Decolorization of Corn Fiber Arabinoxylan Extract with (MN102) Resin: Adsorption Performance and Film-Forming Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Resin Preparation
2.2.2. Adsorption Studies
- Effect of pH
- Effect of Resin Dosage
- Resin Regeneration
2.2.3. Adsorption Isotherms
2.3. Fixed-Bed Column Dynamic Adsorption Experiments
- Breakthrough curve modeling
- General logistic model
- Yan model
- log-Gompertz model
2.4. Film Formulation and Characterization
2.4.1. Arabinoxylan Film Preparation
2.4.2. Film Characterization
- Color measurement
- Solubility in water
- Mechanical Properties—Perforation
- Statistical Analysis
3. Results and Discussion
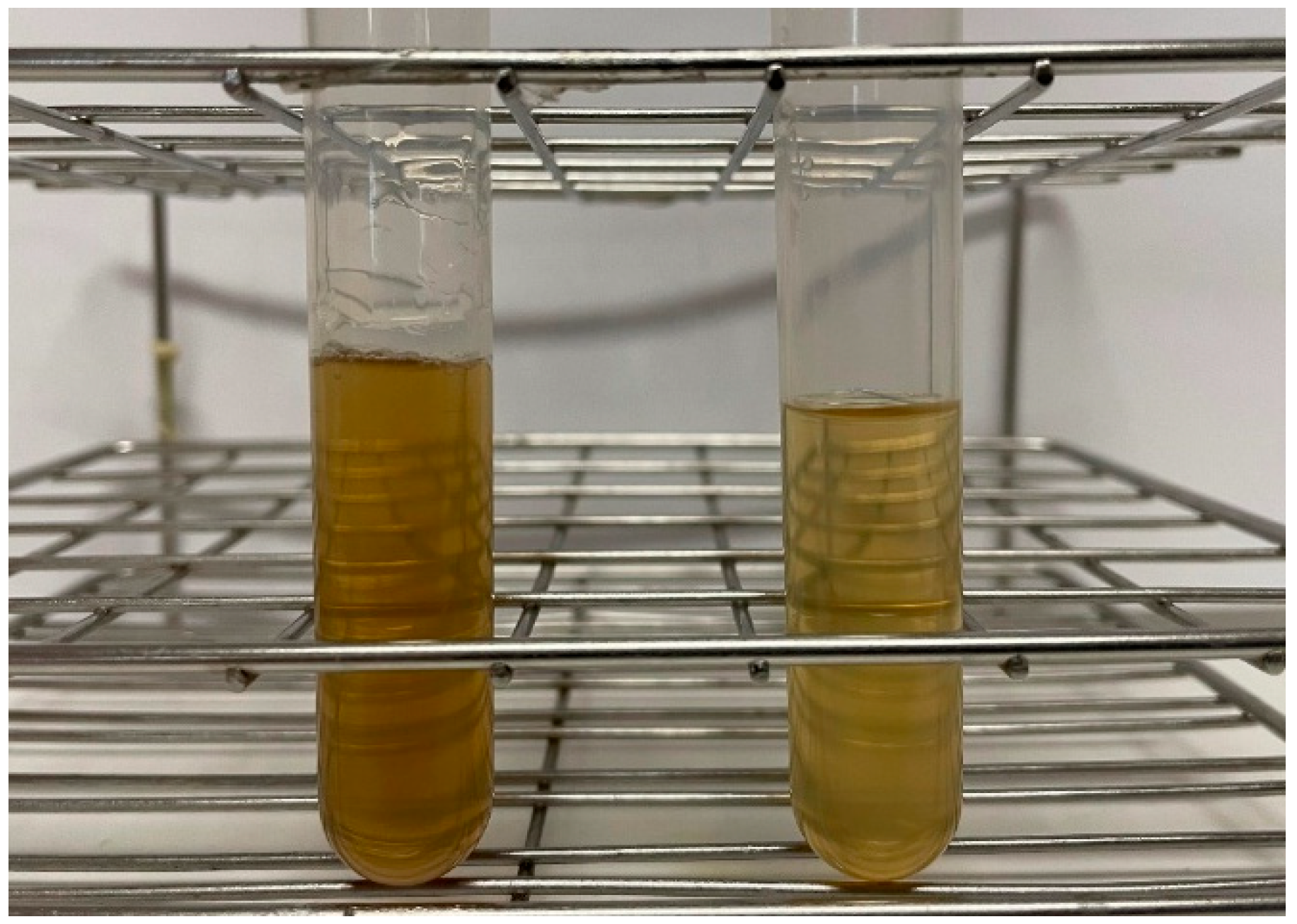
3.1. Arabinoxylan Extract Studies
3.1.1. Effect of Resin Dosage
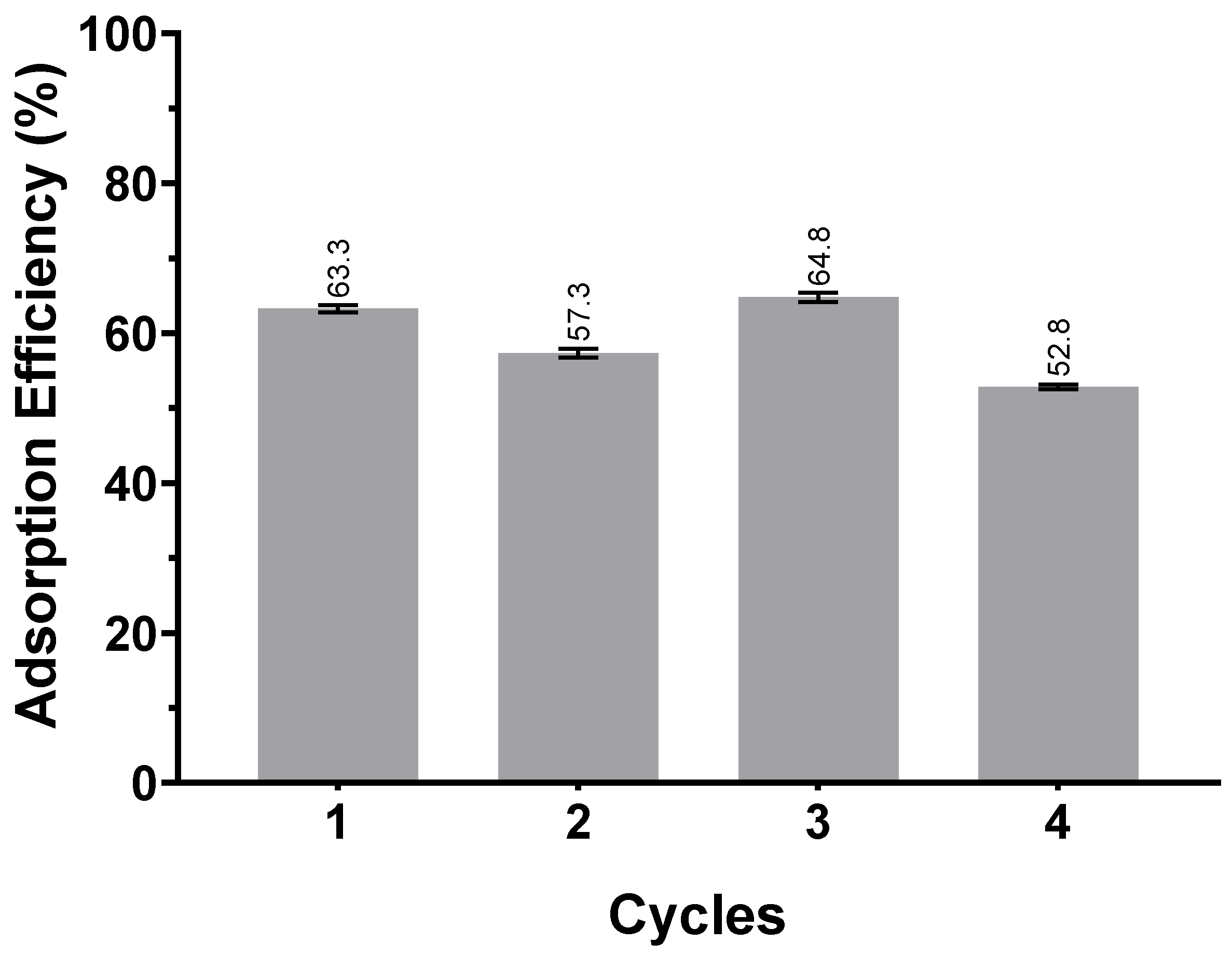
3.1.2. Resin Regeneration
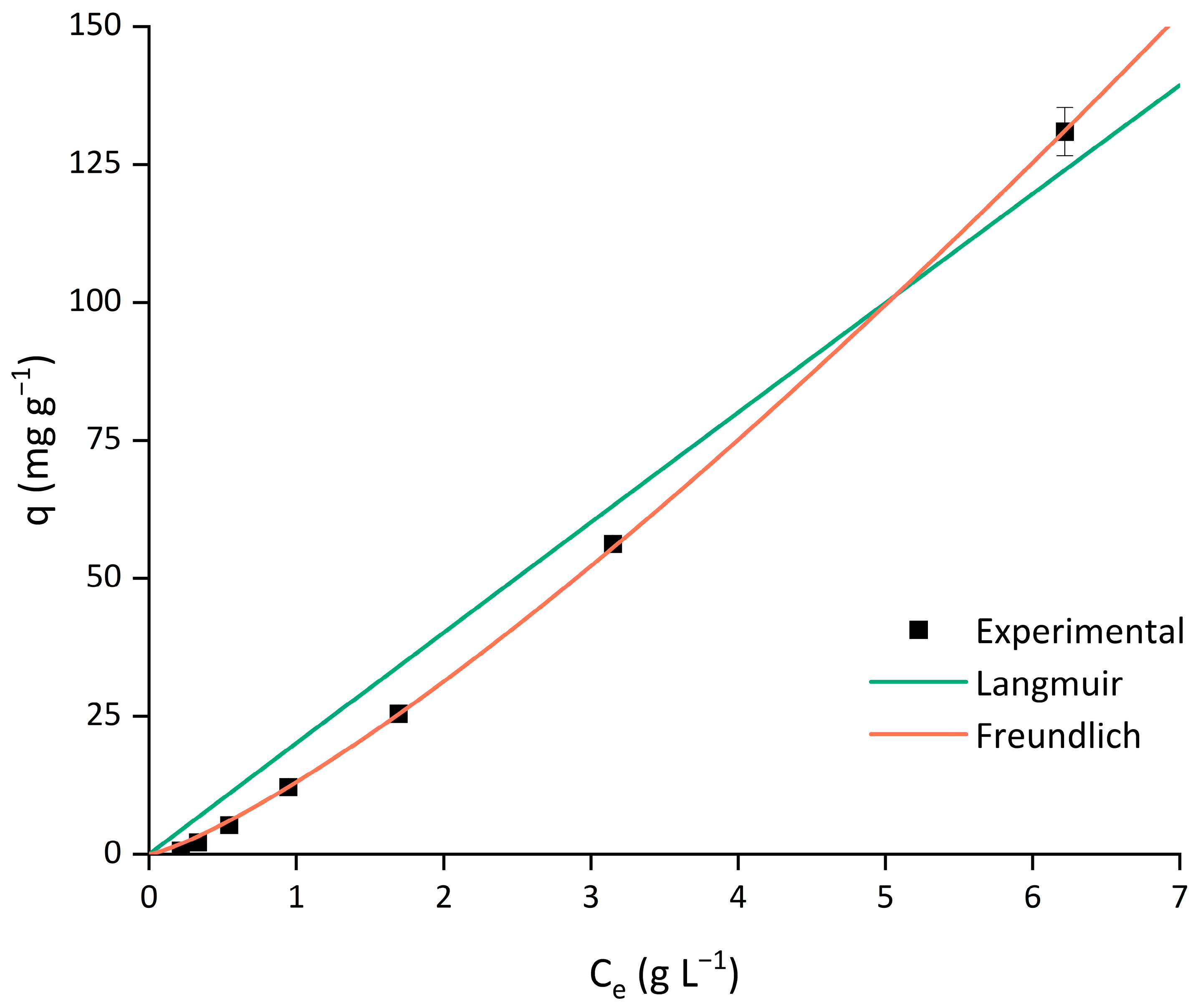
3.1.3. Adsorption Isotherm Modeling
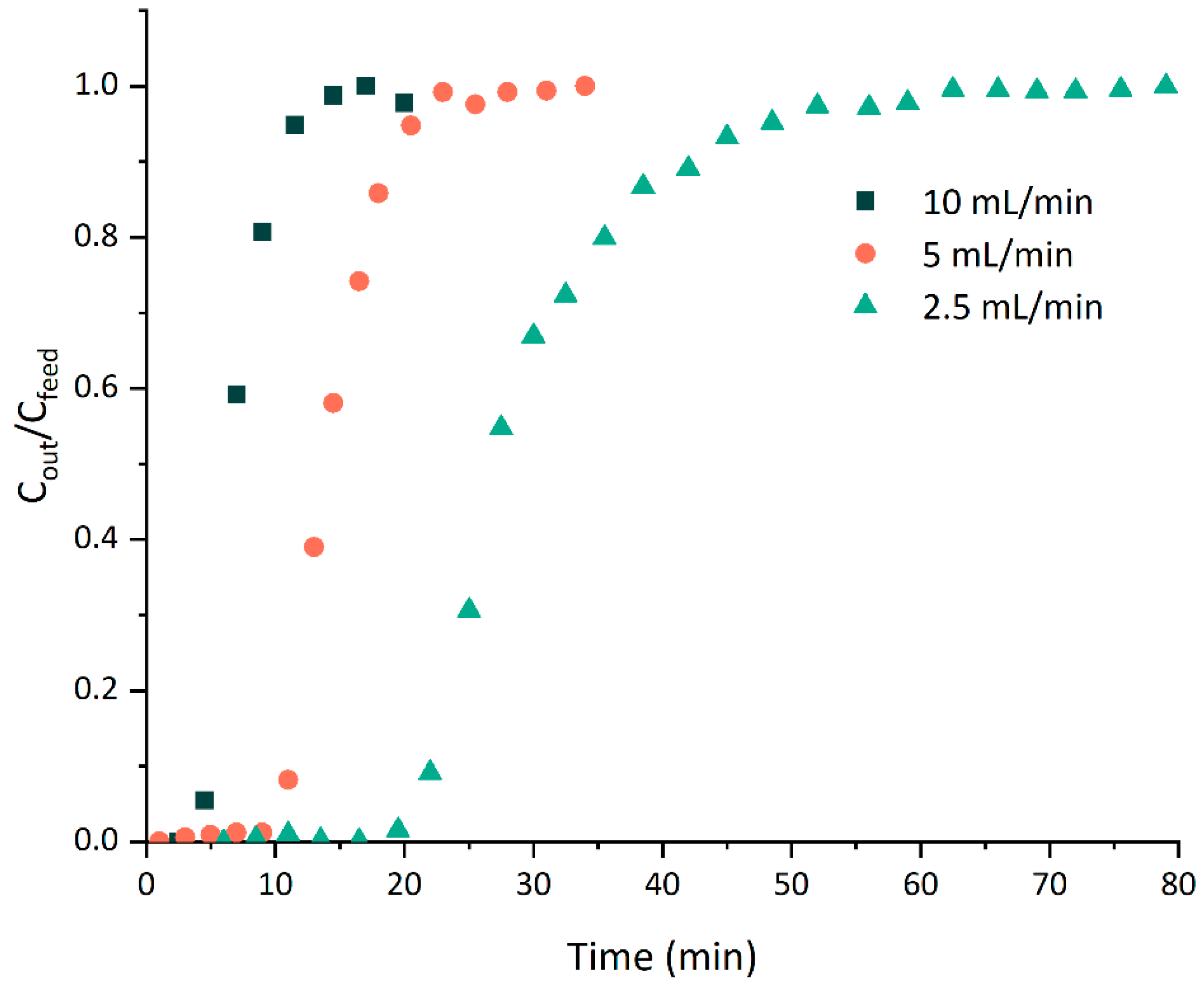
3.1.4. Dynamic Adsorption Experiments
3.1.5. Breakthrough Curve Modeling
3.1.6. Scaled-Up Process
3.2. Arabinoxylan Film and Characterization
3.2.1. Arabinoxylan Films
3.2.2. Color Measurement
3.2.3. Solubility in Water
3.2.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, R.; Sharma, M. Cereal Polysaccharides as Sources of Functional Ingredient for Reformulation of Meat Products: A Review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G.; Larsson, A.; Ström, A. Thermoplastic and Flexible Films from Arabinoxylan. ACS Appl. Polym. Mater. 2019, 1, 1443–1450. [Google Scholar] [CrossRef]
