Preparation and Performance Evaluation of Graphene Oxide-Based Self-Healing Gel for Lost Circulation Control
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation Method
- (1)
- Preparation of GO
- (2)
- Preparation of SG gel
2.3. Characterization
2.4. Mechanical and Rheological Property Tests
2.5. Self-Healing and Expansion Performance Tests
2.6. Self-Healing and Expansion Performance Tests
3. Results and Discussion
3.1. Synthesis of SG Gel
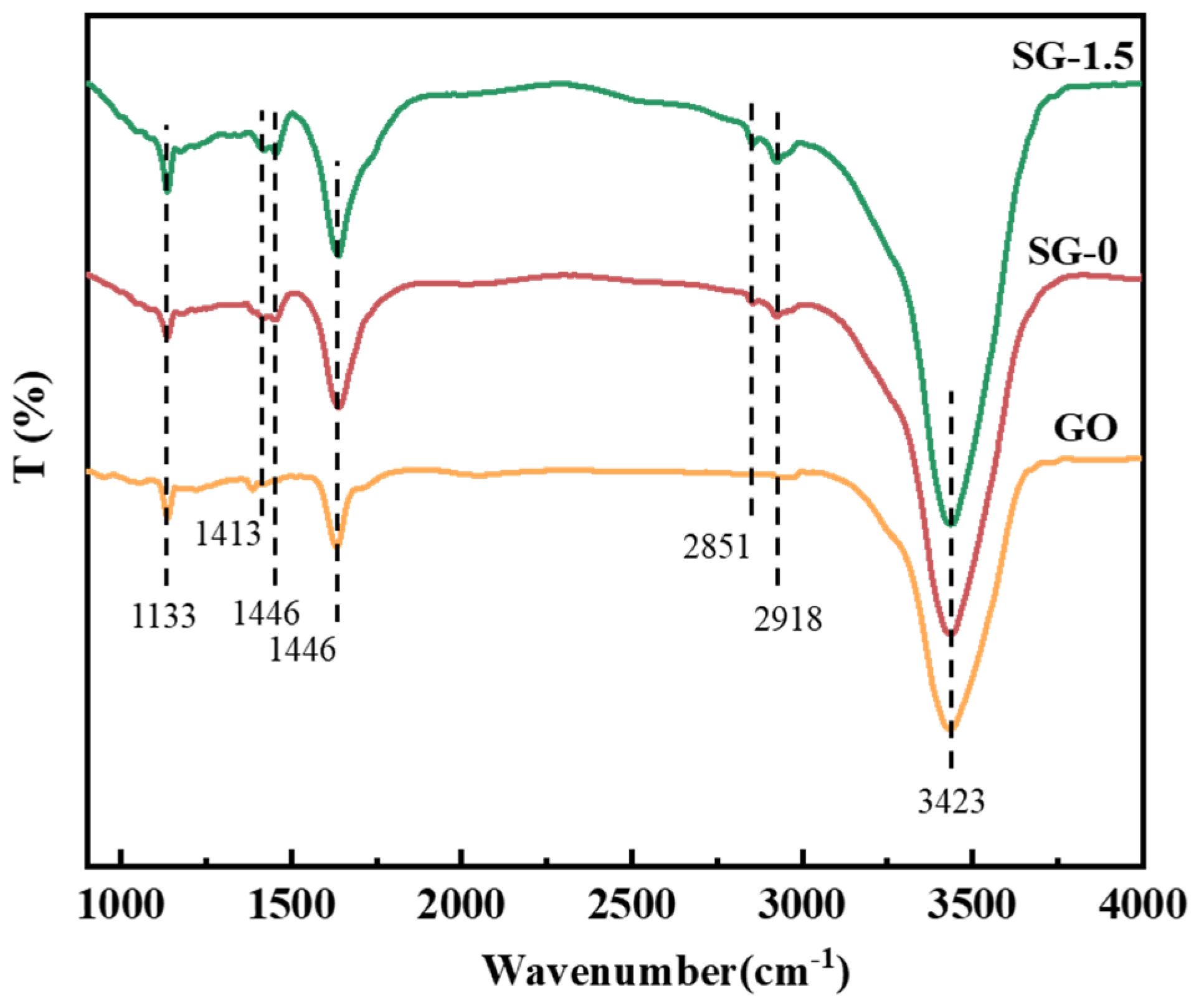
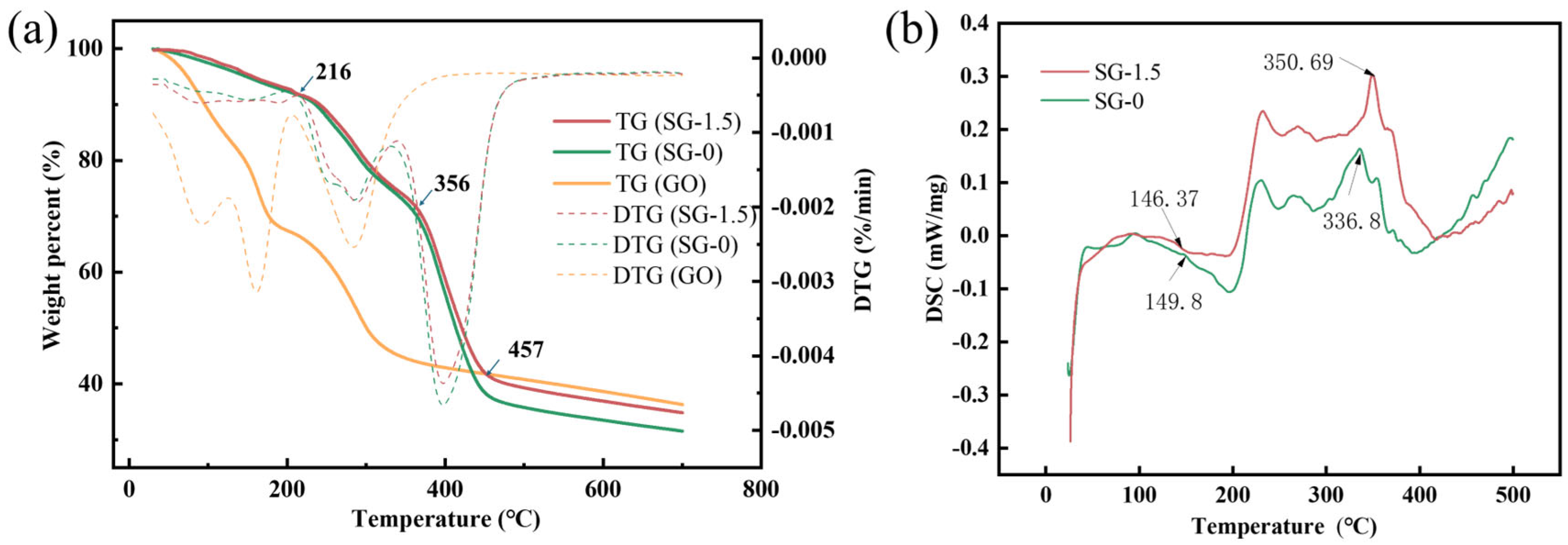
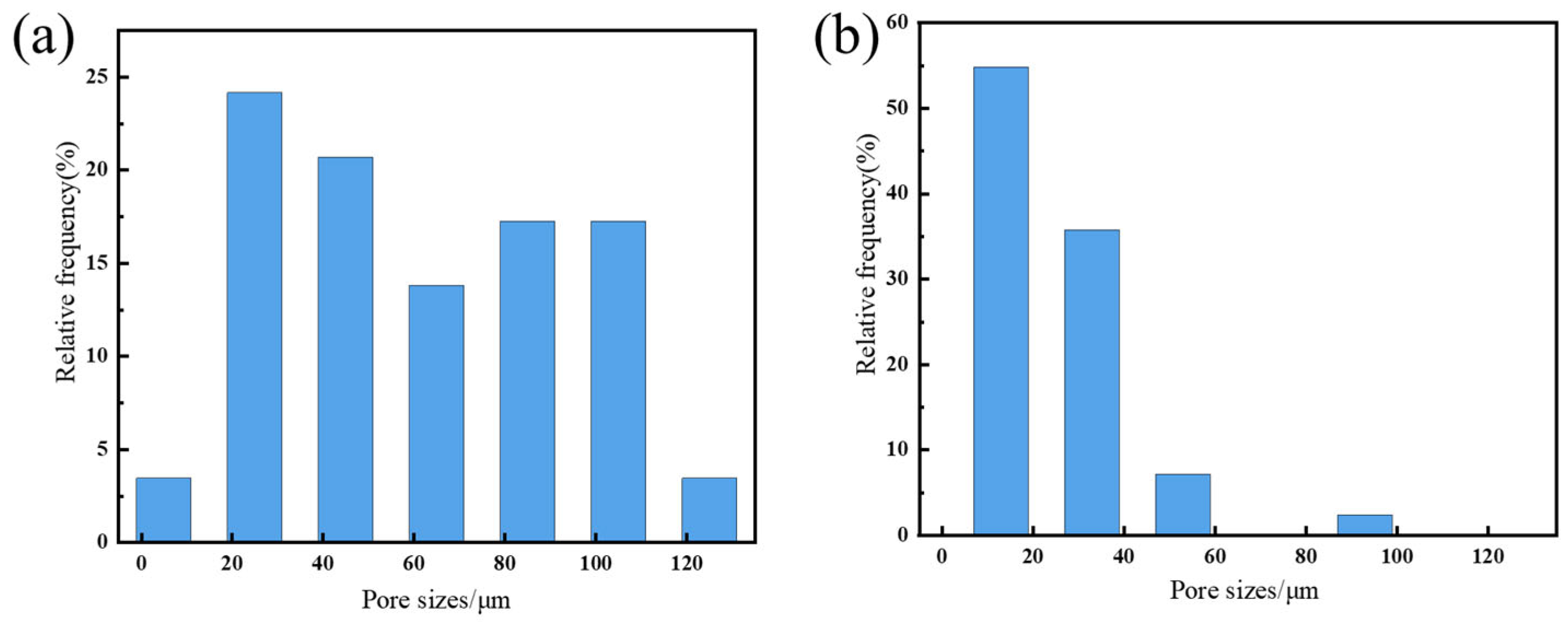
3.2. Characterization of SG Gel
3.3. Performance Evaluation of SG Gels
- (1)
- Swelling properties
- (2)
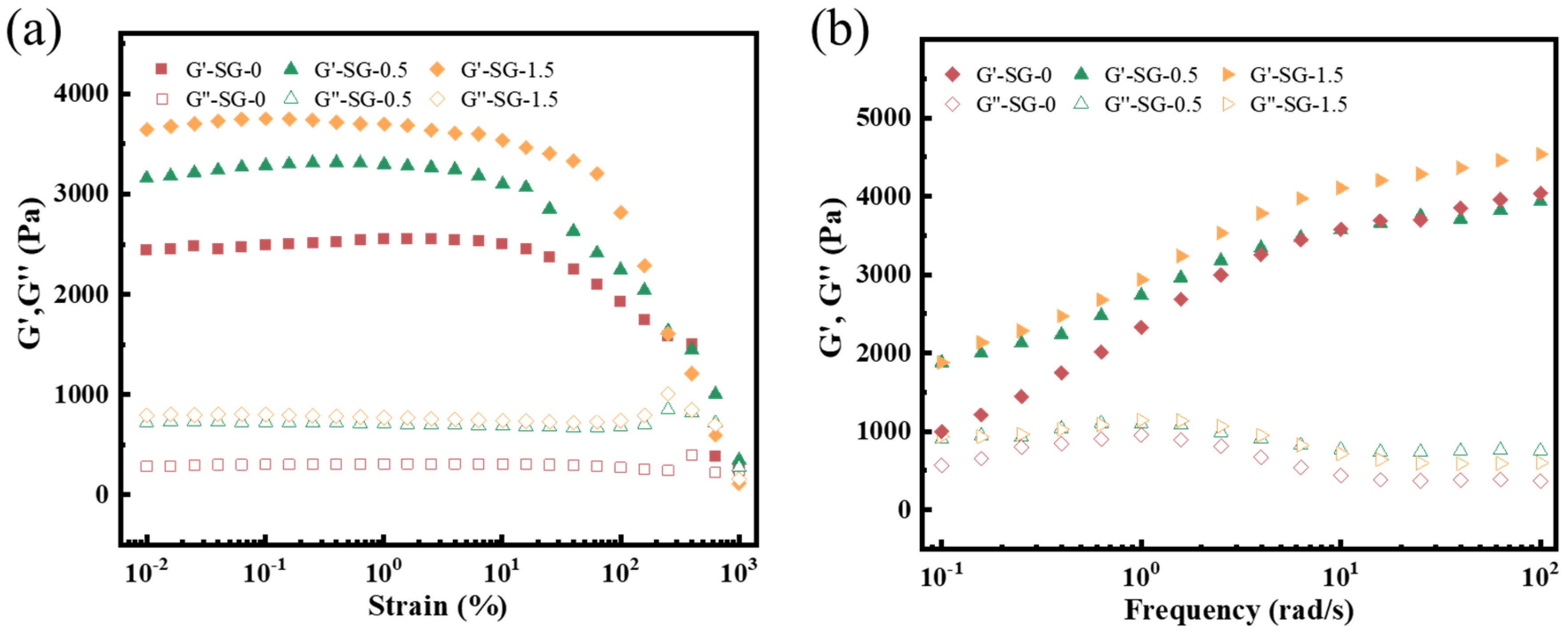
- Rheological properties
- (3)
- Mechanical properties
- (4)
- Adhesion properties
- (5)
- Self-healing properties
- (6)
- Plugging performance
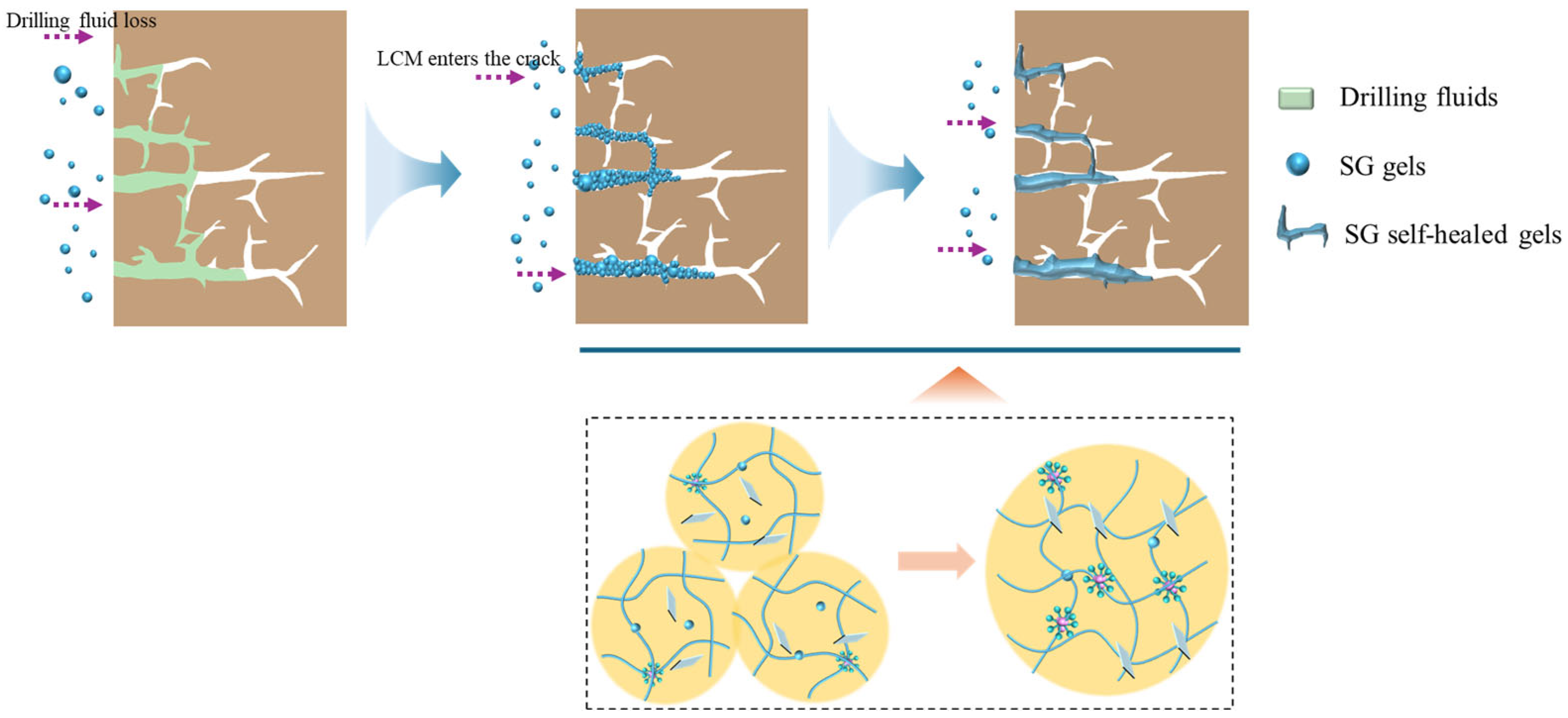
3.4. Plugging Mechanism of SG Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.B.; Bai, Y.R.; Sun, J.S.; Lv, K.H. Curing kinetics and plugging mechanism of high strength curable resin plugging material. Pet. Sci. 2024, 21, 3446–3463. [Google Scholar] [CrossRef]
- Alhaidari, S.A.; Alarifi, S.A.; Bahamdan, A. Plugging efficiency of flaky and fibrous lost circulation materials in different carrier fluid systems. Front. Phys. 2022, 10, 1065526. [Google Scholar] [CrossRef]
