Polyacrylamide-Based Solutions: A Comprehensive Review on Nanomaterial Integration, Supramolecular Design, and Sustainable Approaches for Integrated Reservoir Management
Abstract
1. Introduction
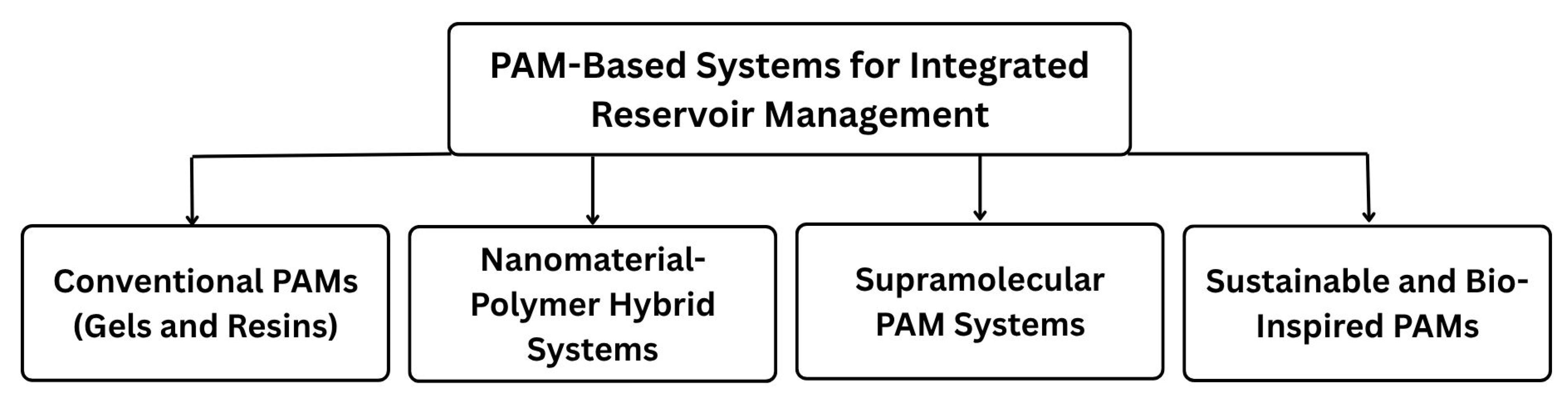
2. Chemistry and Applications of Key Polyacrylamide Derivatives
3. Historical Evolution of Polyacrylamide-Based Systems
4. Conventional PAM Gels for Conformance Control and Early Sand Stabilization
4.1. In Situ Monomer-Based Gels
4.2. In Situ Polymer-Based Gels
4.3. Preformed Particle Gels (PPG)
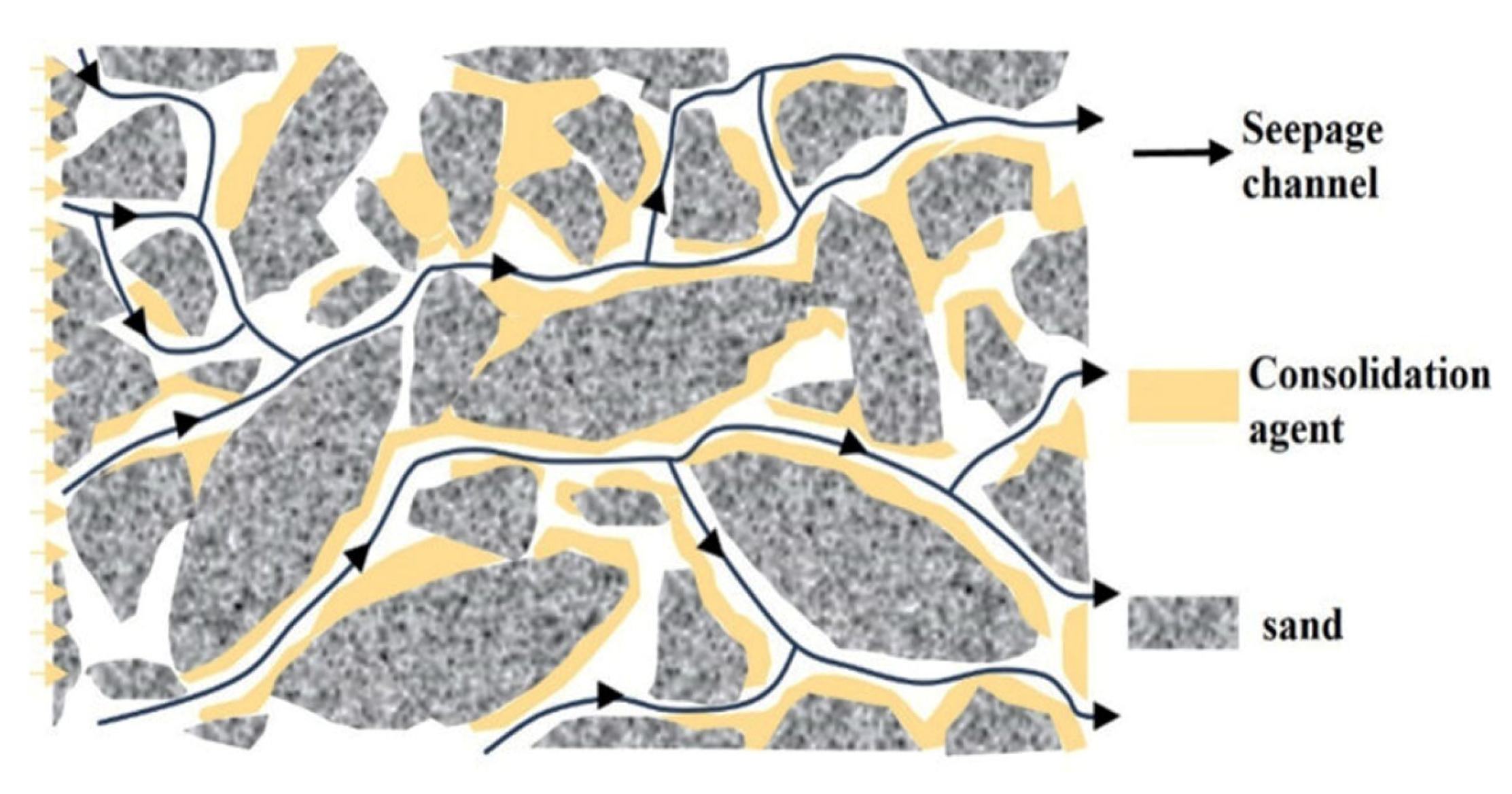
4.4. Early Chemical Sand Stabilization (Resins)
5. Nanoparticle-Enhanced PAM Systems
5.1. PAM–Nanoparticle Gels
5.2. Nanoparticle–Polymer-Surfactant (NPS) Formulations
5.3. Mechanisms of Nanoparticle Interaction and Contribution
6. Adaptive Polymer Networks for Harsh Environments: Supramolecular and Dynamic Covalent Approaches
6.1. Supramolecular PAMs Based on Physical Crosslinking
6.2. Dynamic Covalent PAMs Based on Reversible Chemical Bonds
7. Sustainable and Bio-Inspired Polyacrylamide-Based Systems
7.1. Bio-Inspired Materials and Composites for Enhanced Performance
7.2. Biodegradation of Polyacrylamide for Environmental Mitigation
7.3. PAM Gels in Biogas Purification: A Green Energy Application
8. Integrated Reservoir Management Strategies and Practical Considerations
8.1. Chemical–Mechanical Hybrid Sand Control
8.2. Damage Prevention and Remediation in Conformance Control
8.3. Monitoring and Prediction Technologies for Optimized Reservoir Management
9. Key Properties, Characterization Techniques, and Mechanistic Insights
10. Challenges and Future Outlook
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| cp | Centipoise (unit of dynamic viscosity) |
| mL | Milliliter (unit of volume) |
| g | Gram (unit of mass) |
| mPa·s | Millipascal-second (unit of dynamic viscosity) |
| G′ | Storage modulus (unit: Pascal, Pa) |
| mN/m | Millinewtons per meter (unit of surface tension) |
| G″ | Loss modulus (unit: Pascal, Pa) |
| nm | Nanometer (unit of length) |
| J/mol | Joules per mole (unit of energy) |
| Pa | Pascal (unit of pressure/stress) |
| K | Equilibrium constant (dimensionless) |
| ppm | Parts per million (unit of concentration) |
| kPa | Kilopascal (unit of pressure) |
| psi | Pounds per square inch (unit of pressure) |
| Mw | Weight-average molecular weight |
| PV | Pore volume (dimensionless) |
| Mn | Number-average molecular weight |
| s−1 | Reciprocal seconds (unit of shear rate/frequency) |
| mD | Millidarcy (unit of permeability) |
| wt% | Weight percent (unit of concentration) |
| mg/kg | Milligrams per kilogram (unit of concentration) |
| % | Percent (dimensionless) |
| mg/L | Milligrams per liter (unit of concentration) |
| µm | Micrometer (unit of length) |
| Acronyms | |
| AI | Artificial intelligence |
| ML | Machine learning |
| A-PAM | Anionic polyacrylamide |
| mMWCNT | Modified multiwalled carbon nanotubes |
| ASL-PVA | Amphoteric lignin-based poly(vinyl alcohol) |
| NMR | Nuclear magnetic resonance |
| ATBS | Acrylamido-tertiary-butyl sulfonic acid |
| NPs | Nanoparticles |
| CDG | Colloidal dispersion gels |
| OOIP | Original oil in place |
| CDs | Cyclodextrins |
| PAM | Polyacrylamide |
| CFD | Computational fluid dynamics |
| PEI | Polyethyleneimine |
