1. Introduction
The provision of safe, potable water represents one of the most significant challenges for contemporary society, exacerbated by accelerating industrialization and demographic growth [
1,
2,
3]. Unregulated or inadequately treated effluents from industrial and municipal sources have led to the accumulation of toxic and hazardous substances in surface and groundwater bodies [
4,
5], posing significant risks to both the environment and human beings. Consequently, the development of robust treatment schemes capable of removing or degrading these pollutants is of paramount importance [
6,
7]. Traditional physicochemical and biological remediation methods have limited efficacy, high operational costs, and the potential to form secondary pollutants [
8,
9,
10]. Thus, as nanomaterials developed for environmental applications, magnetic NPs offer additional advantages, such as their ability to be produced on a large scale, low toxicity, and eco-compatibility [
11,
12,
13,
14,
15,
16,
17]. The most common magnetic NPs are composed of metals, as well as their oxides, such as nickel, iron, and cobalt [
18,
19,
20].
Among the various mineral materials studied, such as clays, zeolites, aluminosilicates, sulphates, and iron hydroxides, zero-valent iron (ZVI) NPs have demonstrated exceptional performance [
21]. Through electron transfer processes with adsorbed contaminants, ZVI facilitates advanced reductive degradation of organic compounds and transforms metal ions into less soluble oxidation states, thus promoting contaminant sequestration.
Through electron transfer processes with adsorbed contaminants, these NPs facilitate the advanced reductive degradation of organic compounds and transform metal ions into less soluble oxidation states, thus promoting contaminant sequestration. Naturally dissolved iron in water is oxidized, either by dissolved oxygen or by residual disinfectants such as chlorine, to form ferric hydroxide precipitates. These precipitates induce turbidity and promote the accumulation of iron oxide crusts in distribution networks. Such a crust is particularly exacerbated during periods of stagnation, for example, following maintenance-induced flow interruptions or hydraulic redirection. At the same time, the interaction of water laden with pollutants can accelerate the degradation of pipe materials (ductile iron (DF), high-density polyethylene (HDPE), or glass-fibre-reinforced polyester with sand incrustations (GFRP)) and can compromise hydraulic integrity. For pipes made of polymeric and composite materials, additional protective measures are required to safeguard against mechanical and chemical damage. Damage to DF distribution networks often occurs due to the solubilization of iron salts in acidic environments and mineral deposits, as well as microbiological corrosion, which accelerates bacterial colonisation [
21,
22].
Progressive deterioration driven by dissolved iron species—ranging from ferrous (Fe
2+) to ferric (Fe
3+) forms—has a negative impact on water quality (taste, odour, colour, and turbidity), while promoting particulate and biofilm formation that impacts treatment efficacy [
23].
As is known, iron oxides exist in different crystalline phases, such as magnetite (Fe
3O
4), maghemite (γ-Fe
3O
4), and hematite (α-Fe
3O
4), magnetite being the most widespread type [
24] with the highest magnetism of all iron oxides. Maghemite, on the other hand, is a mixture of the composition and structure of magnetite and hematite, reflecting structural similarities of both forms of iron oxides. γ-Fe
3O
4 is a reddish-brown material that forms on soil due to the corrosion of magnetite, and α-Fe
3O
4 is the oldest known form of iron oxide [
25].
Some recent studies have used magnetic NPs to help remediate oil-contaminated soils [
26,
27]. The use of Fe
3O
4 NPs could reduce reliance on conventional methods, thereby reducing treatment costs and environmental risks [
28].
In this work, a computational simulation was used to predict and optimize the interaction, at the molecular level, between Fe
3O
4 NPs and target pollutants, providing insights into the synthesis of polymer composite films with water purification applications and improved catalytic and adsorption properties. Thus, by comparative analysis of the coupling of geologically analysed polymeric material selection with a computer simulation, we aim to establish a scalable and efficient platform for the mitigation of emerging contaminants in public water supplies [
29]. This analysis allows us to directly correlate the predictions of the computer simulation (e.g., binding energies, adsorption sites, and electronic structure changes) with the experimental observations obtained from SEM, EDX, and BET analyses and aims to confirm the effectiveness of the material in capturing and degrading contaminants. Thus, this work aims to develop a method for purifying tap water, associated with algorithms that can provide a complete picture of its contamination, in terrestrial areas, presenting certain advantages over the other methods studied to date [
30,
31,
32,
33].
This approach is helpful in identifying areas requiring intervention, as well as in establishing criteria for preventing exposure to contaminated water. The deposition of ferric oxide precipitates and other chemical contaminants, including synthetic organic pollutants, on distribution network pipes adsorbs the residual chlorine from the water, thus reducing the chlorination effect. Therefore, it is necessary to monitor water parameters to maintain them within the permissible limits. By comparative analysis of the efficiency of wastewater decontamination using LDPE/Fe
3O
4-type polymeric films through computer modeling (DFT, DFTB+) and experimental validation (SEM, EDX, BET), it was demonstrated that these materials maintain their structural integrity, offer high magnetic reactivity, and exhibit improved adsorption of heavy metal ions [
34,
35,
36,
37].
