Polymeric Composite Thin Films Deposited by Laser Techniques for Antimicrobial Applications—A Short Overview
Abstract
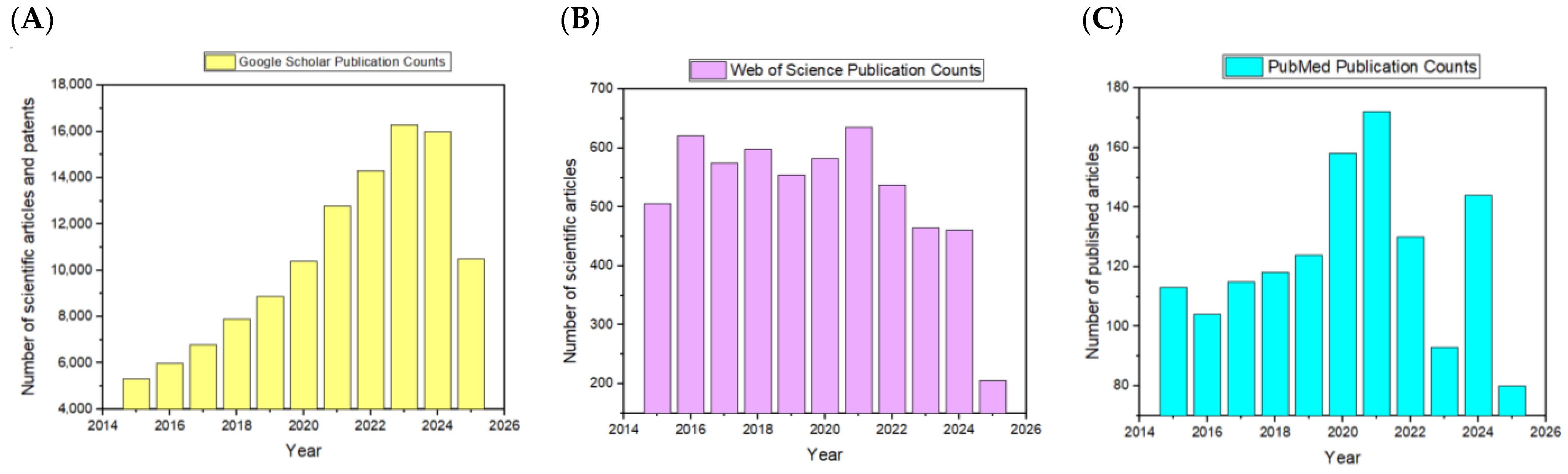
1. Introduction
2. Laser Deposition Techniques for Antimicrobial Polymeric Composite Thin Films
2.1. Mechanical Considerations in Laser-Deposited Flexible Films
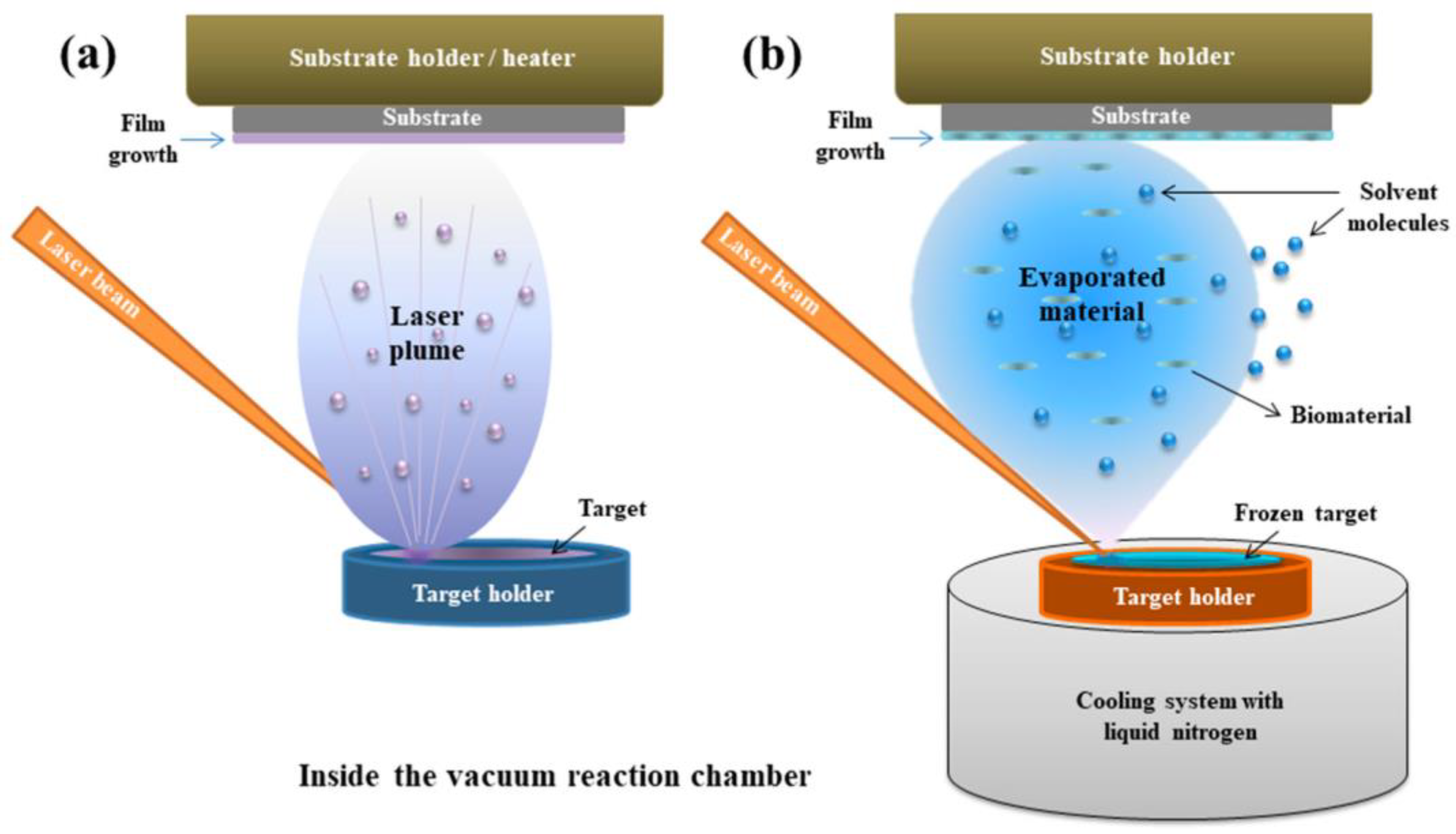
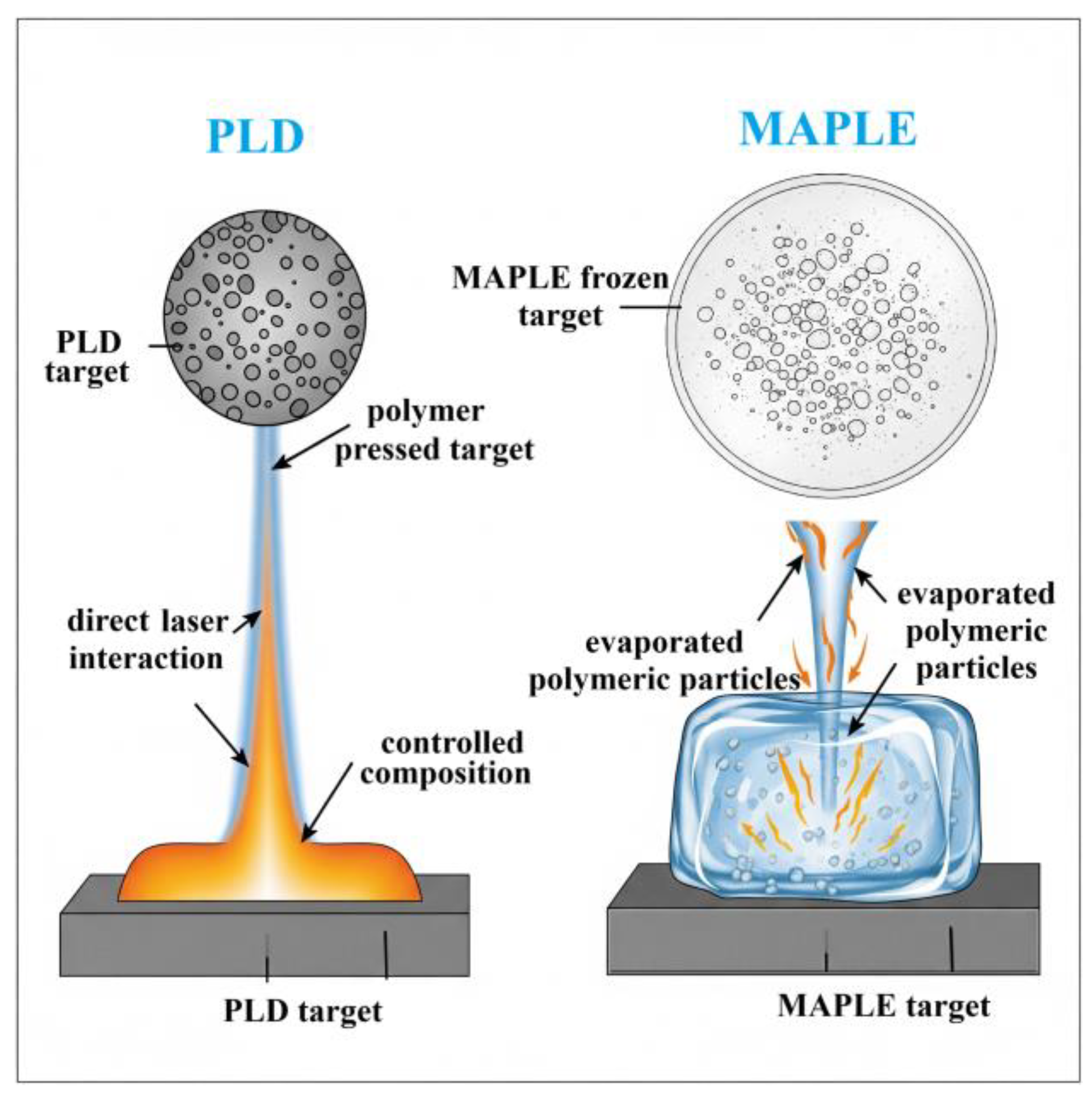
2.2. PLD and MAPLE
2.2.1. Principle
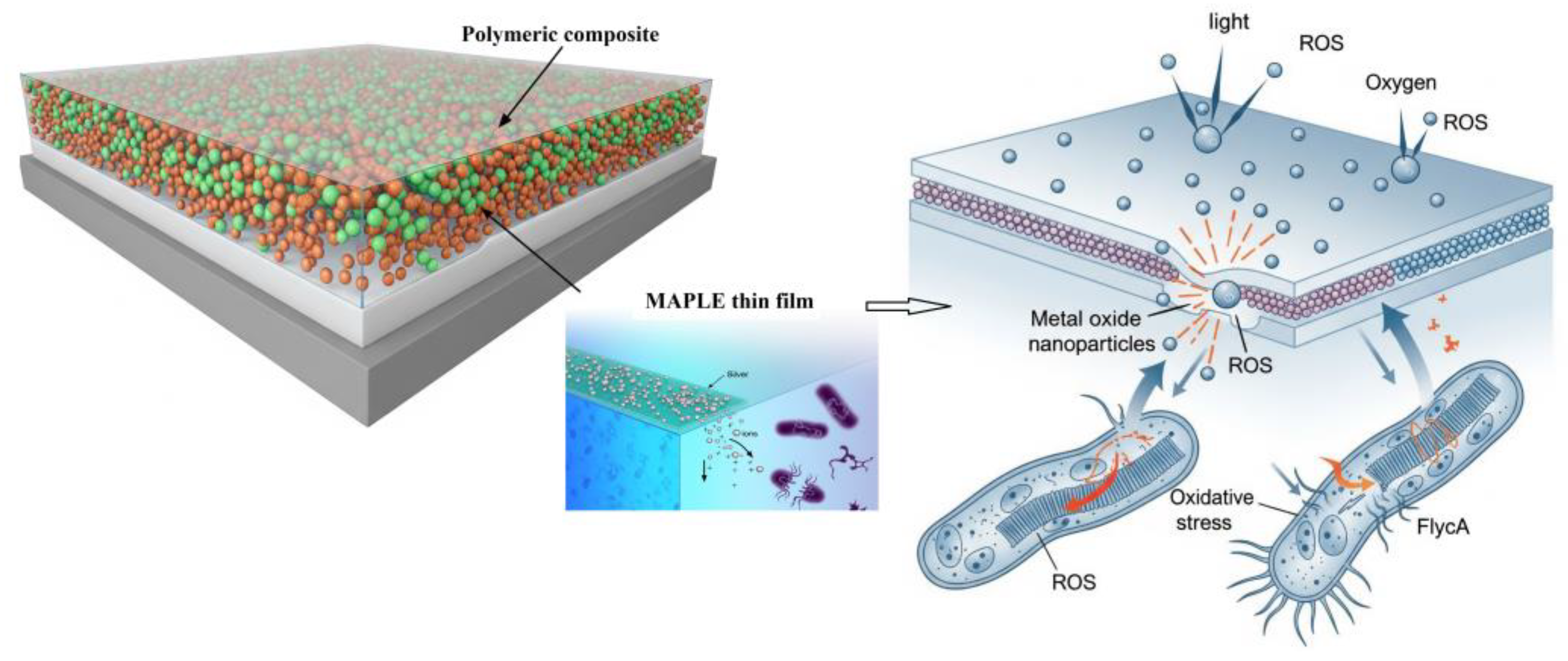
2.2.2. Application to Polymeric Composite Thin Films
2.2.3. Key Parameters Influencing PLD and MAPLE for Polymeric Composite Thin Films
3. Composition of Laser-Deposited Antimicrobial Thin Films
3.1. Polymeric Composite Classification
Polymer Matrix Type (Synthetic vs. Natural)
- Synthetic polymers
- Natural/biocompatible polymers
3.2. Type of Antimicrobial Agent
3.2.1. Metal and Metal Oxide NPs
3.2.2. Organic Antimicrobial Molecules
4. Antimicrobial Efficacy of Laser-Deposited Polymeric Composite Thin Films
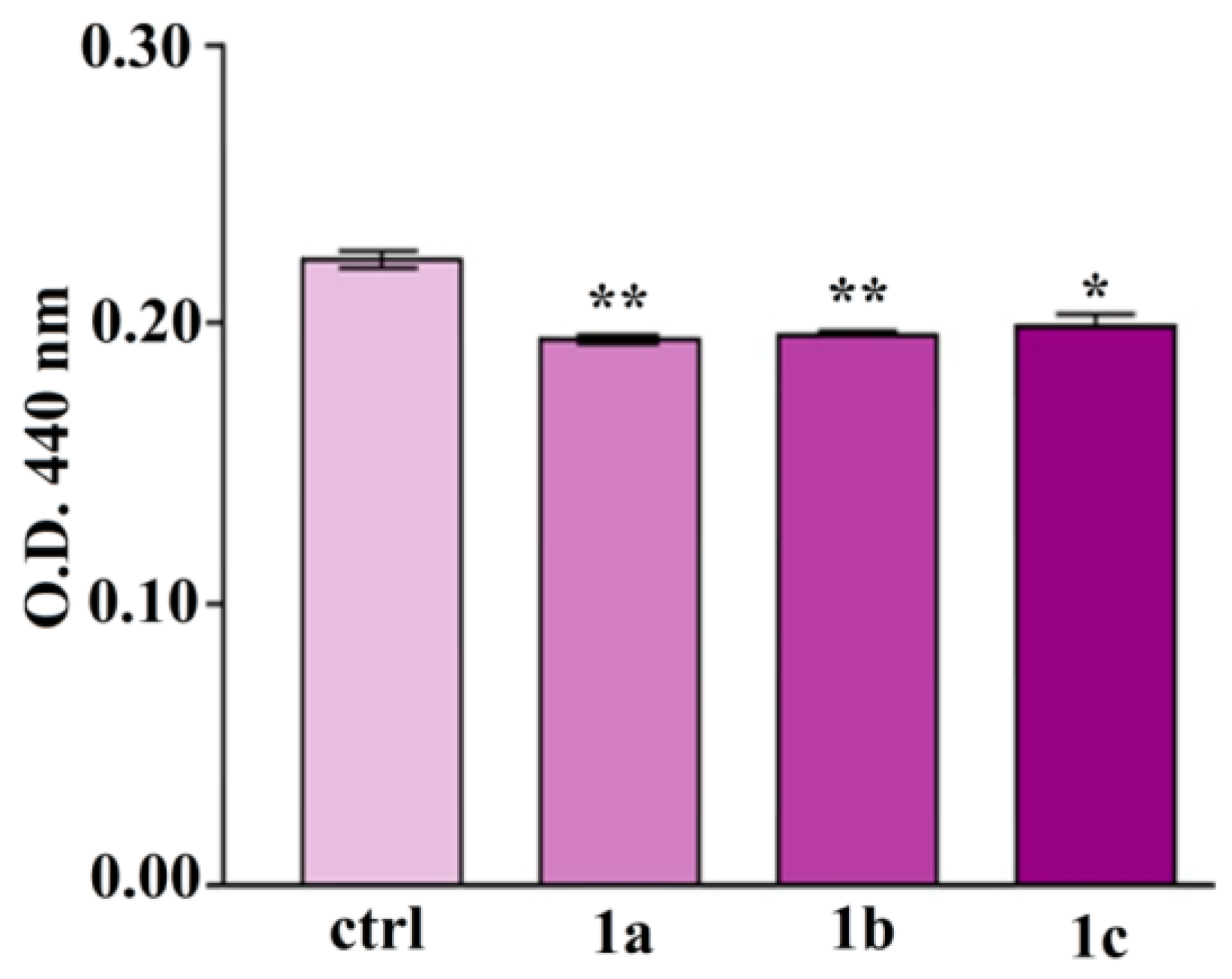
4.1. Antimicrobial Efficacy Testing
4.2. Mechanisms of Antimicrobial Action
- (i)
- The release of active antimicrobial agents into the surrounding environment
- (ii)
- Direct contact interaction with microbial cells, leading to their disruption.