| CT | Computed tomography |
| PDI | Polydispersity index |
| DEM | Discrete element method |
| PPG | Preformed particle gels |
| DPR | Disproportionate permeability reduction |
| S-S | Disulfide bonds |
| EOR | Enhanced oil recovery |
| SGP | Squeeze gravel pack |
| FTIR | Fourier-transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| HPAM | Partially hydrolyzed polyacrylamide |
| SP | Surfactant–polymer |
| HQ | Hydroquinone |
| SMRF | Sulfomethylated resorcinol–formaldehyde |
| HMTA | Hexamethylenetetramine |
| TGA | Thermogravimetric analysis |
| IFT | Interfacial tension |
| TDS | Total dissolved solids |
| L-cPAM | Lignin-crosslinked polyacrylamide |
| TEM | Transmission electron microscopy |
| LSW | Low-salinity water |
| XRD | X-ray diffraction |
References
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Zuo, C.; Liu, P.; Du, J.; Wu, G.; Chen, X.; Liu, J. A review: Supramolecular polymers used in high-temperature fracturing fluids research status and prospects on the coupling relationship between molecular conformation and temperature resistance mechanism. Geoenergy Sci. Eng. 2025, 247, 213699. [Google Scholar] [CrossRef]
- Muhammad, M.; Rasol, A.A.A. Advances and challenges of sand production and control in oilfields: A review. Results Eng. 2025, 25, 104596. [Google Scholar] [CrossRef]
- Salem, K.G.; Tantawy, M.A.; Gawish, A.A.; Salem, A.M.; Gomaa, S.; El-hoshoudy, A.N. Key aspects of polymeric nanofluids as a new enhanced oil recovery approach: A comprehensive review. Fuel 2024, 368, 131515. [Google Scholar] [CrossRef]
- Li, J.; Usman, M.; Arslan, M.; Gamal El-Din, M. Molecular and microbial insights towards anaerobic biodegradation of anionic polyacrylamide in oil sands tailings. Water Res. 2024, 258, 121757. [Google Scholar] [CrossRef]
- Mörtl, M. Methods of Diverting Water Based Resins for Sand Consolidation; Mining University of Leoben: Leoben, Austria, 2009. [Google Scholar]
- Saghandali, F.; Salehi, M.B.; Hosseinzadehsemnani, R.; Moghanloo, R.G.; Taghikhani, V. A Review on Chemical Sand Production Control Techniques in Oil Reservoirs. Energy Fuels 2022, 36, 5185–5208. [Google Scholar] [CrossRef]
- Song, Y.; Dong, C.; He, H.; Peng, Z.; Zhan, X.; Bai, H. Experimental evaluation of chemical sand stabilization and its optimization of composite sand control with squeeze gravel pack. Geoenergy Sci. Eng. 2024, 237, 212804. [Google Scholar] [CrossRef]
- Suleimanov, B.A.; Abbasov, H.F.; Ismailov, S.Z. A comprehensive review on sand control in oil and gas wells. Part II. Chemical treatment and sand management. SOCAR Proc. 2024, 4, 27–41. [Google Scholar] [CrossRef]
- De Morais, S.C.; Marques, N.D.N.; da Câmara, P.C.F.; de Souza, E.A.; de Carvalho Balaban, R. Versatile hydrolyzed polyacrylamide-polyethyleneimine gels reinforced with glycerol for plugging and abandonment of oil wells. J. Mol. Liq. 2023, 388, 122748. [Google Scholar] [CrossRef]
- Kalof, A.N.; Evans, M.F.; Dantey, K.; Cooper, K. Special Diagnostic Techniques in Surgical Pathology. In Gattuso’s Differential Diagnosis in Surgical Pathology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–40. [Google Scholar] [CrossRef]
- Doble, M.; Kumar, A. Degradation of Polymers. In Biotreatment of Industrial Effluents; Elsevier: Amsterdam, The Netherlands, 2005; pp. 101–110. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Recent Insights in Acrylamide as Carcinogen in Foodstuffs. Adv. Mol. Toxicol. 2012, 6, 163–193. [Google Scholar] [CrossRef]