Computer simulations highlight the electronic and magnetic properties of Fe3O4 NPs embedded in the polymer matrix, which lead to an increase in the affinity of the composite for heavy metals and organic pollutants. Experimental tests validate these predictions by demonstrating the effectiveness of the chosen solution, establishing a clear link between the computer simulation and the experimental results obtained. Water abstracted from the aquifer is often contaminated simultaneously with several pollutants, which can interact, resulting in profound effects on human health; therefore, the choice of material for replacing pipes and its adaptation to the network connection are issues that are intensively studied. The novelty of this work consists in the selection (after a careful and detailed analysis of these materials) and treatment of the polymeric composite material for an improvement of its anti-corrosive characteristics.
The anti-corrosion coating, made of a blend of low-density polyethylene (LDPE) and Fe3O4 NP filler (NPFE), exhibits strong adhesion, low shrinkage, and good chemical stability in water. The role of the filler (Fe3O4 NPs) is to improve the adhesion of the polymer film and its durability and to prevent microcracking.
Overall, the synergy between computer modeling and experimental validation allows for a more rational and efficient design of polymer composites tailored for enhanced water purification, providing an understanding of the mechanisms that occur and how these materials interact with contaminants and improve water quality.
3. Results
3.1. Characteristics of the Deferrization Treatment
The samples collected from the water distribution network in the analyzed area were treated at the laboratory level, and the experimental results obtained indicate a fluctuation in corrosivity and provide useful information for monitoring: the corrosion rate in the distribution network and water quality. Turbidity is a crucial index for assessing the quality of drinking water in terms of the degree of obstruction to the passage of light through a solution. This phenomenon is closely related to the content of impurities, such as sediment, clay, plankton, and microorganisms [
49]. The characteristics of deionized water and the recorded turbidity data are presented in
Table 4.
Therefore, the water is delivered with the required drinking water characteristics, including turbidity below the maximum permissible limit of 5 NTU [
50]. The life cycles of functional groups and excited ions produce significant stress on the polymer structure, which can be highlighted with the BIOVIA Materials Studio DFTB+ simulation program, based on the functional density and electronic properties of these groups.
Using the method configured by the Materials Studio DFTB+ model, defects and complex interactions of atomic orbitals between the organic and inorganic surfaces of the composite were studied. The recorded parameter values in the DFTB+ database were compared with the Slater–Koster parameter libraries, which contain specific values of the interactions between the reactive elements in the analyzed polymer composite material. If elements appear that are not identified, the Materials Studio DFTB+ module provides specific comparative data or support for the development of new sets of values, allowing the extension of experimental data to new systems that detailed predictions can analyze. In addition to the usual repulsive and short-range repulsive electronuclear terms, the Kohn–Sham approximate final energy additionally includes a Coulomb interaction between charge fluctuations. Electrostatic forces are considered between two-point charges at varying distances and also include self-interaction of atoms if the charges are located on the same atom.
3.2. Determination of Optimal Molecular Geometries
Based on the molecular dynamics of different optimized geometries, one can determine the density of the polymer band, the energy of the orbitals in the layers, and some mechanical properties of the durable structure.
The oxidation potential of excited spinel molecules for the calculation of photosensitization adsorption parameters is given by the following relation:
where:
Pox = oxidation potential.
λ
max = the wavelength of maximum adsorption of the lowest-energy electronic transition in the UV/vis adsorption spectrum of the excited molecule (Mex), cumulated with the photosensitizing energy (E
photo).
where:
Γ = band dipole moment correction factor.
where:
EPL = photosensitive coating efficiency.
eVphoto = open-circuit photo voltage correlated with the level of sensitization, as follows:
F = oscillating power as the corresponding UV/vis adsorption band value.
η = overall efficiency, η as a function of photosensitive cell energy.
The optimal molecular geometries for the polymer film structures were calculated using approximate estimates of solvation energies, calculated for metal ions as the difference between the total energies in solvents used for recovery from the residual effluent (tetrahydrofuran (THF) and ethanol (EtOH)) and the solution, and are reported in
Table 5. Simulations have complemented experimental data, providing new insights into polymer–turbulence interactions, in particular the suppression of turbulent vortices by elastic stresses [
51,
52]. Tests performed in flow systems (pipes and channels) have clarified the interaction between turbulence modulation and fluid elasticity, correlating pressure drop, velocity profiles, and elastic shear stress with the efficiency of flow resistance reduction [
53,
54].