- Electrostatic interactions: Cationic polymers or functional groups (e.g., quaternary ammonium, polyaniline) can interact electrostatically with bacterial membranes, disrupting membrane integrity [183].
- Hydrophobic interactions: Hydrophobic patches on the film can destabilize bacterial membranes, especially for Gram-negative bacteria with outer lipid membranes [184].
- Mechanical puncture or deformation: Nanostructures, such as sharp nanopillars or ridges, can physically rupture membranes, similar to a “bed-of-nails” effect [182].
4.3. Influence of Key Environmental Factors
- a.
- Hydrophobicity/hydrophilicity
- b.
- pH
- c.
- Temperature
- d.
- Nanostructures and surface topography
- Increased surface area at the nanoscale facilitates stronger contact interactions between the film and microbial cells, improving contact-killing efficiency and diffusion-controlled drug release [203].
- Nanostructured surfaces increase the likelihood of direct contact between bacteria and bioactive agents (e.g., metal NPs or antibiotics). This proximity improves membrane disruption, ion release, and interaction with microbial cell walls. Greater surface area allows better dispersion and embedding of antimicrobial agents within polymer matrices, enabling diffusion-driven release profiles suited for sustained antimicrobial action [204].
- e.
- Combined influence of environmental factors
- f.
- Biocompatibility of composite polymeric coatings as an important factor
4.4. Polymeric Antimicrobial Coatings for Combating MDR Pathogens and AMR
5. Biodegradability and Long-Term Stability of Antimicrobial Polymeric Coatings
5.1. Biodegradability
5.1.1. Structural Integrity and Functional Preservation
5.1.2. Tunability of Degradation Rates
5.1.3. Nanocomposite Enhancements
5.2. Long-Term Structural and Functional Stability
6. Challenges and Future Perspectives Regarding Scalability and Industrial Translation of Laser-Based Deposition Techniques
- Adoption of validated international standards (e.g., ISO 22196, ASTM E2149, ISO 7581) to ensure methodological consistency [284].
- Comprehensive reporting of methodological parameters, including strain identification, CFU/mL inoculum, incubation times, and environmental conditions [285].
- Implementation of multi-method validation (e.g., CFU in conjunction with live/dead staining) to increase confidence in reported outcomes [286].
- Use of dynamic flow systems or ex vivo models that simulate clinically relevant environments, such as saliva or wound exudate [287].
- Inclusion of benchmark controls, such as well-characterized silver-coated or antibiotic-loaded reference materials, to contextualize results [288].
7. Conclusions
- Laser-based deposition methods such as PLD and MAPLE enable precise, clean, and efficient fabrication of antimicrobial coatings with controllable properties. The choice between MAPLE and PLD depends on factors such as the nature of the polymer and antimicrobial agent, desired film properties (thickness, morphology, uniformity), substrate material, and considerations of scalability and cost-effectiveness. PLD offers higher deposition rates but risks thermal damage; MAPLE preserves bioactivity but has lower throughput.
- Polymeric matrices embedded with antimicrobial agents (e.g., metal NPs, antibiotics, natural compounds) enhance surface functionality and broaden the antimicrobial spectrum. Ag NPs provide rapid bactericidal effects but raise cytotoxicity concerns; antibiotics (e.g., ciprofloxacin) offer targeted action but may induce resistance.
- Hybrid systems (e.g., Ag NPs + chitosan) balance efficacy and safety, with MAPLE being optimal for delicate biomolecules.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fasiku, V.O.; Hassan, D.; Owonubi, S.J.; Mukwevho, E.; Olajide, J.L. Antibiotic Polymer for Biomedical Applications. In Antibiotic Materials in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–49. [Google Scholar]
- Olaru, I.; Stefanache, A.; Gutu, C.; Lungu, I.I.; Mihai, C.; Grierosu, C.; Calin, G.; Marcu, C.; Ciuhodaru, T. Combating Bacterial Resistance by Polymers and Antibiotic Composites. Polymers 2024, 16, 3247. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart bandages: The future of wound care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Suo, H.; Xie, G.; Lyu, Q.; Mo, M.; Xie, Z.; Zhou, N.; Zhang, L.; Tao, J.; Zhu, J. Self-powered and photothermal electronic skin patches for accelerating wound healing. Nano Energy 2022, 93, 106906. [Google Scholar] [CrossRef]
- Warwick, K. Neuroengineering and neuroprosthetics. Brain Neurosci. Adv. 2018, 2, 2398212818817499. [Google Scholar] [CrossRef] [PubMed]
- Bussooa, A.; Neale, S.; Mercer, J.R. Future of smart cardiovascular implants. Sensors 2018, 18, 2008. [Google Scholar] [CrossRef] [PubMed]
- Chuang, A.T.; Margo, C.E.; Greenberg, P.B. Retinal implants: A systematic review. Br. J. Ophthalmol. 2014, 98, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Fletter, P.C.; Zaszczurynski, P.J.; Damaser, M.S. Urothelial Biomechanics: Submucosal Sensing of Intravesical Pressure. In Proceedings of the Summer Bioengineering Conference, Marco Island, FL, USA, 25–29 June 2008; pp. 761–762. [Google Scholar]
- Li, Z.; Oo, M.Z.; Nalam, V.; Thang, V.D.; Ren, H.; Kofidis, T.; Yu, H. Design of a novel flexible endoscope—Cardioscope. J. Mech. Robot. 2016, 8, 051014. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Z.; Wu, R.; Zang, J.; Lu, B.; Du, C.; Yu, Y. Direct fabrication of flexible strain sensor with adjustable gauge factor on medical catheters. J. Sci. Adv. Mater. Devices 2023, 8, 100558. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Mercado, A.; Allmond, C.; Hoekstra, J.; Fitz-Gerald, J. Pulsed laser deposition vs. matrix assisted pulsed laser evaporation for growth of biodegradable polymer thin films. Appl. Phys. A 2005, 81, 591–599. [Google Scholar] [CrossRef]
- Popescu, R.-C.; Fufa, O.; Apostol, A.I.; Popescu, D.; Grumezescu, A.M.; Andronescu, E. Antimicrobial thin coatings prepared by laser processing. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 223–236. [Google Scholar]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ.-Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Liu, Z.; Liu, H.; Chen, Y.; Li, S.; Guo, S.; Xiu, B.; Dong, X.; Cao, H. Temperature Dependence on Microstructure, Crystallization Orientation, and Piezoelectric Properties of ZnO Films. Sensors 2025, 25, 242. [Google Scholar] [CrossRef] [PubMed]
- Olmos, D.; González-Benito, J. Polymeric materials with antibacterial activity: A review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Finina, B.F.; Mersha, A.K. Nano-enabled antimicrobial thin films: Design and mechanism of action. RSC Adv. 2024, 14, 5290–5308. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Pinho, A.C.; Piedade, A.P. Polymeric coatings with antimicrobial activity: A short review. Polymers 2020, 12, 2469. [Google Scholar] [CrossRef] [PubMed]
- Corradini, C.; Alfieri, I.; Cavazza, A.; Lantano, C.; Lorenzi, A.; Zucchetto, N.; Montenero, A. Antimicrobial films containing lysozyme for active packaging obtained by sol–gel technique. J. Food Eng. 2013, 119, 580–587. [Google Scholar] [CrossRef]
- Nicolaus, M.; Schäpers, M. Fundamentals of Thin-film Technology. In Modern Surface Technology; Wiley-VCH: Weinheim, Germany, 2006; pp. 31–50. [Google Scholar]
- Shu, H.; Chen, P.; Yang, R. Advances in antibacterial polymer coatings synthesized via chemical vapor deposition. Chem Bio Eng. 2024, 1, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Mihaiescu, D.; Socol, G.; Stamatin, I.; Mihailescu, I.; Chrisey, D. Deposition of biopolymer thin films by matrix assisted pulsed laser evaporation. Appl. Phys. A 2004, 79, 1023–1026. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin-Ince, G.; Coclite, A.M.; Gleason, K.K. CVD of polymeric thin films: Applications in sensors, biotechnology, microelectronics/organic electronics, microfluidics, MEMS, composites and membranes. Rep. Prog. Phys. 2011, 75, 016501. [Google Scholar] [CrossRef] [PubMed]
- Ubhale, Y.S.; More, A.P. Antimicrobial sol–gel coating: A review. J. Coat. Technol. Res. 2024, 22, 527–548. [Google Scholar] [CrossRef]
- Tusher, M.M.H.; Imam, A.; Shuvo, M.S.I. Future and challenges of coating materials. In Coating Materials: Computational Aspects, Applications and Challenges; Springer: Berlin/Heidelberg, Germany, 2023; pp. 229–251. [Google Scholar]
- Thangaraju, P.; Varthya, S.B. ISO 10993: Biological evaluation of medical devices. In Medical Device Guidelines and Regulations Handbook; Springer: Berlin/Heidelberg, Germany, 2022; pp. 163–187. [Google Scholar]
- Socol, M.; Preda, N.; Breazu, C.; Costas, A.; Petre, G.; Stanculescu, A.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G. Organic thin films based on DPP-DTT: C60 blends deposited by MAPLE. Nanomaterials 2020, 10, 2366. [Google Scholar] [CrossRef] [PubMed]
- Murariu, A.C.; Cocard, M.; Geana, A.A.; Socol, G. Study on the Parameters of MAPLE and PLD Coating Technologies for Optimal Corrosion Resistance of MnTa2O6 Pseudo-Binary Oxide and 5, 10-(4-Carboxy-Phenyl)-15, 20-(4-Phenoxy-Phenyl)-Porphyrin Thin Film Coating System. Solid State Phenom. 2023, 349, 43–54. [Google Scholar] [CrossRef]
- Axente, E.; Socol, G. Special Issue “Pulsed Laser Deposition of Thin Films: Recent Advances and Challenge”. Coatings 2022, 12, 368. [Google Scholar] [CrossRef]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Rothman, L. Properties of thin polyimide films. J. Electrochem. Soc. 1980, 127, 2216. [Google Scholar] [CrossRef]
- Deshmukh, R.R.; Bhat, N.V. The mechanism of adhesion and printability of plasma processed PET films. Mater. Res. Innov. 2003, 7, 283–290. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.S.; Kolekar, P.; Negi, P.; Anure, A.; Ajit, K.; Kolekar, S.V.; Singh, S.K.; Disouza, J.; Patravale, V.B. 4 Synthesis of. In Commercial Scale Production of Nanomedicines; CRC Press: Boca Raton, FL, USA, 2025; Volume 96. [Google Scholar]
- Ong, S.-E.; Zhang, S. Amorphous Carbon Coatings for Biological Applications. In Biological and Biomedical Coatings Handbook. Volume 2: Applications; CRC Press: Boca Raton, FL, USA, 2012; pp. 45–111. [Google Scholar]
- Bull, S. Interface engineering and graded films: Structure and characterization. J. Vac. Sci. Technol. A 2001, 19, 1404–1414. [Google Scholar] [CrossRef]
- Zulfikar, B.; Aydemir, N. Fabrication and characterization of flexible graphene films with kirigami-inspired structural configurations. J. Mater. Sci. Mater. Electron. 2025, 36, 543. [Google Scholar] [CrossRef]
