1. Introduction
The demand for synthetic dyes in various industries (textile, pharmaceutical, paper, food, among others) is increasing significantly worldwide, contaminating water resources through wastewater discharge [
1]. Current regulations, such as the Environmental Quality Standards and the Maximum Permissible Levels in the case of Peru, are transversal; however, the lack of industrial water treatment plants aggravates the problem.
The annual production of industrial dyes amounts to thousands of tons. The most commonly used dyes are alcian blue, basic fuchsin, crystal violet, toluidine blue, and methylene blue (MB), which are cationic dyes. Other commonly used dyes are aniline blue, Congo red, methyl orange, acid black, and acid red, which are anionic dyes [
2,
3]. Although the use of anionic dyes is between 60% and 70% compared with cationic dyes, the latter cause greater environmental damage due to their low degradability, especially in water bodies. Therefore, several studies have focused on proposing materials that can remove these dyes, such as methylene blue [
4,
5,
6,
7,
8,
9,
10,
11,
12].
MB is the most widely used dye and one of the most studied due to health side effects such as increased heart rate, vomiting, respiratory toxicity, mucous membrane irritation, diarrhea, and skin cancer [
13]. Similarly, in aquatic environments, even at low concentrations, they inhibit the metabolic and photosynthetic processes of living organisms, causing deterioration in aquatic ecosystems [
14,
15].
Traditional methods for wastewater treatment, such as chemical precipitation, filtration, and reverse osmosis, are expensive, so the aim is to apply emerging methods that are easy to operate and economical, such as adsorption, which also allows the use of ecological adsorbents such as biomass waste and activated clay from sustainable, renewable, and abundant sources [
14,
16,
17].
Several studies have been conducted on inorganic materials (mainly clays, zeolites, and metal oxides such as TiO
2 and Fe
2O
3), organic materials (such as lignocellulosic biomass, activated carbon, chitosan, and their modifications), and synthetic materials (mainly polyacrylamide, alginates, polyethyleneimine, and ion exchange resins) for MB adsorption [
6,
18]. Organic materials are inexpensive and sustainable, but their capacity depends on the treatment, and their stability is limited. Inorganic materials are cheap and abundant, although sensitive to pH and generate non-treatable waste. Synthetic materials offer high efficiency and specificity but are expensive and can release toxic waste [
19,
20]. Furthermore, they are subject to limitations such as rapid saturation, dye desorption, and complex regeneration. Therefore, ensuring adequate adsorption depends on cost, efficiency, effluent conditions, and environmental sustainability criteria, and these aspects can be improved through the synergy of these individual materials [
21,
22,
23,
24].
Clay is used as an efficient alternative in the removal of anionic and cationic dyes due to its high ion exchange capacity and specific surface area, low cost, abundance, and eco-friendliness, although it has some disadvantages, such as complex regeneration and limited selectivity, as well as requiring chemical modification to optimize removal efficiency [
25,
26,
27]. On the other hand, many materials of biological origin have been used for the removal of dyes in aqueous media, including algae such as Chlorella, Spirulina, and Sargassum, due to their functional groups (carboxyl, amino, and hydroxyl), offering advantages of biodegradability, environmental friendliness, recyclability, and promotion of green chemistry and contributing to sustainable development, although their advantages are limited by their limited stability, complex separation, and sensitivity to the pH and temperature of the aqueous medium [
28,
29]. Nostoc sphaericum is a high Andean algae that requires minimal nutrient demand, grows above 4000 m altitude, and has qualities that make its use as an adsorbent of cationic dyes potential due to its good stability in aqueous media, its anionic functional groups, and, above all, the extraction of its hydrocolloid does not require processes that involve the use of chemical substances [
30,
31,
32].
The fusion of new adsorbent materials such as atomized
Nostoc sphaericum (NS) and activated nanoclays (NC) is a promising proposal to improve the adsorption capacity of MB, due to their properties. NS is a new material in the world of adsorption; however, NC would stand out as a good adsorbent due to its surface area, structural properties, and functional groups of anionic character (such as -OH, -COOH, and -NH
2), which can establish electrostatic attraction and bind cationic molecules [
25,
33].
Many studies of inorganic–organic materials in the adsorption of dyes confirm high percentages of contaminant removal in water, since they offer superior performance to their components due to their unique properties and structural diversity [
34], making these materials potential low-cost and environmentally friendly adsorbents.