- Alkinani, H.H.; Al-Hameedi, A.T.T.; Dunn-Norman, S.; Flori, R.E.; Alsaba, M.T.; Amer, A.S.; Hilgedick, S.A. Using data mining to stop or mitigate lost circulation. J. Pet. Sci. Eng. 2019, 173, 1097–1108. [Google Scholar] [CrossRef]
- Ghanbari, S.; Naderifar, A. Enhancing the physical plugging behavior of colloidal silica nanoparticles using binomial size distribution. J. Nat. Gas Sci. Eng. 2016, 30, 213–220. [Google Scholar] [CrossRef]
- Ezeakacha, C.P.; Salehi, S.; Kiran, R. Lost circulation and filter cake evolution: Impact of dynamic wellbore conditions and wellbore strengthening implications. J. Pet. Sci. Eng. 2018, 171, 1326–1337. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, J.P.; Sun, J.S.; Lv, K.H.; Wang, Z.L.; Xu, Z.; Sun, Y.W.; Weiss, C.K. Novel Modified Styrene-Based Microspheres for Enhancing the Performance of Drilling Fluids at High Temperatures. Gels 2023, 9, 9090763. [Google Scholar] [CrossRef]
- Yao, L.; Quan, X.H.; Zhang, Y.J.; Huang, S.M.; Feng, Q.; Zhang, X. Preparation and Performance Evaluation of High-Temperature Polymer Nano-Plugging Agents for Water-Based Drilling Fluids Systems Applicable to Unconventional Reservoirs. Polymers 2025, 17, 17050588. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.S.; Long, Y.F.; Peng, L.; Li, Y.Y.; Wang, R.; Qu, Y.Z.; Yang, X.M. Rapid self-healing nanocomposite gel crosslinked by LDH for lost circulation control. Colloids Surf. A 2024, 695, 134207. [Google Scholar] [CrossRef]
