Preparation of Self-Healing Antifogging Hard Coatings Using Carboxy-Functionalized Polysilsesquioxanes and Oligo(ethylene glycol)s
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Preparation of PSQ-2C
2.4. Ethyl Esterification of the Carboxy Groups in PSQ-2C
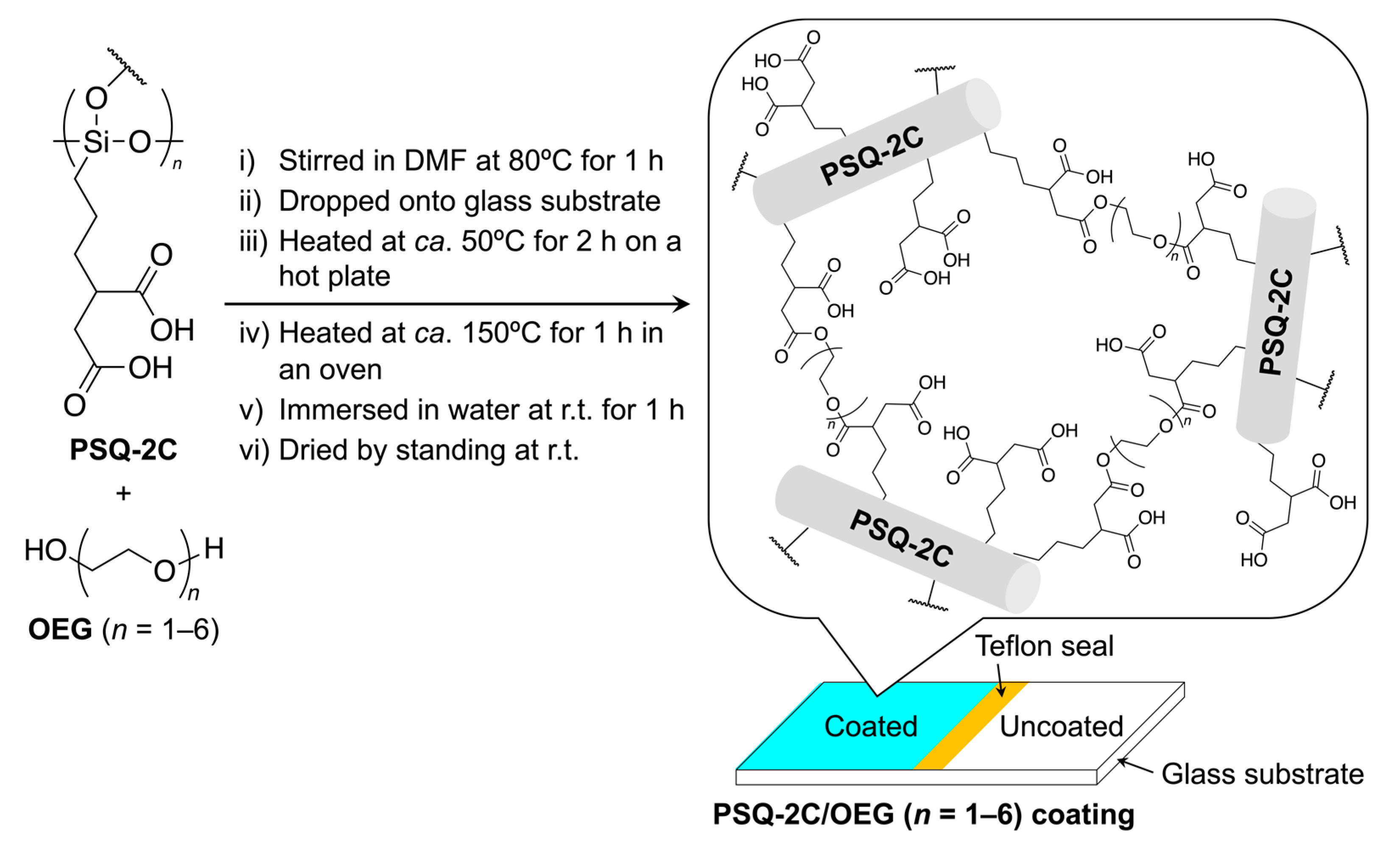
2.5. Preparation of Coatings
3. Results and Discussion
3.1. Preparation of PSQ-2C/OEG Coatings
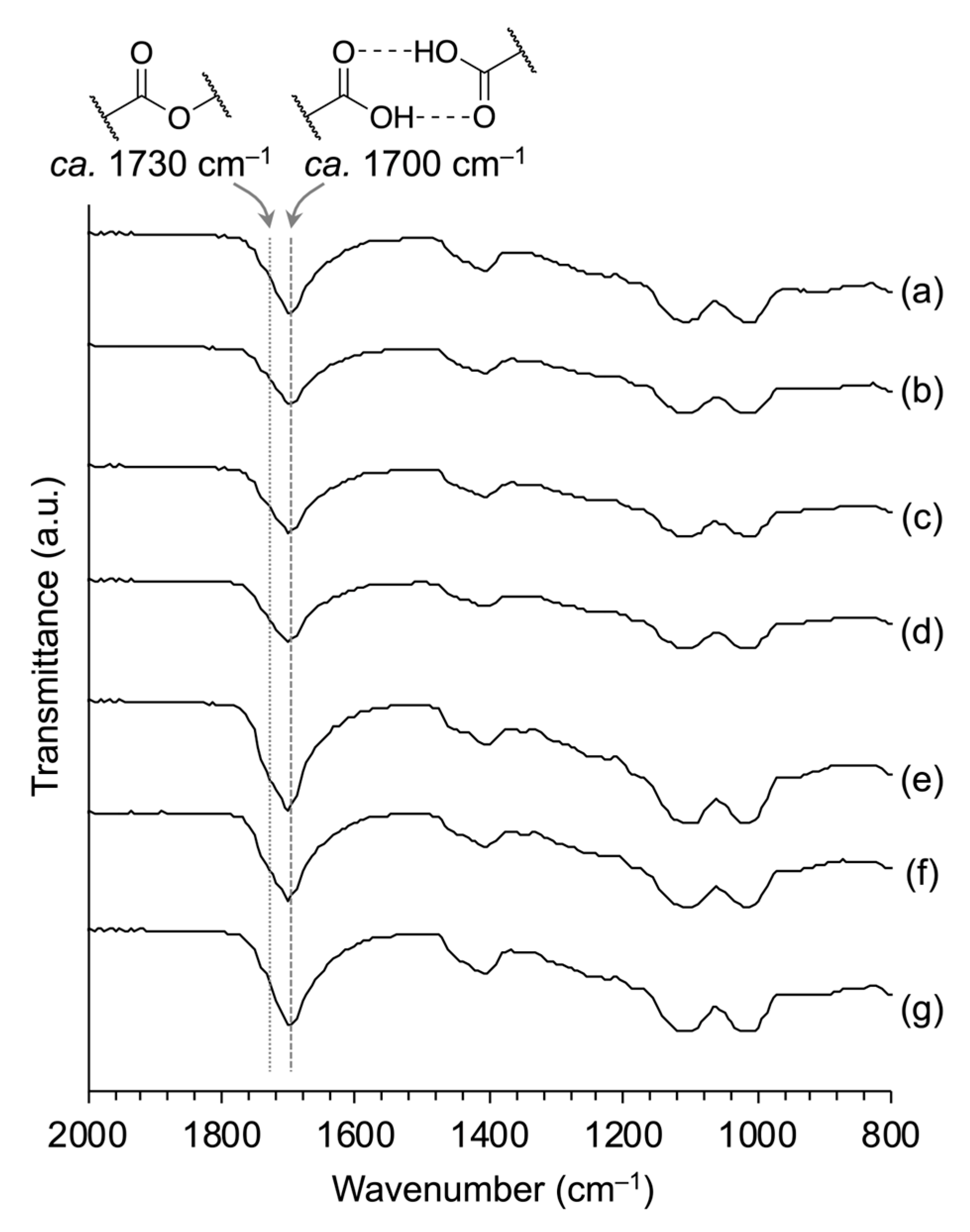
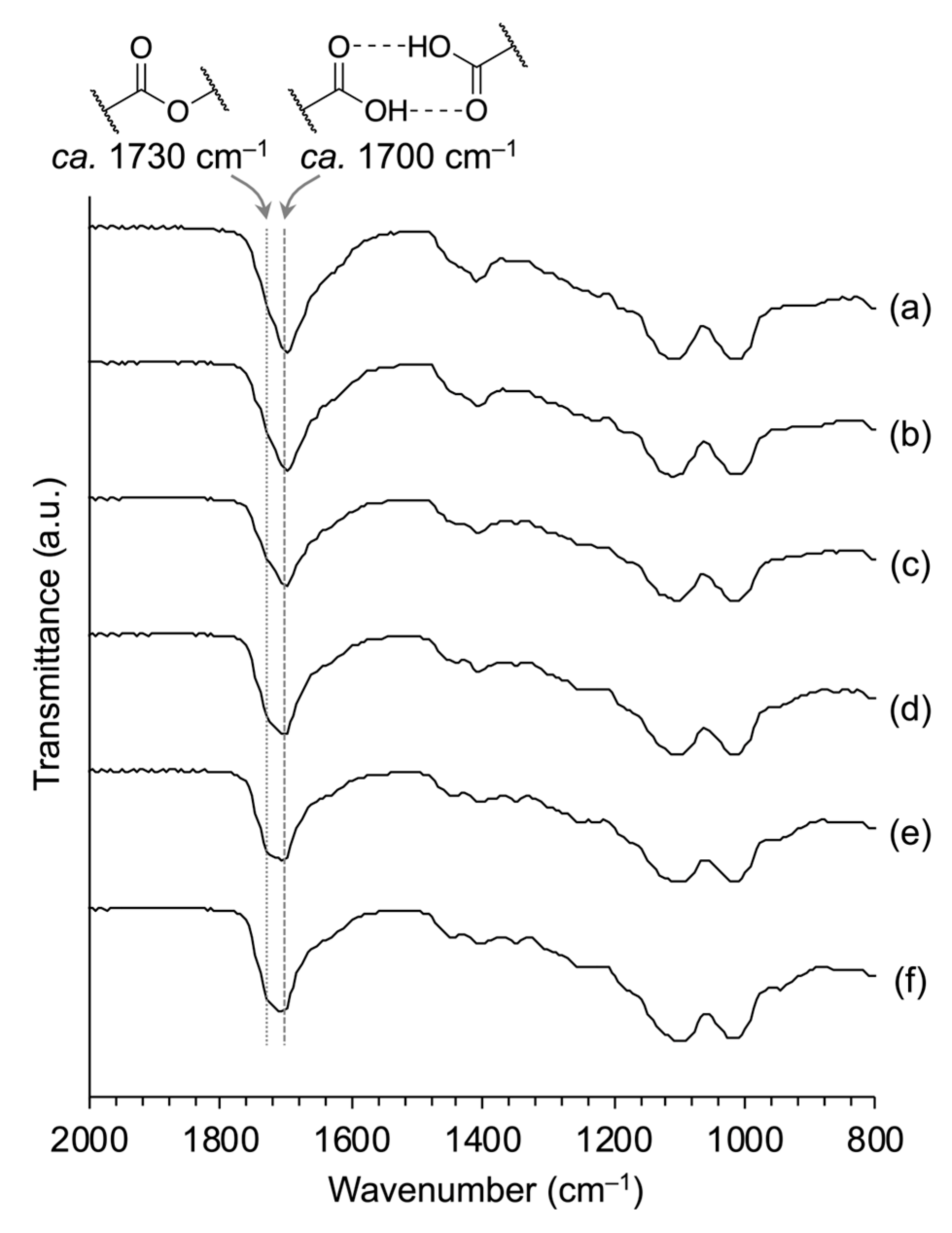
3.2. Water Resistance of PSQ-2C/OEG Coatings
3.3. Surface Hardness of PSQ-2C/OEG Coatings
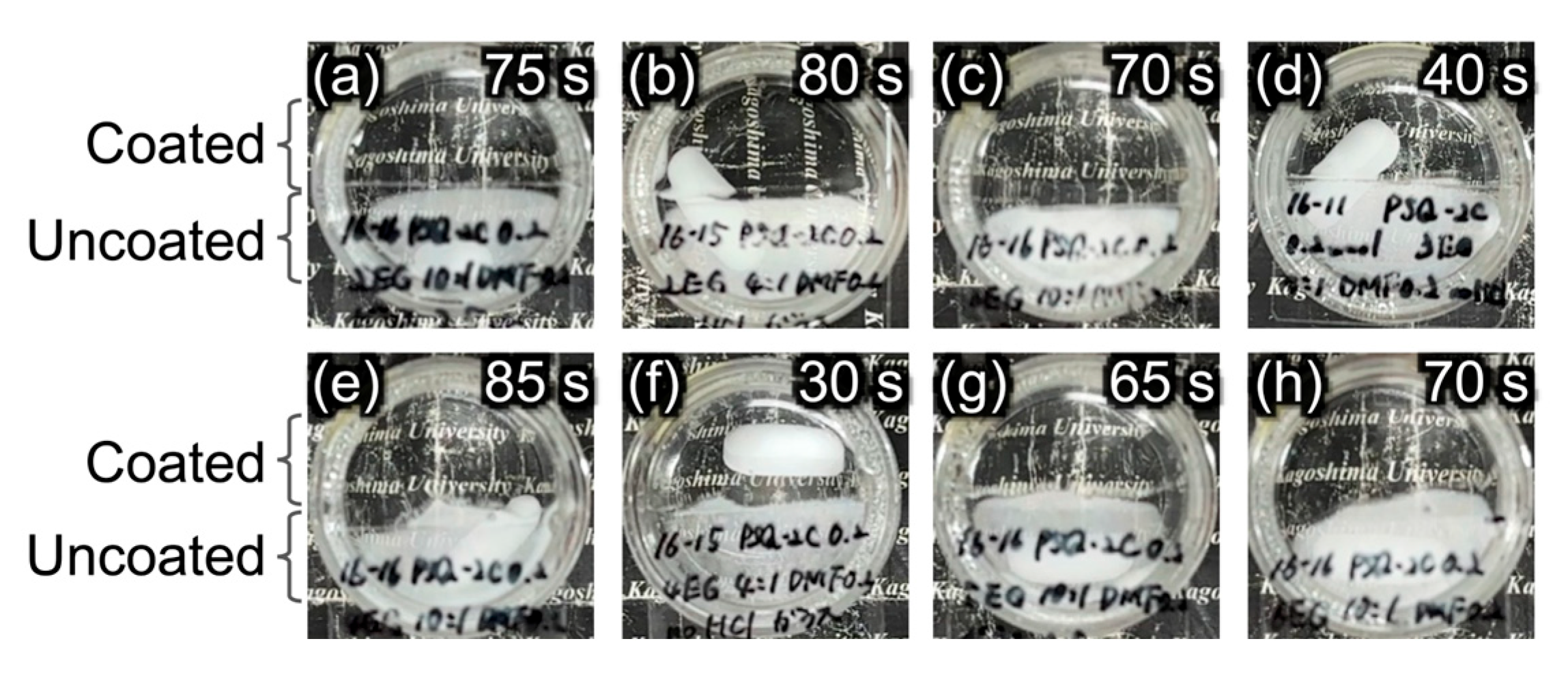
3.4. Antifogging Property of PSQ-2C/OEG Coatings
3.5. Water Contact Angles of PSQ-2C/OEG Coatings
3.6. Self-Healing Property of PSQ-2C/OEG Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SQ | Silsesquioxane |
| PSQ | Polysilsesquioxane |
| POSS | Polyhedral oligomeric silsesquioxane |
| POSS-C | Carboxy-functionalized polyhedral oligomeric silsesquioxane |
| TESPSA | [3-(Triethoxysilyl)propyl]succinic anhydride |
| PSQ-2C | Carboxy-functionalized polysilsesquioxane |
| OEG | Oligo(ethylene glycol) |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| THF | Tetrahydrofuran |
| PEG1000 | Polyethylene glycol with an average molecular weight of 1000 |
| FTIR/ATR | Fourier-transform infrared spectroscopy/attenuated total reflectance |
| GPC | Gel permeation chromatography |
| DSC | Differential scanning calorimetry |
References
- Durán, I.R.; Laroche, G. Current trends, challenges, and perspectives of anti-fogging technology: Surface and material design, fabrication strategies, and beyond. Prog. Mater. Sci. 2019, 99, 106–186. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X. Special oleophobic and hydrophilic surfaces: Approaches, mechanisms, and applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Ming, W. Antifogging and frost-resisting polymeric surfaces. Adv. Polym. Sci. 2018, 284, 185–214. [Google Scholar]
- Chen, Y.; Zhang, Y.; Shi, L.; Li, J.; Xin, Y.; Yang, T.; Guo, Z. Transparent superhydrophobic/superhydrophilic coatings for self-cleaning and anti-fogging. Appl. Phys. Lett. 2012, 101, 033701. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J.H.; Dou, S.X. Fly-eye inspired superhydrophobic anti-fogging inorganic nanostructures. Small 2014, 10, 3001–3006. [Google Scholar] [CrossRef]