Obtaining the adsorbent from organic matter requires treatment and activation processes that optimize its removal capacity. Clay, an abundant and easily accessible material, has been shown in numerous studies to be suitable for the removal of dyes from wastewater. Similarly,
Nostoc sphaericum, a seaweed available in high Andean lagoons during the rainy season, is a promising material for the removal of cationic dyes in aqueous media because it is composed primarily of proteins and polysaccharides, which contain negatively charged groups such as carboxyl, carbonyl, and hydroxyl groups [
30,
35,
36,
37]. Its advantage lies in its biodegradable and sustainable nature; however, it has limitations due to its limited reusability. The use of bio-based materials allows for the removal of MB above 90% due to their chemical composition, as demonstrated by chitosan, alginate, hydrogels, and modified cellulose [
38,
39,
40].
Therefore, the study aimed to elaborate an NC/ANS adsorbent in different formulations and to evaluate the adsorption capacity of MB at different pH and adsorbent doses; likewise, to know its adsorption capacity, the adsorption kinetics were studied for optimum conditions.
2. Materials and Methods
2.1. Raw Materials and Reagents
Clay was collected from a quarry in the Huancabamba zone, Andahuaylas, Peru (13°43′58″ S, 73°20′38″ W, 3682 m of altitude). NS was collected at Huamanilla Lagoon, Andahuaylas, Peru, at coordinates 13°47′52″ S, 73°17′55″ W and 4251 m of altitude, during the rainy season of 2023.
Solutions of phosphoric acid (H3PO4) (Oxford, Maharashtra, India), methylene blue from Spectrum Chemical Mfg. Corp. (New Brunswick, NJ, USA), sodium chloride (NaCl), potassium bromide (KBr), and hydrochloric acid (HCl) (EMSURE, Darmstadt, Germany) were used.
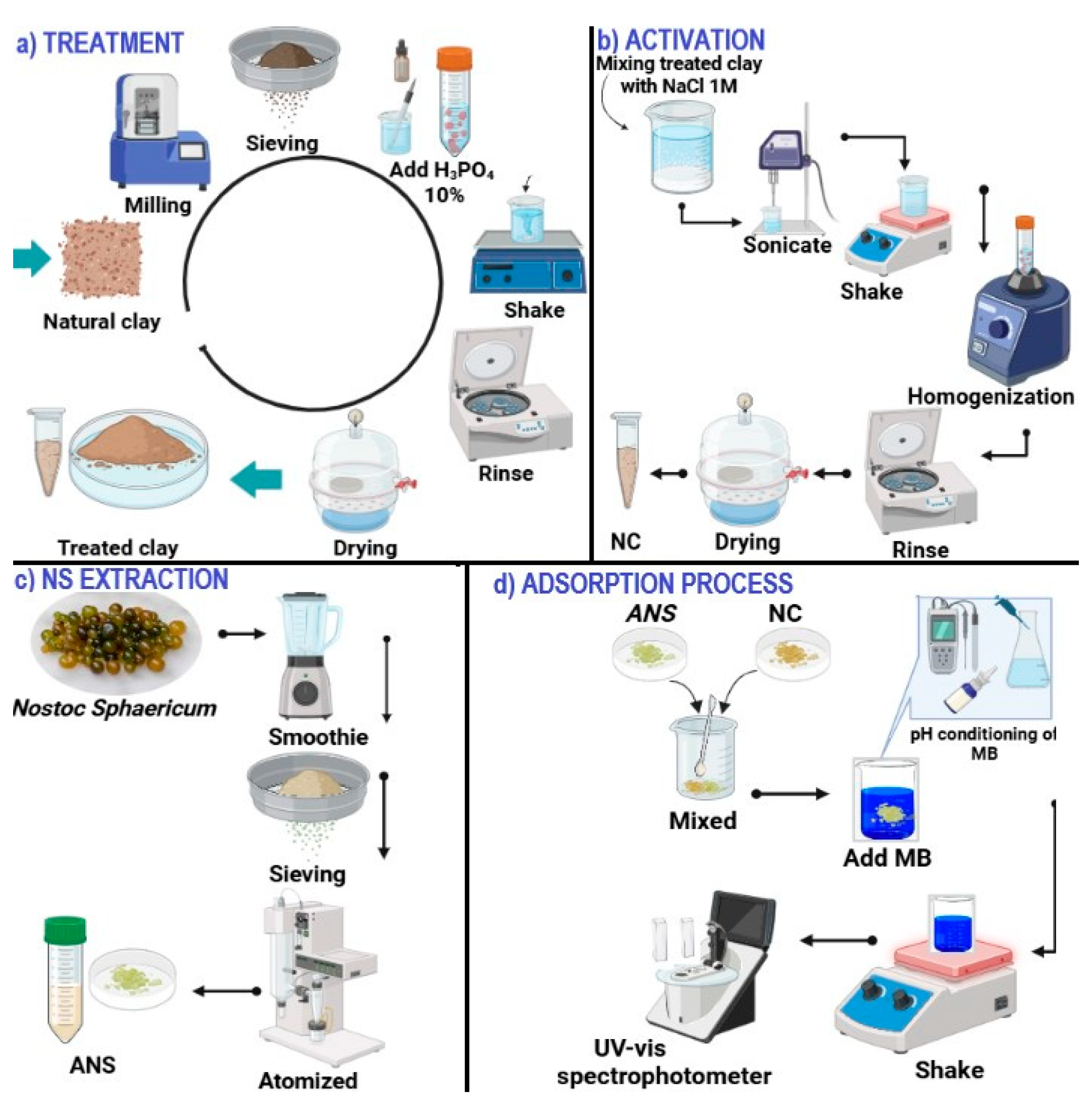
2.2. Clay Treatment and Activation
The ground and sieved clay was treated with 10% H
3PO
4, stirring at 300 rpm for 6 h. It was washed with distilled water until neutral and dried at 60 °C in a Binder Model BD56 oven (Tuttlingen, Germany), thus obtaining treated clay (