- Bai, X.D.; Wang, M.B.; Chen, Y.; Wu, L.C.; Yu, J.Y.; Luo, Y.M. Synthesis and properties of self-healing hydrogel plugging agent. J. Appl. Polym. Sci. 2024, 141, 55005. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.S.; Long, Y.F.; Wang, R.; Qu, Y.Z.; Peng, L.; Ren, H.; Gao, S.F. A re-crosslinkable composite gel based on curdlan for lost circulation control. J. Mol. Liq. 2023, 371, 121010. [Google Scholar] [CrossRef]
- Singh, R.; Mahto, V. Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. Pet. Sci. 2017, 14, 765–779. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Peplinska, B.; Przysiecka, L.; Coy, E.; Jarek, M.; Chybczynska, K.; Jurga, S. Nanocomposite Gel as Injectable Therapeutic Scaffold: Microstructural Aspects and Bioactive Properties. ACS Appl. Mater. Interfaces 2020, 12, 7840–7853. [Google Scholar] [CrossRef]
- Asadizadeh, S.; Ayatollahi, S.; ZareNezhad, B. Fabrication of a highly efficient new nanocomposite polymer gel for controlling the excess water production in petroleum reservoirs and increasing the performance of enhanced oil recovery processes. Chin. J. Chem. Eng. 2021, 32, 385–392. [Google Scholar] [CrossRef]
- Gao, M.Z.; Zhang, P.H.; Wang, F.F.; Li, L.; Li, Z.Y. The Relationship Between Dielectric Properties and Nanoparticle Dispersion of Nano-SILICA/Epoxy Composites. In Proceedings of the IEEE Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Shenzhen, China, 20–23 October 2013; pp. 234–237. [Google Scholar]
