Synthesis, Thermal Behavior and Mechanical Property of Fully Biobased Poly(hexamethylene Furandicarboxylate-co-hexamethylene Thiophenedicarboxylate) Copolyesters
Abstract
1. Introduction
2. Experimental
2.1. Materials
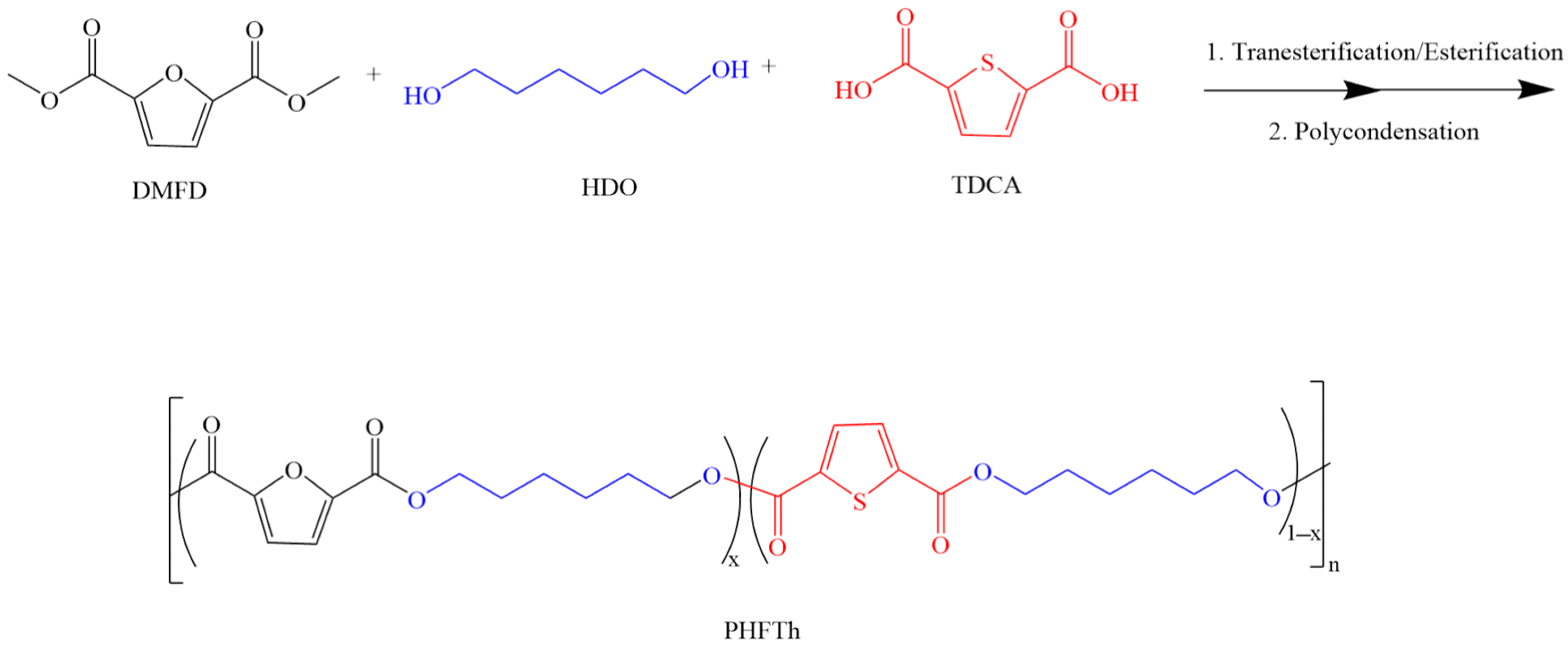
2.2. Synthesis of the PHFTh Copolyesters
2.3. Characterization
3. Results and Discussion
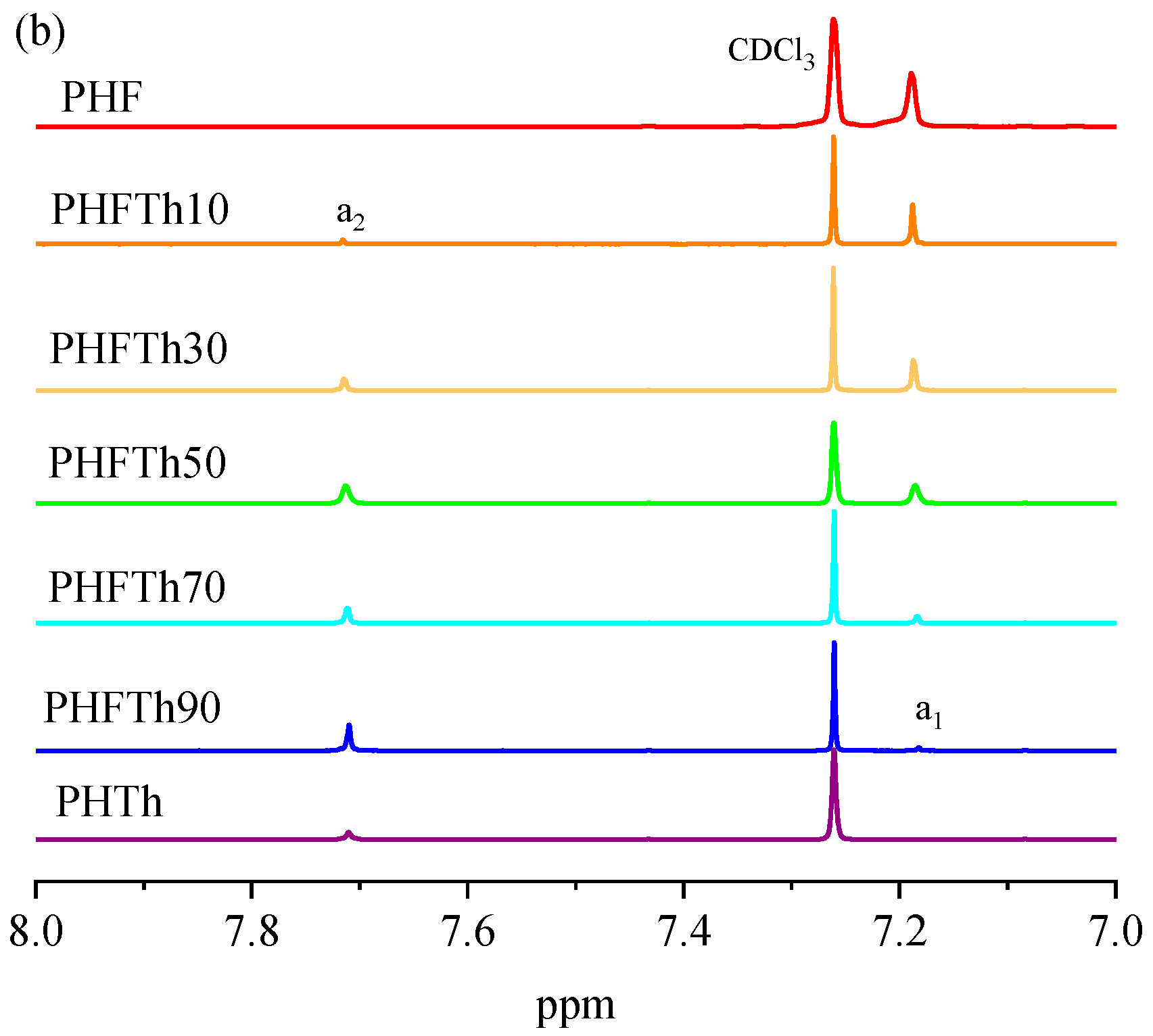
3.1. Chemical Structure and Composition
3.2. Thermal Stability
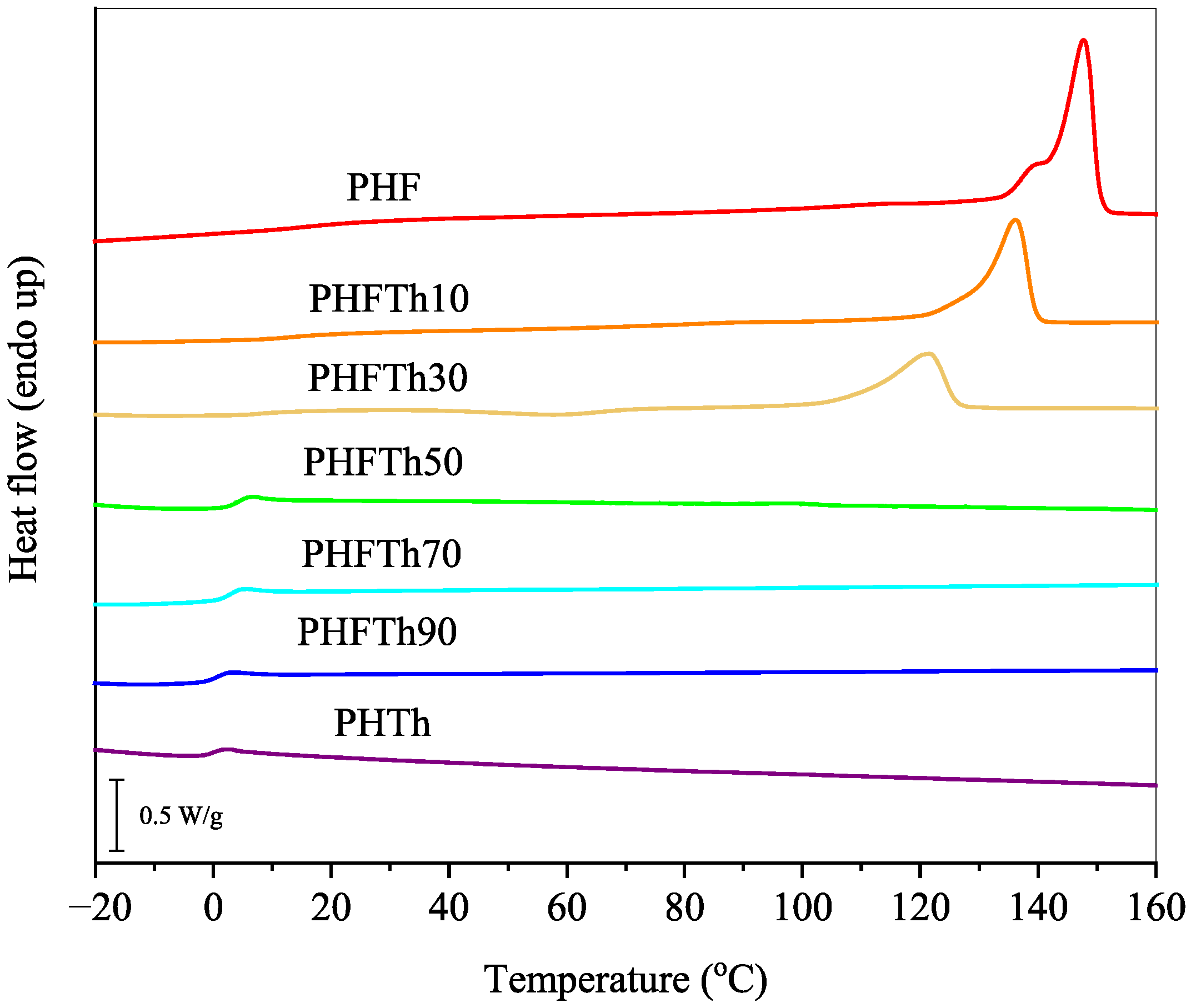
3.3. Basic Thermal Parameters and Crystallization Behaviors
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Song, M.; Xu, Y.; Wang, W.; Wang, Z.; Zhang, L. Bio-based polyesters: Recent progress and future prospects. Prog. Polym. Sci. 2021, 120, 101430. [Google Scholar] [CrossRef]
- Brandolese, A.; Kleij, A. Catalyst engineering empowers the creation of biomass-derived polyesters and polycarbonates. Acc. Chem. Res. 2022, 55, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jonnalagadda, S.; Ramanujachary, K.; Mugweru, A. Selective oxidation of 5-hydroxymethyl-2-furfural to furan-2,5-dicarboxylic acid over spinel mixed metal oxide catalyst. Catal. Commun. 2015, 58, 179–182. [Google Scholar] [CrossRef]
- Baniamerian, H.; Høj, M.; Beier, M.; Jensen, A. Catalytic conversion of sugars and polysaccharides to glycols: A review. Appl. Catal. B Environ. 2023, 330, 122650. [Google Scholar] [CrossRef]
- Faveere, W.; Van Praet, S.; Vermeeren, B.; Dumoleijn, K.; Moonen, K.; Taarning, E.; Sels, B. Toward replacing ethylene oxide in a sustainable world: Glycolaldehyde as a bio-based C2 platform molecule. Angew. Chem. Int. Ed. 2021, 60, 12204–12223. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Metal-free oxidative synthesis of succinic acid from biomass-derived furan compounds using a solid acid catalyst with hydrogen peroxide. Appl. Catal. A Gen. 2013, 458, 55–62. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Khouri, N.; Bahú, J.; Blanco-Llamero, C.; Severino, P.; Concha, V.; Souto, E. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Focarete, M.; Ceccorulli, G.; Scandola, M.; Kowalczuk, M. Further evidence of crystallinity-induced biodegradation of synthetic atactic poly(β-hydroxybutyrate) by PHB-depolymerase a from pseudomonas lemoignei. Blends of atactic poly(β-hydroxybutyrate) with crystalline polyesters. Macromolecules 1998, 31, 8485–8492. [Google Scholar] [CrossRef]
- Ilhami, S.; Rahman, S.; Iqhrammullah, M.; Hamid, Z.; Chai, Y.; Lam, M. Polyhydroxyalkanoates production from microalgae for sustainable bioplastics: A review. Biotechnol. Adv. 2025, 79, 108529. [Google Scholar] [CrossRef]
- Eid, N.; Ameduri, B.; Boutevin, B. Synthesis and properties of furan derivatives for epoxy resins. ACS Sustain. Chem. Eng. 2021, 9, 8018–8031. [Google Scholar] [CrossRef]
- Kainulainen, T.; Hukka, T.; Özeren, H.; Sirviö, J.; Hedenqvist, M.; Heiskanen, J. Utilizing furfural-based bifuran diester as monomer and comonomer for high-performance bioplastics: Properties of poly(butylene furanoate), poly(butylene bifuranoate), and their copolyesters. Biomacromolecules 2020, 21, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.; Petersen, G. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(butylene 2,5-thiophenedicarboxylate): An added value to the class of high gas barrier biopolyesters. Polymers 2018, 10, 167. [Google Scholar] [CrossRef]
- Tian, S.; Du, Y.; Wang, P.; Chen, T.; Xu, J.; Yu, H.; Guo, B. Effects of different isomers of thiophenedicarboxylic acids on the synthesis and properties of thiophene-based sustainable polyesters. ACS Sustain. Chem. Eng. 2023, 11, 6652–6664. [Google Scholar] [CrossRef]
- Carlos Morales-Huerta, J.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef]
- Knoop, R.; Vogelzang, W.; Van Haveren, J.; Van Es, D. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Miah, M.; Dong, Y.; Wang, J.; Zhu, J. Recent progress on sustainable 2,5-furandicarboxylate-based polyesters: Properties and applications. ACS Sustain. Chem. Eng. 2024, 12, 2927–2961. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Tsanaktsis, V.; Papageorgiou, D.