The values on the energy shift of solvents (essential in modifying the photophysical properties) lead to increased electronic excitation in adsorption reactions in the photosensitization processes of functional groups and lattice ions, which is in agreement with UV/Vis spectra and the adsorption–desorption isotherms of metal ions and some functional groups. The eVphoto values indicate that the more significant the adsorption–desorption phenomenon, the more efficient the sensitization, so the solvent has a smaller effect on the charge transfer. The calculated average intramolecular charge value of 0.68 indicates that there is an equilibrium at the transitions and that this could be associated with possible defects in the structure when there is a tendency for the energy to increase on specific facets of the structure. Thus, spontaneous deformation energies appear at the surface of structures, including functional groups and excited ions, which means that the solvent has an increased effect on the functional structures. Possible adsorption configurations may occur at the interaction between the two surfaces of the organic–organic system as the temperature slowly rises. The additional local energy phenomenon shows an increased adsorption of carboxylate functional groups or oxido-metallic groups, marked by their adsorption energies at the surface of the configurations. The adsorption and deformation energies indicate that there are configurations of adsorbed functional groups that are influenced by the excitation energies and their direction of adhesion (horizontal or vertical), and they also suggest that their adsorption can be spontaneous. Still, the direction of adsorption–desorption is a function of the nature of the functional group, the pore-filling gradient, and the temperature.
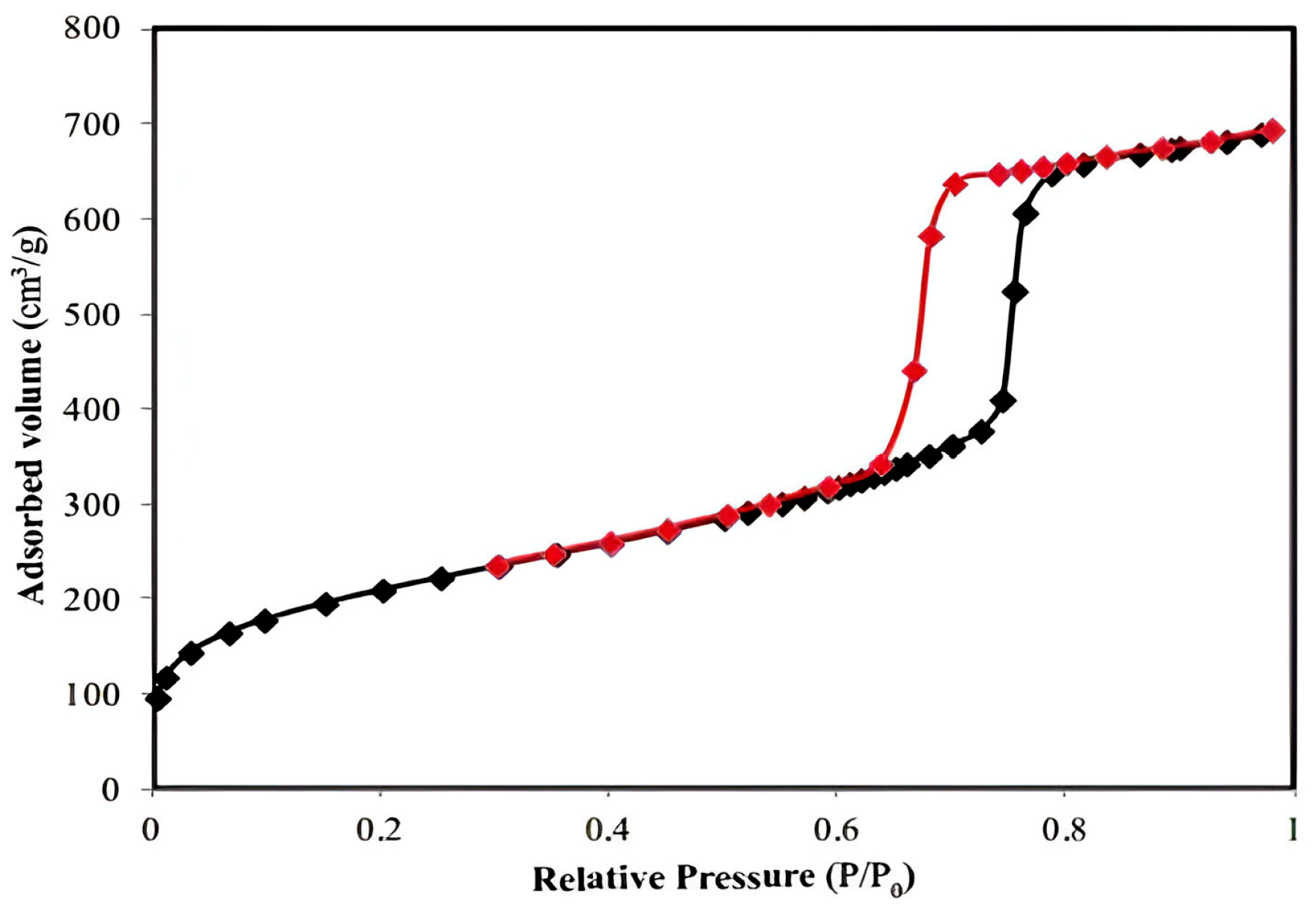
3.3. Magnetic Tests
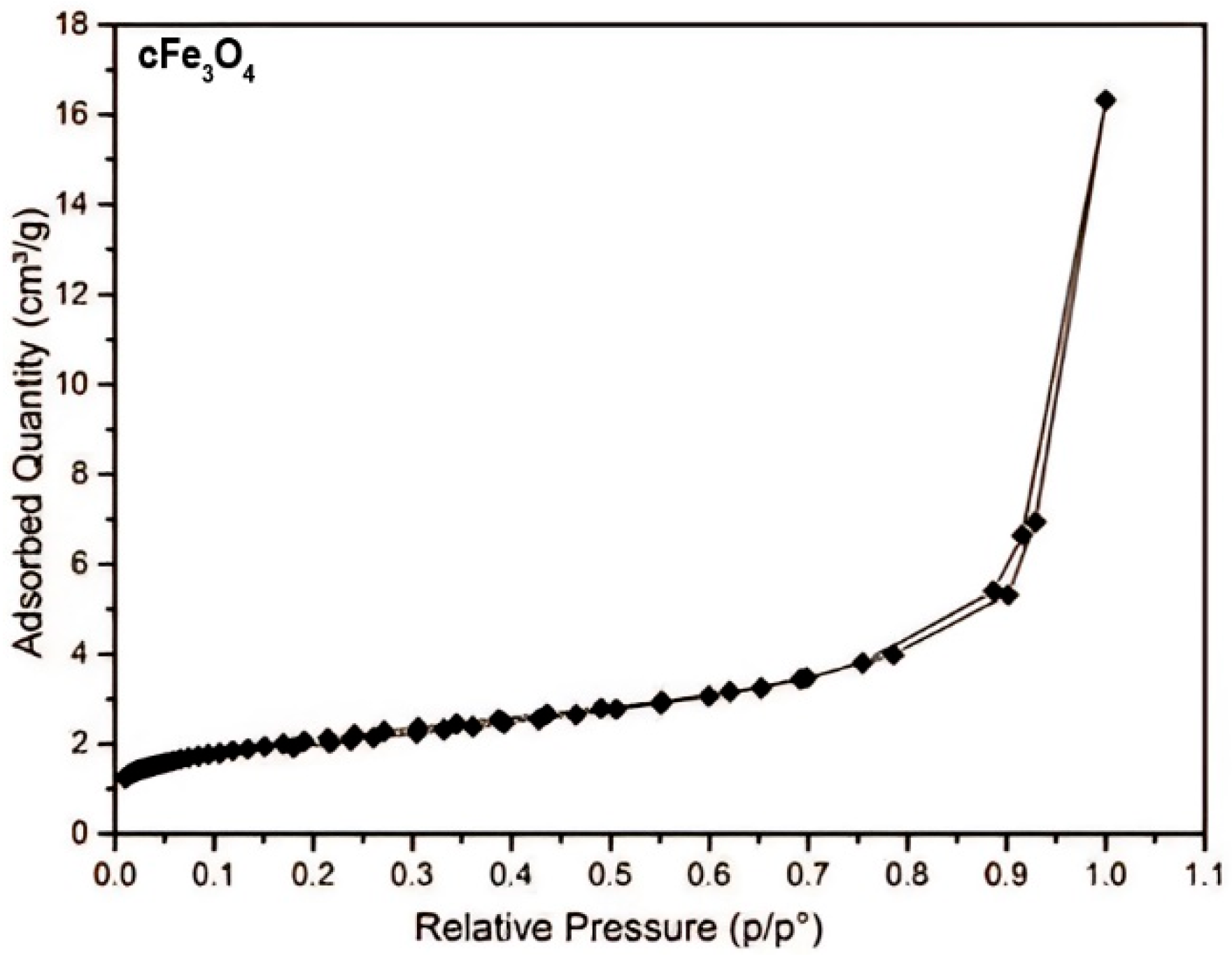
The hysteresis loop is characteristic of ordered mesoporous solids with a stable pore geometry, and the steep configuration suggests that the Fe
3O
4 polymeric film structure has a narrow pore size distribution—the Barrett–Joyner–Halenda (BJH) model—which, according to the literature [
55], increases their relative filling pressure (
Figure 1).