Figure 1a). NaCl was then added at 1 M (5:1
v/
w) and stirred at 200 rpm for 24 h, after which it was washed with ultrapure water until obtaining a conductivity <10 μS/cm. Then it was sonicated for 10 min at 740 W power, 750 kJ energy, and 40% amplitude in a Sonics VCX750 equipment (Newtown, CT, USA) and dried at 60 °C for 24 h. It was then ground in a planetary ball mill (PM10-Resch, Haan, Germany) at 3000 rpm for 5 min and sieved through a 45 µm mesh (model AS200, Resch, Haan, Germany), obtaining activated nanoclay (NC) [
33] (
Figure 1b).
2.3. Nostoc Sphaericum Extraction
The NS with an average moisture content of 98% was washed with abundant distilled water, then homogenized with distilled water (1:1
w/
w) in a domestic blender, and sieved at 120 µm. It was then atomized in a Buchi mini spray dryer, model B-290 (Flawil, Switzerland) at 100 °C, air flow 650 L/h, 85% suction, and feed rate 5 mL/min to obtain atomized
Nostoc sphaericum (ANS) (
Figure 1c) [
41].
2.4. Point of Zero Charge (PZC), Zeta Potential (ζ), and Particle Size Determination
For this, a 50 mL solution was prepared at pH 2 to 12, 0.05 g of adsorbent was added (1 ANS/4 NC by weight), and stirred at 60 rpm for 24 h at 20 °C. The initial and final pH values were then plotted to determine the PZC. The ζ was determined by dynamic light scattering (DLS) by preparing a suspension of 4 mg adsorbent in 50 mL ultrapure water, stirring at 1000 rpm for 5 min, and sonicating for 10 min. An aliquot was added to the size determinator (model ZSU3100, Malvern Instruments, Worcestershire, UK) at 25 °C, 632.8 nm, scattering angle of 14.14°, and electric field strength of 5 V/cm.
2.5. FTIR Analysis
Tablets of the NC, ANS, ANS/NC (1:4 w/w), and NC/ANS-MB (after adsorption) were prepared at 0.1% KBr. Readings were performed on the transmission module of the Nicolet IS50 FTIR spectrometer, Thermo Fisher (Waltham, MA, USA), with a wavelength of 4000–400 cm−1, a resolution of 8 cm−1, and 32 scans.