- Gupta, M.; Chowdhury, F.R.; Barlage, D.; Tsui, Y.Y. Optimization of pulsed laser deposited ZnO thin-film growth parameters for thin-film transistors (TFT) application. Appl. Phys. A 2013, 110, 793–798. [Google Scholar] [CrossRef]
- Lee, M.H.; Arcidiacono, J.A.; Bilek, A.M.; Wille, J.J.; Hamill, C.A.; Wonnacott, K.M.; Wells, M.A.; Oh, S.S. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng. Part B Rev. 2010, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L. Fatigue Testing and Analysis: Theory and Practice; Butterworth-Heinemann: Oxford, UK, 2005; Volume 13. [Google Scholar]
- Wei, Z.; Attarilar, S.; Ebrahimi, M.; Li, J. Corrosion and wear behavior of additively manufactured metallic parts in biomedical applications. Metals 2024, 14, 96. [Google Scholar] [CrossRef]
- Udriște, A.S.; Burdușel, A.C.; Niculescu, A.-G.; Rădulescu, M.; Grumezescu, A.M. Coatings for cardiovascular stents—An up-to-date review. Int. J. Mol. Sci. 2024, 25, 1078. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.; Jakešová, M.; Mao, G.; Nikić, M.; Kaltenbrunner, M.; Đerek, V.; Głowacki, E.D. Scalable microfabrication of folded parylene-based conductors for stretchable electronics. Adv. Electron. Mater. 2021, 7, 2001236. [Google Scholar] [CrossRef]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.J.; Alawsi, T.; Haider, M.J.; Taha, B.A.; Marhoon, H.A. A comprehensive review on pulsed laser deposition technique to effective nanostructure production: Trends and challenges. Opt. Quantum Electron. 2022, 54, 488. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A practical guide to pulsed laser deposition. Chem. Soc. Rev. 2023, 52, 2294–2321. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Abate, F.; Ambrogi, V.; Califano, V.; Cerruti, P.; Pepe, G.P.; Vicari, L.R.; Ausanio, G. Smart coatings prepared via MAPLE deposition of polymer nanocapsules for light-induced release. Molecules 2021, 26, 2736. [Google Scholar] [CrossRef] [PubMed]
- Aruta, C.; Amoruso, S.; Bruzzese, R.; Wang, X.; Maccariello, D.; Miletto Granozio, F.; Scotti di Uccio, U. Pulsed laser deposition of SrTiO3/LaGaO3 and SrTiO3/LaAlO3: Plasma plume effects. Appl. Phys. Lett. 2010, 97, 252105. [Google Scholar] [CrossRef]
- Hill, M.; Wagenaars, E. Modelling of Plasma Temperatures and Densities in Laser Ablation Plumes of Different Metals. Photonics 2022, 9, 937. [Google Scholar] [CrossRef]
- Itina, T. Plume dynamics and nanoparticle formation in ultra-short laser ablation of metals. arXiv 2014, arXiv:1405.3439. [Google Scholar]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Ristoscu, C.; Mihailescu, I.N. Thin films and nanoparticles by pulsed laser deposition: Wetting, adherence, and nanostructuring. In Pulsed Laser Ablation; Jenny Stanford Publishing: Singapore, 2018; pp. 245–276. [Google Scholar]
- Visan, A.I.; Popescu-Pelin, G.F. Advanced Laser Techniques for the Development of Nature-Inspired Biomimetic Surfaces Applied in the Medical Field. Coatings 2024, 14, 1290. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.-F.; Ristoscu, C.-G.; Badiceanu, M.; Mihailescu, I.N. Protected laser evaporation/ablation and deposition of organic/biological materials: Thin films deposition for nano-biomedical applications. In Laser Ablation—From Fundamentals to Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Popescu, A.; Ulmeanu, M.; Ristoscu, C.; Mihailescu, I. Deposition and surface modification of thin solid structures by high-intensity pulsed laser irradiation. In Laser Surface Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 287–313. [Google Scholar]
- Mihailescu, I.N.; Bigi, A.; Gyorgy, E.; Ristoscu, C.; Sima, F.; Oner, E.T. Biomaterial thin films by soft pulsed laser technologies for biomedical applications. In Lasers in Materials Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 271–294. [Google Scholar]
- Schaaf, P. Laser Processing of Materials: Fundamentals, Applications and Developments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; Volume 139. [Google Scholar]
- Nelea, V.; Jelinek, M.; Mihailescu, I. Biomaterials: New issues and breakthroughs for biomedical applications. In Pulsed Laser Deposition of Thin Films: Applications-Lead Growth of Functional Materials; Eason, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 421–459. [Google Scholar]
- Ristoscu, C.; Mihailescu, I.N. Biomimetic coatings by pulsed laser deposition. In Laser Technology in Biomimetics: Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 163–191. [Google Scholar]
- Mihailescu, I.N.; Caricato, A.P. Pulsed Laser Ablation: Advances and Applications in Nanoparticles and Nanostructuring Thin Films; Jenny Stanford Publishing: New York, NY, USA, 2018. [Google Scholar]
- Cristescu, R.; Negut, I.; Visan, A.I.; Nguyen, A.K.; Sachan, A.; Goering, P.L.; Chrisey, D.B.; Narayan, R.J. Matrix-assisted pulsed laser evaporation-deposited rapamycin thin films maintain antiproliferative activity. Int. J. Bioprinting 2020, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Surdu, A.; Grumezescu, A.; Oprea, A.; Trusca, R.; Vasile, O.; Dorcioman, G.; Visan, A.; Socol, G.; Mihailescu, I.; et al. Microbial colonization of biopolymeric thin films containing natural compounds and antibiotics fabricated by MAPLE. Appl. Surf. Sci. 2015, 336, 234–239. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, K.; Monnier, X.; Jeong, H.; Chowdhury, M.; Cangialosi, D.; Loo, Y.-L.; Priestley, R.D. Tunable properties of MAPLE-deposited thin films in the presence of suppressed segmental dynamics. ACS Macro Lett. 2019, 8, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Gudi, D.; Sen, P.; Forero Pico, A.A.; Nandi, D.; Gupta, M. Optimization of growth parameters to obtain epitaxial large area growth of molybdenum disulfide using pulsed laser deposition. AIP Adv. 2022, 12, 065027. [Google Scholar] [CrossRef]
- Wang, Y.; Jeong, H.; Chowdhury, M.; Arnold, C.B.; Priestley, R.D. Exploiting physical vapor deposition for morphological control in semi-crystalline polymer films. Polym. Cryst. 2018, 1, e10021. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, V.; Socol, G.; Ficai, A. Nanoarchitectonics prepared by laser processing and their biomedicinal applications. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–53. [Google Scholar]
- Mostafa, A.M. The Influence of Various Parameters on the Ablation and Deposition Mechanisms in Pulsed Laser Deposition. Plasmonics 2025, 20, 5627–5645. [Google Scholar] [CrossRef]
- Garrido, J.M.C.; Silveyra, J.M. A review of typical PLD arrangements: Challenges, awareness, and solutions. Opt. Lasers Eng. 2023, 168, 107677. [Google Scholar] [CrossRef]
- Negut, I.; Visan, A.I.; Popescu, C.; Cristescu, R.; Ficai, A.; Grumezescu, A.M.; Chifiriuc, M.C.; Boehm, R.D.; Yamaleyeva, D.; Taylor, M.; et al. Successful release of voriconazole and flavonoids from MAPLE deposited bioactive surfaces. Appl. Sci. 2019, 9, 786. [Google Scholar] [CrossRef]
- Sima, F.; Davidson, P.M.; Dentzer, J.; Gadiou, R.; Pauthe, E.; Gallet, O.; Mihailescu, I.N.; Anselme, K. Inorganic–organic thin implant coatings deposited by lasers. ACS Appl. Mater. Interfaces 2015, 7, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Sima, F.; Mihailescu, I.N. Biomimetic assemblies by matrix-assisted pulsed laser evaporation. In Laser Technology in Biomimetics: Basics and Applications; Springer: Berlin, Heidelberg, 2014; pp. 111–141. [Google Scholar]
- Capuccini, C.; Sima, F.; Axente, E.; Boanini, E.; Gazzano, M.; Bigi, A.; Mihailescu, I. Strontium-substituted hydroxyapatite thin films grown by pulsed laser deposition. In Functionalized Nanoscale Materials, Devices and Systems; Springer: Berlin/Heidelberg, Germany, 2008; pp. 389–398. [Google Scholar]
- Bigi, A.; Boanini, E.; Capuccini, C.; Fini, M.; Mihailescu, I.N.; Ristoscu, C.; Sima, F.; Torricelli, P. Biofunctional alendronate–hydroxyapatite thin films deposited by matrix assisted pulsed laser evaporation. Biomaterials 2009, 30, 6168–6177. [Google Scholar] [CrossRef] [PubMed]
- Chichkov, B.N.; Momma, C.; Nolte, S.; Von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G. Pulsed Laser Deposition of Thin Films; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Pique, A.; Chrisey, D.B. Laser Direct-Write. Direct-Write Technologies for Rapid Prototyping: Sensors, Electronics, and Integrated Power Sources; Academic Press: Cambridge, MA, USA, 2002; Volume 385. [Google Scholar]
- Bloembergen, N. Laser-Material Interactions-Fundamentals and Applications. AIP Conf. Proc. 1993, 288, 3–10. [Google Scholar]
- Pronko, P.; Dutta, S.; Squier, J.; Rudd, J.; Du, D.; Mourou, G. Machining of sub-micron holes using a femtosecond laser at 800 nm. Opt. Commun. 1995, 114, 106–110. [Google Scholar] [CrossRef]
- Balling, P.; Schou, J. Femtosecond-laser ablation dynamics of dielectrics: Basics and applications for thin films. Rep. Prog. Phys. 2013, 76, 036502. [Google Scholar] [CrossRef] [PubMed]
- Gamaly, E.G.; Rode, A.V.; Luther-Davies, B.; Tikhonchuk, V.T. Ablation of solids by femtosecond lasers: Ablation mechanism and ablation thresholds for metals and dielectrics. Phys. Plasmas 2002, 9, 949–957. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Fujihara, A. Self-assembled spontaneous structures induced by a pulsed laser on a surface of azobenzene polymer film. J. Appl. Phys. 2007, 101, 033110. [Google Scholar] [CrossRef]
- Bulgakova, N.M. Fundamentals of ultrafast laser processing. In Ultrafast Laser Processing: From Micro-to Nanoscale; Jenny Stanford Publishing: New York, NY, USA, 2013; pp. 99–182. [Google Scholar]
- Bonse, J.; Kirner, S.V.; Krüger, J. Laser-induced periodic surface structures (LIPSS). In Handbook of Laser Micro-and Nano-Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–59. [Google Scholar]
- Sugioka, K.; Cheng, Y. Ultrafast lasers—Reliable tools for advanced materials processing. Light Sci. Appl. 2014, 3, e149. [Google Scholar] [CrossRef]
- Watanabe, W.; Li, Y.; Itoh, K. [INVITED] Ultrafast laser micro-processing of transparent material. Opt. Laser Technol. 2016, 78, 52–61. [Google Scholar] [CrossRef]
- Solomon, K.D.; de Castro, L.E.F.; Sandoval, H.P.; Biber, J.M.; Groat, B.; Neff, K.D.; Ying, M.S.; French, J.W.; Donnenfeld, E.D.; Lindstrom, R.L.; et al. LASIK world literature review: Quality of life and patient satisfaction. Ophthalmology 2009, 116, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Iocozzia, J.; Chen, Y.; Zhao, S.; Cui, X.; Wang, W.; Yu, H.; Lin, S.; Lin, Z. From precision synthesis of block copolymers to properties and applications of nanoparticles. Angew. Chem. Int. Ed. 2018, 57, 2046–2070. [Google Scholar] [CrossRef] [PubMed]
- Jawahir, I.; Kaynak, Y.; Lu, T. The impact of novel material processing methods on component quality, life and performance. Procedia CIRP 2014, 22, 33–44. [Google Scholar] [CrossRef]