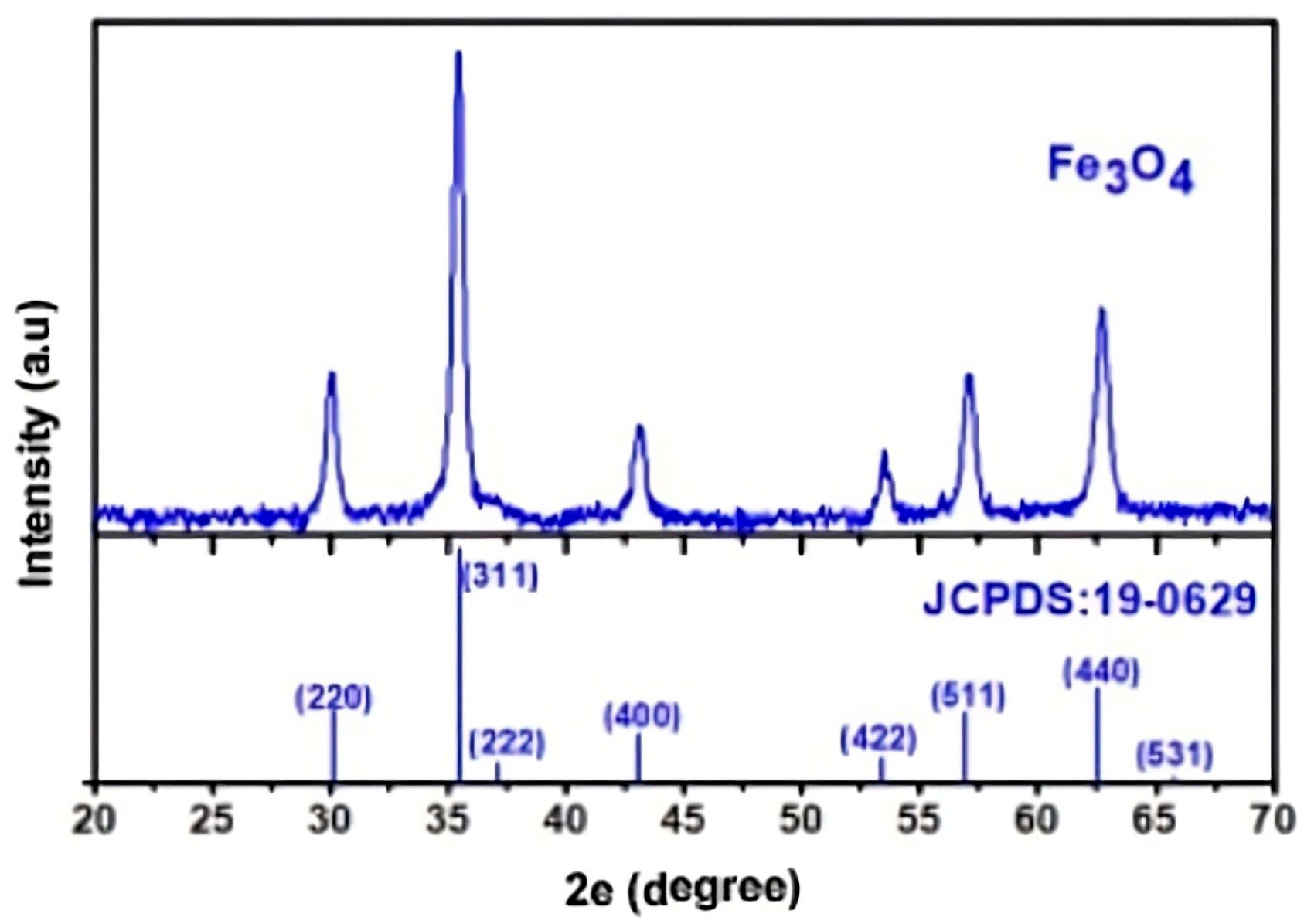
3.4. Structural Analyses
SEM was used to characterize the morphology of oxide nanopowders (Fe
3O
4) after adsorption of Cu
2+ and Ni
2+ ions from contaminated aqueous solutions (
Figure 2). The composition of these metal ion-loaded powders is highlighted by the EDX spectrum (energy-dispersive X-ray spectroscopy). It demonstrates that the process of metal ion incorporation and adsorption into the oxide powder structure results in the formation of complex structures.
The authors also had concerns in performing SEM analyses of polymer composite materials with metal fillers [
56,
57,
58] (
Figure 3), as well as in the effect of the extremely low-frequency electric field on living matter in wastewater [
59,
60]. The loading with metal ions of the Fe
3O
4 oxide nanopowder is shown in
Figure 4.