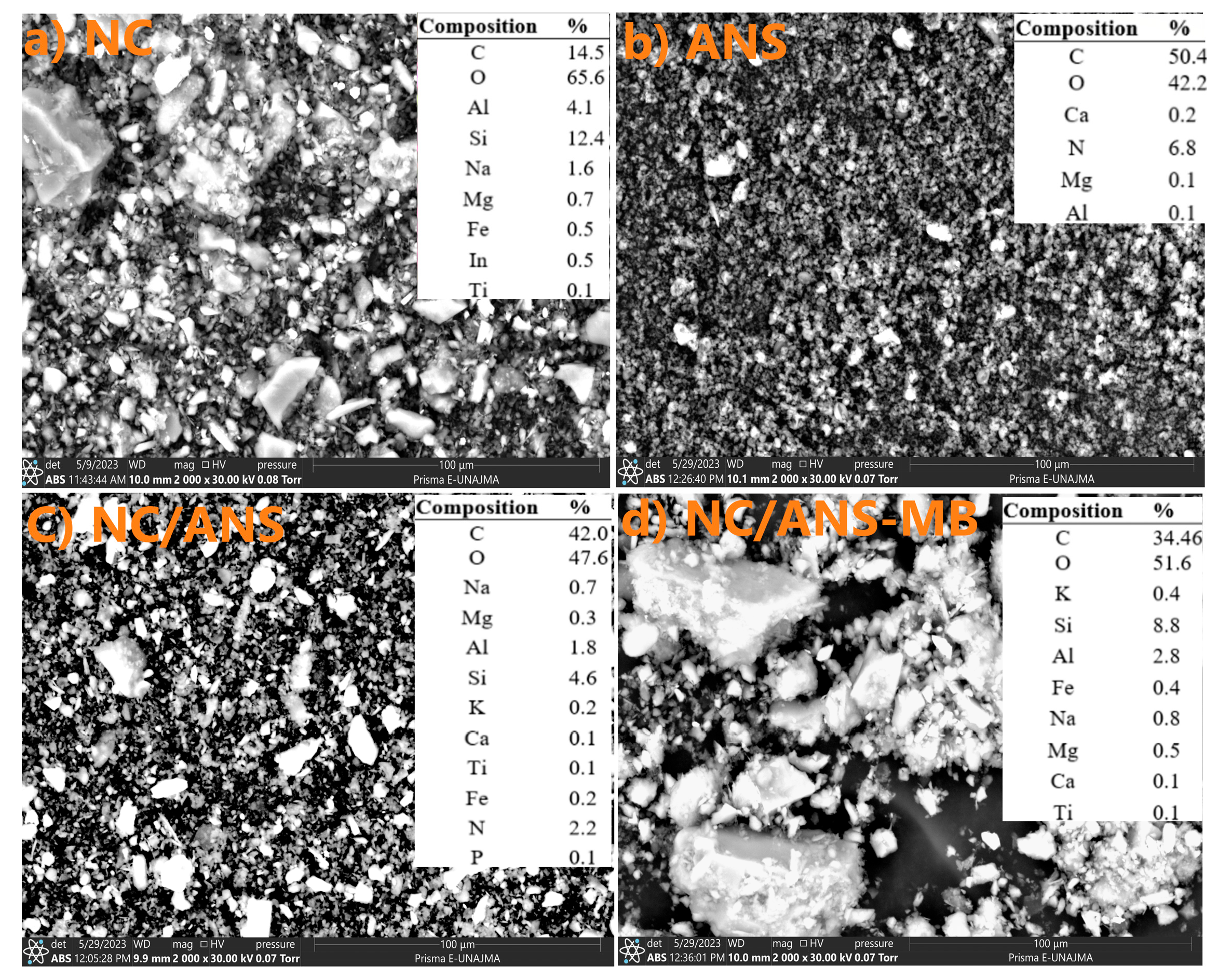
2.6. SEM and EDX Analysis
Samples of NC, ANS, ANS/NC (1:4 w/w), and NC/ANS-MB were fixed with carbon tapes, and their morphology was determined using a Prism E scanning electron microscope, Thermo Fisher (Massachusetts, MA, USA) at 25 kV acceleration and 1000× magnification. The elemental composition was also measured by X-ray energy dispersive spectroscopy in the same equipment.
