Development of a Reflective Electrochromic Zinc-Ion Battery Device for Infrared Emissivity Control Using Self-Doped Polyaniline Films
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of SP(ANI-MA) Electrodes
2.3. Preparation of Gel Electrolytes
2.4. Assembly Procedure of Electrochromic Film Devices
2.5. Characterization
3. Results and Discussion
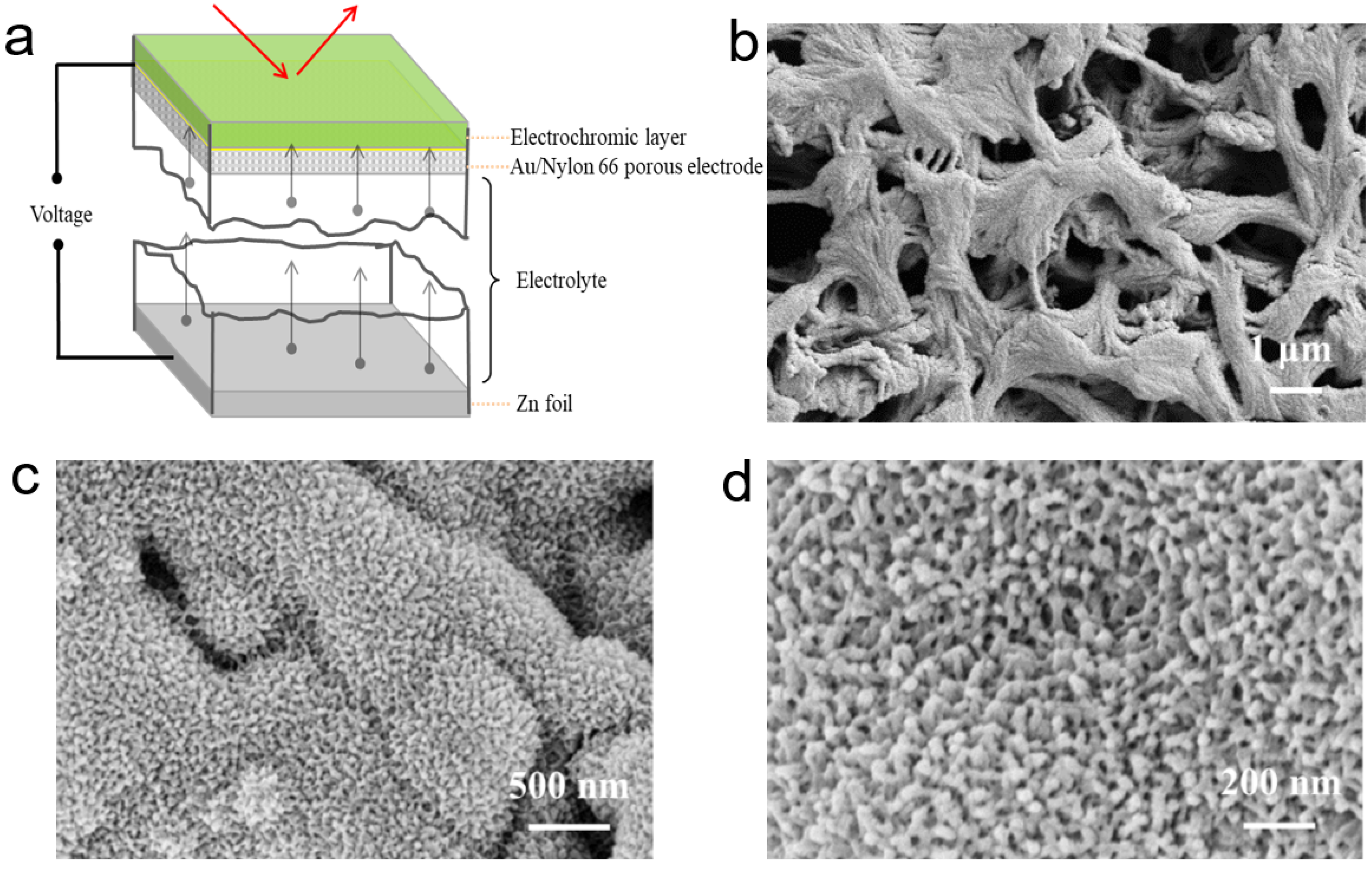
3.1. Characterization and Analysis of the Surface Encapsulation Layer and SP(ANI-MA) Active Material in HWEC-ZIB
3.2. Study on the Infrared Emissivity Modulation Performance of Electrochromic Film Devices
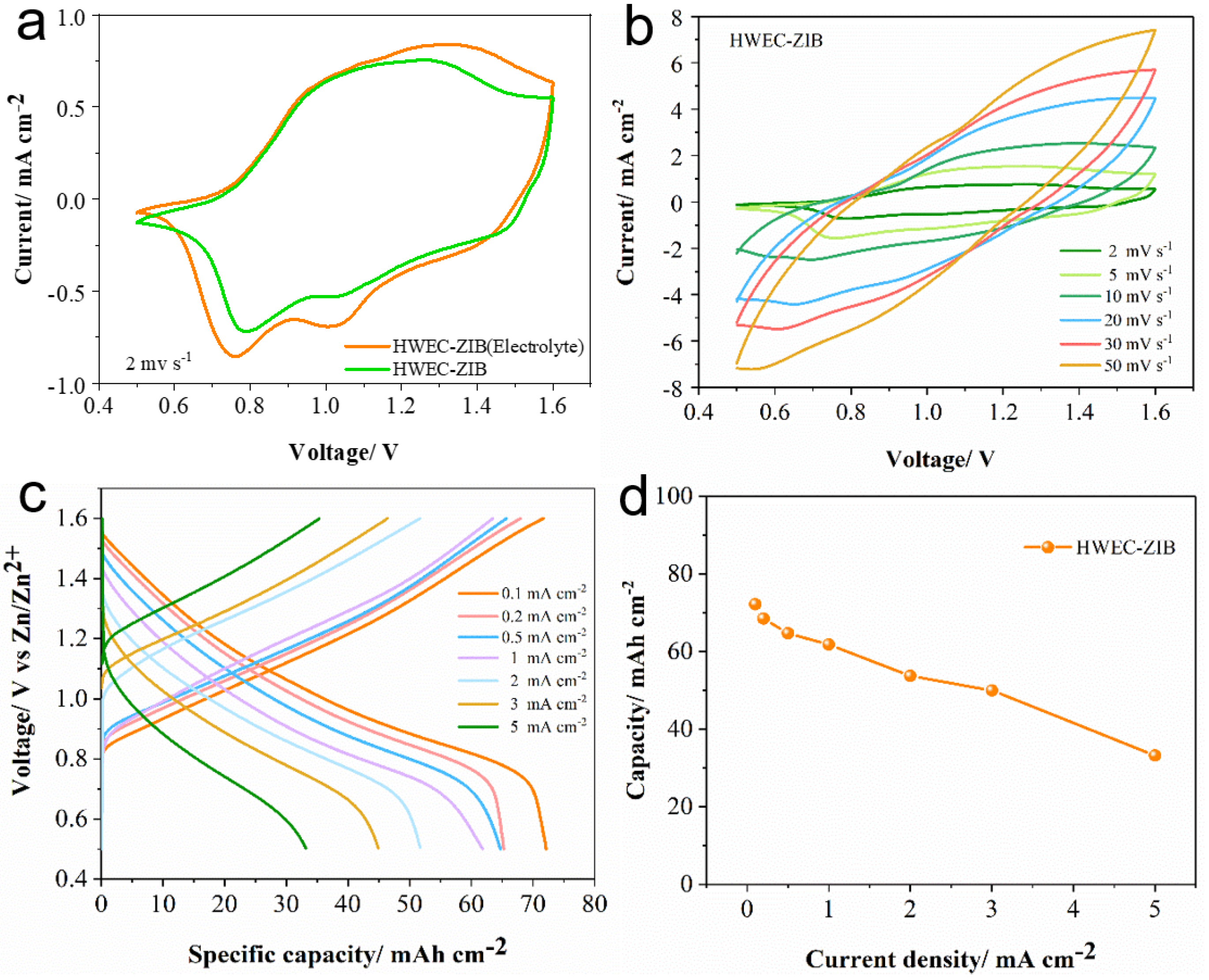
3.3. Electrochemical Energy Storage and Electrochromic Performance of the Film Device
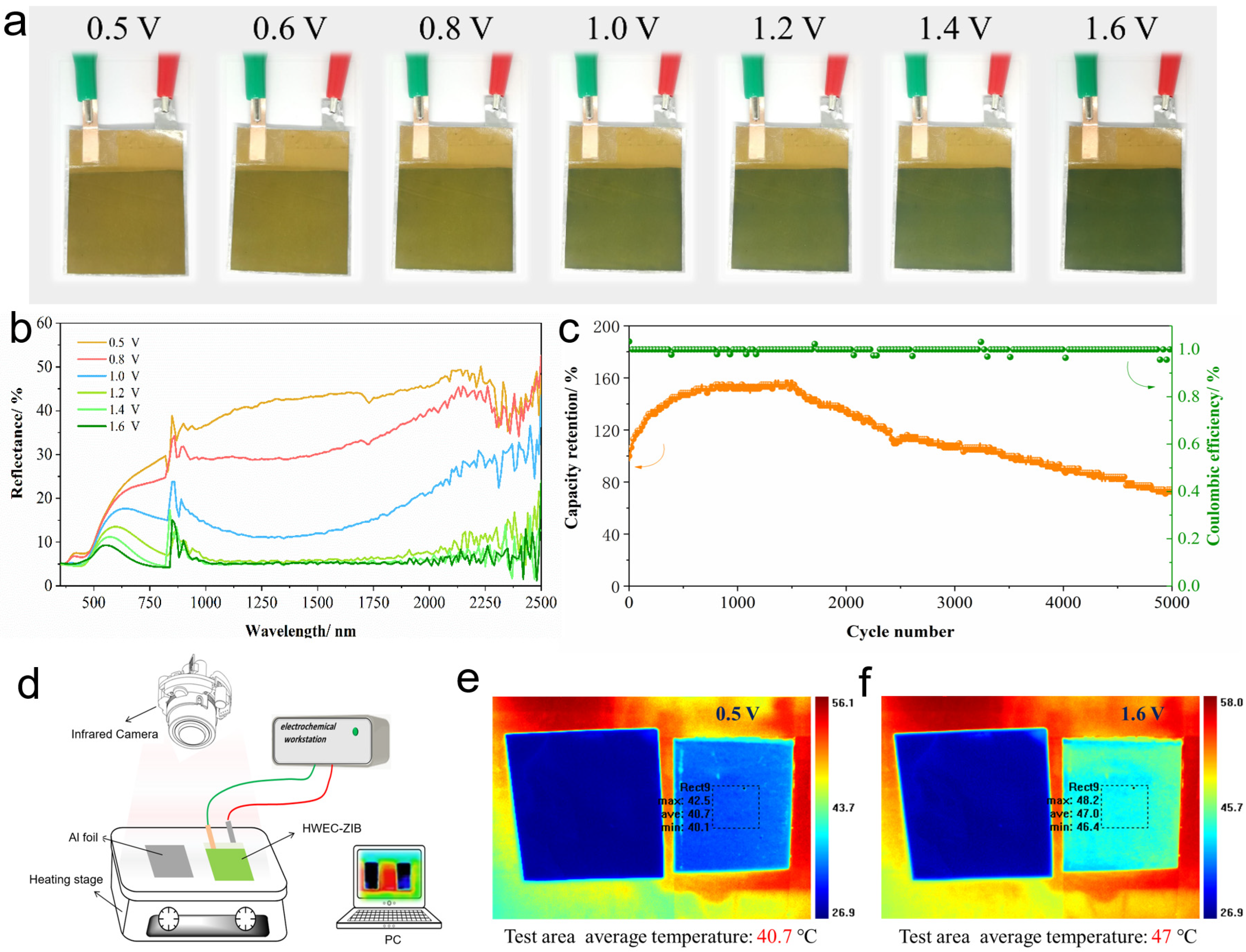
3.4. Investigation of Infrared Emissivity Modulation Performance and Applications of Film Devices
| Device Structure | Active Material | Wavelength Range (μm)IR Modulation Range (Δε) | Response Time (s)Tb/Tc | Energy Storage Capacity (mAh/cm2) | Reference |
|---|---|---|---|---|---|
| SP(ANI-MA)//Zn | Self-doped PANI (SP(ANI-MA)) | 0.28 @3–5 μm 0.19 @8–14 μm | 9.25 s/13.3 s | 72.15 @ 0.1 mA/cm2 | This work |
| WO3//WO3 | WO3/Au/nylon 66 porous | Reflectance modulation of 38.9% @250–2500 nm | ~0.32 s | / | [2] |
| DBSA-PANI//DBSA-PANI | DBSA-doped PANI films | 0.43 @8–14 μm 0.4 @2.5–25 μm | / | / | [30] |
| Reflective-type Zn-ions electrochromic devices WO3//Zn | WO3 | 0.26 @3–5 μm 0.31 @8–14 μm | 7.2 s/11.7 s | / | [20] |
| TENG for building self-powered infrared detector | PANI with a sulfuric acid dopant | Average infrared reflectance contrast of 46% @8–14 μm | / | / | [31] |
| CSA-doped//CSA-PANI | CSA doped-PANI film | 0.225 @3–5 μm 0.399 @8–12 μm 0.426 @2.5–25 μm | 2.5 s/6 s | / | [15] |
| H2SO4-doped PANI//H2SO4-doped-PANI | H2SO4-doped PANI EC films | 0.4 @8–14 μm 0.3 @2.5–25 μm | / | / | [27] |
| PANI/Au//PANI/Au | PANI/Au composite film | 0.402 @8–14 μm | / | / | [32] |