- Dan, L.; Duan-Ming, Z. Vaporization and plasma shielding during high power nanosecond laser ablation of silicon and nickel. Chin. Phys. Lett. 2008, 25, 1368. [Google Scholar] [CrossRef]
- Naghilou, A.; Armbruster, O.; Kautek, W. Laser-induced non-thermal processes. In Handbook of Laser Micro-and Nano-Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 61–82. [Google Scholar]
- Grumezescu, V.; Socol, G.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Truşcǎ, R.; Bleotu, C.; Balaure, P.C.; Cristescu, R.; Chifiriuc, M.C. Functionalized antibiofilm thin coatings based on PLA–PVA microspheres loaded with usnic acid natural compounds fabricated by MAPLE. Appl. Surf. Sci. 2014, 302, 262–267. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Piqué, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser deposition of polymer and biomaterial films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.G.; Giwa, A.S.; Ahmad, J. Nanotechnology and thin films: Explores nanoscale thin films, nanocomposites, nanoparticles, and their application. In Thin Films and Coatings: Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2025; pp. 187–215. [Google Scholar]
- Lee, D.; Cohen, R.E.; Rubner, M.F. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 2005, 21, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
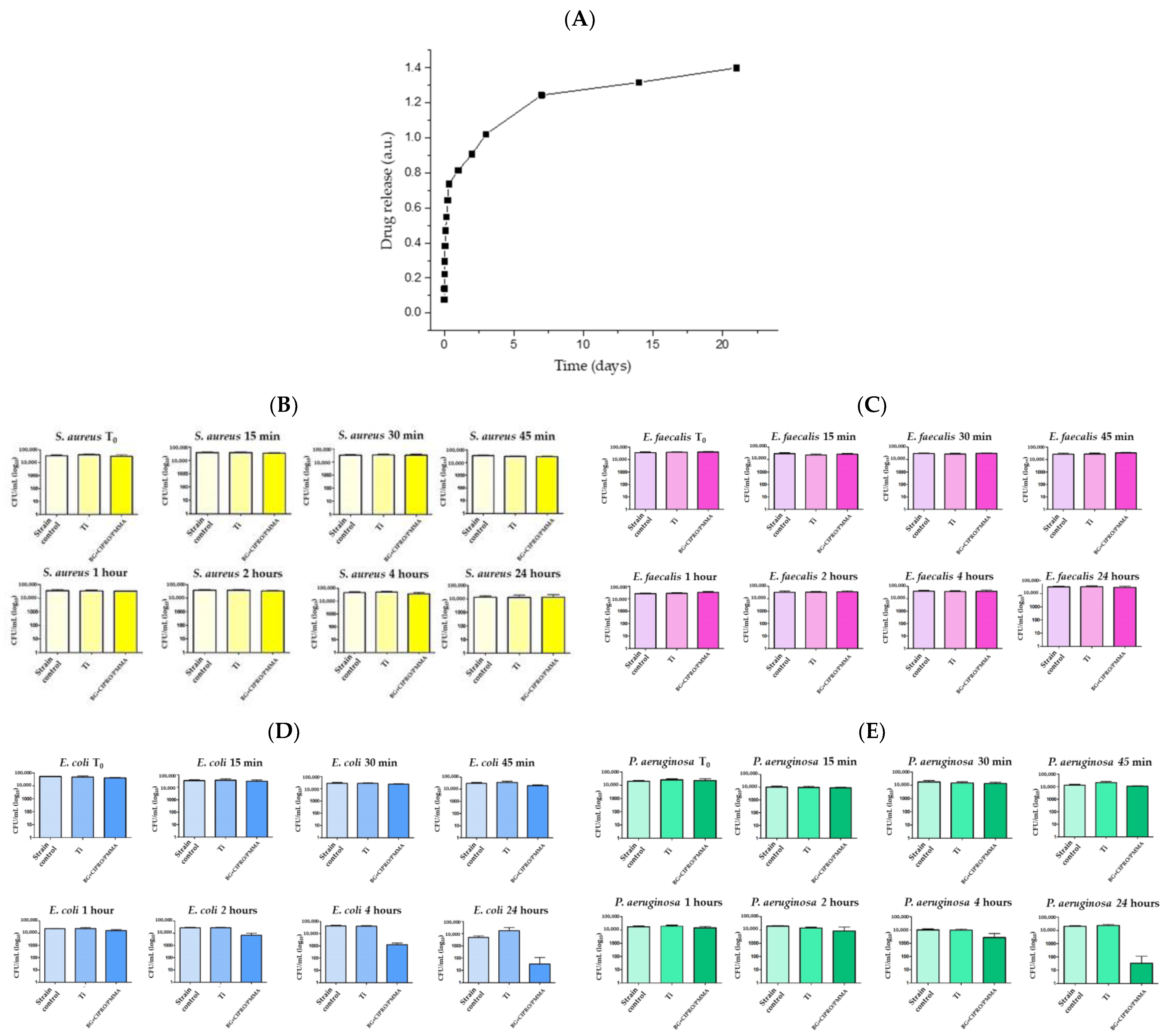
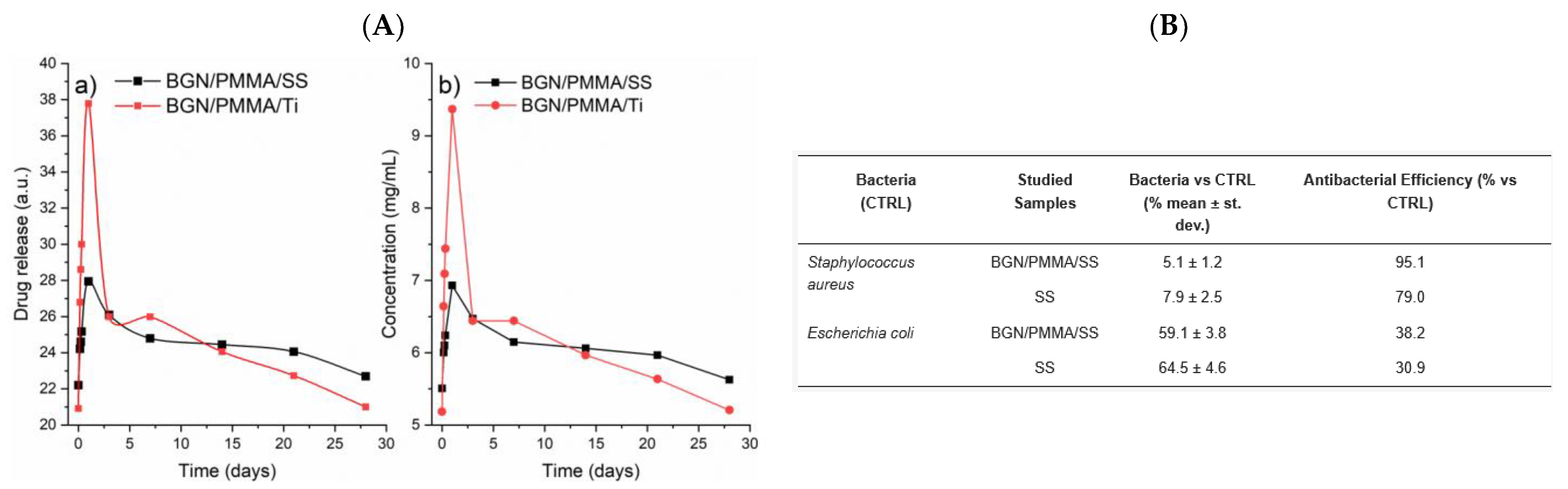
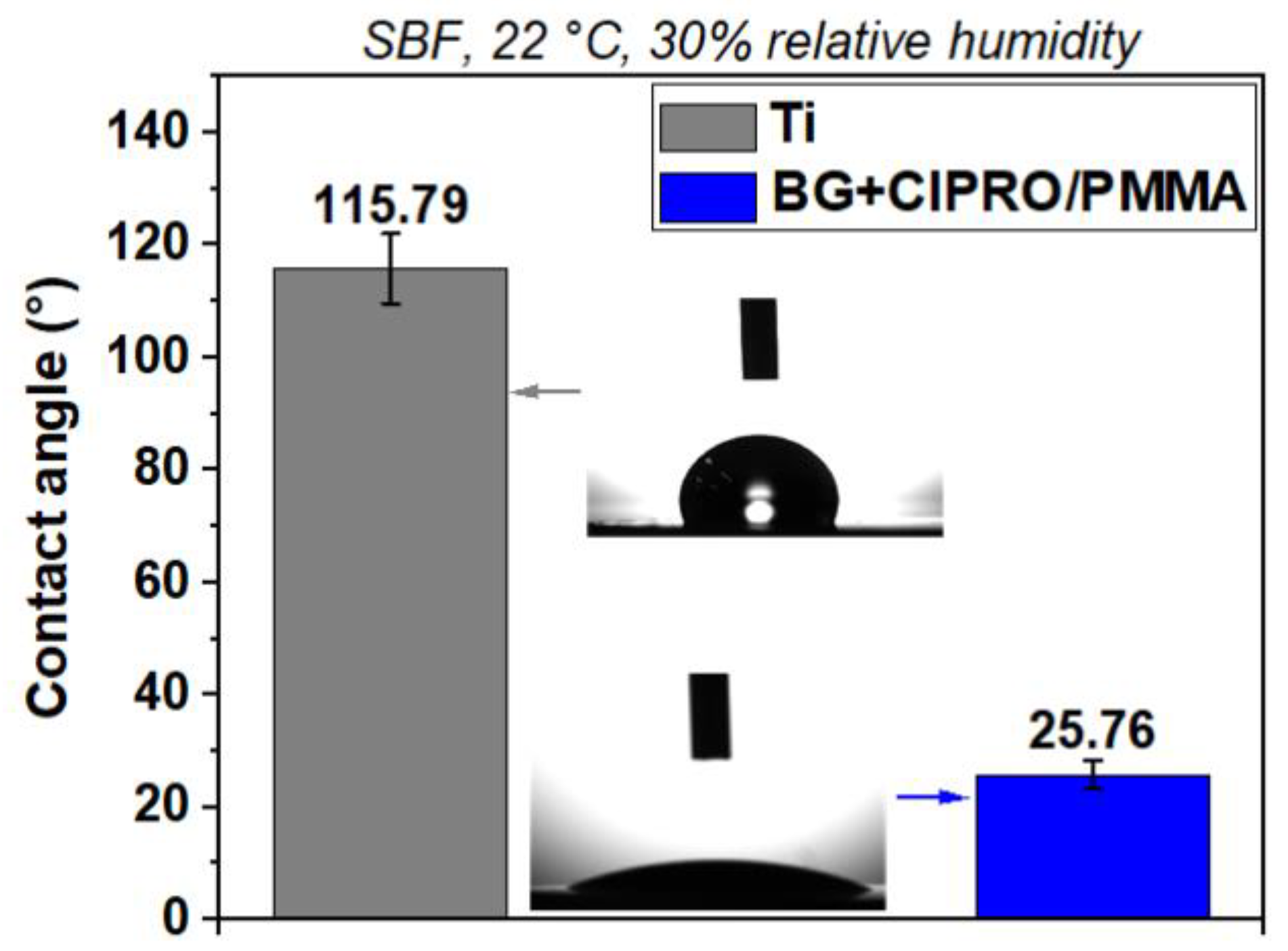
- Negut, I.; Ristoscu, C.; Tozar, T.; Dinu, M.; Parau, A.C.; Grumezescu, V.; Hapenciuc, C.; Popa, M.; Stan, M.S.; Marutescu, L.; et al. Implant surfaces containing bioglasses and ciprofloxacin as platforms for bone repair and improved resistance to microbial colonization. Pharmaceutics 2022, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Sakthinathan, S.; Meenakshi, G.A.; Vinothini, S.; Yu, C.-L.; Chen, C.-L.; Chiu, T.-W.; Vittayakorn, N. A Review of Thin-Film Growth, Properties, Applications, and Future Prospects. Processes 2025, 13, 587. [Google Scholar] [CrossRef]
- Jang, Y.; Park, S.; Char, K. Functionalization of polymer multilayer thin films for novel biomedical applications. Korean J. Chem. Eng. 2011, 28, 1149–1160. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R.J., Jr.; Wang, Y.-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Shunxi, J. Synergistic Effects of Nanoparticles and Polymers on Depletion and Structural Interactions. Langmuir 2013, 29, 15159–15167. [Google Scholar] [CrossRef] [PubMed]
- Badiceanu, M.; Anghel, S.; Mihailescu, N.; Visan, A.I.; Mihailescu, C.N.; Mihailescu, I.N. Coatings functionalization via laser versus other deposition techniques for medical applications: A comparative review. Coatings 2022, 12, 71. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Dorcioman, G.; Miroiu, F.; Socol, G.; Mihailescu, I.; Gittard, S.; Miller, P.; Narayan, R.; Enculescu, M.; et al. Antimicrobial activity of biopolymer–antibiotic thin films fabricated by advanced pulsed laser methods. Appl. Surf. Sci. 2013, 278, 211–213. [Google Scholar] [CrossRef]
- Califano, V.; Bloisi, F.; Vicari, L.R.; Yunos, D.M.; Chatzistavrou, X.; Boccaccini, A.R. Matrix assisted pulsed laser evaporation (MAPLE) of poly (D, L lactide)(PDLLA) on three dimensional bioglass (R) structures. Adv. Eng. Mater. 2009, 11, 685–689. [Google Scholar] [CrossRef]
- Alfe, M.; Minopoli, G.; Tartaglia, M.; Gargiulo, V.; Ausanio, G. Biocompatible Hybrid Graphenic Thin Coatings on Flexible Substrates through Matrix-Assisted Pulsed Laser Evaporation (MAPLE). ACS Appl. Mater. Interfaces 2024, 16, 38956–38967. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Popescu, C.; Popescu, A.; Grigorescu, S.; Duta, L.; Mihailescu, I.; Caraene, G.; Albulescu, R.; Albulescu, L.; Andronie, A.; et al. Functionalized polyvinyl alcohol derivatives thin films for controlled drug release and targeting systems: MAPLE deposition and morphological, chemical and in vitro characterization. Appl. Surf. Sci. 2009, 255, 5600–5604. [Google Scholar] [CrossRef]
- Bloisi, F.; Vicari, L.; Papa, R.; Califano, V.; Pedrazzani, R.; Bontempi, E.; Depero, L.E. Biomaterial thin film deposition and characterization by means of MAPLE technique. Mater. Sci. Eng. C 2007, 27, 1185–1190. [Google Scholar] [CrossRef]
- Bubb, D.; Ringeisen, B.; Callahan, J.; Galicia, M.; Vertes, A.; Horwitz, J.; McGill, R.; Houser, E.; Wu, P.; Piqué, A.; et al. Vapor deposition of intact polyethylene glycol thin films. Appl. Phys. A 2001, 73, 121–123. [Google Scholar] [CrossRef]
- Dumitrescu, L.N.; Neacsu, P.; Necula, M.G.; Bonciu, A.; Marascu, V.; Cimpean, A.; Moldovan, A.; Rotaru, A.; Dinca, V.; Dinescu, M. Induced hydrophilicity and in vitro preliminary osteoblast response of polyvinylidene fluoride (PVDF) coatings obtained via maple deposition and subsequent thermal treatment. Molecules 2020, 25, 582. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Frank, R.; Krinke, D.; Jahnke, H.-G.; Robitzki, A.A. Novel PMMA based 96-well microelectrode arrays for bioelectronic high throughput monitoring of cells in a live mode. Biosens. Bioelectron. 2022, 202, 114012. [Google Scholar] [CrossRef] [PubMed]
- Plokhotnichenko, A.; Karachevtsev, V.; Pashynska, V.; Kuzema, P. PMMA and PVP blended nanofibers with incorporated antimicrobial agent: Spectroscopy and mass spectrometry characterization. Low Temp. Phys. 2024, 50, 215–221. [Google Scholar] [CrossRef]
- Sava, F.; Cristescu, R.; Socol, G.; Radvan, R.; Savastru, R.; Savastru, D. Structure of bulk and thin films of poly-methyl-methacrylate (PMMA) polymer prepared by pulsed laser deposition. J. Optoelectron. Adv. Mater. 2002, 4, 965–970. [Google Scholar]
- Liu, Y.; Wang, X.; Wu, Q.; Pei, W.; Teo, M.J.; Chen, Z.S.; Huang, C. Application of lignin and lignin-based composites in different tissue engineering fields. Int. J. Biol. Macromol. 2022, 222, 994–1006. [Google Scholar] [CrossRef] [PubMed]
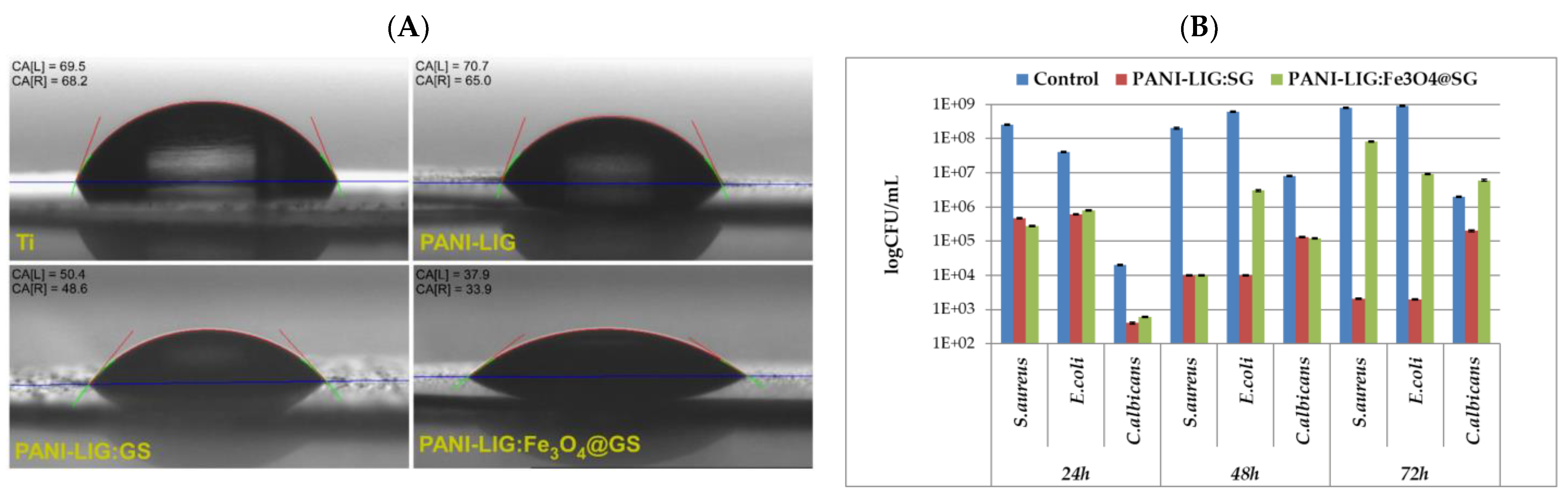
- Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Grumezescu, V.; Socol, M.; Zgura, I.; Florica, C.; Popescu, R.C.; Savu, D.; Holban, A.M.; et al. Laser processed antimicrobial nanocomposite based on polyaniline grafted lignin loaded with Gentamicin-functionalized magnetite. Polymers 2019, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.; Stan, G.E.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Besleaga, C.; Luculescu, C.; Chifiriuc, M.C.; Hussien, M.; Marsan, O.; et al. Combinatorial MAPLE deposition of antimicrobial orthopedic maps fabricated from chitosan and biomimetic apatite powders. Int. J. Pharm. 2016, 511, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Gyorgy, E.; Radu, M.; Costache, M.; Dinischiotu, A.; Le Coustumer, P.; Lafdi, K.; Predoi, D. Biomedical properties and preparation of iron oxide-dextran nanostructures by MAPLE technique. Chem. Cent. J. 2012, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Skopinska-Wisniewska, J. Influence of glycosaminoglycans on the properties of thin films based on chitosan/collagen blends. J. Mech. Behav. Biomed. Mater. 2018, 80, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, J.; Chen, K.-R.; Donato, J.; Geohegan, D.; Liu, C.; Puretzky, A.; Wood, R. Modeling of plume dynamics in laser ablation processes for thin film deposition of materials. Phys. Plasmas 1996, 3, 2203–2209. [Google Scholar] [CrossRef]
- Papavlu, A.P.; Dinca, V.; Filipescu, M.; Dinescu, M. Matrix-Assisted Pulsed Laser Evaporation of Organic Thin Films: Applications in Biology and Chemical Sensors. In Laser Ablation: From Fundamentals to Applications; IntechOpen: London, UK, 2017; Volume 171. [Google Scholar]
- Khan, M.; Gittard, S.D.; Narayan, R.J.; Bubb, D.M. Antimicrobial Testing, Morphological Characterization, and Surface Potential Mapping of Silver-Poly-(Methyl Methacrylate) Nanocomposite Films Made Through Matrix-Assisted Pulsed Laser Deposition againsts. Aureus. Nano Life 2010, 1, 145–152. [Google Scholar] [CrossRef]
- Frippiat, T.; Art, T.; Delguste, C. Silver Nanoparticles as Antimicrobial Agents in Veterinary Medicine: Current Applications and Future Perspectives. Nanomaterials 2025, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.B.; Hubenthal, F.; Träger, F. Shaping nanoparticles with laser light: A multi-step approach to produce nanoparticle ensembles with narrow shape and size distributions. J. Phys. Conf. Ser. 2007, 59, 240. [Google Scholar] [CrossRef]
- DeJournett, T.J.; Spicer, J.B. Laser-induced, in situ, nanoparticle shell synthesis in polymer matrix nanocomposites. Phys. Chem. Chem. Phys. 2013, 15, 19753–19762. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, M.; González-Rodríguez, L.; Gontad, F.; Teixeira-Santos, R.; Doiro, M.; Álvarez-Gómez, L.; Mergulhão, F.; González, P.; Serra, J. Dual pulsed laser deposition of Ag nanoparticles on calcium phosphate coatings for biomedical applications. Biomed. Phys. Eng. Express 2022, 8, 065019. [Google Scholar] [CrossRef] [PubMed]
- Orgiani, P.; Chaluvadi, S.; Chalil, S.P.; Mazzola, F.; Jana, A.; Dolabella, S.; Rajak, P.; Ferrara, M.; Benedetti, D.; Fondacaro, A.; et al. Dual pulsed laser deposition system for the growth of complex materials and heterostructures. Rev. Sci. Instrum. 2023, 94, 033903. [Google Scholar] [CrossRef] [PubMed]
- Cocean, G.; Cocean, A.; Garofalide, S.; Pelin, V.; Munteanu, B.S.; Pricop, D.A.; Motrescu, I.; Dimitriu, D.G.; Cocean, I.; Gurlui, S. Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae. Polymers 2023, 15, 3953. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, I.N.; Bociaga, D.; Socol, G.; Stan, G.E.; Chifiriuc, M.-C.; Bleotu, C.; Husanu, M.A.; Popescu-Pelin, G.; Duta, L.; Luculescu, C.R.; et al. Fabrication of antimicrobial silver-doped carbon structures by combinatorial pulsed laser deposition. Int. J. Pharm. 2016, 515, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Mirza, I.; O’Connell, G.; Wang, J.; Lunney, J. Comparison of nanosecond and femtosecond pulsed laser deposition of silver nanoparticle films. Nanotechnology 2014, 25, 265301. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Polonskyi, O.; Hinz, A.; Mollea, C.; Bosco, F.; Strunskus, T.; Balagna, C.; Perero, S.; Faupel, F.; Ferraris, M. Antibacterial, highly hydrophobic and semi transparent Ag/plasma polymer nanocomposite coating on cotton fabric obtained by plasma based co-deposition. Cellulose 2019, 26, 8877–8894. [Google Scholar] [CrossRef]