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D. Furan-based polyesters from renewable resources: Crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Burgess, S.; Kriegel, R.; Koros, W. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.; Karvan, O.; Johnson, J.; Kriegel, R.; Koros, W. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Araujo, C.; Nolasco, M.; Ribeiro-Claro, P.; Rudić, S.; Silvestre, A.; Vaz, P.; Sousa, A. Inside PEF: Chain conformation and dynamics in crystalline and amorphous domains. Macromolecules 2018, 51, 3515–3526. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. Poly(alkylene 2,5-thiophenedicarboxylate) polyesters: A new class of bio-based high-performance polymers for sustainable packaging. Polymers 2021, 13, 2460. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Salatelli, E.; Gazzano, M.; Siracusa, V.; Munari, A. Tailoring poly(butylene 2,5-thiophenedicarboxylate) features by the introduction of adipic acid co-units: Biobased and biodegradable aliphatic/aromatic polyesters. Polymer 2018, 145, 11–20. [Google Scholar] [CrossRef]
- Gigli, M.; Quartinello, F.; Soccio, M.; Pellis, A.; Lotti, N.; Guebitz, G.; Licoccia, S.; Munari, A. Enzymatic hydrolysis of poly(1,4-butylene 2,5-thiophenedicarboxylate) (PBTF) and poly(1,4-butylene 2,5-furandicarboxylate) (PBF) films: A comparison of mechanisms. Environ. Int. 2019, 130, 104852. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.; Jiang, M.; Zhang, Q.; Wang, R.; Wang, H.; Zhou, G. Synthesis and characterization of bio-based polyesters from 2,5-thiophenedicarboxylic acid. Polym. Degrad. Stab. 2019, 168, 108942. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.; Dubois, P. Biobased Poly(ethylene-co-hexamethylene 2,5-furandicarboxylate) (PEHF) copolyesters with superior tensile properties. Ind. Eng. Chem. Res. 2018, 57, 13094–13102. [Google Scholar] [CrossRef]