- Shang, Q.; Zhou, Y. Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceram. Int. 2016, 42, 8706–8712. [Google Scholar] [CrossRef]
- Syafiq, A.; Vengadaesvaran, B.; Ahmed, U.; Rahim, N.A.; Pandey, A.K.; Bushroa, A.R.; Ramesh, K.; Ramesh, S. Facile synthesize of transparent hydrophobic nano-CaCO3 based coatings for self-cleaning and anti-fogging. Mater. Chem. Phys. 2020, 239, 121913. [Google Scholar] [CrossRef]
- Qiu, Z.; Lin, H.; Zeng, L.; Liang, Y.; Zeng, C.; Hong, R. Ultra-scratch-resistant, hydrophobic and transparent organosilicon-epoxy-resin coating with a double cross-link structure. Appl. Sci. 2022, 12, 4854. [Google Scholar] [CrossRef]
- Watanabe, T.; Nakajima, A.; Wang, R.; Minabe, M.; Koizumi, S.; Fujishima, A.; Hashimoto, K. Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass. Thin Solid Films 1999, 351, 260–263. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Pratsinis, S.E. Anti-fogging nanofibrous SiO2 and nanostructured SiO2-TiO2 films made by rapid flame deposition and in situ annealing. Langmuir 2009, 25, 12578–12584. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based coatings for self-cleaning and anti-fogging. J. Mater. Chem. 2012, 22, 7420–7426. [Google Scholar] [CrossRef]
- Park, J.T.; Kim, J.H.; Lee, D. Excellent anti-fogging dye-sensitized solar cells based on superhydrophilic nanoparticle coatings. Nanoscale 2014, 6, 7362–7368. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.Q.; Chen, Z.; Chi, L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef]
- Chemin, J.B.; Bulou, S.; Baba, K.; Fontaine, C.; Sindzingre, T.; Boscher, N.D.; Choquet, P. Transparent anti-fogging and self-cleaning TiO2/SiO2 thin films on polymer substrates using atmospheric plasma. Sci. Rep. 2018, 8, 9603. [Google Scholar] [CrossRef] [PubMed]
- Xi, R.; Wang, Y.; Wang, X.; Lv, J.; Li, X.; Li, T.; Zhang, X.; Du, X. Ultrafine nano-TiO2 loaded on dendritic porous silica nanoparticles for robust transparent antifogging self-cleaning nanocoatings. Ceram. Int. 2020, 46, 23651–23661. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Self-cleaning and next generation anti-fog surfaces and coatings. Macromol. Rapid. Commun. 2008, 29, 455–466. [Google Scholar] [CrossRef]