- Babutan, I.; Lucaci, A.-D.; Botiz, I. Antimicrobial polymeric structures assembled on surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Morillas-Becerril, L.; Franco-Ulloa, S.; Fortunati, I.; Marotta, R.; Sun, X.; Zanoni, G.; De Vivo, M.; Mancin, F. Specific and nondisruptive interaction of guanidium-functionalized gold nanoparticles with neutral phospholipid bilayers. Commun. Chem. 2021, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Poletti, A.; Fracasso, G.; Conti, G.; Pilot, R.; Amendola, V. Laser generated gold nanocorals with broadband plasmon absorption for photothermal applications. Nanoscale 2015, 7, 13702–13714. [Google Scholar] [CrossRef] [PubMed]
- Lunin, L.S.; Lunina, M.L.; Kravtsov, A.A.; Sysoev, I.A.; Blinov, A.V. Synthesis and study of thin TiO2 films doped with silver nanoparticles for the antireflection coatings and transparent contacts of photovoltaic converters. Semiconductors 2016, 50, 1231–1235. [Google Scholar] [CrossRef]
- Sripriya, J.; Anandhakumar, S.; Achiraman, S.; Antony, J.J.; Siva, D.; Raichur, A.M. Laser receptive polyelectrolyte thin films doped with biosynthesized silver nanoparticles for antibacterial coatings and drug delivery applications. Int. J. Pharm. 2013, 457, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, Z.; Cai, R.; Yang, W.; He, H.; Wang, Y. Rational design of Ag/ZnO hybrid nanoparticles on sericin/agarose composite film for enhanced antimicrobial applications. Int. J. Mol. Sci. 2020, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for efficient photocatalyst: Fabrication and activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Stan, G.; Duta, L.; Dorcioman, G.; Iordache, O.; Dumitrescu, I.; Pasuk, I.; Mihailescu, I. Influence of a hydrophobin underlayer on the structuring and antimicrobial properties of ZnO films. J. Mater. Sci. 2013, 48, 8329–8336. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for wound healing applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Popescu, C.; Socol, G.; Visan, A.; Mihailescu, I.; Gittard, S.; Miller, P.; Martin, T.; Narayan, R.; Andronie, A.; et al. Deposition of antibacterial of poly (1, 3-bis-(p-carboxyphenoxy propane)-co-(sebacic anhydride)) 20: 80/gentamicin sulfate composite coatings by MAPLE. Appl. Surf. Sci. 2011, 257, 5287–5292. [Google Scholar] [CrossRef]
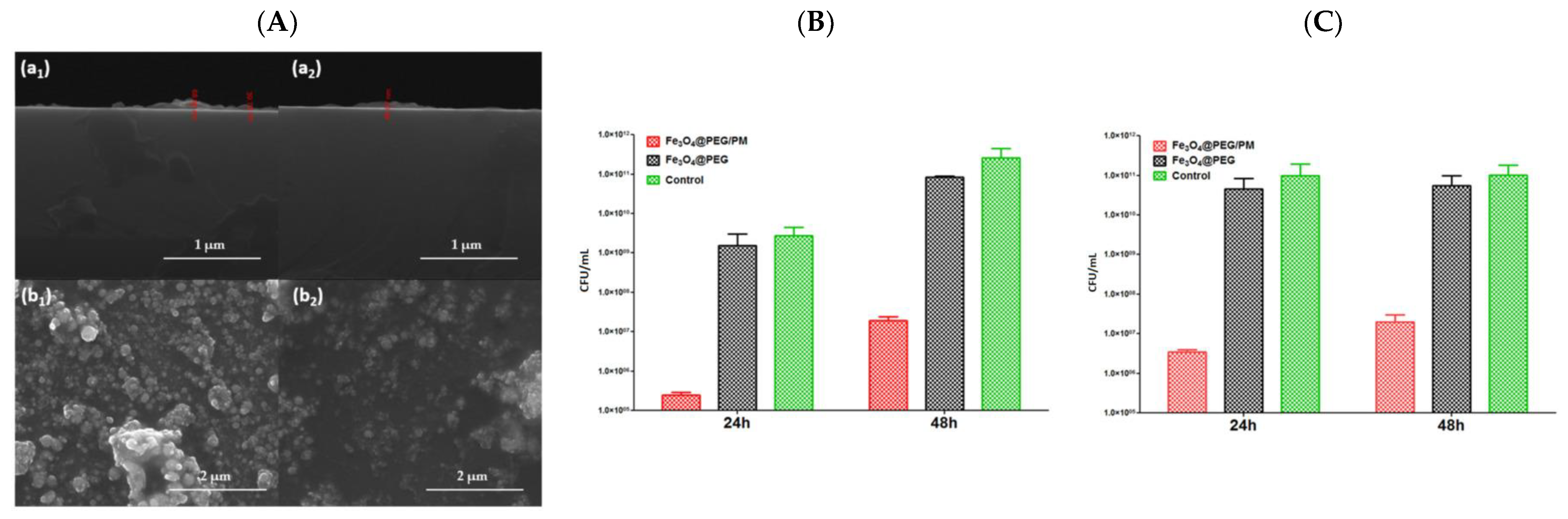
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Mogoantă, L.; Mogoşanu, G.D.; Grumezescu, A.M.; Stănculescu, A.; Socol, G.; Iordache, F.; Maniu, H.; et al. MAPLE fabrication of thin films based on kanamycin functionalized magnetite nanoparticles with anti-pathogenic properties. Appl. Surf. Sci. 2015, 336, 188–195. [Google Scholar] [CrossRef]
- Rădulescu, D.; Grumezescu, V.; Andronescu, E.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Oprea, A.E.; Rădulescu, M.; Surdu, A.; Trusca, R.; et al. Biocompatible cephalosporin-hydroxyapatite-poly (lactic-co-glycolic acid)-coatings fabricated by MAPLE technique for the prevention of bone implant associated infections. Appl. Surf. Sci. 2016, 374, 387–396. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.C.; Urzica, I.; Dyia, H.M.; Bleotu, C.; et al. Functionalized antimicrobial composite thin films printing for stainless steel implant coatings. Molecules 2016, 21, 740. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Visan, A.; Grumezescu, V.; Kuncser, V.; Kuncser, A.; Iacob, N.; Schinteie, G.; Socol, M.; Florica, C.; Zgura, I.; et al. MAPLE deposition of hybrid PLGA-Fe3O4-Cypress-PEDOT: PSS coatings. Giant 2024, 18, 100250. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Truong, V.K.; Wang, F.; Pushpamali, W.A.A.; Wang, J.Y.; Ellis, A.V.; Berndt, C.C.; Crawford, R.J.; Ivanova, E.P. Plasma-enhanced synthesis of bioactive polymeric coatings from monoterpene alcohols: A combined experimental and theoretical study. Biomacromolecules 2010, 11, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Visan, A.; Socol, G.; Surdu, A.; Oprea, A.; Grumezescu, A.; Chifiriuc, M.; Boehm, R.; Yamaleyeva, D.; Taylor, M.; et al. Antimicrobial activity of biopolymeric thin films containing flavonoid natural compounds and silver nanoparticles fabricated by MAPLE: A comparative study. Appl. Surf. Sci. 2016, 374, 290–296. [Google Scholar] [CrossRef]
- Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Mirza Rosca, J.C.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional bioglass—Biopolymer double nanostructure for natural antimicrobial drug extracts delivery. Nanomaterials 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Mihaiescu, D.; Cristescu, R.; Dorcioman, G.; Popescu, C.; Nita, C.; Socol, G.; Mihailescu, I.; Grumezescu, A.; Tamas, D.; Enculescu, M.; et al. Functionalized magnetite silica thin films fabricated by MAPLE with antibiofilm properties. Biofabrication 2012, 5, 015007. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Negut, I.; Cristescu, R.; Grumezescu, A.M.; Holban, A.M.; Iordache, F.; Chifiriuc, M.C.; Narayan, R.J.; Chrisey, D.B. Isoflavonoid-antibiotic thin films fabricated by MAPLE with improved resistance to microbial colonization. Molecules 2021, 26, 3634. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, N.; Haskoylu, M.E.; Ristoscu, C.; Bostan, M.S.; Sopronyi, M.; Eroğlu, M.S.; Chifiriuc, M.C.; Mustaciosu, C.C.; Axente, E.; Oner, E.T.; et al. Gradient multifunctional biopolymer thin film assemblies synthesized by combinatorial MAPLE. Appl. Surf. Sci. 2019, 466, 628–636. [Google Scholar] [CrossRef]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Grumezescu, V.; Negut, I.; Grumezescu, A.M.; Ficai, A.; Dorcioman, G.; Socol, G.; Iordache, F.; Truşcă, R.; Vasile, B.S.; Holban, A.M. MAPLE fabricated coatings based on magnetite nanoparticles embedded into biopolymeric spheres resistant to microbial colonization. Appl. Surf. Sci. 2018, 448, 230–236. [Google Scholar] [CrossRef]
- Campos, M.D.; Zucchi, P.C.; Phung, A.; Leonard, S.N.; Hirsch, E.B. The activity of antimicrobial surfaces varies by testing protocol utilized. PLoS ONE 2016, 11, e0160728. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Völpel, A.; Ewald, A.; Remesch, M.; Kuever, J.; Bauer, J.; Griesheim, S.; Hauser, C.; Thielmann, J.; Tonndorf-Martini, S.; et al. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLoS ONE 2018, 13, e0194339. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, M.; Grotenhuis, A.; van de Belt-Gritter, B.; Roest, S.; Loontjens, T.J.; Busscher, H.J.; van der Mei, H.C.; Ren, Y. Comparison of methods to evaluate bacterial contact-killing materials. Acta Biomater. 2017, 59, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Maitz, S.; Poelzl, S.; Dreisiebner, D.; Zarschenas, E.; Kittinger, C. Antimicrobial non-porous surfaces: A comparison of the standards ISO 22196: 2011 and the recently published ISO 7581: 2023. Front. Microbiol. 2024, 15, 1400265. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Popescu, A.C. Current research in pulsed laser deposition. Coatings 2021, 11, 274. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of antimicrobial properties of titanium dioxide nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef] [PubMed]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver nanoparticles (AgNPs): Comprehensive insights into bio/synthesis, key influencing factors, multifaceted applications, and toxicity—A 2024 update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef] [PubMed]
- Juan Carlos, F.-A.; Rene, G.-C.; German, V.-S.; Laura Susana, A.-T. Antimicrobial poly (methyl methacrylate) with silver nanoparticles for dentistry: A systematic review. Appl. Sci. 2020, 10, 4007. [Google Scholar] [CrossRef]
- Rojas, A.; Velásquez, E.; Patiño Vidal, C.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Active PLA packaging films: Effect of processing and the addition of natural antimicrobials and antioxidants on physical properties, release kinetics, and compostability. Antioxidants 2021, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Floroian, L.; Ristoscu, C.; Candiani, G.; Pastori, N.; Moscatelli, M.; Mihailescu, N.; Negut, I.; Badea, M.; Gilca, M.; Chiesa, R.; et al. Antimicrobial thin films based on ayurvedic plants extracts embedded in a bioactive glass matrix. Appl. Surf. Sci. 2017, 417, 224–233. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; Algahtani, F.D.; Othman, M.S.; Ahmad, K.; Maysara, S.; Al-Najjar, M.A.; El-Morsy, M.; Menazea, A. Laser deposited ultra-thin silver nanoparticles on CMC-PVA blend film as sheet for wound dressings. Mater. Chem. Phys. 2024, 318, 129246. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Pasuk, I.; Stan, G.E.; Stan, M.S.; Popa, M.; Chifiriuc, M.C.; Hapenciuc, C.; Oktar, F.N.; et al. Fish bone derived bi-phasic calcium phosphate coatings fabricated by pulsed laser deposition for biomedical applications. Mar. Drugs 2020, 18, 623. [Google Scholar] [CrossRef] [PubMed]
- Birdeanu, M.; Fratilescu, I.; Epuran, C.; Murariu, A.C.; Socol, G.; Fagadar-Cosma, E. Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser-Type Approaches. Nanomaterials 2022, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Sopronyi, M.; Nita, C.; Le Meins, J.-M.; Vidal, L.; Jipa, F.; Axente, E.; Ghimbeu, C.M.; Sima, F. Laser-assisted synthesis of carbon coatings with cobalt oxide nanoparticles embedded in gradient of composition and sizes. Surf. Coat. Technol. 2021, 419, 127301. [Google Scholar] [CrossRef]
- Cocean, G.; Cocean, A.; Postolachi, C.; Garofalide, S.; Bulai, G.; Munteanu, B.S.; Cimpoesu, N.; Cocean, I.; Gurlui, S. High-power laser deposition of chitosan polymers: Medical and environmental applications. Polymers 2022, 14, 1537. [Google Scholar] [CrossRef] [PubMed]
- Bonciu, A.; Cremer, L.; Calugaru, A.; Vlase, E.; Coman, C.; Palla-Papavlu, A.; Cristian, D.A.; Grama, F. Laser engineered polymer thin films as drug delivery systems. Appl. Phys. A 2023, 129, 327. [Google Scholar] [CrossRef]
- Paula, K.T.; Santos, M.V.; Facure, M.H.; Andrade, M.B.; Araujo, F.L.; Correa, D.S.; Ribeiro, S.J.; Mendonca, C.R. Laser patterning and induced reduction of graphene oxide functionalized silk fibroin. Opt. Mater. 2020, 99, 109540. [Google Scholar] [CrossRef]
- Heng, P.W. Controlled release drug delivery systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Kalwar, K.; Shan, D. Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism–a mini review. Micro Nano Lett. 2018, 13, 277–280. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cui, H.; Shi, R.; Guo, J.; Wang, B.; Xu, Y.; Ding, Y.; Mao, H.; Yan, F. Antimicrobial anionic polymers: The effect of cations. Eur. Polym. J. 2018, 107, 181–188. [Google Scholar] [CrossRef]
- Cardoso, M.H.; de la Fuente-Nunez, C.; Santos, N.C.; Zasloff, M.A.; Franco, O.L. Influence of antimicrobial peptides on the bacterial membrane curvature and vice versa. Trends Microbiol. 2024, 32, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhang, J.; Hu, X.; Li, Z.; Fa, K.; Liu, H.; Waigh, T.A.; McBain, A.; Lu, J.R. Hydrophobic control of the bioactivity and cytotoxicity of de novo-designed antimicrobial peptides. ACS Appl. Mater. Interfaces 2019, 11, 34609–34620. [Google Scholar] [CrossRef] [PubMed]