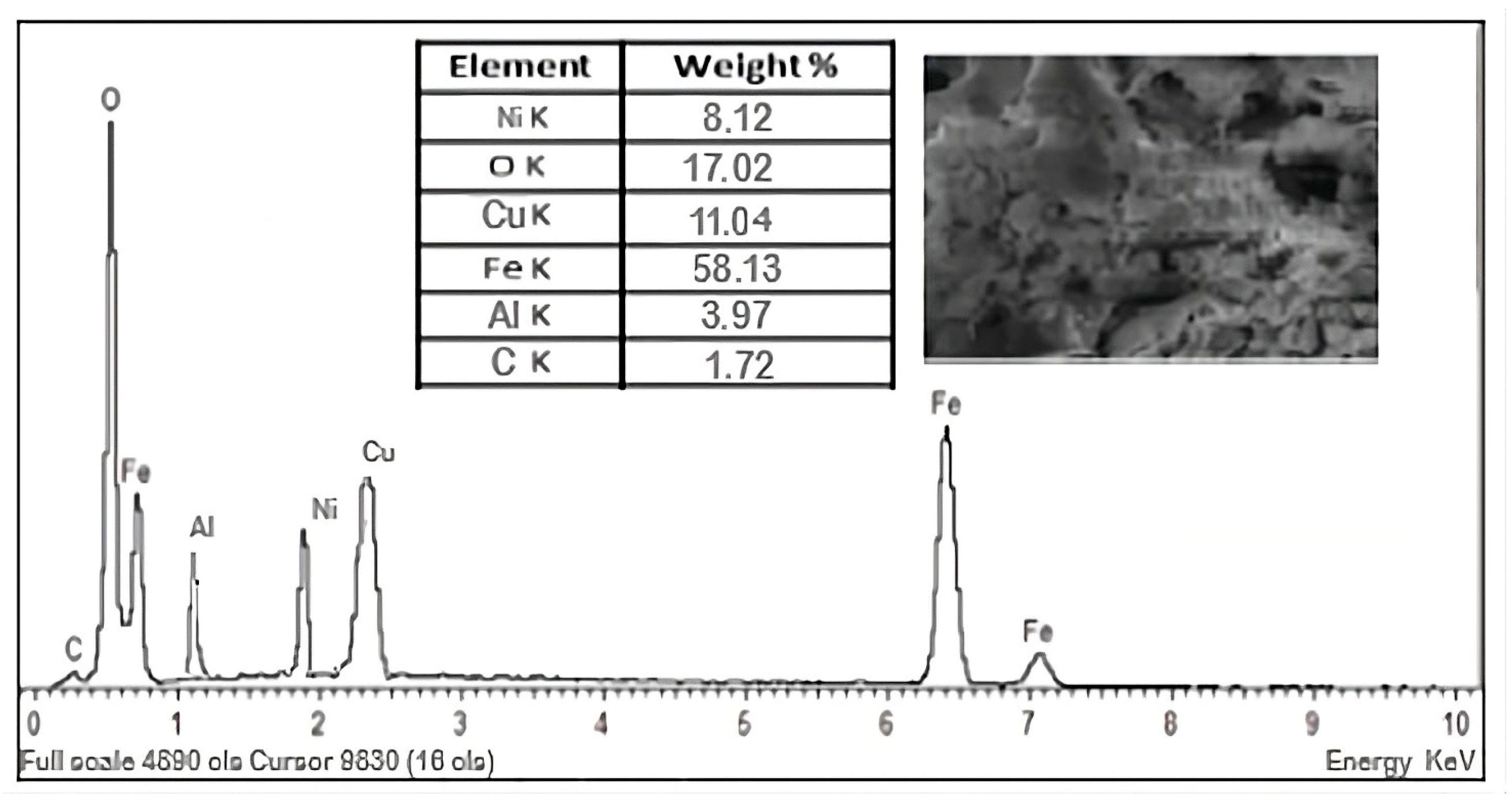
Table 6 shows date regarding the magnetic measurements and EDX analyses.
EDX analysis of these nanopowders indicates the presence of oxygen, iron, nickel, copper, and aluminum (
Figure 5). Additionally, the presence of a minor peak for carbon (C) was observed in the EDX spectrum.
The interaction of nickel and copper ions with the iron oxide structure led to a shift in the spinel structure of Fe3O4. The average particle size of Cu: Fe3O4 and Ni: Fe3O4 is 78 ± 0.2 nm and 11 ± 0.1 nm, respectively. The spherical morphology of the Fe3O4 oxide structure presents elongated, irregular shapes, which shows that the adsorption of heavy metal ions influences the oxide shape of the powder, and the pore size is slightly different according to the BET isotherm (relative pressure P/P: 0.001–1 mmHg). Cu and Ni ions immobilized by the Fe3O4 nanopowder were detected by EDX analysis. Adsorption kinetics were performed by varying the concentrations of Ni2+ and Cu2+ in the solution to be analyzed to evaluate the decontamination efficiency of the Fe3O4 nanopowder sample.