- Mohyaldinn, M.E.; Solomon, E.B.L.; Mohamed, M.H.; Alakbari, F.S.; Ayoub, M.A. A polymer-crosslinker-nanoparticles formulation for effective sand consolidation in loose sandstone formations. J. Pet. Explor. Prod. Technol. 2025, 15, 125. [Google Scholar] [CrossRef]
- Bajpai, P. Papermaking Chemistry. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 207–236. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Usama, M.; Bal, D.K. Effectiveness of polyacrylamide gel formulation for CO2 and H2S mitigation in biogas purification. Fuel 2025, 401, 135856. [Google Scholar] [CrossRef]
- Beteta, A.; Nurmi, L.; Rosati, L.; Hanski, S.; McIver, K.; Sorbie, K.; Toivonen, S. Polymer Chemical Structure and its Impact on EOR Performance. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 18–22 April 2020. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Wang, X.; Wang, X.; Zheng, L.; Wen, J.; Yang, H.; Zhang, H. Modulation of syneresis rate and gel strength of PAM-PEI gels by nanosheets and their mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135525. [Google Scholar] [CrossRef]
- Xu, B.; Hou, Y.; Yang, Z.; Fang, J.; Wu, X.; Qi, J.; Li, H. Management of saline-alkali sandy soils by amphoteric lignin-based sand fixation. Int. J. Biol. Macromol. 2025, 311, 144018. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Zhang, J.; Zhang, T.; Gao, Y.-Q.; Guo, S.; Yang, Y.-L.; Lu, J.-M. Damage mechanism analysis of polymer gel to petroleum reservoirs and development of new protective methods based on NMR technique. Pet. Sci. 2025, 22, 1225–1233. [Google Scholar] [CrossRef]
- Lew, J.H.; Matar, O.K.; Müller, E.A.; Maung, M.T.M.; Luckham, P.F. Adsorption of Hydrolysed Polyacrylamide onto Calcium Carbonate. Polymers 2022, 14, 405. [Google Scholar] [CrossRef] [PubMed]
- Marandi, S.Z.; Salehi, M.B.; Moghadam, A.M. Sand control: Experimental performance of polyacrylamide hydrogels. J. Pet. Sci. Eng. 2018, 170, 430–439. [Google Scholar] [CrossRef]
- Pandey, A.; Qamar, S.F.; Das, S.; Basu, S.; Kesarwani, H.; Saxena, A.; Sharma, S.; Sarkar, J. Advanced multi-wall carbon nanotube-optimized surfactant-polymer flooding for enhanced oil recovery. Fuel 2024, 355, 129463. [Google Scholar] [CrossRef]
- Saw, R.K.; Singh, A.; Maurya, N.K.; Mandal, A. A mechanistic study of low salinity water-based nanoparticle-polymer complex fluid for improved oil recovery in sandstone reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131308. [Google Scholar] [CrossRef]
- Soleimani, M.; Abdalisousan, A.; KhaksarManshad, A.; Sajadiyan, V.A. Synthesis, characterization, and mechanistic study of a new highly-stable comb-like polymeric surfactant in enhanced oil recovery. Geoenergy Sci. Eng. 2024, 234, 212542. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- El-Karsani, K.S.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer Systems for Water Shutoff and Profile Modification: A Review Over the Last Decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Seright, R.S.; Wang, D. Polymer flooding: Current status and future directions. Pet. Sci. 2023, 20, 730–921. [Google Scholar] [CrossRef]