| PANI//MXene/PANI/PVDF | PANI | 0.39 @8–14 μm | / | / | [33] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Liu, Y.; Luo, W.; Zeng, X.; Cheng, L.; Zhang, Y. Electrochromic devices and smart window applications of near-infrared electrochromic thienoviologens polymer properties. ACS Appl. Mater. Interfaces 2025, 17, 28472–28483. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Li, X.; Wang, L.; Sun, W.; Zhang, H.; Wang, J.; Zhao, J.; Li, Y. Bio-inspired electrochromic skin based on tungsten oxide. Sol. Energy Mater. Sol. Cells 2021, 230, 111195. [Google Scholar] [CrossRef]
- Lin, K.; Wu, C.; Zhou, Y.; Li, J.; Chen, X.; Wang, Y.; Lu, B. A multicolored polymer for dynamic military camouflage electrochromic devices. Sol. Energy Mater. Sol. Cells 2024, 278, 113180. [Google Scholar] [CrossRef]
- Lv, X.; Dong, J.; Shao, M.; Li, J.; Cui, J.; Shi, Y.; He, Y.; Ouyang, M.; Zhang, C. Flexible all-solid-state zinc-based electrochromic energy storage devices for adaptive military camouflage. ACS Appl. Polym. Mater. 2024, 6, 13171–13181. [Google Scholar] [CrossRef]
- Li, Y.; Jia, C.; Liu, Y.; Duan, W.; Qing, X.; Zhang, J.; Weng, X. Evaluation of electrochromic film camouflage effect based on visual perception. Displays 2025, 89, 103066. [Google Scholar] [CrossRef]
- Zhao, G.-f.; Wang, W.-q.; Wang, X.-l.; Xia, X.-h.; Gu, C.-d.; Tu, J.-p. A multicolor electrochromic film based on a SnO2/V2O5 core/shell structure for adaptive camouflage. J. Mater. Chem. C 2019, 7, 5702–5709. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, L.; Dong, Y.; Zhang, C.; Li, W. Recent advances in electrochromic materials and devices for camouflage applications. Mater. Chem. Front. 2023, 7, 2337–2358. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Mao, Y.; Huang, C.; Huang, J.; Ma, X.; Qi, Y.; Liu, Y.; Lin, H.; Luo, X. Visible-infrared compatible and independent camouflage with multicolor patterns and tunable emissivity. Nanophotonics 2024, 13, 3123–3133. [Google Scholar] [CrossRef]