- Tahk, D.; Kim, T.I.; Yoon, H.; Choi, M.; Shin, K.; Suh, K.Y. Fabrication of antireflection and antifogging polymer sheet by partial photopolymerization and dry etching. Langmuir 2010, 26, 2240–2243. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, P.; Turgeon, S.; Sarra-Bournet, C.; Turcotte, R.; Laroche, G. Characterization of multilayer anti-fog coatings. ACS Appl. Mater. Interfaces 2011, 3, 750–758. [Google Scholar] [CrossRef]
- Lee, H.; Alcaraz, M.L.; Rubner, M.F.; Cohen, R.E. Zwitter-wettability and antifogging coatings with frost-resisting capabilities. ACS Nano 2013, 7, 2172–2185. [Google Scholar] [CrossRef]
- Zhao, J.; Meyer, A.; Ma, L.; Ming, W. Acrylic coatings with surprising antifogging and frost-resisting properties. Chem. Commun. 2013, 49, 11764–11766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Meyer, A.; Ma, L.; Wang, X.; Ming, W. Terpolymer-based SIPN coating with excellent antifogging and frost-resisting properties. RSC Adv. 2015, 5, 102560–102566. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, S.; Sun, J. Antifogging and frost-resisting polyelectrolyte coatings capable of healing scratches and restoring transparency. Chem. Mater. 2015, 27, 8058–8065. [Google Scholar] [CrossRef]
- Ezzat, M.; Huang, C.J. Zwitterionic polymer brush coatings with excellent anti-fog and anti-frost properties. RSC Adv. 2016, 6, 61695–61702. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Wang, Y.; Ma, B.; Sun, J. Highly transparent and water-enabled healable antifogging and frost-resisting films based on poly(vinyl alcohol)-nafion complexes. Chem. Mater. 2016, 28, 6975–6984. [Google Scholar] [CrossRef]
- Hong, J.W.; Cheon, H.K.; Kim, S.H.; Hwang, K.H.; Kim, H.K. Synthesis and characterization of UV curable urethane acrylate oligomers containing ammonium salts for anti-fog coatings. Prog. Org. Coat. 2017, 110, 122–127. [Google Scholar] [CrossRef]
- Yao, B.; Zhao, H.; Wang, L.; Liu, Y.; Zheng, C.; Li, H.; Sun, C. Synthesis of acrylate-based UV/thermal dual-cure coatings for antifogging. J. Coat. Technol. Res. 2018, 15, 149–158. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Zhang, R.; Li, C.; Zhu, K.; Sun, P.; Zhao, Y.; Ren, L.; Yuan, X. Enhancing antifogging/frost-resisting performances of amphiphilic coatings via cationic, zwitterionic or anionic polyelectrolytes. Chem. Eng. J. 2019, 357, 667–677. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, L.; Lin, N.; Wang, J.; Wang, Y.; Wu, T.; Song, P. Highly transparent, healable, and durable anti-fogging coating by combining hydrophilic pectin and tannic acid with poly(ethylene terephthalate). Green Chem. 2019, 21, 5405–5413. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, P.; Song, L.; Tian, L.; Ming, W.; Ren, L. Highly efficient antifogging and frost-resisting acrylic coatings from one-step thermal curing. Colloids Surf. A 2020, 585, 124160. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, X.; Yu, B.; Zhou, S. Fast UV-curable zwitter-wettable coatings with reliable antifogging/frost-resisting performances. Biomimetics 2022, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, L.; Ye, H.; Liu, H.; Li, W. Modifying a waterborne polyacrylate coating with a silica sol for enhancing anti-fogging performance. RSC Adv. 2016, 6, 92252–92258. [Google Scholar] [CrossRef]
- Kaya, A.S.T.; Cengiz, U. Fabrication and application of superhydrophilic antifog surface by sol–gel method. Prog. Org. Coat. 2019, 126, 75–82. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Z.M.; Cheng, L.P. Preparation of superhydrophilic nanosilica/polyacrylate hard coatings on plastic substrate for antifogging and frost-resistant applications. J. Appl. Polym. Sci. 2019, 136, 48144. [Google Scholar] [CrossRef]
- Shi, J.; Xu, L.; Qiu, D. Effective antifogging coating from hydrophilic/hydrophobic polymer heteronetwork. Adv. Sci. 2022, 9, 2200072. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzukit, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Kaneko, Y.; Iyi, N.; Kurashima, K.; Matsumoto, T.; Fujita, T.; Kitamura, K. Hexagonal-structured polysiloxane material prepared by sol–gel reaction of aminoalkyltrialkoxysilane without using surfactants. Chem. Mater. 2004, 16, 3417–3423. [Google Scholar] [CrossRef]
- Kaneko, Y.; Iyi, N.; Matsumoto, T.; Kitamura, K. Synthesis of rodlike polysiloxane with hexagonal phase by sol–gel reaction of organotrialkoxysilane monomer containing two amino groups. Polymer 2005, 46, 1828–1833. [Google Scholar] [CrossRef]
- Toyodome, H.; Kaneko, Y.; Shikinaka, K.; Iyi, N. Preparation of carboxylate group-containing rod-like polysilsesquioxane with hexagonally stacked structure by sol–gel reaction of 2-cyanoethyltriethoxysilane. Polymer 2012, 53, 6021–6026. [Google Scholar] [CrossRef]