- Uranta, K.G.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the Effectiveness of Polyacrylamide (PAM) Application in Hydrocarbon Reservoirs at Different Operational Conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef]
- JOUENNE, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Seright, R.S.; Campbell, A.R.; Mozley, P.S.; Han, P. Stability of Partially Hydrolyzed Polyacrylamides at Elevated Temperatures in the Absence of Divalent Cations. SPE J. 2010, 15, 341–348. [Google Scholar] [CrossRef]
- Ji, K.; Jia, W.; He, G.; Chen, G.; Yu, L. Rheological behavior of hydrophobic association polyacrylamides with high salt resistance and corrosion inhibition: Cross-linking modification based on trace dopamine hydrochloride derivatives. J. Mol. Liq. 2024, 400, 124520. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Jiang, H.; Bi, W.; Pan, S.; Li, H.; Sarsenbekuly, B.; Kang, W.; Zhang, X. Study on the rheological properties and salt resistance mechanism of an amphiphilic polymer with twin-tailed group. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134748. [Google Scholar] [CrossRef]
- Mao, H.; Liu, J.; Zhang, W.; Qiu, W.; Zhang, Q.; Wang, J.; Zhang, D.; Shao, Z. Self-Soluble Hydrophobically Associating Polyacrylamide Inverse Emulsions with Extraordinary Thickening Properties. ACS Appl. Polym. Mater. 2024, 6, 3748–3755. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Lecourtier, J.; Chauveteau, G.; Muller, G. Salt-induced extension and dissociation of a native double-stranded xanthan. Int. J. Biol. Macromol. 1986, 8, 306–310. [Google Scholar] [CrossRef]
- Peng, C.; Gou, S.; Wu, Q.; Zhou, L.; Zhang, H.; Fei, Y. Modified acrylamide copolymers based on β-cyclodextrin and twin-tail structures for enhanced oil recovery through host–guest interactions. New J. Chem. 2019, 43, 5363–5373. [Google Scholar] [CrossRef]
- Taylor, K.C.; Nasr-El-Din, H.A. Water-soluble hydrophobically associating polymers for improved oil recovery: A literature review. J. Pet. Sci. Eng. 1998, 19, 265–280. [Google Scholar] [CrossRef]
- Pye, D.J. Improved Secondary Recovery by Control of Water Mobility. J. Pet. Technol. 1964, 16, 911–916. [Google Scholar] [CrossRef]
- Sandiford, B.B. Laboratory and Field Studies of Water Floods Using Polymer Solutions to Increase Oil Recoveries. J. Pet. Technol. 1964, 16, 917–922. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef]
- Wang, D.; Dong, H.; Lv, C.; Fu, X.; Nie, J. Review of Practical Experience by Polymer Flooding at Daqing. SPE Reserv. Eval. Eng. 2009, 12, 470–476. [Google Scholar] [CrossRef]
- Sydansk, R.D. A New Conformance-Improvement-Treatment Chromium(III) Gel Technology. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Society of Petroleum Engineers, Tulsa, Oklahoma, 16–21 April 1988. [Google Scholar] [CrossRef]
- Levitt, D.B.; Pope, G.A. Selection and Screening of Polymers for Enhanced-Oil Recovery. In SPE Symposium on Improved Oil Recovery; SPE: Tulsa, OK, USA, 2008. [Google Scholar] [CrossRef]
- Gaillard, N.; Giovannetti, B.; Favero, C. Improved Oil Recovery using Thermally and Chemically Protected Compositions Based on co- and ter-polymers Containing Acrylamide. In SPE Improved Oil Recovery Symposium; SPE: Tulsa, OK, USA, 2010. [Google Scholar] [CrossRef]
