Preparation of Inverse-Loaded MWCNTs@Fe2O3 Composites and Their Impact on Glycidyl Azide Polymer-Based Energetic Thermoplastic Elastomer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Samples
- (1)
- Preparation of MWCNTs@Fe2O3
- (2)
- Preparation of MWCNTs@Fe2O3/GAP-ETPE
- (3)
- Preparation of MWCNTs@Fe2O3/GAP-ETPE-Based Propellants
2.3. Measurements and Characterizations
3. Results and Discussion
3.1. Morphological Characterization of MWCNTs@Fe2O3 Composites
3.2. Thermal Analysis of MWCNTs@Fe2O3/GAP-ETPE Composites
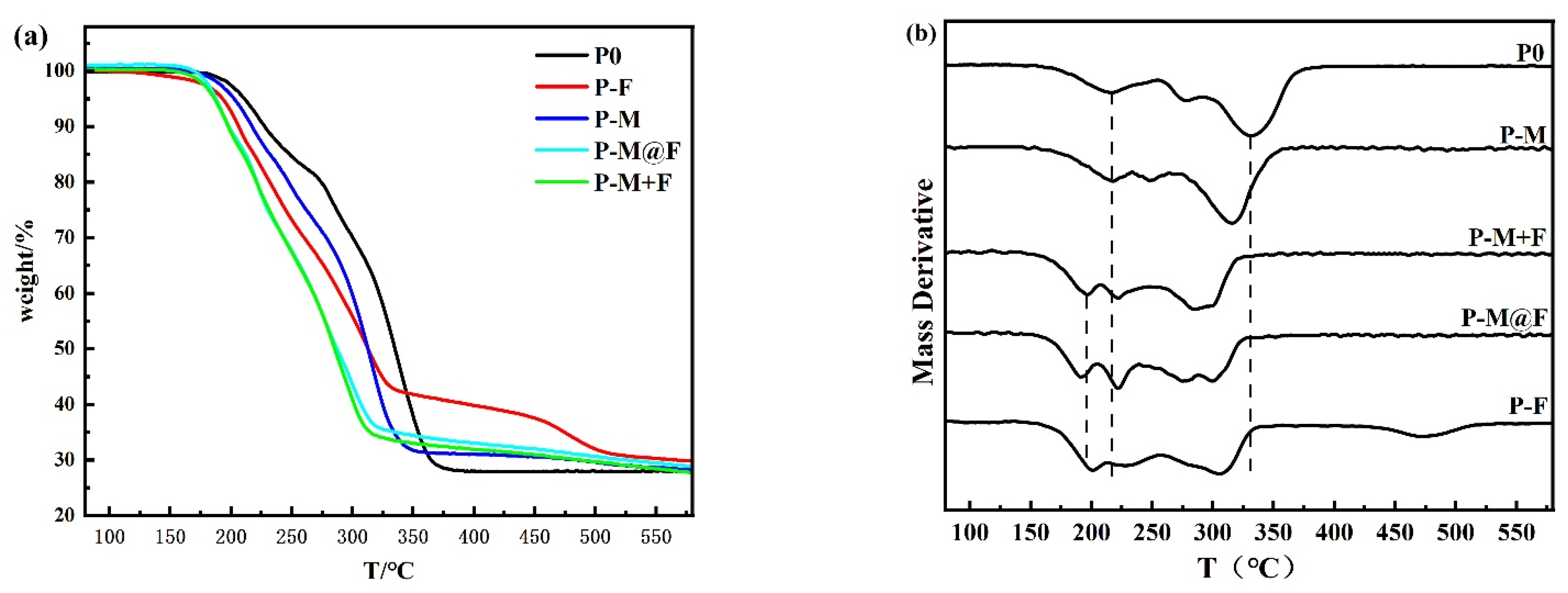
3.3. Characteristics of GAP-ETPE-Based Propellants
4. Conclusions
- (i)
- Pristine Fe2O3 nanoparticles.
- (ii)
- Unmodified MWCNTs.
- (iii)
- Physically mixed MWCNTs+Fe2O3 counterparts.