- Kaneko, Y.; Toyodome, H.; Mizumo, T.; Shikinaka, K.; Iyi, N. Preparation of a sulfo-group-containing rod-like polysilsesquioxane with a hexagonally stacked structure and its proton conductivity. Chem. Eur. J. 2014, 20, 9394–9399. [Google Scholar] [CrossRef]
- Harada, A.; Shikinaka, K.; Ohshita, J.; Kaneko, Y. Preparation of a one-dimensional soluble polysilsesquioxane containing phosphonic acid side-chain groups and its thermal and proton-conduction properties. Polymer 2017, 121, 228–233. [Google Scholar] [CrossRef]
- Kaneko, Y. Ionic silsesquioxanes: Preparation, structure control, characterization, and applications. Polymer 2018, 144, 205–224. [Google Scholar] [CrossRef]
- Kaneko, Y.; Shoiriki, M.; Mizumo, T. Preparation of cage-like octa(3-aminopropyl)silsesquioxane trifluoromethanesulfonate in higher yield with a shorter reaction time. J. Mater. Chem. 2012, 22, 14475–14478. [Google Scholar] [CrossRef]
- Imai, K.; Kaneko, Y. Preparation of ammonium-functionalized polyhedral oligomeric silsesquioxanes with high proportions of cagelike decamer and their facile separation. Inorg. Chem. 2017, 56, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kaneko, Y. Preparation of polyhedral oligomeric silsesquioxanes containing carboxyl side-chain groups and isolation of a cage-like octamer using clay mineral. Bull. Chem. Soc. Jpn. 2018, 91, 1120–1127. [Google Scholar] [CrossRef]
- Kozuma, T.; Kaneko, Y. Preparation of carboxyl-functionalized polyhedral oligomeric silsesquioxane by a structural transformation reaction from soluble rod-like polysilsesquioxane. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2511–2518. [Google Scholar] [CrossRef]
- Ishii, T.; Enoki, T.; Mizumo, T.; Ohshita, J.; Kaneko, Y. Preparation of imidazolium-type ionic liquids containing silsesquioxane frameworks and their thermal and ion-conductive properties. RSC Adv. 2015, 5, 15226–15232. [Google Scholar] [CrossRef]
- Maeda, D.; Ishii, T.; Kaneko, Y. Effect of lengths of substituents in imidazolium groups on the preparation of imidazolium-salt-type ionic liquids containing polyhedral oligomeric silsesquioxane structures. Bull. Chem. Soc. Jpn. 2018, 91, 1112–1119. [Google Scholar] [CrossRef]
- Kaneko, Y. Superacid-catalyzed preparation of ionic polyhedral oligomeric silsesquioxanes and their properties, polymerization, and hybridization. J. Sol–Gel Sci. Technol. 2022, 104, 588–598. [Google Scholar] [CrossRef]