- Haruna, M.A.; Gardy, J.; Yao, G.; Hu, Z.; Hondow, N.; Wen, D. Nanoparticle modified polyacrylamide for enhanced oil recovery at harsh conditions. Fuel 2020, 268, 117186. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Huo, X.; Liu, X. Bioinspired Self-Assembly Polymer Based on Nucleobase for Enhanced Oil Recovery. J. Polym. Environ. 2024, 32, 5406–5421. [Google Scholar] [CrossRef]
- Yan, D.; Lai, L.; Xiao, X.; Zhang, L.; Zhao, Z.; Zhao, J. Water Consolidation Performance of Acrylic-Polymer-Modified Materials and Their Concrete Impermeability Repair Characteristics. Gels 2023, 9, 764. [Google Scholar] [CrossRef]
- Needham, R.B.; Threlkeld, C.B.; Gall, J.W. Control of Water Mobility Using Polymers and Multivalent Cations. In SPE Improved Oil Recovery Symposium; SPE: Tulsa, OK, USA, 1974. [Google Scholar] [CrossRef]
- Sydansk, R.D. Acrylamide-Polymer/Chromium(III)-Carboxylate Gels for Near Wellbore Matrix Treatments. SPE Adv. Technol. Ser. 1993, 1, 146–152. [Google Scholar] [CrossRef]
- Moradi-Aragbx, A.; Bjornson, G.; Doe, P.H. Thermally Stable Gels for Near-Wellbore Permeability Contrast Corrections. SPE Adv. Technol. Ser. 1993, 1, 140–145. [Google Scholar] [CrossRef]
- Yegane, M.M.; Boukany, P.E.; Zitha, P. Fundamentals and Recent Progress in the Flow of Water-Soluble Polymers in Porous Media for Enhanced Oil Recovery. Energies 2022, 15, 8575. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for Enhanced Oil Recovery Technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef]
- Mack, J.C.; Smith, J.E. In-Depth Colloidal Dispersion Gels Improve Oil Recovery Efficiency. In SPE/DOE Improved Oil Recovery Symposium; SPE: Tulsa, OK, USA, 1994. [Google Scholar] [CrossRef]
- Ding, F.; Dai, C.; Sun, Y.; Zhao, G.; You, Q.; Liu, Y. Gelling Behavior of PAM/Phenolic Crosslinked Gel and Its Profile Control in a Low-Temperature and High-Salinity Reservoir. Gels 2022, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, K.L.N.P.; de Oliveira, P.F.; Mansur, C.R.E. A comprehensive review of in situ polymer hydrogels for conformance control of oil reservoirs. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2020, 75, 8. [Google Scholar] [CrossRef]
- Zhuang, Y.; Pandey, S.N.; McCool, C.S.; Willhite, G.P. Permeability Modification With Sulfomethylated Resorcinol-Formaldehyde Gel System. SPE Reserv. Eval. Eng. 2000, 3, 386–393. [Google Scholar] [CrossRef]
- Du, J.; Huang, Q.; Liu, P.; Fu, Y.; Liu, J.; Chen, X.; Yuan, Y. Review of Recent Advances in Chemical Sand Production Control: Materials, Methods, Mechanisms, and Future Perspectives. Energy Fuels 2024, 38, 21845–21872. [Google Scholar] [CrossRef]
- Wang, K.; Wen, J.; Zhang, S.; Yang, L.; Yang, H.; Yu, X.; Zhang, H. Magnetic polyacrylamide-based gel with tunable structure and properties and its significance in conformance control of oil reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135093. [Google Scholar] [CrossRef]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef]
- Fouji, M.; Sheng, J.J.; Ganat, T.; Esmaeilnezhad, E. A performance evaluation of magnetic nanoparticle, biosurfactant, and polymer fluids for enhanced oil recovery under magnetic field stimulation. J. Mol. Liq. 2025, 431, 127823. [Google Scholar] [CrossRef]
- Kalgaonkar, R.; Alnoaimi, K.; Wagle, V. Novel Nanoparticle Based Applications in Oilfield: From Sand Consolidation to Water Shutoff. In Proceedings of the SPE Gas & Oil Technology Showcase and Conference, Tulsa, OK, USA, 21–23 October 2019. [Google Scholar] [CrossRef]