- Lee, S.B.; Jeong, B.H.; Yi, J.W.; Lee, W.; Um, M.K. Quantitative Dispersion Evaluation of Carbon Nanotubes Reinforced Polymer Nano-composites. Polymer 2011, 35, 60–65. [Google Scholar]
- Zhang, J.J.; Huang, Q.L.; Du, C.G.; Peng, R.; Hua, Y.T.; Li, Q.; Hu, A.L.; Zhou, J.H. Preparation and Anti-Mold Properties of Nano-ZnO/Poly (N-isopropylacrylamide) Composite Hydrogels. Molecules 2020, 25, 4135. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Ma, C.B.; Wang, Y.Q.; Li, X.P.; Yu, G.H. A Conductive Self-Healing Hybrid Gel Enabled by Metal-Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.Y.; Wei, Z.Q.; Zhang, B.Z.; Weng, X.S. Advances and Progress in Self-Healing Hydrogel and Its Application in Regenerative Medicine. Materials 2023, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.N.; Zhang, Y.L.; Liu, Y.Q.; Han, D.D.; Mao, J.W.; Zhang, J.R.; Zhao, W.C.; Sun, H.B. Heterogeneous self-healing assembly of MXene and graphene oxide enables producing free-standing and self-reparable soft electronics and robots. Sci. Bull. 2022, 67, 501–511. [Google Scholar] [CrossRef]
- Jirickova, A.; Jankovsky, O.; Sofer, Z.; Sedmidubsky, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Zheng, W.J.; Gao, Y.; Fan, X.X.; Cui, X.F.; Zou, W.; Zheng, D.; Yan, J.; Li, B. Physically Linked PVA/P (AAm-SMA) Hydrogels: Tough, Fast Recovery, Antifatigue, and Self-Healing Soft Materials. Macromol. Mater. Eng. 2018, 303, 1800104. [Google Scholar] [CrossRef]