- Maeda, T.; Hamada, T.; Tsukada, S.; Katsura, D.; Okada, K.; Ohshita, J. Antifogging hybrid materials based on amino-functionalized polysilsesquioxanes. ACS Appl. Polym. Mater. 2021, 3, 2568–2575. [Google Scholar] [CrossRef]
- Hamada, T.; Sugimoto, T.; Maeda, T.; Katsura, D.; Mineoi, S.; Ohshita, J. Robust and transparent antifogging polysilsesquioxane film containing a hydroxy group. Langmuir 2022, 38, 5829–5837. [Google Scholar] [CrossRef]
- Kozuma, T.; Mihata, A.; Kaneko, Y. Preparation of soluble poss-linking polyamide and its application in antifogging films. Materials 2021, 14, 3178. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, J.; Morinaga, S.; Kaneko, Y. Preparation of antifog hard coatings based on carboxy-functionalized polyhedral oligomeric silsesquioxane cross-linked with oligo(ethylene glycol)s. ACS Omega 2024, 9, 28895–28902. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Benedetti, A.V. Self-healing ability based on hydrogen bonds in organic coatings for corrosion protection of AA1200. Corros. Sci. 2020, 177, 108984. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Zhang, D.; Lou, Y.; Huang, L.; Ma, L.; Hao, X.; Dong, L.; Rosei, F.; Lau, W.M. Ultrafast and high-efficient self-healing epoxy coatings with active multiple hydrogen bonds for corrosion protection. Corros. Sci. 2021, 187, 109485. [Google Scholar] [CrossRef]
- Bai, Z.G.; Bai, Y.Y.; Zhang, G.P.; Wang, S.Q.; Zhang, B. A Hydrogen bond based self-healing superhydrophobic octadecyltriethoxysilane−lignocellulose/silica coating. Prog. Org. Coat. 2021, 151, 106104. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-healing multiphase polymers via dynamic metal–ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Zhou, S.; Yuan, J.Y. Self-healing elastomers and coatings via metal coordination bonds. Chem. Eur. J. 2025, 31, e202404038. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host–guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Lu, P.; Xu, J.; Tian, W.; Zhang, C.; Niu, S.; Zhao, J.; Ming, W.; Ren, L. Robust antifogging coatings with ultra-fast self-healing performances through host–guest strategy. Chem. Eng. J. 2023, 465, 142868. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chuo, T.W. Self-healing polymers based on thermally reversible diels–alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic covalent polymer networks based on degenerative imine bond exchange: Tuning the malleability and self-healing properties by solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, H.; Zhang, Z.; Shao, Y.; Yu, Q.; Cao, X.; Pan, S.; Mu, X.; Gao, Z.; Wang, D.; et al. Self-healing polyurethane coatings based on dynamic chemical bond synergy under conditions of photothermal response. Chem. Eng. J. 2023, 474, 145811. [Google Scholar] [CrossRef]
- Wang, H.; Kuwayama, M.; Fujisawa, Y.; Oshima, Y.; Yamauchi, Y.; Wu, J.; Aida, T. Poly(thioether thiourea)s as novel room-temperature self-healable glassy polymers. CCS Chem. 2025, 7, 1305–1314. [Google Scholar] [CrossRef]
- Outwater, J.O.; Gerry, D.J. On the fracture energy, rehealing velocity and refracture energy of cast epoxy resin. J. Adhes. 1969, 1, 290–298. [Google Scholar] [CrossRef]
- Fang, L.; Chen, J.; Zou, Y.; Xu, Z.; Lu, C. Thermally-induced self-healing behaviors and properties of four epoxy coatings with different network architectures. Polymers 2017, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Hornat, C.C.; Urban, M.W. Entropy and interfacial energy driven self-healable polymers. Nat. Commun. 2020, 11, 1028. [Google Scholar] [CrossRef]
- England, M.W.; Urata, C.; Dunderdale, G.J.; Hozumi, A. Anti-fogging/self-healing properties of clay-containing transparent nanocomposite thin films. ACS Appl. Mater. Interfaces 2016, 8, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Fan, Z.; Yang, H.; Wang, Q.; Cai, Z. Facile preparation of a high-transparency anti-fogging/frost-resisting poly(amps-co-aa) coating with self-healing property. Prog. Org. Coat. 2021, 151, 106053. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, L.; Li, X.; Cai, Z. Hydrophilic/hydrophobic poly(AA-co-BA-co-BPA) anti-fog coating with excellent water resistance and self-healing properties. Prog. Org. Coat. 2024, 187, 108071. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Z.; Jia, E.; Zhang, G.; Su, Z. Transparent and scratch-resistant antifogging coatings with rapid self-healing capability. ACS Appl. Mater. Interfaces 2019, 11, 30300–30307. [Google Scholar] [CrossRef] [PubMed]



| Run | Coating | Feed Functional Group Ratio COOH/OH a | Water Resistance b | Surface Hardness c | Time to Keep Antifogging d (s) | Water Contact Angle e |
|---|---|---|---|---|---|---|
| 1 | PSQ-2C/OEG (n = 1) | 10:1 | sticky | – | – | – |