- Tanasa, E.; Zaharia, C.; Radu, I.C.; Surdu, V.A.; Vasile, B.S.; Damian, C.M.; Andronescu, E. Novel Nanocomposites Based on Functionalized Magnetic Nanoparticles and Polyacrylamide: Preparation and Complex Characterization. Nanomaterials 2019, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Cheraghian, G.; Nezhad, S.S.K.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Effect of nanoclay on improved rheology properties of polyacrylamide solutions used in enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2015, 5, 189–196. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano. Lett. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability Alteration of Sandstone Cores by Alumina-Based Nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Li, X.; Fu, M.; Hu, J. Preparation and Performance Evaluation of Temperature-Resistant and Salt-Resistant Gels. Gels 2024, 10, 337. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; El-Frarrge, A.F.M.; Ismail, D.A.; Abd-Elaal, A.A. Enhancement of the Surface Activity for Some Monomeric and Polymeric Thiol Surfactants Using Silver Nanoparticles. J. Dispers. Sci. Technol. 2011, 32, 816–821. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Wu, C.; Zhao, H. Surface-Active Gold Nanoparticles with Mixed Polymer Brushes as Surfactants in the Preparation of Polystyrene Colloid Particles. Macromol. Rapid Commun. 2008, 29, 45–51. [Google Scholar] [CrossRef]
- Zell, Z.A.; Isa, L.; Ilg, P.; Leal, L.G.; Squires, T.M. Adsorption Energies of Poly(ethylene oxide)-Based Surfactants and Nanoparticles on an Air–Water Surface. Langmuir 2014, 30, 110–119. [Google Scholar] [CrossRef]
- Abramenko, N.; Demidova, T.B.; Krutyakov, Y.A.; Zherebin, P.M.; Krysanov, E.Y.; Kustov, L.M.; Peijnenburg, W.J.G.M. The effect of capping agents on the toxicity of silver nanoparticles to Danio rerio embryos. Nanotoxicology 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Blkoor, S.O.; Norddin, M.N.A.M.; Ismail, I.; Oseh, J.O.; Risal, A.R.; Basaleh, S.S.; Mohamed, M.H.; Duru, U.I.; Ngouangna, E.N.; Yahya, M.N. Enhanced cutting transport performance of water-based drilling muds using polyethylene glycol/nanosilica composites modified by sodium dodecyl sulphate. Geoenergy Sci. Eng. 2023, 230, 212276. [Google Scholar] [CrossRef]
- Oseh, J.O.; Blkoor, S.O.; Norddin, M.N.A.M.; Ismail, I.; Gbadamosi, A.O.; Agi, A.; Risal, A.R.; Mohamed, M.H. Modification and Performance Evaluation of the Viscosity of Water-Based Mud at Different Hole Angles for Cuttings Transport Using Sodium Dodecyl Sulfate Modified Polyethylene Glycol-Nanosilica Composite. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Lew, J.H.; Matar, O.K.; Müller, E.A.; Luckham, P.F.; Santos, A.S.; Myo Thant, M.M. Atomic Force Microscopy of Hydrolysed Polyacrylamide Adsorption onto Calcium Carbonate. Polymers 2023, 15, 4037. [Google Scholar] [CrossRef] [PubMed]
- Hue, K.Y.; Lew, J.H.; Matar, O.K.; Luckham, P.F.; Müller, E.A. Parametric Studies of Polyacrylamide Adsorption on Calcite Using Molecular Dynamics Simulation. Molecules 2025, 30, 285. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.H.S.; Moreno, R.B.Z.L. Polyacrylamide Adsorption and Readsorption in Sandstone Porous Media. SPE J. 2020, 25, 497–514. [Google Scholar] [CrossRef]