- Weng, V.; Cardeira, M.; Bento-Silva, A.; Serra, A.T.; Brazinha, C.; Bronze, M.R. Arabinoxylan from Corn Fiber Obtained through Alkaline Extraction and Membrane Purification: Relating Bioactivities with the Phenolic Compounds. Molecules 2023, 28, 5621. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Simsek, S. What Are the Characteristics of Arabinoxylan Gels? Food Nutr. Sci. 2018, 9, 818–833. [Google Scholar] [CrossRef]
- Rudjito, R.C.; Matute, A.C.; Jiménez-Quero, A.; Olsson, L.; Stringer, M.A.; Krogh, K.B.R.M.; Eklöf, J.; Vilaplana, F. Integration of Subcritical Water Extraction and Treatment with Xylanases and Feruloyl Esterases Maximises Release of Feruloylated Arabinoxylans from Wheat Bran. Bioresour. Technol. 2024, 395, 130387. [Google Scholar] [CrossRef]
- Weng, V.; Brazinha, C.; Coelhoso, I.M.; Alves, V.D. Decolorization of a Corn Fiber Arabinoxylan Extract and Formulation of Biodegradable Films for Food Packaging. Membranes 2021, 11, 321. [Google Scholar] [CrossRef]
- Serra, M.; Weng, V.; Coelhoso, I.M.; Alves, V.D.; Brazinha, C. Purification of Arabinoxylans from Corn Fiber and Preparation of Bioactive Films for Food Packaging. Membranes 2020, 10, 95. [Google Scholar] [CrossRef]
- Yan, J.; Jia, X.; Feng, L.; Yadav, M.; Li, X.; Yin, L. Rheological and Emulsifying Properties of Arabinoxylans from Various Cereal Brans. J. Cereal Sci. 2019, 90, 102844. [Google Scholar] [CrossRef]