- Latag, G.V.; Nakamura, T.; Palai, D.; Mondarte, E.A.Q.; Hayashi, T. Investigation of three-dimensional bacterial adhesion manner on model organic surfaces using quartz crystal microbalance with energy dissipation monitoring. ACS Appl. Bio Mater. 2023, 6, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ghosh, A.; Saha, B.; Bhadury, P.; De, P. Surface Charge-Switchable Antifouling Block Copolymer with Bacteriostatic Properties. Langmuir 2024, 40, 5314–5325. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Schujman, G.; Gramajo, H. Regulation of membrane lipid homeostasis in bacteria. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 509–517. [Google Scholar]
- Gamage, Y.I.; Pan, J. Nanoscale Perturbations of Lipid Bilayers Induced by Magainin 2: Insights from AFM Imaging and Force Spectroscopy. Chem. Phys. Lipids 2024, 263, 105421. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Ishihara, K. Reduction of protein adsorption on well-characterized polymer brush layers with varying chemical structures. Colloids Surf. B Biointerfaces 2010, 81, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Comenge, J.; Puntes, V.F. The Role of PEG Conformation in Mixed Layers: From Protein Corona Substrate to Steric Stabilization Avoiding Protein Adsorption. Sci. Res. 2015, 1–10. [Google Scholar] [CrossRef]
- Psarra, E.; König, U.; Ueda, Y.; Bellmann, C.; Janke, A.; Bittrich, E.; Eichhorn, K.-J.; Uhlmann, P. Nanostructured biointerfaces: Nanoarchitectonics of thermoresponsive polymer brushes impact protein adsorption and cell adhesion. ACS Appl. Mater. Interfaces 2015, 7, 12516–12529. [Google Scholar] [CrossRef] [PubMed]
- Legeay, G.; Poncin-Epaillard, F.; Arciola, C.R. New surfaces with hydrophilic/hydrophobic characteristics in relation to (no) bioadhesion. Int. J. Artif. Organs 2006, 29, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Ahn, S.-H.; Wang, Y. A multiscale adhesion model for deposition prediction in laser enhanced nanoparticle deposition process. Acta Mater. 2021, 208, 116740. [Google Scholar] [CrossRef]
- Wang, X.; Deng, A.; Cao, W.; Li, Q.; Wang, L.; Zhou, J.; Hu, B.; Xing, X. Synthesis of chitosan/poly (ethylene glycol)-modified magnetic nanoparticles for antibiotic delivery and their enhanced anti-biofilm activity in the presence of magnetic field. J. Mater. Sci. 2018, 53, 6433–6449. [Google Scholar] [CrossRef]
- Yu, Q.; Ge, W.; Atewologun, A.; López, G.P.; Stiff-Roberts, A.D. RIR-MAPLE deposition of multifunctional films combining biocidal and fouling release properties. J. Mater. Chem. B 2014, 2, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Socol, M.; Preda, N.; Costas, A.; Breazu, C.; Stanculescu, A.; Rasoga, O.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G. Hybrid organic-inorganic thin films based on zinc phthalocyanine and zinc oxide deposited by MAPLE. Appl. Surf. Sci. 2020, 503, 144317. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Wang, H.; Liu, Z.; Yang, J.; Zhang, H.; Liang, H.; Bai, L. Innovative temperature-responsive membrane with an elastic interface for biofouling mitigation in industrial circulating cooling water treatment. Water Res. 2024, 267, 122528. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-Y.; Zhang, J.-H.; Huang, H.-L. Effects of laser texture oxidation and high-temperature annealing of TiV alloy thin films on mechanical and antibacterial properties and cytotoxicity. Materials 2018, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- Paneysar, J.S.; Barton, S.; Ambre, P.; Coutinho, E. Novel temperature responsive films impregnated with silver nano particles (Ag-NPs) as potential dressings for wounds. J. Pharm. Sci. 2022, 111, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Ganjian, M.; Modaresifar, K.; Ligeon, M.R.; Kunkels, L.B.; Tümer, N.; Angeloni, L.; Hagen, C.W.; Otten, L.G.; Hagedoorn, P.L.; Apachitei, I.; et al. Nature helps: Toward bioinspired bactericidal nanopatterns. Adv. Mater. Interfaces 2019, 6, 1900640. [Google Scholar] [CrossRef]
- Tagawa, N.; Takada, M.; Mori, A.; Sawada, H.; Kawahara, K. Development of contact sliders with nanotextures by femtosecond laser processing. Tribol. Lett. 2006, 24, 143–149. [Google Scholar] [CrossRef]
- Chen, S.; Popovich, J.; Iannuzo, N.; Haydel, S.E.; Seo, D.-K. Silver-ion-exchanged nanostructured zeolite X as antibacterial agent with superior ion release kinetics and efficacy against methicillin-resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 39271–39282. [Google Scholar] [CrossRef] [PubMed]
- Jaligam, M.M.; Takahashi, C.; Heidt, B.; Shen, A.Q. Enhanced antibacterial efficacy: Rapid analysis of silver-decorated azithromycin-infused Soluplus®® nanoparticles against E. coli and S. epidermidis biofilms. Nanoscale 2024, 16, 17877–17885. [Google Scholar] [CrossRef] [PubMed]
- Sinev, D.A.; Yuzhakova, D.S.; Moskvin, M.K.; Veiko, V.P. Formation of the submicron oxidative LIPSS on thin titanium films during nanosecond laser recording. Nanomaterials 2020, 10, 2161. [Google Scholar] [CrossRef] [PubMed]
- Santillan, R.; Wong, A.; Segovia, P.; Camacho-Lopez, M.; Camacho-Lopez, S. Femtosecond laser-induced periodic surface structures formation on bismuth thin films upon irradiation in ambient air. Opt. Mater. Express 2020, 10, 674–681. [Google Scholar] [CrossRef]
- Kane, R.; Ma, P.X. Mimicking the nanostructure of bone matrix to regenerate bone. Mater. Today 2013, 16, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Eriberto, B.; Barbara, Z. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Y.; Li, T.; Ding, S.; Zhang, W.; Gu, Y.; Liu, C. Facilitated receptor-recognition and enhanced bioactivity of bone morphogenetic protein-2 on magnesium-substituted hydroxyapatite surface. Sci. Rep. 2016, 6, 24323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bai, L.; Zhang, Y.; Yao, R.; Sun, Y.; Hang, R.; Chen, X.; Wang, H.; Yao, X.; Xiao, Y.; et al. Type I collagen decorated nanoporous network on titanium implant surface promotes osseointegration through mediating immunomodulation, angiogenesis, and osteogenesis. Biomaterials 2022, 288, 121684. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, X.; Xia, W.; Wang, Z.; Zhang, P.; Xia, L.; Lin, K.; Zhu, M. Nano-structure designing promotion osseointegration of hydroxyapatite coated Ti–6Al–4V alloy implants in diabetic model. J. Biomed. Nanotechnol. 2019, 15, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.R.; Hong, M.-H. Improved Biocompatibility and Osseointegration of Nanostructured Calcium-Incorporated Titanium Implant Surface Treatment (XPEED®). Materials 2024, 17, 2707. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Mattmann, M.; Von Marttens, A.; Caviedes, P.; Arriagada, C.; Valenzuela, F.; Rodríguez, J.P.; Corral, C. Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Appl. Surf. Sci. 2016, 363, 286–295. [Google Scholar] [CrossRef]
- Dhinasekaran, D.; Kaliaraj, G.S.; Jagannathan, M.; Rajendran, A.R.; Prakasarao, A.; Ganesan, S.; Subramanian, B. Pulsed laser deposition of nanostructured bioactive glass and hydroxyapatite coatings: Microstructural and electrochemical characterization. Mater. Sci. Eng. C 2021, 130, 112459. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, S.; He, F.; Guo, Z.; Hu, P.; Zhao, R.; Huang, Y.; Chen, Q.; Ji, P.; Chu, L.; et al. Promotion of osseointegration using protamine/alginate/bone morphogenic protein 2 biofunctionalized composite coating on nanopolymorphic titanium surfaces. J. Biomed. Nanotechnol. 2018, 14, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-H.; Yu, X.-W.; Zeng, S.-X.; Du, R.-L.; Hu, Y.-H.; Yuan, Z.; Lu, E.-Y.; Dai, K.-R.; Tang, T.-T. Enhanced osteointegration of orthopaedic implant gradient coating composed of bioactive glass and nanohydroxyapatite. J. Mater. Sci. Mater. Med. 2010, 21, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Quarrington, R.D.; Tsangari, H.; Starczak, Y.; Mulaibrahimovic, A.; Burzava, A.L.; Christou, C.; Barker, A.J.; Morel, J.; Bright, R.; et al. A novel nanostructured surface on titanium implants increases osseointegration in a sheep model. Clin. Orthop. Relat. Res. 2022, 480, 2232–2250. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Coelho, P.G.; Vandeweghe, S.; Schwartz-Filho, H.O.; Hayashi, M.; Ono, D.; Andersson, M.; Wennerberg, A. Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomater. 2011, 7, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Andronescu, E.; Negut, I.; Bîrcă, A.C.; Gălățeanu, B.; Hudiță, A. Bioactive coatings loaded with osteogenic protein for metallic implants. Polymers 2021, 13, 4303. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zhang, Y.; Cao, Q.; Li, Y. Nanopillar-Driven Antibacterial Surfaces: Elucidating Bactericidal Mechanisms and Engineering Nanostructures for Enhanced Efficacy. arXiv 2025, arXiv:2501.11727. [Google Scholar]
- Sinha, S.; Bhattacharya, R.; Ray, S.; Manna, I. Influence of deposition temperature on structure and morphology of nanostructured SnO2 films synthesized by pulsed laser deposition. Mater. Lett. 2011, 65, 146–149. [Google Scholar] [CrossRef]
- Hasan, F.A.; Hussein, M.T.; Abdulsattar, M.A. Structural, Optical, and Morphological Study of the Zinc Oxide Nano-Thin Films with Different Thickness Prepared by Pulsed Laser Deposition Technique. Iraqi J. Sci. 2022, 63, 5242–5254. [Google Scholar] [CrossRef]
- Dorcioman, G.; Grumezescu, V.; Stan, G.E.; Chifiriuc, M.C.; Gradisteanu, G.P.; Miculescu, F.; Matei, E.; Popescu-Pelin, G.; Zgura, I.; Craciun, V.; et al. Hydroxyapatite thin films of marine origin as sustainable candidates for dental implants. Pharmaceutics 2023, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Popok, V.N.; Jeppesen, C.M.; Fojan, P.; Kuzminova, A.; Hanuš, J.; Kylián, O. Comparative study of antibacterial properties of polystyrene films with TiOx and Cu nanoparticles fabricated using cluster beam technique. Beilstein J. Nanotechnol. 2018, 9, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Vladkova, T.; Angelov, O.; Stoyanova, D.; Gospodinova, D.; Gomes, L.; Soares, A.; Mergulhao, F.; Ivanova, I. Magnetron co-sputtered TiO2/SiO2/Ag nanocomposite thin coatings inhibiting bacterial adhesion and biofilm formation. Surf. Coat. Technol. 2020, 384, 125322. [Google Scholar] [CrossRef]
- Gomaa, H.M.; AlAbdulaal, T.; Yahia, I.; Ismail, A.; Mohammmed, M.; Zahran, H.; Zyoud, S.H.; Abdel-wahab, M.S.; Zahran, M.; Ibrahim, M.A. Exploring the optical and electrical properties of 70% PVP/30% PVA blend polymer doping with graphene thin films for optoelectronics applications. J. Electron. Mater. 2022, 51, 5897–5907. [Google Scholar] [CrossRef]