2.7. Effect of pH and Adsorbent Dosage
Solutions of 10 mg/L MB were prepared at pH 5, 6, and 8. 20, 30, and 50 mg/L of adsorbent (1 ANS/4 NC) were added to each solution and stirred at 60 rpm for 60 min (
Figure 1d). Readings were taken in a UV–Vis spectrophotometer at 664 nm (model GENESYS, Thermo Fisher, Waltham, MA, USA). The standard curve (R
2 = 0.9994) was performed previously.
The percentage removal rate (%R) and adsorption capacity were calculated using Equations (1) and (2) [
34].
where
Ci and
Cf are the initial and final concentrations of MB, respectively (mg/L); m is the mass of the adsorbent (g);
V is the volume of the solution (L);
qe is the adsorption capacity (mg/g);
R is the percentage of removal (%).
2.8. Optimization of the Adsorption Process
Empirical models were constructed according to Equation (3). Linear, interaction, and quadratic models were proposed and evaluated using the Excel Solver application. The restrictions were the maximum and minimum pH value and adsorbent dose, while the objective function was to maximize R.
where
Y is the predicted response expressed in percentage (%), X
i and X
j are the independent variables (pH and adsorbent dose),
β0 is the constant, and
βi,
βii, and
βij are the linear, quadratic, and interaction coefficients of the independent variables, respectively.
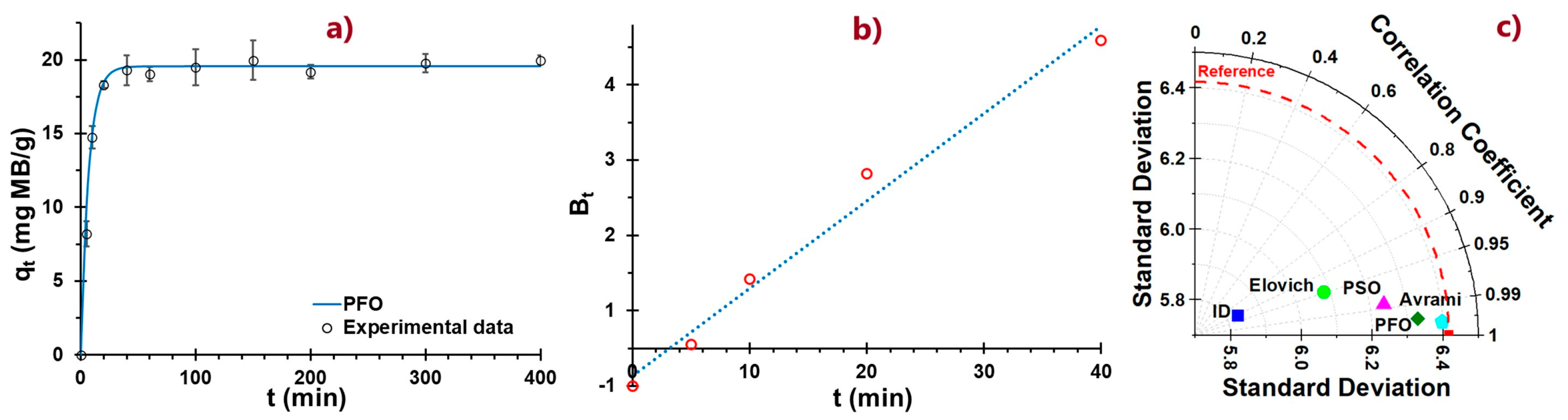
2.9. Adsorption Kinetics Study
A 100 mL solution containing 10 mg/L of MB was prepared at the pH values and the optimum dosage. Adsorption was evaluated at different times (0, 5, 20, 40, 60, 100, 150, 200, 300, and 400 min) and under constant agitation at 60 rpm and 20 °C. An aliquot was taken to the spectrophotometer at 664 nm, and the concentration of MB was reported.
The adsorption kinetics were fitted using the model:
- (a).
Pseudo-first order (PFO) (Equation (5)) kinetic models [
42,
43,
44].
The integration of the equation, for time t = 0 to t = t, the concentration of MB in the adsorbent is q = 0 to q = qt, and has the form
- (b).
Pseudo second order (PSO) [
45] (Equation (7))
Considering the boundary conditions of the pseudo-first-order equation, we obtain
- (c).