- Qin, Z.H.; Niu, R.; Tang, C.J.; Xia, J.; Ji, F.; Dong, D.Y.; Zhang, H.T.; Zhang, S.; Li, J.J.; Yao, F.L. A Dual-Crosslinked Strategy to Construct Physical Hydrogels with High Strength, Toughness, Good Mechanical Recoverability, and Shape-Memory Ability. Macromol. Mater. Eng. 2018, 303, 1700396. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhou, T.Y.; Yu, Z.Y.; Wang, F.; Shi, D.J.; Ni, Z.B.; Chen, M.Q. Effects of surfactant and ionic concentration on properties of dual physical crosslinking self-healing hydrogels by hydrophobic association and ionic interactions. New J. Chem. 2020, 44, 4061–4070. [Google Scholar] [CrossRef]
- Etou, M.; Yonezu, K.; Inoue, T.; Yokoyama, T. Interaction between Al3+ and Poly (acrylic acid) (PAA) in Aqueous Solution-Formation Modes for the Al-PAA Complexes. J. Chem. Eng. Jpn. 2022, 55, 275–280. [Google Scholar] [CrossRef]
- Zeng, R.P.; Qi, C.Y.; Lu, S.X.; Xu, J.B.; Zhao, C.; Dong, Z.X.; Lei, C.H. Hydrophobic association and ionic coordination dual crossed-linked conductive hydrogels with self-adhesive and self-healing virtues for conformal strain sensors. J. Polym. Sci. 2022, 60, 812–824. [Google Scholar] [CrossRef]
- You, X.; Feng, Q.; Yang, J.S.; Huang, K.; Hu, J.B.; Dong, S.M. Preparation of high concentration graphene dispersion with low boiling point solvents. J. Nanopart. Res. 2019, 21, 19. [Google Scholar] [CrossRef]
- Jo, G.; Sanetuntikul, J.; Shanmugam, S. Boron and phosphorous-doped graphene as a metal-free electrocatalyst for the oxygen reduction reaction in alkaline medium. RSC Adv. 2015, 5, 53637–53643. [Google Scholar] [CrossRef]