- Kasmi, N.; Ainali, N.; Agapiou, E.; Papadopoulos, L.; Papageorgiou, G.; Bikiaris, D. Novel high Tg fully biobased poly(hexamethylene-co-isosorbide-2,5-furan dicarboxylate) copolyesters: Synergistic effect of isosorbide insertion on thermal performance enhancement. Polym. Degrad. Stab. 2019, 169, 108983. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, Z.; Qiu, Z. Synthesis, thermal behavior, and mechanical properties of fully biobased poly(hexamethylene 2,5-furandicarboxylate-co-sebacate) copolyesters. Polymers 2022, 15, 85. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Qiu, Z. Novel biobased poly(hexamethylene-co-diethylene glycol furandicarboxylate) copolyesters with improved mechanical properties and hydrolytic degradation rates. Polym. Chem. 2025, 16, 2133–2142. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Z.; Chen, C.; Rwei, S. Bio-based poly(hexamethylene 2,5-furandicarboxylate-co-2,6-naphthalate) copolyesters: A study of thermal, mechanical, and gas-barrier properties. Soft Matt. 2022, 18, 7631–7641. [Google Scholar] [CrossRef]
- Meng, H.; Li, Z.; Wu, L. Poly(ethylene 2,5-thiophenedicarboxylate-co-2,5-furandicarboxylate) copolyesters with improved ductility and PEF-comparable high performance. Eur. Polym. J. 2023, 196, 112284. [Google Scholar] [CrossRef]
- Solomon, O.; Ciuta, I. Détermination de La Viscosité Intrinsèque de Solutions de Polymères Par Une Simple Détermination de La Viscosité. J. Appl. Polym. Sci. 1962, 6, 683–686. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Y.; Hao, X.; Zhang, L.; Sun, R. Bio-based poly(butylene furandicarboxylate-co-butylene 2,5-thiophenedicarboxylate): Synthesis, thermal properties, crystallization properties and mechanical properties. Polym. Bull. 2023, 80, 5373–5395. [Google Scholar] [CrossRef]
- Wunderlich, B. A classification of molecules, phases, and transitions as recognized by thermal analysis. Thermochim. Acta 1999, 340–341, 37–52. [Google Scholar] [CrossRef]