| 2 | PSQ-2C/OEG (n = 1) | 4:1 | sticky | – | – | – |
| 3 | PSQ-2C/OEG (n = 1) | 2:1 | peeled off | – | – | – |
| 4 | PSQ-2C/OEG (n = 2) | 10:1 | not peeled off, not dissolved | 2H | 75 | 76° |
| 5 | PSQ-2C/OEG (n = 2) | 4:1 | not peeled off, not dissolved | 3H | 80 | 67° |
| 6 | PSQ-2C/OEG (n = 2) | 2:1 | peeled off | – | – | – |
| 7 | PSQ-2C/OEG (n = 3) | 10:1 | not peeled off, not dissolved | 2H | 70 | 78° |
| 8 | PSQ-2C/OEG (n = 3) | 4:1 | not peeled off, not dissolved | 4H | 40 | 67° |
| 9 | PSQ-2C/OEG (n = 3) | 2:1 | peeled off | – | – | – |
| 10 | PSQ-2C/OEG (n = 4) | 10:1 | not peeled off, not dissolved | 4H | 85 | 76° |
| 11 | PSQ-2C/OEG (n = 4) | 4:1 | not peeled off, not dissolved | 4H | 30 | 67° |
| 12 | PSQ-2C/OEG (n = 4) | 2:1 | peeled off | – | – | – |
| 13 | PSQ-2C/OEG (n = 5) | 10:1 | not peeled off, not dissolved | 4H | 65 | 78° |
| 14 | PSQ-2C/OEG (n = 5) | 4:1 | peeled off | – | – | – |
| 15 | PSQ-2C/OEG (n = 5) | 2:1 | peeled off | – | – | – |
| 16 | PSQ-2C/OEG (n = 6) | 10:1 | not peeled off, not dissolved | 4H | 70 | 77° |
| 17 | PSQ-2C/OEG (n = 6) | 4:1 | peeled off | – | – | – |
| 18 | PSQ-2C/OEG (n = 6) | 2:1 | peeled off | – | – | – |
| 19 | PSQ-2C | – | dissolved | 3H | >120 | 46° |
| 20 | PEG1000 | – | dissolved | <2B | >120 | 4° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morinaga, S.; Baba, R.; Fujii, C.; Kaneko, Y. Preparation of Self-Healing Antifogging Hard Coatings Using Carboxy-Functionalized Polysilsesquioxanes and Oligo(ethylene glycol)s. Polymers 2025, 17, 2491. https://doi.org/10.3390/polym17182491
Morinaga S, Baba R, Fujii C, Kaneko Y. Preparation of Self-Healing Antifogging Hard Coatings Using Carboxy-Functionalized Polysilsesquioxanes and Oligo(ethylene glycol)s. Polymers. 2025; 17(18):2491. https://doi.org/10.3390/polym17182491
Chicago/Turabian StyleMorinaga, Seiya, Rione Baba, Chino Fujii, and Yoshiro Kaneko. 2025. "Preparation of Self-Healing Antifogging Hard Coatings Using Carboxy-Functionalized Polysilsesquioxanes and Oligo(ethylene glycol)s" Polymers 17, no. 18: 2491. https://doi.org/10.3390/polym17182491
APA StyleMorinaga, S., Baba, R., Fujii, C., & Kaneko, Y. (2025). Preparation of Self-Healing Antifogging Hard Coatings Using Carboxy-Functionalized Polysilsesquioxanes and Oligo(ethylene glycol)s. Polymers, 17(18), 2491. https://doi.org/10.3390/polym17182491