- Egüés, I.; Stepan, A.M.; Eceiza, A.; Toriz, G.; Gatenholm, P.; Labidi, J. Corncob Arabinoxylan for New Materials. Carbohydr. Polym. 2014, 102, 12–20. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, J.; Lan, W.; Yu, L.; Bi, Y.; Song, S.; Xiong, B.; Wang, H. Polysaccharide Decolorization: Methods, Principles of Action, Structural and Functional Characterization, and Limitations of Current Research. Trends Food Sci. Technol. 2023, 138, 284–296. [Google Scholar] [CrossRef]
- Valério, R.; Torres, C.A.V.; Brazinha, C.; da Silva, M.G.; Coelhoso, I.M.; Crespo, J.G. Purification of Ferulic Acid from Corn Fibre Alkaline Extracts for Bio-Vanillin Production Using an Adsorption Process. Sep. Purif. Technol. 2022, 298, 121570. [Google Scholar] [CrossRef]
- Dávila-Guzman, N.E.; Cerino-Córdova, F.J.; Diaz-Flores, P.E.; Rangel-Mendez, J.R.; Sánchez-González, M.N.; Soto-Regalado, E. Equilibrium and Kinetic Studies of Ferulic Acid Adsorption by Amberlite XAD-16. Chem. Eng. J. 2012, 183, 112–116. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, C.P.; Huang, G.Q.; Xiao, J.X. Fractionation and Structural Characterization of Polysaccharides Derived from Red Grape Pomace. Process. Biochem. 2021, 109, 37–45. [Google Scholar] [CrossRef]
- Wang, W.; Dong, S.; Zhang, W.; Qi, Z.; Liu, X. Study on Adsorption/Resolution Properties, Enrichment and Purification of Phenolic Substances of Inonotus Hispidus by Macroporous Adsorption Resin. Ind. Crops Prod. 2024, 216, 118661. [Google Scholar] [CrossRef]