- Song, T.W.; Luo, W.J.; Mu, J.L.; Cai, Y.; Wei, J.; Li, H.F. Preparation of polyacrylic-acidipalygorskite composite particles via inverse-suspension polymerization for efficient separation of Ce3+ from aqueous solution. J. Colloid Interface Sci. 2019, 535, 371–379. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Xie, F.; Cai, J. Strong and tough cellulose–graphene oxide composite hydrogels by multi-modulus components strategy as photothermal antibacterial platform. Chem. Eng. J. 2022, 431, 133964. [Google Scholar] [CrossRef]
- Ye, J.J.; Qi, L.; Liu, B.B.; Xu, C.X. Facile preparation of hexagonal tin sulfide nanoplates anchored on graphene nanosheets for highly efficient sodium storage. J. Colloid Interface Sci. 2018, 513, 188–197. [Google Scholar] [CrossRef]
- Cao, J.; He, G.; Ning, X.; Chen, X.; Fan, L.; Yang, M.; Yin, Y.; Cai, W. Preparation and properties of O-chitosan quaternary ammonium salt/polyvinyl alcohol/graphene oxide dual self-healing hydrogel. Carbohydr. Polym. 2022, 287, 119318. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, K.; Hu, J.Y.; Zhang, Y.C.; Dai, Y.; Xia, F. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J. Mater. Chem. A 2020, 8, 25390–25401. [Google Scholar] [CrossRef]
- Bai, Y.R.; Zhang, Q.T.; Sun, J.S.; Jiang, G.C.; Lv, K.H. Double network self-healing hydrogel based on hydrophobic association and ionic bond for formation plugging. Pet. Sci. 2022, 19, 2150–2164. [Google Scholar] [CrossRef]