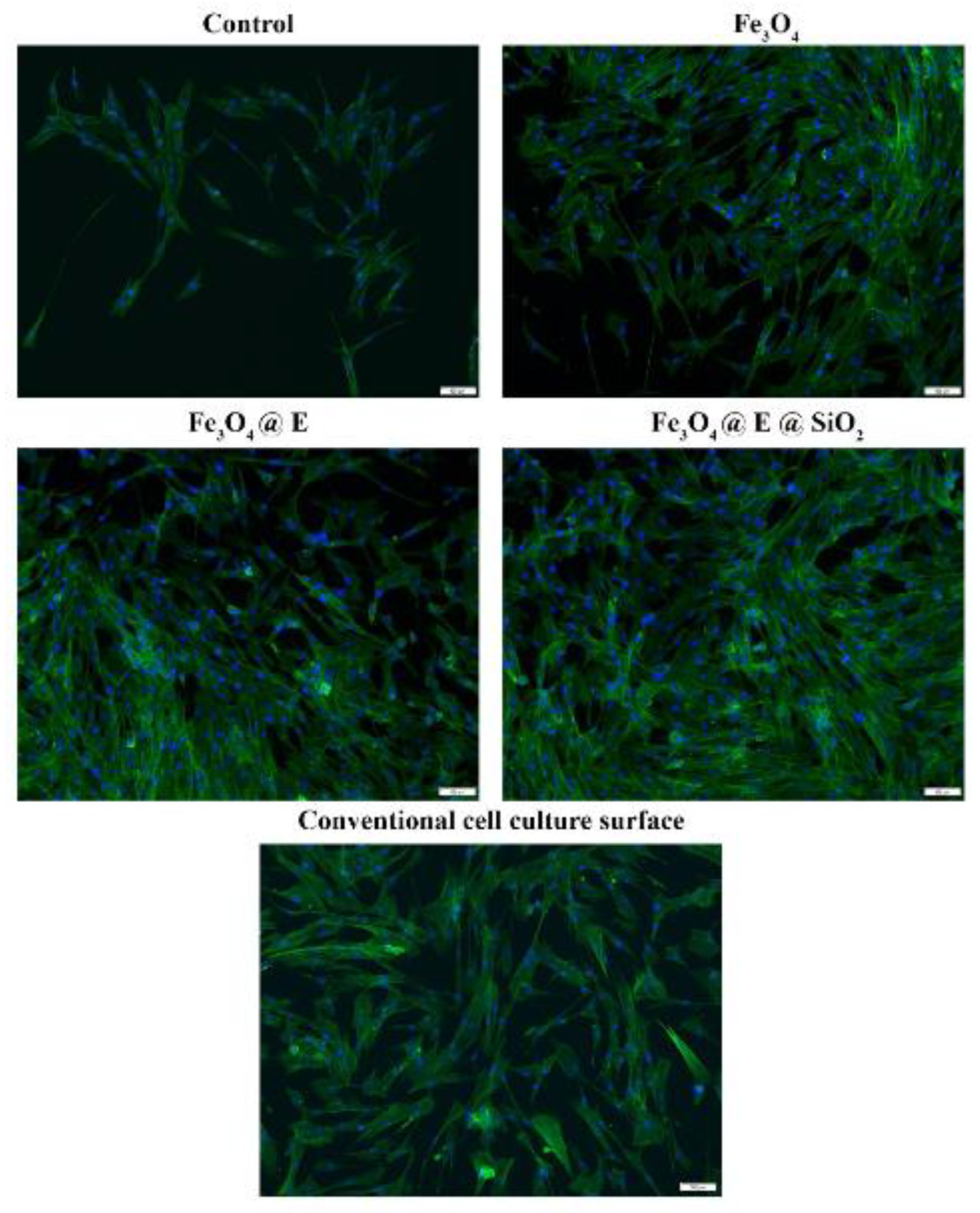
- Cristescu, R.; Popescu, C.; Socol, G.; Iordache, I.; Mihailescu, I.; Mihaiescu, D.; Grumezescu, A.; Balan, A.; Stamatin, I.; Chifiriuc, C.; et al. Magnetic core/shell nanoparticle thin films deposited by MAPLE: Investigation by chemical, morphological and in vitro biological assays. Appl. Surf. Sci. 2012, 258, 9250–9255. [Google Scholar] [CrossRef]
- Liu, Q.; Jia, H.; Ouyang, W.; Mu, Y.; Wu, Z. Fabrication of antimicrobial multilayered nanofibrous scaffolds-loaded drug via electrospinning for biomedical application. Front. Bioeng. Biotechnol. 2021, 9, 755777. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.; Hans, M.; Gachot, C.; Thome, A.; Bonk, S.; Mücklich, F. Direct laser interference patterning: Tailoring of contact area for frictional and antibacterial properties. Lubricants 2016, 4, 2. [Google Scholar] [CrossRef]
- Lavric, R.; Vreme, C.; Busuioc, C.; Isopencu, G.-O.; Nicoara, A.-I.; Oprea, O.-C.; Banciu, D.-D.; Constantinoiu, I.; Musat, A.-M.-R. The effect of silver and samarium on the properties of bioglass coatings produced by pulsed laser deposition and spin coating. J. Funct. Biomater. 2023, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Heitz, J.; Buchberger, G.; Baumgartner, W.; Meyer, M.; Weissbach, M.; Joel, A.-C.; Brajnicov, S.; Palla-Papavlu, A.; Dinescu, M. Change of Adhesion Properties of Bioinspired Laser-Induced Periodic Nanostructures towards Cribellate Spider Nanofiber Threads by Means of Thin Coatings. Coatings 2024, 14, 790. [Google Scholar] [CrossRef]
- Cesaria, M.; Taurino, A.; Cozzoli, P.D.; Arima, V.; Caricato, A.P. Colloidal TiO2 Nanorod Films Deposited Using the MAPLE Technique: Role of the Organic Capping and Absence of Characteristic Surface Patterns. Processes 2023, 11, 2591. [Google Scholar] [CrossRef]
- Birjega, R.; Matei, A.; Marascu, V.; Vlad, A.; Ionita, M.D.; Dinescu, M.; Zăvoianu, R.; Corobea, M.C. Stearic acid/layered double hydroxides composite thin films deposited by combined laser techniques. Molecules 2020, 25, 4097. [Google Scholar] [CrossRef] [PubMed]
- Popescu-Pelin, G.; Sima, F.; Sima, L.; Mihailescu, C.; Luculescu, C.; Iordache, I.; Socol, M.; Socol, G.; Mihailescu, I. Hydroxyapatite thin films grown by pulsed laser deposition and matrix assisted pulsed laser evaporation: Comparative study. Appl. Surf. Sci. 2017, 418, 580–588. [Google Scholar] [CrossRef]
- Holban, A.M.; Andronescu, E.; Grumezescu, V.; Oprea, A.E.; Grumezescu, A.M.; Socol, G.; Chifiriuc, M.C.; Lazar, V.; Iordache, F. Carvone functionalized iron oxide nanostructures thin films prepared by MAPLE for improved resistance to microbial colonization. J. Sol-Gel Sci. Technol. 2015, 73, 605–611. [Google Scholar] [CrossRef]
- Caciandone, M.; Niculescu, A.-G.; Roșu, A.R.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.; Vasile, B.Ș.; Bîrcă, A.C.; Grumezescu, A.M.; et al. PEG-functionalized magnetite nanoparticles for modulation of microbial biofilms on voice prosthesis. Antibiotics 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Tsendzughul, N.T.; Ogwu, A.A. Physicochemical aspects of the mechanisms of rapid antimicrobial contact-killing by sputtered silver oxide thin films under visible light. ACS Omega 2019, 4, 16847–16859. [Google Scholar] [CrossRef] [PubMed]
- Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Mușat, M.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations. Int. J. Mol. Sci. 2022, 23, 7910. [Google Scholar] [CrossRef] [PubMed]
- Puiu, R.A.; Bîrcă, A.C.; Grumezescu, V.; Duta, L.; Oprea, O.C.; Holban, A.M.; Hudiță, A.; Gălățeanu, B.; Balaure, P.C.; Grumezescu, A.M.; et al. Multifunctional polymeric biodegradable and biocompatible coatings based on silver nanoparticles: A comparative in vitro study on their cytotoxicity towards cancer and normal cell lines of cytostatic drugs versus essential-oil-loaded nanoparticles and on their antimicrobial and antibiofilm activities. Pharmaceutics 2023, 15, 1882. [Google Scholar] [PubMed]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
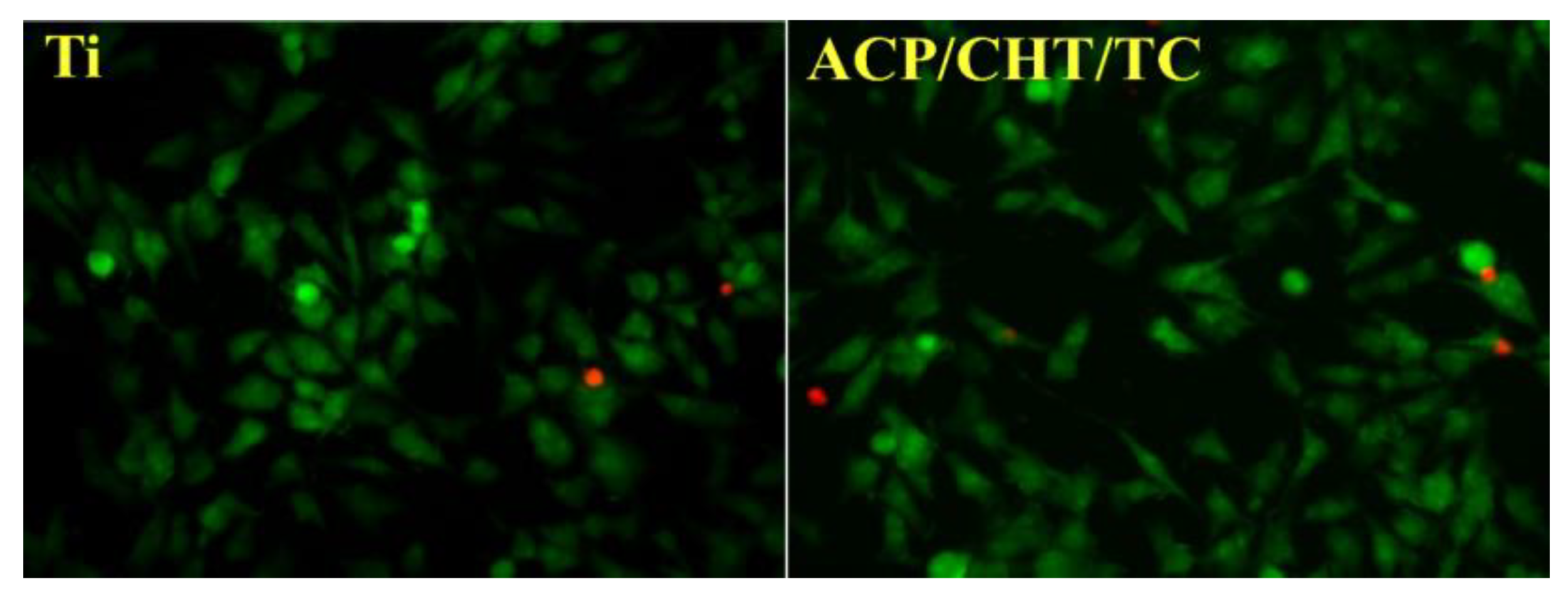
- Visan, A.I.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Matei, C.E.; Socol, G.; Chifiriuc, M.C.; Bleotu, C.; Grossin, D.; Brouillet, F.; et al. Composite Drug Delivery System Based on Amorphous Calcium Phosphate–Chitosan: An Efficient Antimicrobial Platform for Extended Release of Tetracycline. Pharmaceutics 2021, 13, 1659. [Google Scholar] [CrossRef] [PubMed]
- Hudiță, A.; Grumezescu, V.; Gherasim, O.; Grumezescu, A.M.; Dorcioman, G.; Negut, I.; Oprea, O.-C.; Vasile, B.Ș.; Gălățeanu, B.; Curuțiu, C.; et al. MAPLE processed nanostructures for antimicrobial coatings. Int. J. Mol. Sci. 2022, 23, 15355. [Google Scholar] [CrossRef] [PubMed]
- Limban, C.; Missir, A.V.; Grumezescu, A.M.; Oprea, A.E.; Grumezescu, V.; Vasile, B.Ș.; Socol, G.; Trușcă, R.; Caproiu, M.T.; Chifiriuc, M.C.; et al. Bioevaluation of novel anti-biofilm coatings based on PVP/Fe3O4 nanostructures and 2-((4-ethylphenoxy) methyl)-N-(arylcarbamothioyl) benzamides. Molecules 2014, 19, 12011–12030. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Moszyński, D.; Jędrzejewski, R.; Kwiatkowski, K.; Piwowarczyk, J.; Baranowska, J. Chemical structure of EVA films obtained by pulsed electron beam and pulse laser ablation. Polymers 2019, 11, 1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Lazar, M.; Ghiorghita, C.-A.; Raschip, I. Multicomponent hydrogels for controlled drug release and delivery. In Multicomponent Hydrogels: Smart Materials for Biomedical ApplicationsCheck Access; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Kumar, A. Applications of Biotribology in Biomedical Systems; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Smith, J.R.; Lamprou, D.A. Polymer coatings for biomedical applications: A review. Trans. IMF 2014, 92, 9–19. [Google Scholar] [CrossRef]
- Jalageri, M.D.; Kanth, S.; Shetty, S.C.; Patil, P.; Jyothi, K.; Nagaraja, A. Preparation of Biocompatible Antimicrobial Polymer Embedded with Ricinoleic Acid. J. Polym. Environ. 2024, 33, 1216–1231. [Google Scholar] [CrossRef]
- Cheng, J.; Chin, W.; Dong, H.; Xu, L.; Zhong, G.; Huang, Y.; Li, L.; Xu, K.; Wu, M.; Hedrick, J.L.; et al. Biodegradable antimicrobial polycarbonates with in vivo efficacy against multidrug-resistant MRSA systemic infection. Adv. Healthc. Mater. 2015, 4, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Valliammai, A.; Selvaraj, A.; Mathumitha, P.; Aravindraja, C.; Pandian, S.K. Polymeric antibiofilm coating comprising synergistic combination of citral and thymol prevents methicillin-resistant Staphylococcus aureus biofilm formation on titanium. Mater. Sci. Eng. C 2021, 121, 111863. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, C.; Zhang, Z.; Roland, J.D.; Lee, B.P. Antimicrobial property of halogenated catechols. Chem. Eng. J. 2021, 403, 126340. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, P.; Grybaitė, B.; Sapijanskaitė-Banevič, B.; Vaickelionienė, R.; Petraitis, V.; Petraitienė, R.; Naing, E.; Garcia, A.; Grigalevičiūtė, R.; Mickevičius, V. Synthesis of 3-((4-hydroxyphenyl) amino) propanoic acid derivatives as promising scaffolds for the development of antimicrobial candidates targeting multidrug-resistant bacterial and fungal pathogens. Antibiotics 2024, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Paun, I.A.; Moldovan, A.; Luculescu, C.R.; Staicu, A.; Dinescu, M. MAPLE deposition of PLGA: PEG films for controlled drug delivery: Influence of PEG molecular weight. Appl. Surf. Sci. 2012, 258, 9302–9308. [Google Scholar] [CrossRef]
- Paun, I.A.; Ion, V.; Moldovan, A.; Dinescu, M. MAPLE deposition of PEG: PLGA thin films. Appl. Phys. A 2012, 106, 197–205. [Google Scholar] [CrossRef]
- Paun, I.A.; Ion, V.; Luculescu, C.-R.; Dinescu, M.; Canulescu, S.; Schou, J. In vitro studies of PEG thin films with different molecular weights deposited by MAPLE. Appl. Phys. A 2012, 109, 223–232. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Popescu, A.; Grigorescu, S.; Mihailescu, I.; Mihaiescu, D.; Gittard, S.; Narayan, R.; Buruiana, T.; Stamatin, I.; et al. Functional polyethylene glycol derivatives nanostructured thin films synthesized by matrix-assisted pulsed laser evaporation. Appl. Surf. Sci. 2009, 255, 9873–9876. [Google Scholar] [CrossRef]
- Brajnicov, S.; Neacsu, P.; Moldovan, A.; Marascu, V.; Bonciu, A.; Ion, R.; Dinca, V.; Cimpean, A.; Dinescu, M. Tailored biodegradable triblock copolymer coatings obtained by MAPLE: A parametric study. Appl. Phys. A 2017, 123, 707. [Google Scholar] [CrossRef]
- Miroiu, F.M.; Stefan, N.; Visan, A.I.; Nita, C.; Luculescu, C.R.; Rasoga, O.; Socol, M.; Zgura, I.; Cristescu, R.; Craciun, D.; et al. Composite biodegradable biopolymer coatings of silk fibroin–Poly (3-hydroxybutyric-acid-co-3-hydroxyvaleric-acid) for biomedical applications. Appl. Surf. Sci. 2015, 355, 1123–1131. [Google Scholar] [CrossRef]
- Williams, A.; Campbell, A.; Ouchen, F.; Lu, W.; Grant, J.; Grote, J. Investigation of maple-deposited DNA films for graphene-based device applications. In Nanobiosystems: Processing, Characterization, and Applications VI; SPIE: Bellingham, WA, USA, 2013; pp. 52–56. [Google Scholar]
- Mei, Y.; Yu, K.; Lo, J.C.; Takeuchi, L.E.; Hadjesfandiari, N.; Yazdani-Ahmadabadi, H.; Brooks, D.E.; Lange, D.; Kizhakkedathu, J.N. Polymer–nanoparticle interaction as a design principle in the development of a durable ultrathin universal binary antibiofilm coating with long-term activity. ACS Nano 2018, 12, 11881–11891. [Google Scholar] [CrossRef] [PubMed]
- Rusen, L.; Brajnicov, S.; Neacsu, P.; Marascu, V.; Bonciu, A.; Dinescu, M.; Dinca, V.; Cimpean, A. Novel degradable biointerfacing nanocomposite coatings for modulating the osteoblast response. Surf. Coat. Technol. 2017, 325, 397–409. [Google Scholar] [CrossRef]
- Cazan, M.; Sima, L.E.; Dinca, V.; Dinescu, M.; Popescu, C.R. Maple coating of Zirconia with PEG-PCL embedded cyprinol for ear implant applications. Rom. J. Biochem. 2013, 50, 3–14. [Google Scholar]
- Solaiman; Foyez, T.; Monim, S.A.; Rahman, A.; Imran, A.B. Facile Synthesis of Bioactive Silver Nanocomposite Hydrogels with Electro-Conductive and Wound-Healing Properties. Gels 2025, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.C.; de Almeida, R.R.; da Cruz Sousa, A.C.; Martínez, F.N.A.; Denardin, J.C.; de Morais, S.M.; Ricardo, N.M.P.S. Xyloglucan-based hybrid nanocomposite with potential for biomedical applications. Int. J. Biol. Macromol. 2021, 168, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, S.; Jana, S.K.; Pramanik, N. Biomedical application of doxorubicin coated hydroxyapatite—Poly (lactide-co-glycolide) nanocomposite for controlling osteosarcoma therapeutics. J. Nanosci. Nanotechnol. 2020, 20, 3994–4004. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.B.; Prodana, M.; Nartita, R.; Ionita, D.; Simoiu, M. Antibacterial Properties and Long-Term Corrosion Resistance of Bioactive Coatings Obtained by Matrix-Assisted Pulsed Laser Evaporation on TiZrTaAg. Metals 2025, 15, 253. [Google Scholar] [CrossRef]