- Li, S.F.; Li, G.L.; Chen, D.L.; Zhang, L.W. A Green and Simple Method for Enrichment of Major Diterpenoids from the Buds of Wikstroemia Chamaedaphne with Macroporous Resins and Their Activation of Latent Human Immunodeficiency Virus Activity. Int. J. Biol. Macromol. 2024, 272, 132932. [Google Scholar] [CrossRef] [PubMed]
- Macronet MN102 Data Sheet. Available online: https://www.purolite.com/product?normalizedName=mn102 (accessed on 20 November 2024).
- Chavarria-Quicaño, E.; Amaya-Delgado, L.; Arellano-Plaza, M.; Mateos-Díaz, J.C.; Asaff-Torres, A. Improvement of Agave Bagasse Hydrolysates Processing under a Biorefinery Approach. Sep. Purif. Technol. 2024, 347, 127392. [Google Scholar] [CrossRef]
- Davankov, V.; Tsyurupa, M. Sorption of Organic Compounds from Aqueous Solutions. Compr. Anal. Chem. 2011, 56, 411–444. [Google Scholar]
- Alchihab, M.; Aldric, J.M.; Aguedo, M.; Destain, J.; Wathelet, J.P.; Thonart, P. The Use of Macronet Resins to Recover γ-Decalactone Produced by Rhodotorula Aurantiaca from the Culture Broth. J. Ind. Microbiol. Biotechnol. 2010, 37, 167–172. [Google Scholar] [CrossRef]
- Gago, D.; Corvo, M.C.; Chagas, R.; Ferreira, L.M.; Coelhoso, I. Protein Adsorption Performance of a Novel Functionalized Cellulose-Based Polymer. Polymers 2022, 14, 5122. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, X.; Kang, K.; Xie, Y.; Tang, M.; Song, H.; Liang, Y.; Hu, J.; Bai, S. Comparison and Development of the Descriptive Model with Polynomial Structures to Fit Multi-Component Dynamic Breakthrough Curves with Roll-up, Stepwise, and Saddle-Shaped Structure. Sep. Purif. Technol. 2025, 355, 129644. [Google Scholar] [CrossRef]