- Shalaev, E.; Wu, K.; Shamblin, S.; Krzyzaniak, J.; Descamps, M. Crystalline mesophases: Structure, mobility, and pharmaceutical properties. Adv. Drug Deliv. Rev. 2016, 100, 194–211. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Lotti, N.; Siracusa, V.; Gazzano, M.; Munari, A. New multi-block copolyester of 2,5-furandicarboxylic acid containing PEG-like sequences to form flexible and degradable films for sustainable packaging. Polym. Degrad. Stab. 2019, 169, 108963. [Google Scholar] [CrossRef]
- Arandia, I.; Mugica, A.; Zubitur, M.; Arbe, A.; Liu, G.; Wang, D.; Mincheva, R.; Dubois, P.; Müller, A. How composition determines the properties of isodimorphic poly(butylene succinate-ran-butylene azelate) random biobased copolymers: From single to double crystalline random copolymers. Macromolecules 2015, 48, 43–57. [Google Scholar] [CrossRef]
- Pérez-Camargo, R.; Arandia, I.; Safari, M.; Cavallo, D.; Lotti, N.; Soccio, M.; Müller, A. Crystallization of isodimorphic aliphatic random copolyesters: Pseudo-eutectic behavior and double-crystalline materials. Eur. Polym. J. 2018, 101, 233–247. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(propylene 2,5-thiophenedicarboxylate) vs. poly(propylene 2,5-furandicarboxylate): Two examples of high gas barrier bio-based polyesters. Polymers 2018, 10, 785. [Google Scholar] [CrossRef]
- Wan, C.; Heeley, E.L.; Zhou, Y.; Wang, S.; Cafolla, C.T.; Crabb, E.M.; Hughes, D.J. Stress-oscillation behaviour of semi-crystalline polymers: The case of poly(butylene succinate). Soft Matter 2018, 14, 9175–9184. [Google Scholar] [CrossRef]







| Sample | ΦTDCA (mol%) | ΦHTh (mol%) | η (dL/g) |
|---|---|---|---|
| PHF | 0 | 0 | 0.63 |
| PHFTh10 | 10 | 10.0 | 0.74 |
| PHFTh30 | 30 | 28.2 | 0.76 |
| PHFTh50 | 50 | 49.8 | 0.69 |
| PHFTh70 | 70 | 69.9 | 0.74 |
| PHFTh90 | 90 | 89.3 | 0.70 |
| PHTh | 100 | 100 | 0.72 |
| Sample | Tm1 (°C) | ΔHm1 (J/g) | Tm2 (°C) | ΔHm2 (J/g) |
|---|---|---|---|---|
| PHF | 54.5 | 3.4 | 147.5 | 47.2 |
| PHFTh10 | 46.7 | 2.1 | 135.4 | 39.8 |
| PHFTh30 | 48.6 | 1.7 | 120.3 | 30.5 |
| PHFTh50 | 51.7 | 7.0 | 95.5 | 25.7 |
| PHFTh70 | 46.6 | 4.3 | 68.2 | 12.7 |
| PHFTh90 | 45.3 | 3.7 | 90.8 | 25.9 |
| PHTh | 48.1 | 2.3 | 98.5 | 36.3 |