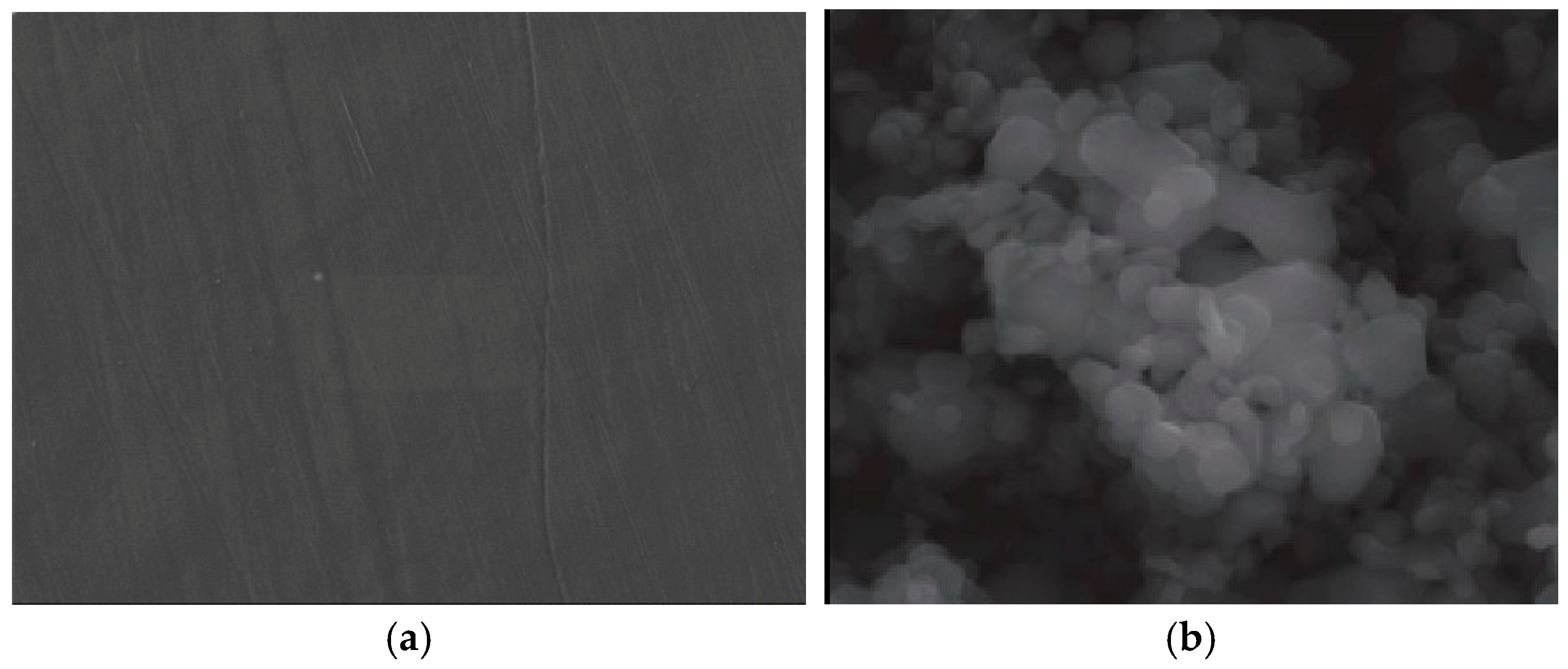
In
Figure 6, the micrographs for the raw materials used were presented, namely the LDPE polymer (
Figure 6a) and Fe
3O
4 (
Figure 6b). The
Figure 7 shows isotherme of adsorption-desorption of Fe
3O
4 nanopowder, and the
Table 7 shows characteristics date about LDPE-g-MA compatibilizer polymer.
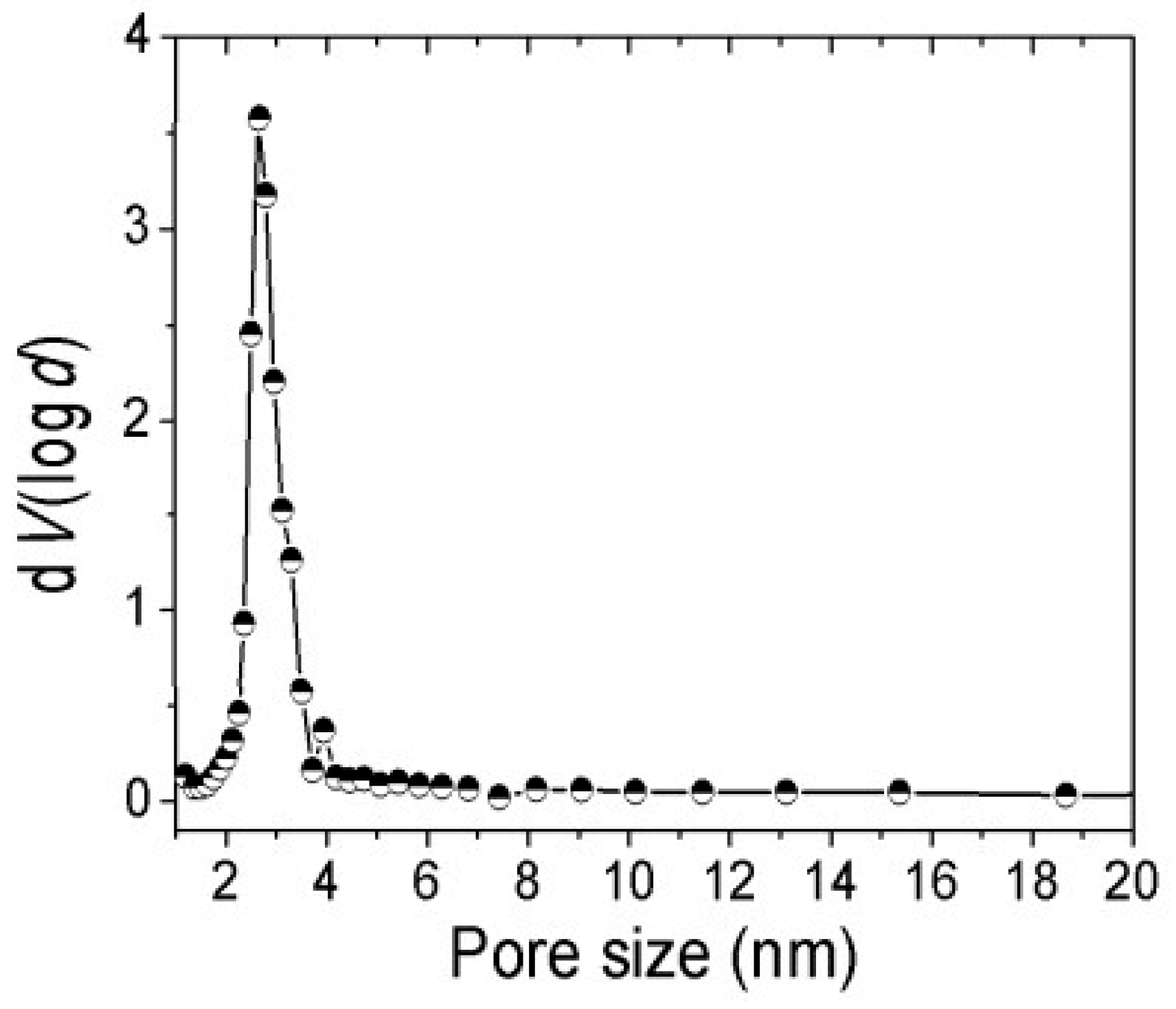
Distribution of the pore size of the Fe
3O
4 oxide nanopowder and compatibilizer polymer (LDPE-g-MA) are graphically represented in the
Figure 8.
The experiments conducted led to the determination of kinetic parameters for Ni2+ and Cu2+ ions, with densities of 6.23 g/cm3 and 9.64 g/cm3, respectively. This resulted in a filling area per cation of 0.0533 nm2 and 0.722 nm2, respectively.
At the laboratory level, tests were conducted on the polymeric composite material film, and composite M5 was chosen, as it exhibited the best properties in terms of water turbidity (treated in five columns of different sizes). The determined average specific surface area of the polymer composite film was 0.2–0.6 m2/g for the removal of ions from tap water in columns with maximum turbidity between 11.64 and 5.33 NTU (above the maximum accepted limit) and 0.55–1.05 m2/g for columns with turbidity values between 5.33 and 2.86 NTU (values within the maximum accepted limit according to legislation).
Depending on the position of the ions, they form nonmagnetic spinels or ferrites with ferromagnetic spinels.
The specific particle surface area, optical properties, and high magnetic susceptibility enable these structures to have multiple industrial applications, as well as applications in water purification and soil decontamination. The use of a polymer or surfactant coating prevents the oxidation and agglomeration of these particles. Thus, the properties of iron oxide, the functionalization of the magnetite (Fe3O4) structure, are chemically stable and can be used in various fields due to its biocompatibility.
The analysis of the obtained results corroborated with the data of the specialized studies [
61,
62,
63,
64,
65,