- Rational design of hybrid nanocatalysts through structural confinement;
- Performance optimization via composition–structure relationships;
- Practical implementation of nanocarbons in next-generation energetic materials.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sikder, A.K.; Reddy, S. Review on energetic thermoplastic elastomers (ETPEs) for military science. Propellants Explos. Pyrotech. 2013, 38, 14–28. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.K.; Gandhe, B.R.; Rao, A.S. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Vo, T.T.; Parrish, D.A.; Shreeve, J.M. Advanced GAP-based Energetic Thermoplastic Elastomers: Molecular Design, Performance Tailoring and Combustion Catalysis. Chem. Eng. J. 2021, 421, 129822. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, S.; Zeng, D.; Tang, G.; Xie, C. MOX (M = Zn, Co, Fe)/AP shell–core nanocomposites for self-catalytical decomposition of ammonium perchlorate. J. Alloys Compd. 2012, 513, 213–219. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Talawar, M.B.; Asthana, S.N.; Mahulikar, P.P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.; Luo, Y. Effect of Metastable Intermolecular Composites on the Thermal Decomposition of Glycidyl Azide Polymer Energetic Thermoplastic Elastomer. Polymers 2024, 16, 2107. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, X.; Yin, G. Mechanical Properties and Thermal Decomposition Mechanism of Glycidyl Azide Polyol Energetic Thermoplastic Elastomer Binder with RDX Composite. Polymers 2024, 16, 2626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, W.; Song, D.; Liu, J.; Li, F. Preparation and catalytic activity of Ni/CNTs nanocomposites using microwave irradiation heating method. Mater. Lett. 2008, 62, 2343–2346. [Google Scholar] [CrossRef]
- Smith, J.A.; Johnson, E.B.; Chen, W.; Müller, M.; Wang, L.; González, C.; Tanaka, H.; Ivanova, N.V.; Zhang, X.; Schmidt, F. Structural and Mechanical Properties of Polymer-Based Composites. J. Mater. Sci. 2023, 58, 789–800. [Google Scholar] [CrossRef]
- Yan, Q.; Gozin, M.; Zhao, F.; Cohen, A.; Pang, S. Highly energetic compositions based on functionalized carbon nanomaterials. ACS Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Xia, X.; Zhao, Y.; Deluca, L.T.; Trache, D.; Ouyang, D.; Meng, S.; Liu, X.; Yu, H. Effect of Carbon Nanotubes (CNTs) on the performance of Solid Rocket Propellants (SRPs): A short review. FirePhysChem. 2023, 3, 227–233. [Google Scholar] [CrossRef]
- Denisyuk, A.; Milekhin, Y.; Demidova, L.; Sizov, V. Effect of Carbon Nanotubes on the Catalysis of Propellant Combustion. Dokl Chem. 2018, 483, 301–303. [Google Scholar] [CrossRef]
- Field, J.; Bourne, N.; Palmer, S.; Walley, S. Hot-spot ignition mechanisms for explosives and propellants. Philos. Trans. R. Soc. Lond. Ser. A Phys. Eng. Sci. 1992, 339, 269–283. [Google Scholar] [CrossRef]
- Dienes, J. Frictional hot-spots and propellant sensitivity. MRS Online Proc. Libr. 1983, 24, 373–381. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Wang, Z.; Zhang, Y.; Ge, Z.; Luo, Y. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 2015, 72, 1835–1847. [Google Scholar] [CrossRef]
- Sang, C.; Chen, K.; Li, G.; Jin, S.; Luo, Y. Facile Mass Preparation and Characterization of Al/Copper Ferrites Metastable Intermolecular Energetic Nanocomposites. RSC Adv. 2021, 11, 7633–7643. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.; Jin, S.; Li, G.; Luo, Y. Preparation of Copper Ferrite by Sol-Gel Method and the Synergistic Catalytic Effect for the Thermal Decomposition of Ammonium Perchlorate. J. Sol-Gel Sci. Technol. 2021, 98, 559–567. [Google Scholar] [CrossRef]