- Ding, M.; Li, W.; Li, A.; Wang, Y.; Liu, J.; Zhang, Q.; Wang, H. Electrochromic fabrics with improved cycling stability via modified polyaniline towards environmentally adaptive camouflage. J. Mater. Chem. C 2025, 13, 4673–4682. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Zou, X.; Tan, Y.; Jia, C.; Weng, X.; Deng, L. Infrared electrochromic materials, devices and applications. Appl. Mater. Today 2021, 24, 101073. [Google Scholar] [CrossRef]
- Ding, Y.; Mei, Z.; Wu, X.; Zhang, W.; Zhang, Y.; Xi, A.; Gao, D.; Lan, F.; Xu, J.; Diao, X.; et al. Integrated multispectral modulator with efficient radiative cooling for innovative thermal camouflage. Adv. Funct. Mater. 2025, 2500122. [Google Scholar] [CrossRef]
- Brooke, R.; Mitraka, E.; Sardar, S.; Sandberg, M.; Sawatdee, A.; Berggren, M.; Crispin, X.; Jonsson, M.P. Infrared electrochromic conducting polymer devices. J. Mater. Chem. C 2017, 5, 5824–5830. [Google Scholar] [CrossRef]
- Hao, Q.; Li, Z.J.; Lu, C.; Sun, B.; Zhong, Y.W.; Wan, L.J.; Wang, D. Oriented two-dimensional covalent organic framework films for near-infrared electrochromic application. J. Am. Chem. Soc. 2019, 141, 19831–19838. [Google Scholar] [CrossRef]
- Mandal, J.; Du, S.; Dontigny, M.; Zaghib, K.; Yu, N.; Yang, Y. Li4Ti5O12: A visible-to-infrared broadband electrochromic material for optical and thermal management. Adv. Funct. Mater. 2018, 28, 1802180. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, G.; Li, X.; Xu, G.; Wang, B.; Li, D.; Gavrilyuk, A.; Zhao, J.; Li, Y. Fabrication of the infrared variable emissivity electrochromic film based on polyaniline conducting polymer. Synth. Met. 2019, 248, 88–93. [Google Scholar] [CrossRef]
- Wang, B.; Xu, G.; Song, S.; Ren, Z.; Liu, D.; Hao, L.; Xing, F.; Wei, H.; Zhang, L.; Li, Y. Flexible, infrared adjustable, thermal radiation control device based on electro-emissive PANI/Ce4+ thin films inspired by chameleon. Chem. Eng. J. 2022, 445, 136819. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, R.; Deng, C.; Peng, Y.; Jiang, T. Tunable Infrared Emissivity in Multilayer Graphene by Ionic Liquid Intercalation. Nanomaterials 2019, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Deng, L.; Zhang, P.; Tang, M.; Li, C.; Xie, H.; Huang, S.; Gao, X. Enhancement of electrochromic properties of polyaniline induced by copper Ions. Nanoscale Res. Lett. 2022, 17, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Zheng, R.; Pan, J.; Niu, J.; Zou, X.; Jia, C. A flexible, electrochromic, rechargeable Zn-ion battery based on actiniae-like self-doped polyaniline cathode. J. Mater. Chem. A 2020, 8, 12799–12809. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Guo, T.; Zhang, Z.; Wang, M.; Li, C.; Li, N.; Xue, C.; Diao, X.; Hu, S. High-performance reflective-type Zn-ions electrochromic devices toward visible-to-infrared broadband dynamic regulation. Chem. Eng. J. 2025, 504, 159117. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, J.; Wang, Y.; Hu, L.; Tang, S.; Wan, Z.; Jia, C.; Weng, X.; Deng, L. A light-weight, thin-thickness, flexible multifunctional electrochromic device integrated with variable optical, thermal management and energy storage. Electrochim. Acta 2022, 435, 141274. [Google Scholar] [CrossRef]
- Tanyi, E.K.; Burton, B.T.; Narimanov, E.E.; Noginov, M.A. Thermal radiation of Er doped dielectric crystals: Probing the range of applicability of the Kirchhoff’s law. Sci. Rep. 2017, 7, 2040. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Li, X.; Xu, G.; Dou, S.; Zhang, X.; Chen, X.; Zhao, J.; Zhang, K.; Li, Y. Further understanding of the mechanisms of electrochromic devices with variable infrared emissivity based on polyaniline conducting polymers. J. Mater. Chem. C 2019, 7, 9878–9891. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Li, X.; Wang, B.; Xu, G.; Zhao, X.; Xia, G.; Gavrilyuk, A.; Zhao, J.; Li, Y. Further explore on the behaviors of IR electrochromism of a double layer constructed by proton acid-doped polyaniline film and ITO layer. Dye. Pigment. 2019, 170, 107570. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Zay, B.J.; Lawrence, D.; Caldwell, E.; Sheth, R.; Stephan, R.; Cornwell, J. Variable-emittance infrared electrochromic skins combining unique conducting polymers, ionic liquid electrolytes, microporous polymer membranes, and semiconductor/polymer coatings, for spacecraft thermal control. J. Appl. Polym. Sci. 2014, 131, 40850. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Xu, G.; Wang, L.; Pan, M.; Ren, F.; Chen, X.; Li, X.; Li, Y. Novel aniline and haloaniline binary copolymer films for electro-emissive devices. Mater. Chem. Phys. 2020, 248, 122866. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, L.; Wang, B.; Chen, X.; Dou, S.; Pan, M.; Ren, F.; Li, X.; Li, Y. A visible-to-infrared broadband flexible electrochromic device based polyaniline for simultaneously variable optical and thermal management. Sol. Energy Mater. Sol. Cells 2020, 208, 110356. [Google Scholar] [CrossRef]
- Long, C.; Wei, T.; Yan, J.; Jiang, L.; Fan, Z. Supercapacitors based on graphene supported iron nanosheets as negative electrode materials. ACS Nano 2013, 7, 11325–11332. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, J.; Yuan, M.; Yu, S.; Ma, W.; Hu, Z.; Xiang, H.; Zhu, M. A flexible long-wave infrared radiation modulator integrated with electrochromic behavior for dual-band camouflage. ACS Appl. Mater. Interfaces 2024, 16, 30421–30429. [Google Scholar] [CrossRef]
- Song, S.; Xu, G.; Wang, B.; Gu, J.; Wei, H.; Ren, Z.; Zhang, L.; Zhao, J.; Li, Y. Highly-flexible monolithic integrated infrared electrochromic device based on polyaniline conducting polymer. Synth. Met. 2021, 278, 116822. [Google Scholar] [CrossRef]
- Sun, J.; Pu, X.; Jiang, C.; Du, C.; Liu, M.; Zhang, Y.; Liu, Z.; Zhai, J.; Hu, W.; Wang, Z.L. Self-powered electrochromic devices with tunable infrared intensity. Sci. Bull. 2018, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, G.; Song, S.; Ren, Z.; Li, X.; Li, J.; Xu, X.; Zhou, W.; Li, H.; Zhang, L.; et al. Electro-emissive device based on novel PANI/Au composite films with neoteric mosaic structure for infrared stealth and thermal radiation control. Electrochim. Acta 2021, 390, 138891. [Google Scholar] [CrossRef]
