, H.-S. Recent Advances in 3D Printed Electrode Materials for Electrochemical Energy Storage Devices. J. Energy Chem. 2023, 81, 272–312. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D Printing Technologies for Electrochemical Energy Storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Zhou, S.; Li, M.; Wang, P.; Cheng, L.; Chen, L.; Huang, Y.; Yu, S.; Mo, F.; Wei, J. Printed Solid-State Batteries. Electrochem. Energy Rev. 2023, 6, 34. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Chen, L.; Hu, S. A Strategy to Build High-Performance Thick Electrodes for Lithium-Ion Batteries with Enhanced Compressive Modulus and Regulated Tortuosity in the Phase-Inversion Process. J. Mater. Chem. A 2024, 12, 16537–16545. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Y.; Tao, L.; Yang, Z.; Liu, P.; Wang, Q.; Wang, T. Developments and Challenges in Direct Ink Writing for High-Performance Polymers. Addit. Manuf. Front. 2025, 4, 200218. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Xu, Q.; Ullah, M.W.; Zahoor; Sethupathy, S.; Morales, G.M.; Sun, J.; Zhu, D. Lignin-Based Additive Materials: A Review of Current Status, Challenges, and Future Perspectives. Addit. Manuf. 2023, 74, 103711. [Google Scholar] [CrossRef]
- McGregor, M.; Patel, S.; McLachlin, S. Mihaela Vlasea Architectural Bone Parameters and the Relationship to Titanium Lattice Design for Powder Bed Fusion Additive Manufacturing. Addit. Manuf. 2021, 47, 102273. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, M.; Yang, X.; Sun, R.; Zhang, J.; Sun, X. Emerging Application of 3D-Printing Techniques in Lithium Batteries: From Liquid to Solid. Mater. Today 2022, 59, 161–181. [Google Scholar] [CrossRef]
- Pramanik, S.; Tasche, L.; Hoyer, K.-P.; Schaper, M. Investigating the Microstructure of an Additively Manufactured FeCo Alloy: An Electron Microscopy Study. Addit. Manuf. 2021, 46, 102087. [Google Scholar] [CrossRef]
- Yang, M.; Cao, C.; Wang, J. Fabrication of High-Quality Platinum Coating Using Explosive Welding Technology and Its Microstructure Evolution Mechanisms. Mater. Des. 2023, 235, 112372. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Tang, L.; Shi, D.; Lam, E.; Bae, J. 3D Shape Morphing of Stimuli-Responsive Composite Hydrogels. Mater. Chem. Front. 2023, 7, 5989–6034. [Google Scholar] [CrossRef]
- Liu, Z.; He, H.; Luo, Z.; Wang, X.; Zeng, J. 3D Printing of Customized MnO2 Cathode for Aqueous Zinc-Ion Batteries. Trans. Nonferrous Met. Soc. China 2023, 33, 1193–1204. [Google Scholar] [CrossRef]
- Ding, H.; He, Y.; Yu, X.; Chen, L.; Chen, M.; Luo, Y.; Li, J.; Wei, S. A Novel 3D Framework Loaded with MnO2 for High-Performance Aqueous Zinc-Ion Battery Cathode. J. Electroanal. Chem. 2025, 986, 119101. [Google Scholar] [CrossRef]
- Peng, J.; Gou, W.; Jiang, T.; Ding, K.; Yu, A.; Fan, Q.; Xu, Q. 3D Printed Reticular Manganese Dioxide Cathode with High Areal Capacity for Aqueous Zinc Ion Batteries. J. Alloys Compd. 2024, 998, 174772. [Google Scholar] [CrossRef]
- Nie, N.; Wang, F.; Yao, W. Fabrication of a 3D Structure MnO2 Electrode with High MnO2 Mass Loading as the Cathode for High-Performance Aqueous Zinc-Ion Batteries. Electrochim. Acta 2023, 472, 143423. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Nagaraju, G.; Sokolikova, M.; Quintin-Baxendale, R.; Mattevi, C. 3D Printing of Layered Vanadium Disulfide for Water-in-Salt Electrolyte Zinc-Ion Batteries. Nanoscale Horiz. 2024, 9, 742–751. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.-F.; Li, Z.-X.; Wang, P.-F.; Xie, Y.; Yi, T.-F. Carbon-Based Nanomaterials for Stabilizing Zinc Metal Anodes towards High-Performance Aqueous Zinc-Ion Batteries. Energy Storage Mater. 2024, 67, 103300. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fu, Y.; Dang, H.; Wang, D.; Ran, F. Optimization Strategies toward Advanced Aqueous Zinc-Ion Batteries: From Facing Key Issues to Viable Solutions. Nano Energy 2023, 116, 108858. [Google Scholar] [CrossRef]