- Apiratikul, R.; Chu, K.H. Improved Fixed Bed Models for Correlating Asymmetric Adsorption Breakthrough Curves. J. Water Process Eng. 2021, 40, 101810. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A New Model for Heavy Metal Removal in a Biosorption Column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Santos, M.V.; Zaritzky, N. Experimental Study and Modelling of an Azo Colourant Dynamic Adsorption onto Functional Cross-Linked Chitosan/Ceramic Particles in a Fixed Bed Column. Can. J. Chem. Eng. 2023, 101, 5941–5955. [Google Scholar] [CrossRef]
- Sukatis, F.F.; Looi, L.J.; Lim, H.N.; Abdul Rahman, M.B.; Mohd Zaki, M.R.; Aris, A.Z. Fixed-Bed Adsorption Studies of Endocrine-Disrupting Compounds from Water by Using Novel Calcium-Based Metal-Organic Frameworks. Environ. Pollut. 2024, 341, 122980. [Google Scholar] [CrossRef] [PubMed]
- Torkaman, R.; Asadollahzadeh, M. Effectively Trapping Lanthanum Ions with the NWPP Polymeric Adsorbent through Gamma Ray Modification Procedure in Continuous Bed Column. Sep. Purif. Technol. 2025, 358, 130305. [Google Scholar] [CrossRef]
- Kimani, P.K. Asymmetrical Fixed-Bed Breakthrough Curve Modelling: Comparing Simplistic, Log-Modified, Fractal-like, and Probability Distribution Function Models. Chem. Eng. Res. Des. 2024, 201, 446–456. [Google Scholar] [CrossRef]