- Tabar, M.A.; Bagherzadeh, H.; Shahrabadi, A.; Dahim, S. A comprehensive research in chemical consolidator/stabilizer agents on sand production control. J. Pet. Explor. Prod. Technol. 2021, 11, 4305–4324. [Google Scholar] [CrossRef]
- Cheraghian, G. Effects of nanoparticles on wettability: A review on applications of nanotechnology in the enhanced Oil recovery. Int. J. Nano Dimens. 2015, 6, 443–452. [Google Scholar]
- Lehn, J.-M. Toward Self-Organization and Complex Matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro. Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Otsuka, H. Reorganization of polymer structures based on dynamic covalent chemistry: Polymer reactions by dynamic covalent exchanges of alkoxyamine units. Polym. J. 2013, 45, 879–891. [Google Scholar] [CrossRef]
- Han, Y.; Yu, Z.; Guan, Z.; Ma, R.; Hu, Y. Hydrophobic association supramolecular gel suitable for oil and gas drilling in fractured formation. Front. Chem. 2024, 12, 1468766. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, X.; Dai, C.; Wu, Y.; Li, L.; Yuan, B. A Supramolecular Reinforced Gel Fracturing Fluid with Low Permeability Damage Applied in Deep Reservoir Hydraulic Fracturing. Gels 2023, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Fan, S.; Chen, X.; He, Z.; Dai, L.; Wen, Z.; Li, M. Preparation and Performance Evaluation of a Supramolecular Polymer Gel-Based Temporary Plugging Agent for Heavy Oil Reservoir. Gels 2024, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Strandman, S.; Zhu, X.X. Self-Healing Supramolecular Hydrogels Based on Reversible Physical Interactions. Gels 2016, 2, 16. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634. [Google Scholar] [CrossRef]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Yang, Z.; Wang, P.; Liu, J.; Xie, Y.; Wu, Y.; Gao, M.; Li, L.; Song, X.; et al. Novel Mussel-Inspired High-Temperature Resistant Gel with Delayed Crosslinking Property for Ultra-Deep Reservoir Fracturing. Adv. Funct. Mater. 2024, 34, 2405111. [Google Scholar] [CrossRef]
- Yang, J.B.; Sun, J.S.; Bai, Y.R.; Lv, K.H.; Li, J.; Li, M.C.; Zhu, Y.C. Preparation and characterization of supramolecular gel suitable for fractured formations. Pet. Sci. 2023, 20, 2324–2342. [Google Scholar] [CrossRef]
- Tian, J.; Mao, J.; Zhang, W.; Yang, X.; Lin, C.; Cun, M.; Mao, J.; Zhao, J. Application of a Zwitterionic Hydrophobic Associating Polymer with High Salt and Heat Tolerance in Brine-Based Fracturing Fluid. Polymers 2019, 11, 2005. [Google Scholar] [CrossRef]
- Zhou, S.; Qi, N.; Zhang, Z.; Jiang, P.; Li, A.; Lu, Y.; Su, X. Recent progress in intrinsic self-healing polymer materials: Mechanisms, challenges and potential applications in oil and gas development. Chem. Eng. J. 2025, 511, 161906. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, J.; Huang, W.; Yuan, Z.; Wei, B.; Wen, Y. Preparation of crosslinked lignin-polyacrylamide hydrogel with high resistance to temperature and salinity. Int. J. Biol. Macromol. 2025, 296, 139730. [Google Scholar] [CrossRef] [PubMed]
- Mulu, E.; M’Arimi, M.M.; Rose, R.C. Purification and upgrade of biogas using biomass-derived adsorbents: Review. In Advances in Phytochemistry, Textile and Renewable Energy Research for Industrial Growth; CRC Press: Boca Raton, FL, USA, 2022; pp. 286–295. [Google Scholar] [CrossRef]