- Boldyrev, V.V.; Alexandrov, V.V.; Boldyreva, A.V.; Gritsan, V.I.; Karpenko, Y.Y.; Korobeinitchev, O.P.; Panfilov, V.N.; Khairetdinov, E.F. On the Mechanism of the Thermal Decomposition of Ammonium Perchlorate. Combust. Flame 1970, 15, 71–77. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Kuibida, L.; Volkov, E.; Shmakov, A. Mass spectrometric study of combustion and thermal decomposition of GAP. Combust. Flame 2002, 129, 136–150. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, F.; Zhang, J.; Wang, Y.; Chen, X.; An, T.; Qin, Z.; Hao, H. Novel graphene iron organic nanocomposites for enhancing combustion and safety properties of AP-HTPB propellant. Combust. Flame 2024, 263, 113420. [Google Scholar] [CrossRef]




| GAP-ETPE | Catalyst | Catalyst Composition |
|---|---|---|
| ETPE | - | - |
| E-F | F | Fe2O3 |
| E-1 | M@F-1 | MWCNTs@Fe2O3 (MWCNTs content: 1 wt%) |
| E-2 | M@F-2 | MWCNTs@Fe2O3 (MWCNTs content: 2 wt%) |
| E-4 | M@F-4 | MWCNTs@Fe2O3 (MWCNTs content: 4 wt%) |
| E-8 | M@F-8 | MWCNTs@Fe2O3 (MWCNTs content: 8 wt%) |
| E-M | M | MWCNTs |
| E-M+F | M+F | MWCNTs (1 wt%), Fe2O3 (99 wt%) |
| Propellant | GAP-ETPE | RDX | Al | AP | Catalyst | |||
|---|---|---|---|---|---|---|---|---|
| MWCNTs@Fe2O3 | MWCNTs | Fe2O3 | Notes | |||||
| P0 | 20 | 12 | 20 | 48 | 0 | 0 | 0 | |
| P-M | 20 | 11.2 | 19.2 | 47.2 | 0 | 2.4 | 0 | M |
| P-M+F | 20 | 11.2 | 19.2 | 47.2 | 0 | 0.024 | 2.376 | M+F |
| P-M@F | 20 | 11.2 | 19.2 | 47.2 | 2.4 | 0 | 0 | M@F-1 |
| P-F | 20 | 11.2 | 19.2 | 47.2 | 0 | 0 | 2.4 | F |
| Samples | TP1 (°C) | ΔT (°C) | TP (°C) |
|---|---|---|---|
| ETPE | 252.3 | 0 | 248.9 |
| E-M | 250.1 | 2.2 | 247.5 |
| E-8 | 243.2 | 9.1 | 241.2 |
| E-4 | 239.6 | 12.7 | 236.9 |
| E-2 | 242.7 | 9.6 | 241.1 |
| E-1 | 243.2 | 9.1 | 242.1 |
| E-F | 244.1 | 8.2 | 242.5 |
| E-M+F | 248.2 | 4.1 | 246.8 |
| Samples | σm (MPa) | εb (%) | Ρ (g/cm3) |
|---|---|---|---|
| P0 | 3.46 | 14.7 | 1.69 |
| P-M | 8.22 | 12.7 | 1.81 |
| P-M+F | 3.88 | 21.1 | 1.81 |
| P-M@F | 4.95 | 21.6 | 1.77 |
| P-F | 3.83 | 14.8 | 1.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, S.; Lv, Y.; Liu, S.; Sang, C.; Jin, B.; Luo, Y. Preparation of Inverse-Loaded MWCNTs@Fe2O3 Composites and Their Impact on Glycidyl Azide Polymer-Based Energetic Thermoplastic Elastomer. Polymers 2025, 17, 2080. https://doi.org/10.3390/polym17152080
Pang S, Lv Y, Liu S, Sang C, Jin B, Luo Y. Preparation of Inverse-Loaded MWCNTs@Fe2O3 Composites and Their Impact on Glycidyl Azide Polymer-Based Energetic Thermoplastic Elastomer. Polymers. 2025; 17(15):2080. https://doi.org/10.3390/polym17152080
Chicago/Turabian StylePang, Shuo, Yihao Lv, Shuxia Liu, Chao Sang, Bixin Jin, and Yunjun Luo. 2025. "Preparation of Inverse-Loaded MWCNTs@Fe2O3 Composites and Their Impact on Glycidyl Azide Polymer-Based Energetic Thermoplastic Elastomer" Polymers 17, no. 15: 2080. https://doi.org/10.3390/polym17152080
APA StylePang, S., Lv, Y., Liu, S., Sang, C., Jin, B., & Luo, Y. (2025). Preparation of Inverse-Loaded MWCNTs@Fe2O3 Composites and Their Impact on Glycidyl Azide Polymer-Based Energetic Thermoplastic Elastomer. Polymers, 17(15), 2080. https://doi.org/10.3390/polym17152080