66]. It highlights the fact that iron oxide particles can be used for the decontamination of contaminated waters, the removal of toxic elements from network waters, and/or the remediation of contaminated soils. The results obtained demonstrate that the use of polymeric composite material films, made from M5 material, provides an efficient and stable chemical structure for removing heavy metals from network waters and treating groundwater.
4. Conclusions
The behavior of iron oxides, which exhibit characteristics similar to those of polymeric materials, as well as their synergistic combination for the development of new water decontamination techniques and environmental conservation, was analyzed in comparison.
This study demonstrates the strong potential of functionalized spinel ferrites, particularly doped with transition metals (Cu2+, Ni2+), for use in wastewater treatment.
Through computational modeling (DFT, DFTB+) and experimental validation (SEM, EDX, BET), it was shown that these materials maintain structural integrity, offer high magnetic responsiveness, and exhibit enhanced adsorption of heavy metal ions.
Key findings reveal that the adsorption mechanism in the polymer matrix varies depending on the adsorbed cation, as shown by the results of laboratory analytical methods and determinations obtained with the BIOVIA Pipeline Pilot specification.
The magnetite coated with LDPE polymer film proved effective in reducing water turbidity and capturing heavy metals.
Overall, the integration of advanced material design with geochemical insights supports the application of spinel ferrites as sustainable, high-performance agents in water purification and environmental protection.
Iron oxide MNPs are the most preferred nanomaterials in medical sciences, due to their features of minimal toxicity and excellent physicochemical properties such as super paramagnetism, stability in aqueous solutions, and biocompatibility [
67,
68,
69,
70]. In contrast, while zero-valent iron NPs (nZVI) are highly reactive and effective in some water treatment contexts, studies have shown that their application, especially at high concentrations, may raise concerns regarding environmental safety and potential toxicity [
67].
Therefore, our choice to use Fe3O4 NPs was motivated by the goal of developing a more environmentally friendly and stable composite material that retains high reactivity and adsorption efficiency while reducing the potential risks associated with nZVI use.






.jpg)