- Xu, G.; Wang, B.; Song, S.; Ren, Z.; Li, J.; Xu, X.; Zhou, W.; Li, H.; Yang, H.; Zhang, L.; et al. High-performance and robust dual-function electrochromic device for dynamic thermal regulation and electromagnetic interference shielding. Chem. Eng. J. 2021, 422, 130064. [Google Scholar] [CrossRef]




| Electrochromic Device | Emissivity | Infrared Wavelength Range | ||
|---|---|---|---|---|
| 3~5 μm | 8~14 μm | 2.5~15 μm | ||
| HWEC-ZIB | Low emissivity of the device | 0.58 | 0.39 | 0.43 |
| High emissivity of the device | 0.86 | 0.59 | 0.62 | |
| Modulation range (Δε) | 0.28 | 0.20 | 0.19 | |
| HWEC-ZIB(Electrolyte) | Low emissivity of the device | 0.54 | 0.57 | 0.58 |
| High emissivity of the device | 0.80 | 0.66 | 0.67 | |
| Modulation range (Δε) | 0.26 | 0.09 | 0.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Feng, T.; Chen, J.; Lin, E.; Xie, A. Development of a Reflective Electrochromic Zinc-Ion Battery Device for Infrared Emissivity Control Using Self-Doped Polyaniline Films. Polymers 2025, 17, 2110. https://doi.org/10.3390/polym17152110
Wang Y, Wang Z, Feng T, Chen J, Lin E, Xie A. Development of a Reflective Electrochromic Zinc-Ion Battery Device for Infrared Emissivity Control Using Self-Doped Polyaniline Films. Polymers. 2025; 17(15):2110. https://doi.org/10.3390/polym17152110
Chicago/Turabian StyleWang, Yi, Ze Wang, Tong Feng, Jiandong Chen, Enkai Lin, and An Xie. 2025. "Development of a Reflective Electrochromic Zinc-Ion Battery Device for Infrared Emissivity Control Using Self-Doped Polyaniline Films" Polymers 17, no. 15: 2110. https://doi.org/10.3390/polym17152110
APA StyleWang, Y., Wang, Z., Feng, T., Chen, J., Lin, E., & Xie, A. (2025). Development of a Reflective Electrochromic Zinc-Ion Battery Device for Infrared Emissivity Control Using Self-Doped Polyaniline Films. Polymers, 17(15), 2110. https://doi.org/10.3390/polym17152110