- Terech, P.; Yan, M.H.; Maréchal, M.; Royal, G.; Galvez, J.; Velu, S.K.P. Characterization of strain recovery and “self-healing” in a self-assembled metallo-gel. Phys. Chem. Chem. Phys. 2013, 15, 7338–7344. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Long, Y.; Huang, H.; Song, J.; Wang, R.; Qu, Y.; Yang, Z. A Self-Healing Gel with an Organic–Inorganic Network Structure for Mitigating Circulation Loss. Gels 2024, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.C.; Wang, G.L.; Tian, D.; Ma, A.Y.; Wang, W.X.; Bai, L.J.; Chen, H.; Yang, L.X.; Yang, H.W.; Wei, D.L.; et al. Self-healing nanocomposite hydrogels via Janus nanosheets: Multiple effects of metal-coordination and host-guest interactions. React. Funct. Polym. 2021, 165, 104963. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhang, G.Z.; Li, F.B.; Zhang, Y.Q.; Lei, Y.; Xia, Y.H.; Jin, X.H.; Feng, X.Q.; Li, H.J. A self-healable and tough nanocomposite hydrogel crosslinked by novel ultrasmall aluminum hydroxide nanoparticles. Nanoscale 2017, 9, 15470–15476. [Google Scholar] [CrossRef]
- Bai, Y.R.; Zhang, Q.T.; Sun, J.S.; Lv, K.H.; Shang, X.S.; Liu, C.T.; Cheng, R.C.; Wang, F. Self-healing hydrogels and their action mechanism in oil-gas drilling and development engineering: A systematic review and prospect. J. Nat. Gas Sci. Eng. 2021, 96, 104250. [Google Scholar] [CrossRef]