- de Oliveira, J.T.; Costa, L.R.d.C.; Agnol, G.D.; Féris, L.A. Experimental Design and Data Prediction by Bayesian Statistics for Adsorption of Tetracycline in a GAC Fixed-Bed Column. Sep. Purif. Technol. 2023, 319, 124097. [Google Scholar] [CrossRef]
- Chen, X.; Ren, Y.; Qu, G.; Wang, Z.; Yang, Y.; Ning, P. A Review of Environmental Functional Materials for Cyanide Removal by Adsorption and Catalysis. Inorg. Chem. Commun. 2023, 157, 111298. [Google Scholar] [CrossRef]
- Chu, K.H. Fixed Bed Adsorption of Water Contaminants: A Cautionary Guide to Simple Analytical Models and Modeling Misconceptions. Sep. Purif. Rev. 2023, 52, 75–97. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour Difference ∆E-A Survey. Mach. Graph. Vision 2011, 20, 383–411. [Google Scholar]
- Bangar, S.P.; Singh, A.; Trif, M.; Kumar, M.; Kumar, P.; Kaur, R.; Kaur, N. Process Parameter Optimization and Characterization for an Edible Film: Flaxseed Concern. Coatings 2021, 11, 1106. [Google Scholar] [CrossRef]



| Internal diameter, Dc (cm) | 2.50 |
| Total column length, Ltot (cm) | 20.00 |
| Wet resin weight (g) | 15.00 |
| Dry resin mass (g) | 5.75 |
| Bed height, Lc (cm) | 11.00 |
| Bed volume, BV (mL) | 54.00 |
| Lc/Dc | 4.40 |
| Cross-sectional area, Ac (cm2) | 4.91 |
| Model | a | b |
|---|---|---|
| Bohart–Adams | ||
| Thomas | ||
| Yoon–Nelson |
| Sample | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg g−1) | KL (L mg−1) | R2 | χ2 | n | KF (L mg−1) | R2 | χ2 | |
| Arabinoxylan Extract | 1.093 × 104 | 1.845 × 10−6 | 0.9789 | 14.73 | 0.7924 | 2.138 × 10−3 | 0.9997 | 1.307 |
| Flow Rate (mL min−1) | 2.5 | 5 | 10 |
|---|---|---|---|