- Wu, B.; Guo, B.; Chen, Y.; Mu, Y.; Qu, H.; Lin, M.; Bai, J.; Zhao, T.; Zeng, L. High Zinc Utilization Aqueous Zinc Ion Batteries Enabled by 3D Printed Graphene Arrays. Energy Storage Mater. 2023, 54, 75–84. [Google Scholar] [CrossRef]
- He, H.; Zeng, L.; Luo, D.; He, J.; Li, X.; Guo, Z.; Zhang, C. 3D Printing of Electron/Ion-Flux Dual-Gradient Anodes for Dendrite-Free Zinc Batteries. Adv. Mater. 2023, 35, 2211498. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Zhang, K.; Zhao, J.; Deng, S.; Li, Y.; Xu, B.; Pang, H. Microfluidic-Assisted 3D Printing Zinc Powder Anode with 2D Conductive MOF/MXene Heterostructures for High-Stable Zinc−Organic Battery. Adv. Mater. 2024, 36, 2309753. [Google Scholar] [CrossRef]
- Li, B.; Zeng, Y.; Zhang, W.; Lu, B.; Yang, Q.; Zhou, J.; He, Z. Separator Designs for Aqueous Zinc-Ion Batteries. Sci. Bull. 2024, 69, 688–703. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Wang, X.; Wang, Z.; Li, M.; Hu, X.; Wang, Y.; Liu, H.; Wang, Y. 3D Printed Dual Network Cross-Linked Hydrogel Electrolytes for High Area Capacity Flexible Zinc Ion Micro-Batteries. Chem. Eng. J. 2024, 490, 151523. [Google Scholar] [CrossRef]
- Poompiew, N.; Jirawatanaporn, N.; Okhawilai, M.; Qin, J.; Román, A.J.; Aumnate, C.; Osswald, T.A.; Potiyaraj, P. 3D-Printed Polyacrylamide-Based Hydrogel Polymer Electrolytes for Flexible Zinc-Ion Battery. Electrochim. Acta 2023, 466, 143076. [Google Scholar] [CrossRef]
- Su, Y.; Liu, B.; Zhang, Q.; Peng, J.; Wei, C.; Li, S.; Li, W.; Xue, Z.; Yang, X.; Sun, J. Printing-Scalable Ti3C2Tx MXene-Decorated Janus Separator with Expedited Zn2+ Flux toward Stabilized Zn Anodes. Adv. Funct. Mater. 2022, 32, 2204306. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Ma, J.; Wang, X.; Zhang, L.; Das, P.; Wang, K.; Wu, Z.-S. All 3D Printing Shape-Conformable Zinc Ion Hybrid Capacitors with Ultrahigh Areal Capacitance and Improved Cycle Life. Adv. Energy Mater. 2022, 12, 2200341. [Google Scholar] [CrossRef]
- Chen, C.; Lu, K.; Wang, Y.; Cheng, R.; Xiang, T.; Xia, M.; Wang, F.; Lei, W.; Yang, J.; Mathur, S.; et al. 3D Printed Flexible Zinc-Ion Battery for Real-Time Health Monitoring Devices. ACS Appl. Mater. Interfaces 2025, 17, 23860–23871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, W.; Ni, J.; Li, L. High Areal Energy Zinc-Ion Micro-Batteries Enabled by 3D Printing. J. Mater. Sci. Technol. 2024, 196, 183–189. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Zhang, F.; Yang, L.; Jiang, X.; Zhang, K.; Qian, Z.; Yang, J. Improvements and Challenges of Hydrogel Polymer Electrolytes for Advanced Zinc Anodes in Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 21779–21803. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Zhao, Y.-H.; Wu, Y.-M.; Wang, P.-F.; Liu, Z.-L.; Zhang, Q.-Y.; Shu, J.; Yi, T.-F. Everything in Aqueous Zinc-Ion Batteries May Be Prussian Blue Analogues: From Cathode Materials to Electrolyte Additives Applications. Energy Storage Mater. 2025, 78, 104299. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hu, Q.; Hao, T.; Cao, H.; Huang, X.; Liu, Y.; Zhang, Y.; Lin, D.; Tang, Y.; et al. Prussian Blue Analogs Cathodes for Aqueous Zinc Ion Batteries. Mater. Today Energy 2022, 29, 101095. [Google Scholar] [CrossRef]