Elovich model [
46,
47] (Equation (10))
when
qt →0,
→
α, which is the initial adsorption rate (mg/g·min), and
β is the sorption constant. Integrating and applying the limits for
t (0,
t) and
qt (0,
qt), Elovich’s model is
For an equilibrium system,
t >>
, the equation can be written as
- (d).
Intraparticle diffusion (ID) [
48,
49] (Equation (12))
The description of intraparticle sorbate diffusion in the porous particle is based on Fick’s diffusion laws. The diffusion process considering sorbents can be described by the mass balance equation [
48,
49] in the form
The probability of the adsorbate being transported from a concentrated zone to the adsorbent through diffusion is considered, this being the step that limits the speed in many adsorption processes, generally for discontinuous stirring processes, where adsorption varies almost proportionally to t
1/2 instead of the contact time t [
50], according to the following equation:
- (e).
Avrami model
It is applied when it is suspected that the adsorption process involves complex mechanisms such as internal diffusion or surface heterogeneity, as well as the presence of multiple mechanisms or heterogeneous growth of adsorbed phases. It is represented by Equation (13) [
51].
- (f).
Boyd model
It is used to predict the rate-limiting step of adsorption, whether it is by internal (intraparticle) or external (pellicular) diffusion. Its nonlinear form is shown in Equation (14) [
52].
A particular solution for Bt (a mathematical function of F) is represented by Equation (15), for F > 0.85.
where
qe is the equilibrium adsorption capacity (mg/g);
qt is the adsorption capacity at one time (mg/g);
k1 is the first-order kinetic constant (1/min);
k2 is the second-order kinetic constant (g/mg·min);
t is the time (minutes);
β is the number of sites available for adsorption;
α is the initial adsorption rate (mg/g);
ki is the ID rate, and
C is the constant ID rate (if C = 0, adsorption is minimal and diffusion within the adsorbent is the main factor; if C ≠ 0, adsorption occurs both on the surface and inside the adsorbent, and high values of C favor surface adsorption);
kAv is the Avrami rate constant;
n is the Avrami constant (if
n = 1, one-dimensional adsorption;
n = 2, two-dimensional adsorption;