| tb (min) | 20.6 | 10.5 | 4.5 |
| tc (min) | 27.5 | 13.9 | 6.6 |
| te (min) | 46.9 | 21.6 | 12.2 |
| Flow Rate (mL min−1) | ||||
|---|---|---|---|---|
| Model | Parameter | 2.5 | 5 | 10 |
| General logistic | a | 7.25 ± 0.70 | 7.78 ± 0.62 | 6.03 ± 0.98 |
| b (min−1) | 0.257 ± 0.025 | 0.548 ± 0.043 | 0.881 ± 0.139 | |
| χ2 | 0.002 | 0.001 | 0.002 | |
| R2 | 0.988 | 0.995 | 0.988 | |
| Bohart–Adams | kBA × 103 (L mg−1 min−1) | 0.224 ± 0.022 | 0.435 ± 0.034 | 0.725 ± 0.114 |
| N0 (mg L−1) | 1500.2 ± 145.4 | 1656.5 ± 131.3 | 1539.0 ± 250.7 | |
| Thomas | kT × 103 (L mg−1 min−1) | 0.224 ± 0.022 | 0.435 ± 0.034 | 0.725 ± 0.114 |
| q0 (mg g−1) | 14.1 ± 1.4 | 15.6 ± 1.2 | 14.5 ± 2.4 | |
| Yoon–Nelson | kYN (min−1) | 0.257 ± 0.025 | 0.548 ± 0.043 | 0.881 ± 0.139 |
| τ (min) | 28.3 ± 0.4 | 14.2 ± 0.9 | 6.8 ± 0.9 | |
| Flow Rate (mL min−1) | ||||
|---|---|---|---|---|
| Model | Parameter | 2.5 | 5 | 10 |
| Yan | q0 (mg g−1) | 13.9 ± 0.1 | 15.4 ± 0.0 | 14.2 ± 0.2 |
| αY | 6.89 ± 0.42 | 7.88 ± 0.03 | 5.73 ± 0.49 | |
| χ2 | 0.001 | 0.000 | 0.001 | |
| R2 | 0.994 | 0.999 | 0.996 | |
| log-Gompertz | kG1 | 14.5 ± 1.2 | 16.1 ± 1.5 | 7.1 ± 0.4 |
| kG2 (min−1) | 4.5 ± 0.4 | 6.3 ± 0.6 | 4.0 ± 0.2 | |
| χ2 | 0.000 | 0.000 | 0.000 | |
| R2 | 0.997 | 0.998 | 0.999 | |
| Scale-Up Factor | Bed Height (cm) | tc (min) |
|---|---|---|
| 1 | 11 | 6.6 |
| 5 | 55 | 33 |
| 10 | 110 | 66 |
| Film | L* | a* | b* | h° | C* | ΔE*ab |
|---|---|---|---|---|---|---|
| Ax | 68.32 ± 2.15 a | 5.72 ± 1.08 a | 33.54 ± 1.36 a | 80.36 ± 1.44 a | 34.03 ± 1.52 a | 15.39 ± 2.08 |