- Yang, L.L.; Xie, C.L.; Zhang, Y.W.; Jiang, G.C.; Wu, Y.P.; Liu, H.Q.; Dong, T.F.; Guo, C.P. Performance of Self-healing microgel incorporating Nano-Silica as plugging material for drilling fluid. J. Mol. Liq. 2023, 386, 122392. [Google Scholar] [CrossRef]





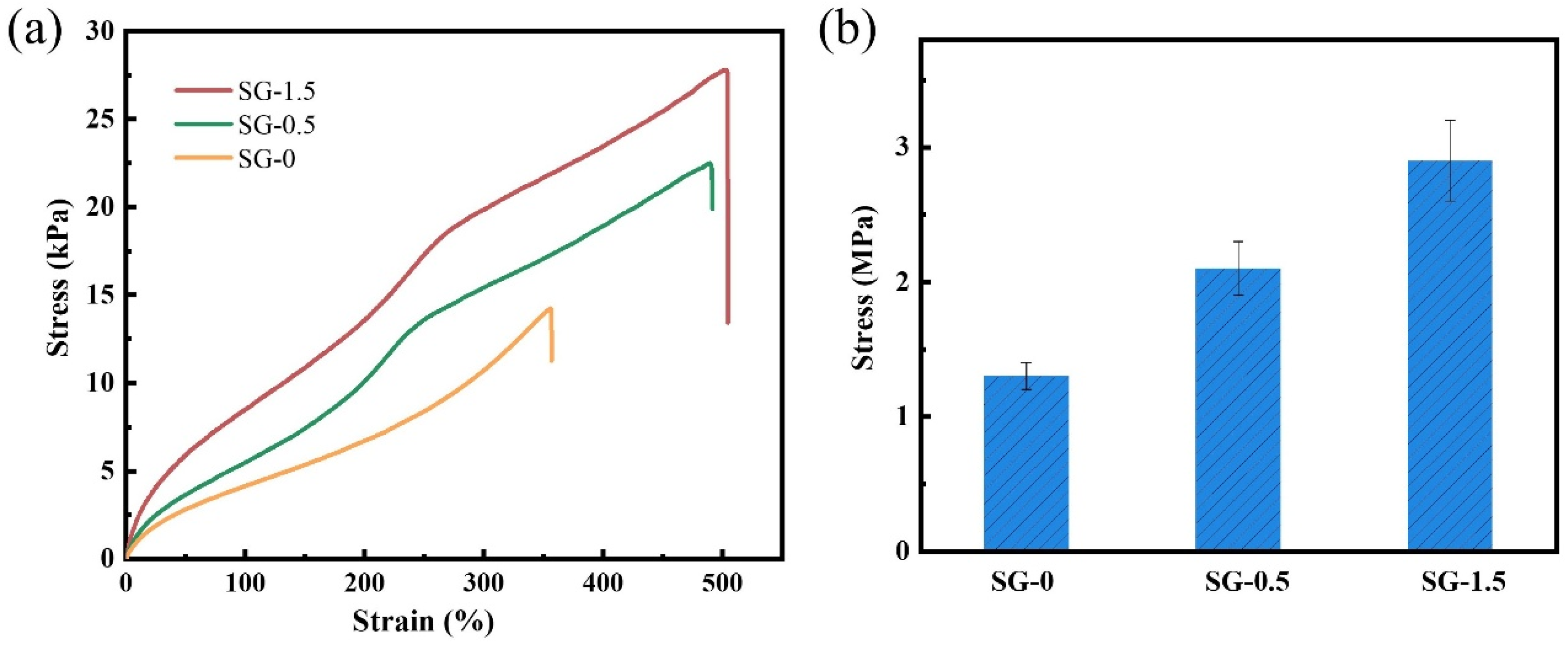
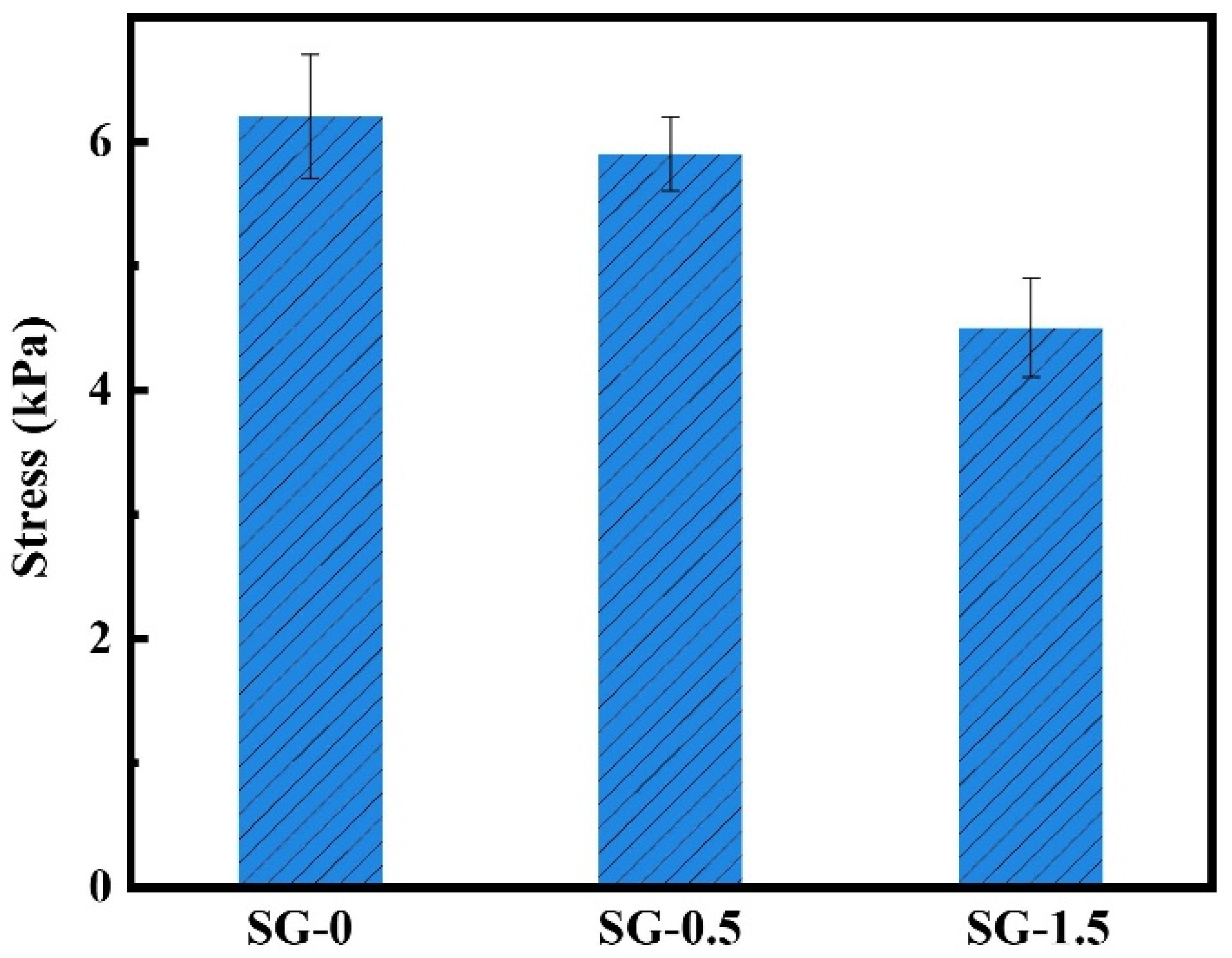
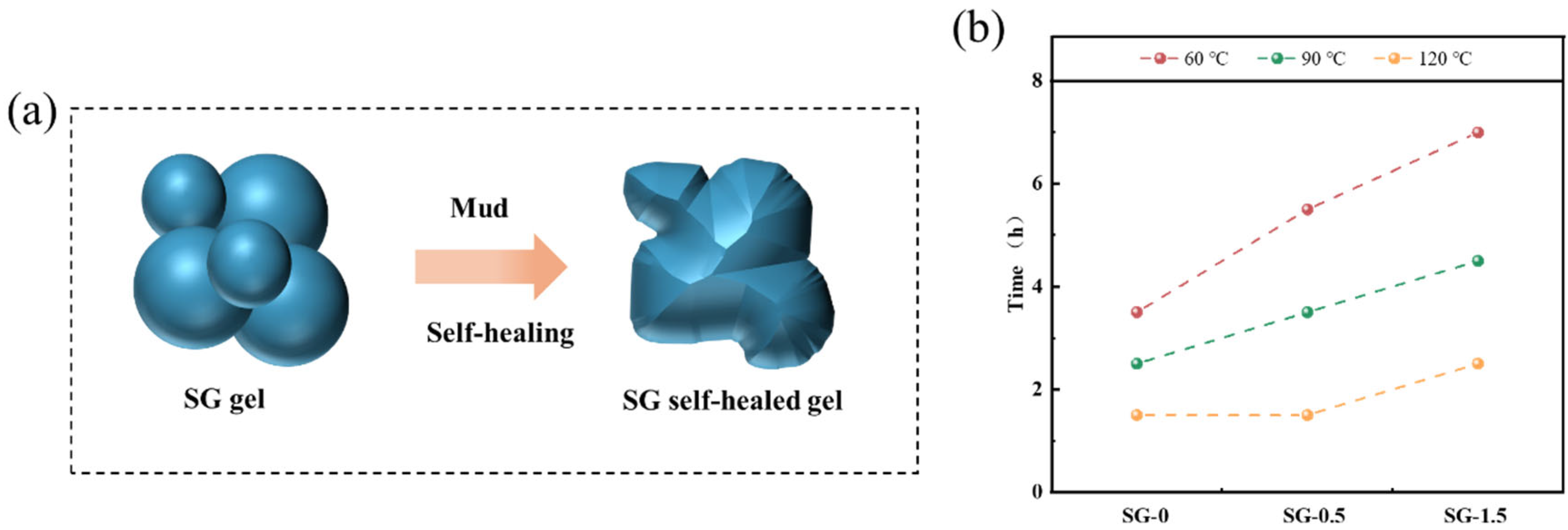
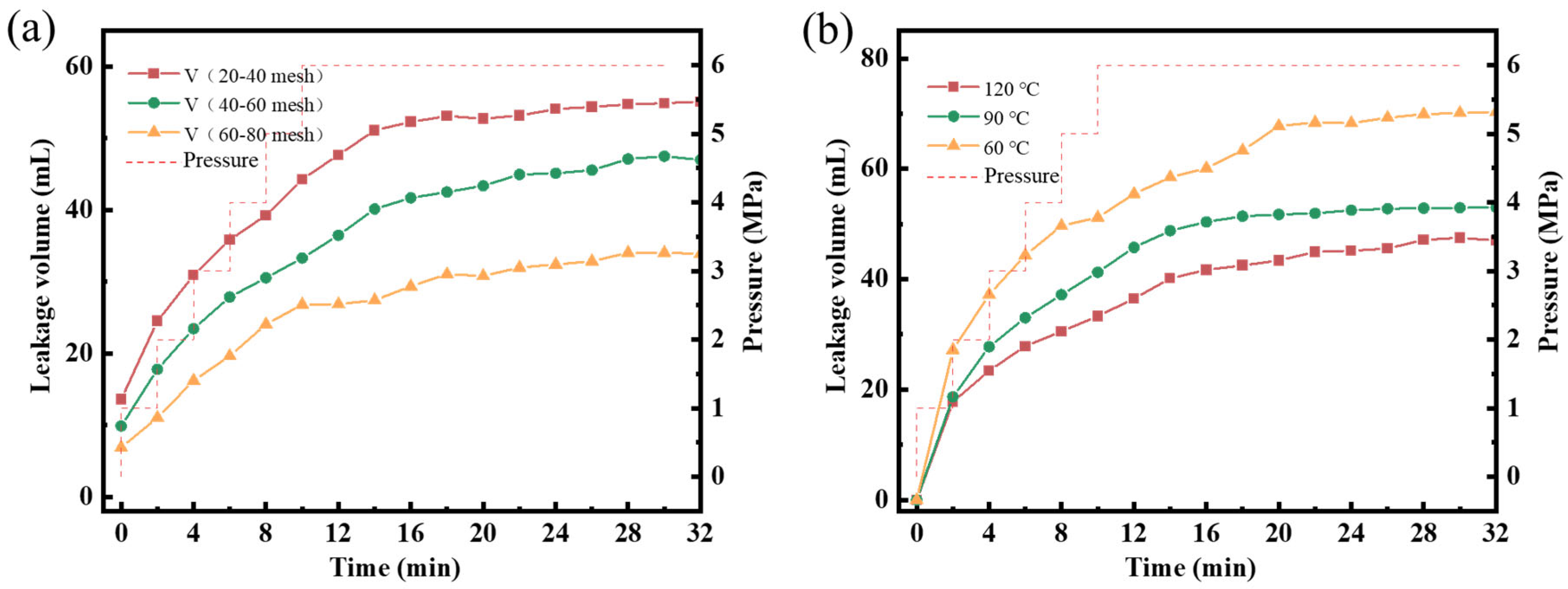
| AV (mPa·s) | PV (mPa·s) | YP (Pa) | FLAPI (mL) | |
|---|---|---|---|---|
| Mud | 13 | 8 | 5 | 28.5 |
| Mud + 4% SG-1.5 | 23.5 | 14 | 9.5 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Luo, P.; Wang, X. Preparation and Performance Evaluation of Graphene Oxide-Based Self-Healing Gel for Lost Circulation Control. Polymers 2025, 17, 1999. https://doi.org/10.3390/polym17151999
Li W, Luo P, Wang X. Preparation and Performance Evaluation of Graphene Oxide-Based Self-Healing Gel for Lost Circulation Control. Polymers. 2025; 17(15):1999. https://doi.org/10.3390/polym17151999
Chicago/Turabian StyleLi, Wenzhe, Pingya Luo, and Xudong Wang. 2025. "Preparation and Performance Evaluation of Graphene Oxide-Based Self-Healing Gel for Lost Circulation Control" Polymers 17, no. 15: 1999. https://doi.org/10.3390/polym17151999
APA StyleLi, W., Luo, P., & Wang, X. (2025). Preparation and Performance Evaluation of Graphene Oxide-Based Self-Healing Gel for Lost Circulation Control. Polymers, 17(15), 1999. https://doi.org/10.3390/polym17151999