n = 3, three-dimensional adsorption; and
n = 4, adsorption with spherical growth); F is the fraction adsorbed at time t (F = q
t/q
e); and Bt is the Boyd function.
2.10. Adsorption Equilibrium Study
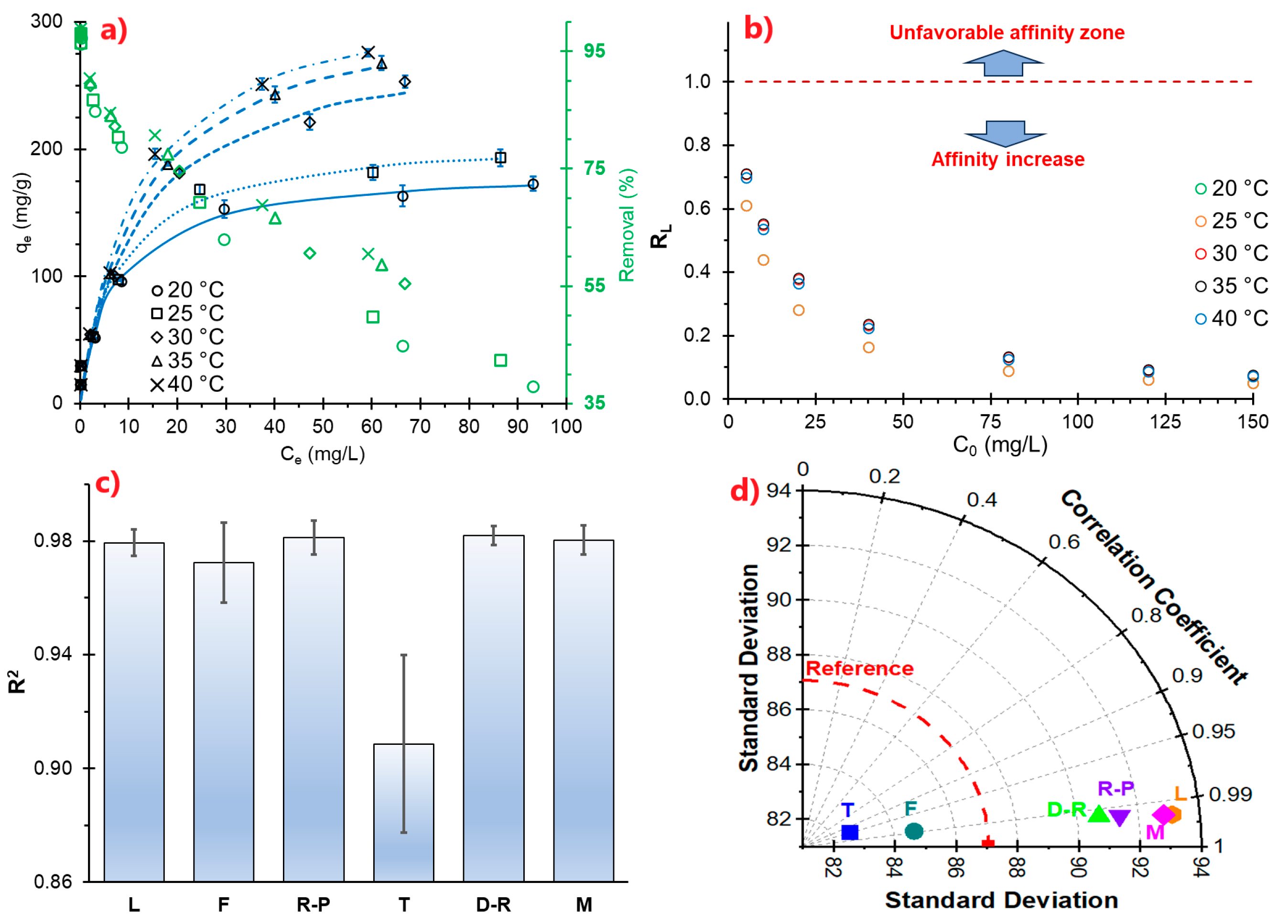
MB solutions with concentrations ranging from 5 to 150 mg/L (adsorbate) were prepared, and the adsorbent was added under optimal adsorption conditions, stirring at 50 rpm for 60 min at 20, 25, 30, 35, and 40 °C. An aliquot was taken and analyzed in a UV–Vis spectrophotometer at 664 nm. The adsorption capacity was calculated using Equation (1). The data obtained were fitted to adsorption isotherm models.
The model (Equation (16)) indicates that the adsorption capacity increases with the equilibrium concentration until it reaches a saturation value corresponding to the total surface area of the adsorbent monolayer, and no further adsorption will occur [
53].
The affinity between the adsorbate and the adsorbent is calculated using the Langmuir separation factor
RL (Equation (17)). If
RL > 1, it is unfavorable; if 0 <
RL < 1, it is favorable (adsorbate retention occurs); if
RL = 0, it is irreversible (the adsorbate is not easily desorbed and is permanently retained) [
54].
The model (Equation (18)) proposes that in an adsorbent with a homogeneous surface, there are two adsorption sites in the system: the original sites of the solid surface for adsorption in the first layer and the adsorbate site for adsorption in the second and subsequent layers (multilayer) [
55].
The model (Equation (19)) proposes that adsorption occurs on a heterogeneous adsorption surface (ideal adsorption) composed of active groups with different adsorption potentials and different adsorption energies [
56,
57].
The model (Equation (20)) explains the heterogeneity of the sorbent through the energy distribution of the active sites. The three-parameter model is formulated by combining the Langmuir and Freundlich models [
58].
The model (Equation (21)) assumes that the heat of adsorption in the adsorbent layers decreases linearly due to adsorbate–adsorbent interactions and that adsorption occurs with uniform binding energies up to a maximum energy. Its parameters allow the variation in adsorption energy to be determined [
59].