| AxR | 81.07 ± 0.61 b | −0.35 ± 0.20 b | 27.40 ± 1.62 b | 90.75 ± 0.46 b | 27.40 ± 1.62 b |
| Film | Solubility (%) |
|---|---|
| Ax | 96 ± 2 a |
| AxR | 91 ± 5 a |
| Film | Thickness (µm) | Tension of Perforation (σ, MPa) | Deformation Upon Perforation (ε, %) |
|---|---|---|---|
| Ax | 81 ± 13 a | 2.71 ± 1.23 a | 32.0 ± 4.5 a |
| AxR | 69 ± 20 a | 2.55 ± 0.85 a | 52.4 ± 5.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, V.; Gago, D.; Brazinha, C.; Alves, V.D.; Coelhoso, I.M. Decolorization of Corn Fiber Arabinoxylan Extract with (MN102) Resin: Adsorption Performance and Film-Forming Capacity. Polymers 2025, 17, 2128. https://doi.org/10.3390/polym17152128
Weng V, Gago D, Brazinha C, Alves VD, Coelhoso IM. Decolorization of Corn Fiber Arabinoxylan Extract with (MN102) Resin: Adsorption Performance and Film-Forming Capacity. Polymers. 2025; 17(15):2128. https://doi.org/10.3390/polym17152128
Chicago/Turabian StyleWeng, Verónica, Diana Gago, Carla Brazinha, Vítor D. Alves, and Isabel M. Coelhoso. 2025. "Decolorization of Corn Fiber Arabinoxylan Extract with (MN102) Resin: Adsorption Performance and Film-Forming Capacity" Polymers 17, no. 15: 2128. https://doi.org/10.3390/polym17152128
APA StyleWeng, V., Gago, D., Brazinha, C., Alves, V. D., & Coelhoso, I. M. (2025). Decolorization of Corn Fiber Arabinoxylan Extract with (MN102) Resin: Adsorption Performance and Film-Forming Capacity. Polymers, 17(15), 2128. https://doi.org/10.3390/polym17152128