| Sample | Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Td (°C) | Tmax (°C) |
|---|---|---|---|---|---|---|---|
| PHF | 12.3 | 109.0 | 59.6 | 147.7 | 66.0 | 374.6 | 393.6 |
| PHFTh10 | 9.7 | 86.5 | 31.1 | 136.1 | 32.7 | 380.5 | 403.8 |
| PHFTh30 | 6.4 | 59.4 | 19.5 | 121.5 | 25.1 | 380.2 | 408.4 |
| PHFTh50 | 4.2 | - | - | - | - | 382.1 | 410.0 |
| PHFTh70 | 3.3 | - | - | - | - | 383.2 | 409.0 |
| PHFTh90 | 1.1 | - | - | - | - | 385.0 | 408.1 |
| PHTh | −0.1 | - | - | - | - | 383.8 | 409.1 |
| Sample | Et (MPa) | σb (MPa) | εb (%) |
|---|---|---|---|
| PHF | 565.6 ± 23.1 | 30.3 ± 1.4 | 261.6 ± 8.3 |
| PHFTh10 | 558.5 ± 22.0 | 40.4 ± 1.4 | 448.7 ± 21.0 |
| PHFTh30 | 443.4 ± 16.4 | 31.8 ± 0.6 | 488.4 ± 23.4 |
| PHFTh50 | 208.5 ± 24.6 | 24.7 ± 1.4 | 481.1 ± 14.6 |
| PHFTh70 | 115.5 ± 3.8 | 27.7 ± 1.4 | 875.8 ± 31.1 |
| PHFTh90 | 167.3 ± 16.6 | 12.4 ± 0.9 | 498.9 ± 23.7 |
| PHTh | 248.1 ± 10.0 | 12.8 ± 1.0 | 435.9 ± 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Feng, S.; Qiu, Z. Synthesis, Thermal Behavior and Mechanical Property of Fully Biobased Poly(hexamethylene Furandicarboxylate-co-hexamethylene Thiophenedicarboxylate) Copolyesters. Polymers 2025, 17, 1997. https://doi.org/10.3390/polym17141997
Yang H, Feng S, Qiu Z. Synthesis, Thermal Behavior and Mechanical Property of Fully Biobased Poly(hexamethylene Furandicarboxylate-co-hexamethylene Thiophenedicarboxylate) Copolyesters. Polymers. 2025; 17(14):1997. https://doi.org/10.3390/polym17141997
Chicago/Turabian StyleYang, Haidong, Shiwei Feng, and Zhaobin Qiu. 2025. "Synthesis, Thermal Behavior and Mechanical Property of Fully Biobased Poly(hexamethylene Furandicarboxylate-co-hexamethylene Thiophenedicarboxylate) Copolyesters" Polymers 17, no. 14: 1997. https://doi.org/10.3390/polym17141997
APA StyleYang, H., Feng, S., & Qiu, Z. (2025). Synthesis, Thermal Behavior and Mechanical Property of Fully Biobased Poly(hexamethylene Furandicarboxylate-co-hexamethylene Thiophenedicarboxylate) Copolyesters. Polymers, 17(14), 1997. https://doi.org/10.3390/polym17141997