- Sha, L.; Sui, B.; Wang, P.; Gong, Z.; Zhang, Y.; Wu, Y.; Zhao, L.; Shi, F. Printing 3D Mesh-like Grooves on Zinc Surface to Enhance the Stability of Aqueous Zinc Ion Batteries. J. Colloid Interface Sci. 2023, 647, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Meng, J.; Shen, H.; Chen, R.; Wang, Y.; Cao, J.; Yao, J.; Li, L.; Liu, H.; Zhang, G.; et al. Beyond Conventional Stabilization: Carbon Materials for next-Generation Aqueous Zinc-Ion Batteries. J. Power Sources 2025, 646, 237250. [Google Scholar] [CrossRef]
- Gao, W.; Michalička, J.; Pumera, M. Hierarchical Atomic Layer Deposited V2O5 on 3D Printed Nanocarbon Electrodes for High-Performance Aqueous Zinc-Ion Batteries. Small 2022, 18, 2105572. [Google Scholar] [CrossRef]
- Zeng, L.; He, H.; Chen, H.; Luo, D.; He, J.; Zhang, C. 3D Printing Architecting Reservoir-Integrated Anode for Dendrite-Free, Safe, and Durable Zn Batteries. Adv. Energy Mater. 2022, 12, 2103708. [Google Scholar] [CrossRef]
| Technology | Core Applicable Battery Components | Electrode Thickness Range | Surface/Layer Accuracy (XY) | Line/Feature Resolution (Min. Line Width) | Core Advantages | Core Limitations |
|---|---|---|---|---|---|---|
| DIW | Thick Electrodes, Porous Structure Electrodes, Solid Electrolyte Structures, Integrated Battery Architectures | 200–800 μm | ±20–100 μm | 100–500 μm | High material freedom, Precise ionic channel construction, Mold-free integrated forming | Electrode cracking due to ink drying shrinkage, Insufficient nanoscale resolution, Slow printing constrains mass production |
| FFF | Battery Casings/Brackets, Current Collector Molds, Molds for Electrode Casting | 50–500 μm (post-casting) | ±100–200 μm | 200–500 μm | Ultra-low cost, Customized shapes, Conductive composites applicable | Interlayer voids reduce electrode density, Thermoplastic matrix limits active material loading, Anisotropy causes delamination failure |
| SLA | Microfluidic Electrolyte Layers, Precision Solid Electrolyte Structures, Miniature Battery Components | 10–200 μm (single layer) | ±5–25 μm | 10–100 μm | Submicron accuracy, Smooth interfaces, Potential for in situ monitoring | Photocurable resin restricts material selection, Post-processing (cleaning, curing) risks damaging thin electrolyte layers, Uneven curing within thick electrodes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ding, H.; Chen, H.; Gao, H.; Yu, J.; Mo, F.; Wang, N. Recent Progress on the Research of 3D Printing in Aqueous Zinc-Ion Batteries. Polymers 2025, 17, 2136. https://doi.org/10.3390/polym17152136
Liu Y, Ding H, Chen H, Gao H, Yu J, Mo F, Wang N. Recent Progress on the Research of 3D Printing in Aqueous Zinc-Ion Batteries. Polymers. 2025; 17(15):2136. https://doi.org/10.3390/polym17152136
Chicago/Turabian StyleLiu, Yating, Haokai Ding, Honglin Chen, Haoxuan Gao, Jixin Yu, Funian Mo, and Ning Wang. 2025. "Recent Progress on the Research of 3D Printing in Aqueous Zinc-Ion Batteries" Polymers 17, no. 15: 2136. https://doi.org/10.3390/polym17152136
APA StyleLiu, Y., Ding, H., Chen, H., Gao, H., Yu, J., Mo, F., & Wang, N. (2025). Recent Progress on the Research of 3D Printing in Aqueous Zinc-Ion Batteries. Polymers, 17(15), 2136. https://doi.org/10.3390/polym17152136