The isotherm (Equation (22)) explains adsorption as a Gaussian energy distribution on a heterogeneous and porous surface, free energy (
E) (Equation (23)), that is, it is the energy required to remove molecules from the adsorbent surface. If E < 8 kJ/mol, physisorption predominates, and if 8 < E < 168 kJ/mol, adsorption is chemical. Adsorption occurs when the micropore of the adsorbent is filled with the dye molecule [
60].
where
qe (mg/g) is the equilibrium adsorption capacity;
kL (L/mg) is the Langmuir constant;
Ce (mg/L) is the equilibrium adsorbate concentration;
qm (mg/g) is the adsorption capacity of the monolayer;
k1 (L/mg) is the adsorption affinity constant for the first layer;
k2 (L/mg) represents the adsorption affinity for subsequent multilayers;
Kf (mg
1−1/n/L
n) and
n are the Freundlich constants, indicators of adsorption capacity and adsorption intensity, respectively;
kR (L/g) indicates the maximum adsorption capacity;
aR (L/mg)
g is the constant parameter of the R–P model;
g is the parameter that varies between 0 and 1;
R is the universal gas constant (8.314 J/mol·K),
T is the temperature (K),
bT (J/mol) is the change in adsorption energy,
AT (L/mg) is the maximum binding constant,
kRD (mmol
2/J
2) is the constant of Dubinin–Radushkevich isotherm, and Ɛ is the Polanyi potential (J/mol).
The kinetic and isotherm models were fitted using nonlinear regression.
R2 (Equation (24)), Average Relative Error (
ARE) (Equation (25)), and Chi-square (
χ2) (Equation (26)) were calculated to evaluate the accuracy of the models and minimize the fractional error [
61].
where
qadj is the reported adsorption capacity,
qexp is the experimental adsorption capacity,
exp is the mean of
qexp, and N is the total number of values or estimates.
2.11. Adsorbent Regeneration Cycle
The method proposed by Auta et al. [
62] was adapted. After the test, the adsorbent was recovered under optimum conditions by filtration at 0.22 µm, rinsed with plenty of distilled water, and dried at 60 °C. The recovered adsorbent was added to 100 mL of a 0.1 M HCl desorption solution and stirred continuously at 60 rpm for 60 min. Then, it was recovered by filtration, rinsed with deionized water, and dried. The adsorption process then proceeded. This operation was repeated five times.
2.12. Statistical Data Processing
The data, represented in triplicate, were presented as mean values, standard deviations, and coefficients of variability in dynamic tables and graphs. A two-factor ANOVA with interaction described in the Pareto diagram was applied. The Taylor plot was also used to analyze the fit and correlation of the kinetic models employing the mean square error. All analyses were performed at 5% significance. Excel spreadsheets, OriginPro 2025, and STATISCA 12.0 were used.
4. Conclusions
Residues of cationic dyes are persistent and toxic to water. The use of environmentally friendly adsorbents is a growing alternative. The study proposed the use of an atomized freshwater alga, Nostoc sphaericum (ANS), and activated clay (AN). Characterization of NC/ANS reveals structural and surface properties favorable for MB adsorption. The pHPZC values (pH 3 and 6) indicate a surface with mixed characteristics, attributed to the combination of NC and ANS. ζ indicates good stability with values between −16.32 and −39.34 mV over a wide pH range (3 to 12), with higher affinity to neutral conditions. The particle size ranges from 532.17 to 5468 nm, exhibiting a heterogeneous morphology with lamellar and filamentous structures and featuring the availability of active functional groups (hydroxyl, carboxyl, and amide) that increase the number of active sites available for interaction with methylene blue. Maximum removal of 97.98% was achieved from an initial concentration of 10 ppm MB, pH 6, and 50 mg/L NC/ANS adsorbent. The optimization process resulted in optimum pH conditions (6.8) and a dose of 32.9 mg/L, from which the adsorption kinetics study was carried out, which showed rapid adsorption during the first 40 min of adsorption. The PFO and PSO models fit the kinetic process adequately, reporting adsorption capacities around 20 mg/g, suggesting that physisorption processes control adsorption at the specific active sites. Isothermal models showed that adsorption mainly occurs at the monolayer level and on a heterogeneous surface, with favorable adsorption of high MB saturation in the range of 20 to 40 °C, through an endothermic (ΔH 21.01 kJ/mol), spontaneous, and thermodynamically favorable process (ΔS 16.89 J/mol·K), reporting removal efficiency up to 37.54% after five regeneration cycles. The NC/ANS adsorbent is projected to be an efficient material due to its low application rate, taking advantage of the synergy of activated clay and the hydrocolloid from the algae Nostoc sphaericum, which confers stability during the removal of cationic dyes in aqueous solutions. However, challenges remain, such as its pilot-scale application, the effect of temperature, and competition from other dissolved ions.