- Zhang, B. Resonant Infrared Matrix-Assisted Pulsed Laser Evaporation: Advancing Methodology and Elucidating Mechanisms for Precise Control of Film Morphology and Composition; Duke University: Durham, NC, USA, 2023. [Google Scholar]
- Visan, A.; Cristescu, R.; Stefan, N.; Miroiu, M.; Nita, C.; Socol, M.; Florica, C.; Rasoga, O.; Zgura, I.; Sima, L.; et al. Antimicrobial polycaprolactone/polyethylene glycol embedded lysozyme coatings of Ti implants for osteoblast functional properties in tissue engineering. Appl. Surf. Sci. 2017, 417, 234–243. [Google Scholar] [CrossRef]
- Chien, C.; Liu, C.; Kuo, T.; Wu, C.; Hong, T. Bioactivity of fluorapatite/alumina composite coatings deposited on Ti6Al4V substrates by laser cladding. Appl. Phys. A 2016, 122, 303. [Google Scholar] [CrossRef]
- Li, M.; Komasa, S.; Hontsu, S.; Hashimoto, Y.; Okazaki, J. Structural characterization and osseointegrative properties of pulsed laser-deposited fluorinated hydroxyapatite films on nano-zirconia for implant applications. Int. J. Mol. Sci. 2022, 23, 2416. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Kocourek, T.; Remsa, J.; Cristescu, R.; Mihailescu, I.; Chrisey, D. MAPLE applications in studying organic thin films. Laser Phys. 2007, 17, 66–70. [Google Scholar] [CrossRef]
- Bloisi, F.; Barra, M.; Cassinese, A.; Vicari, L.R.M. Matrix-Assisted Pulsed Laser Thin Film Deposition by Using Nd: YAG Laser. J. Nanomater. 2012, 2012, 395436. [Google Scholar] [CrossRef]
- Axente, E.; Elena Sima, L.; Sima, F. Biomimetic coatings obtained by combinatorial laser technologies. Coatings 2020, 10, 463. [Google Scholar] [CrossRef]
- Russo, T.; Peluso, V.; Gloria, A.; Gargiulo, V.; Alfe, M.; Ausanio, G. An integrated design strategy coupling additive manufacturing and matrix-assisted pulsed laser evaporation (MAPLE) towards the development of a new concept 3D scaffold with improved properties for tissue regeneration. Nanoscale Adv. 2024, 6, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Mokabber, T.; Cao, H.; Norouzi, N.; Van Rijn, P.; Pei, Y. Antimicrobial electrodeposited silver-containing calcium phosphate coatings. ACS Appl. Mater. Interfaces 2020, 12, 5531–5541. [Google Scholar] [CrossRef] [PubMed]
- García, I.; Trobajo, C.; Amghouz, Z.; Adawy, A. Nanolayered metal phosphates as biocompatible reservoirs for antimicrobial silver nanoparticles. Materials 2021, 14, 1481. [Google Scholar] [CrossRef] [PubMed]
- Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Iosif, A.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Bioactive Hydroxyapatite-Magnesium Phosphate Coatings Deposited by MAPLE for Preventing Infection and Promoting Orthopedic Implants Osteointegration. Materials 2022, 15, 7337. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and antibiofilm coating of dental implants—Past and new perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.D.; Bickner, S.; Carter, P.W.; Fulghum, T.; Luebke, M.; Nordhaus, M.A.; Strathmann, S. Antimicrobial testing for surface-immobilized agents with a surface-separated live–dead staining method. Biotechnol. Bioeng. 2011, 108, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kolter, R. Biofilms in lab and nature: A molecular geneticist’s voyage to microbial ecology. Int. Microbiol. 2010, 13, 1–7. [Google Scholar] [PubMed]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Turner, R.J. Assessing microbial monitoring methods for challenging environmental strains and cultures. Microbiol. Res. 2022, 13, 235–257. [Google Scholar] [CrossRef]
| Technique | Overview | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| MAPLE | Designed for organic materials and polymers. A frozen matrix containing the polymer is irradiated with a laser, ejecting material onto a substrate. |
|
| [32] |
| PLD | A high-power laser ablates a solid target (can be polymeric or composite) in a vacuum or a gas atmosphere. |
|
| [33] |
| Parameter | Effect on Film Properties | Relevant Technique(s) | Reference |
|---|---|---|---|
| substrate temperature | Modulates adhesion, crystallinity, and solvent evaporation; critical for morphology control | PLD | [68] |
| background gas pressure | Influences plasma plume dynamics and energy transfer; affects film density and composition | [69] | |
| matrix solvent | Should absorb laser well and evaporate cleanly; affects analyte preservation and plume quality | MAPLE | [70] |
| active substance concentration | Low concentrations reduce aggregation and enhance uniform molecular transfer | [71] | |
| target-substrate distance | Controls kinetic energy and flux; affects uniformity and deposition rate | PLD, MAPLE | [69] |
| laser wavelength | Affects absorption and ablation efficiency; UV preferred for polymers to minimize damage | [33] | |
| pulse duration and repetition rate | Short pulses reduce thermal damage; repetition rate influences film growth rate and roughness | [72] | |
| laser fluence | Controls amount of material ablated; if too high, may cause damage; if too low, reduces efficiency | [73] |
| Pulse Duration | Primary Mechanism(s) | Thermal Impact | Common Applications | Refs. |
|---|---|---|---|---|
| Ns | Thermal melting and vaporization; plasma shielding | Significant | PLD, MAPLE, bulk material removal (cutting, welding) | [79,94] |
| Ps | Hybrid (transition from thermal to non-thermal); reduced electron–phonon coupling time | Minimized | High-precision micromachining, LIPSS (less common than fs) | [79,95] |
| Fs | Non-thermal (Coulomb explosion, phase explosion, multiphoton ionization) | Negligible | LIPSS, ultra-precision micromachining, transparent material processing, medical applications | [79,95] |
| Component | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Polymer Matrices | -Biocompatible (e.g., PLGA, chitosan) | -Limited thermal stability (degradation in PLD) | [103]. |
| -Tunable drug release kinetics | -Variable mechanical strength | [104] | |
| -Flexible substrate adhesion | -Potential cytotoxicity (e.g., PVP at high doses) | [105] | |
| NPs | -High surface-area-to-volume ratio (e.g., Ag NPs) | -Aggregation risks | [106] |
| -Broad-spectrum antimicrobial activity | -Potential cytotoxicity (e.g., Cu NPs) | [107] | |
| -Synergistic effects with polymers | -Complex synthesis and functionalization | [108] |
| Category | Compound | Application In Thin Films | Reference |
|---|---|---|---|
| Antibiotic | Gentamicin | Incorporated in poly(sebacic anhydride) films via MAPLE; active against E. coli and S. aureus | [147] |
| Kanamycin | Functionalized onto Fe3O4 NPs; MAPLE-deposited films inhibit microbial adhesion and biofilm formation | [148] | |
| Ceftriaxone/Cefuroxime | Loaded into HAp/PLGA coatings; prevent E. coli adhesion and biofilm development on bone implants | [149] | |
| Ciprofloxacin | Embedded in bioglass–polymer composite coatings for titanium implants with antibacterial properties | [100] | |
| Doxycicline | Embedded in bioglass–polymer composite coatings for stainless steel implants with antibacterial properties against S. aureus and E. coli | [150] | |
| Essential oils | Cypress oil | Combined with Fe3O4 nanoparticles in PLGA coatings; antimicrobial against S. aureus, E. coli, and C. albicans | [151] |
| Tea Tree Oil (Terpinen-4-ol) | Used in plasma-polymerized films; inhibits P. aeruginosa biofilm formation | [152] | |
| Natural powders | Propolis | Incorporated into bioactive coatings; shown to enhance antimicrobial performance and healing | [153] |
| Neem | Combined with bioglasses for antimicrobial activity against S. aureus | [154] | |
| Natural extract | Salicylic acid | Combined with silica/magnetite layers; contributes to antibiofilm properties in laser-deposited coatings | [155] |
| Method | Strengths | Limitations | Reference |
|---|---|---|---|
| Zone of Inhibition (ZOI) | Simple, rapid screening tool | Limited to diffusible agents; not applicable for contact-killing surfaces | [160] |
| Colony Forming Unit (CFU) | Quantitative measurement of viable bacteria | Time-consuming; may miss VBNC cells; highly variable based on growth phase and incubation conditions | [161] |
| Live/Dead Staining (SYTO9/PI) | Direct imaging of viable vs. compromised cells | Requires fluorescence microscopy; possible overestimation due to temporary permeability changes | Not standardized |
| Metabolic Assays (MTT, resazurin) | Rapid, adaptable to high-throughput screening | Indirect measure; affected by material-specific interference | Not always reported |
| Biofilm Quantification (CV Assay) | Useful for assessing biofilm biomass over time | Cannot distinguish live vs. dead cells; limited sensitivity | [162] |
| Factor | Mechanism | Influence on Contact Killing | References |
|---|---|---|---|
| Hydrophobicity | Lipid membrane interaction | Moderate hydrophobicity = ↑ membrane rupture | [185] |
| Hydrophilicity | Reduced adhesion, biofilm prevention | ↑ Water-wettability = ↑ contact area | [186] |
| pH | Surface charge and membrane binding | ↑ Protonation at low pH = ↑ cationic interaction | [187] |
| Temperature | Membrane fluidity and surface energy | ↑ Temperature = ↑ membrane fluidity = ↑ vulnerability to rupture | [188] |
| Nanostructures | Mechanical puncture or trapping | ↑ Nanoscale roughness = ↑ disruption efficiency | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visan, A.I.; Negut, I. Polymeric Composite Thin Films Deposited by Laser Techniques for Antimicrobial Applications—A Short Overview. Polymers 2025, 17, 2020. https://doi.org/10.3390/polym17152020
Visan AI, Negut I. Polymeric Composite Thin Films Deposited by Laser Techniques for Antimicrobial Applications—A Short Overview. Polymers. 2025; 17(15):2020. https://doi.org/10.3390/polym17152020
Chicago/Turabian StyleVisan, Anita Ioana, and Irina Negut. 2025. "Polymeric Composite Thin Films Deposited by Laser Techniques for Antimicrobial Applications—A Short Overview" Polymers 17, no. 15: 2020. https://doi.org/10.3390/polym17152020
APA StyleVisan, A. I., & Negut, I. (2025). Polymeric Composite Thin Films Deposited by Laser Techniques for Antimicrobial Applications—A Short Overview. Polymers, 17(15), 2020. https://doi.org/10.3390/polym17152020