- Alakbari, F.S.; Mohyaldinn, M.E.; Muhsan, A.S.; Hasan, N.; Ganat, T. Chemical Sand Consolidation: From Polymers to Nanoparticles. Polymers 2020, 12, 1069. [Google Scholar] [CrossRef]
- Mishra, S.; Ojha, K. Nanoparticle induced chemical system for consolidating loosely bound sand formations in oil fields. J. Pet. Sci. Eng. 2016, 147, 15–23. [Google Scholar] [CrossRef]
- Shabdirova, A.; Kozhagulova, A.; Samenov, Y.; Minh, N.; Zhao, Y. Sand Production Prediction with Machine Learning using Input Variables from Geological and Operational Conditions in the Karazhanbas Oilfield, Kazakhstan. Nat. Resour. Res. 2024, 33, 2789–2805. [Google Scholar] [CrossRef]
- Hue, K.Y.; Damasceno, D.A.; Maung, M.T.M.; Luckham, P.F.; Matar, O.K.; Müller, E.A. Atomistic molecular dynamics simulations of the tensile strength properties of polymer-calcite systems. Comput. Mater. Sci. 2025, 253, 113866. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, P.; Li, Z.; Fan, C.; Wen, J.; Yu, Y.; Jia, L. Efficient removal of gaseous elemental mercury by Fe-UiO-66@BC composite adsorbent: Performance evaluation and mechanistic elucidation. Sep. Purif. Technol. 2025, 372, 133463. [Google Scholar] [CrossRef]







| Derivative | Key Functional Group(s) | Characteristic Properties | Applications | References |
|---|---|---|---|---|
| Non-Ionic Polyacrylamide (Parent Polymer) NPAM | Amide (-CONH2) |
|
| [35,36] |
| Anionic/Partially Hydrolyzed Polyacrylamide APAM/HPAM | Carboxylate (-COO−Na+) Amide (-CONH2) |
|
| [1,26,28] |
| Cationic Polyacrylamide CPAM | Quaternary ammonium (-N+R3Cl−) Amide (-CONH2) |
|
| [37] |
| Hydrophobically Modified Polyacrylamide HMPAM/HAPAM | Hydrophobic groups (e.g., CₙH2ₙ₊1) Anionic/non-ionic backbone |
|
| [32,34,38] |
| Sulfonated Polyacrylamide (AM/AMPS Copolymer) | Sulfonate (-SO3−) Amide (-CONH2) |
|
| [17,30,31] |
| Author and Year | Material | Case Study/Innovation | Technical Details and Key Findings | Application |
|---|---|---|---|---|
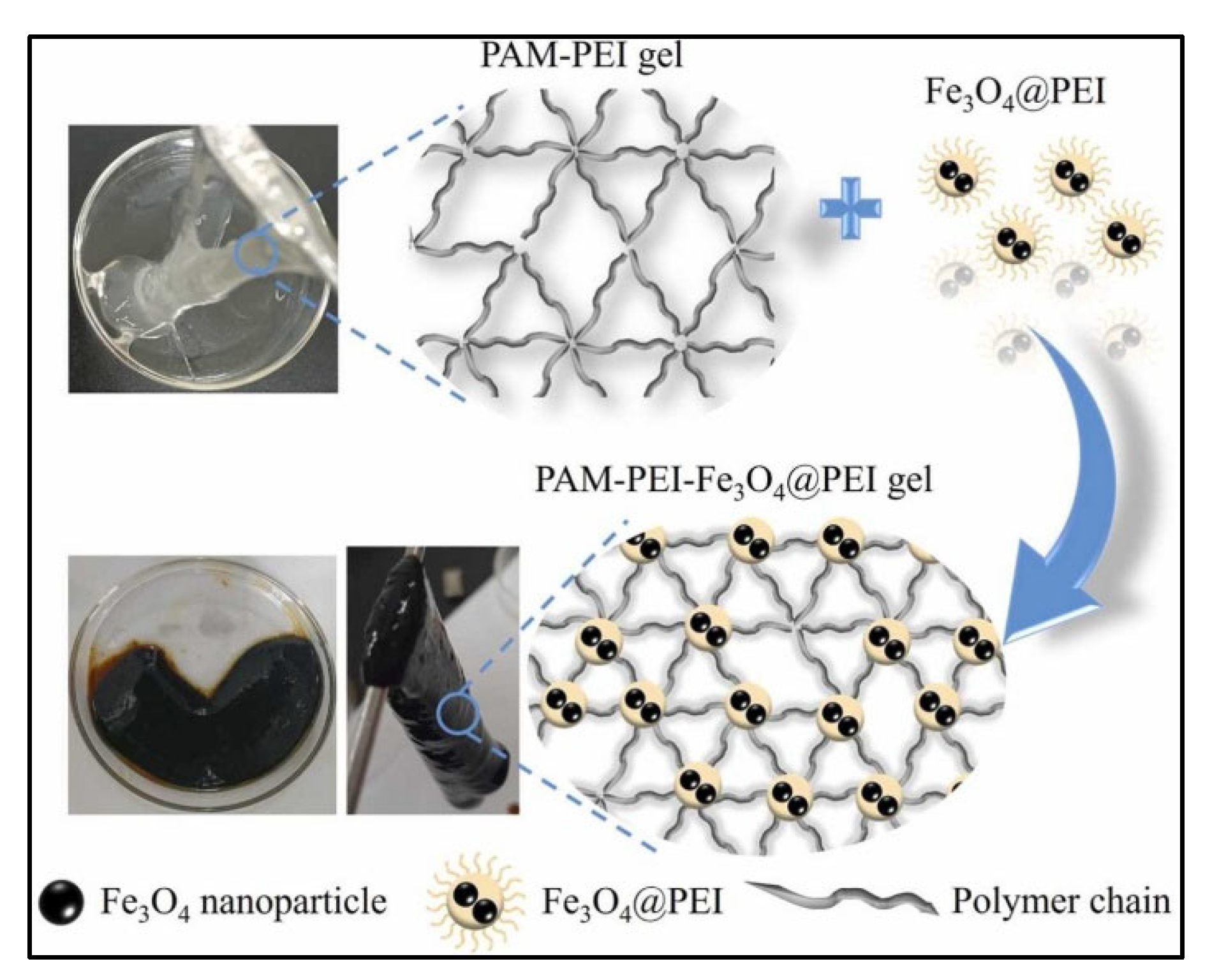
| [59] | Fe3O4@PEI/PAM Smart Polymer Gel | Magnetic smart polymer gel: core–shell architecture enabling magnetic controllability and robust crosslinking. | The system, with NP size of 3.83 nm and density of 2.98 g/cm3, remained solid at 120 °C. Its mechanical qualities included 25,490 Pa storage and 7610 Pa loss modulus. After 1 month at 120 °C, it obtained 126,780 mPa·s gelation strength, 8.56 MPa bearing capacity, and 97.83% plugging efficiency in core displacement trials, increasing water flow fractional enhancement from 6.3% to 97.2%. | Magnetically controllable directional plugging for conformance control in high-temperature oil reservoirs; breakthrough in intelligent reservoir management. |
| [63] | PAM + Functionalized Magnetite Nanoparticles (Fe3O4) | Polyacrylamide–magnetite nanocomposite hydrogels: covalent integration of functionalized NPs as inorganic crosslinkers for enhanced mechanical properties. | Microstructural examination (SEM, HR-TEM) revealed the homogeneous distribution of 5–14 nm spherical Fe3O4 NPs, producing a hybrid organic–inorganic network. This improved mechanical qualities over traditional polymer gels. | Soft tissue engineering and biomedical scaffolds (potential for EOR application via similar strengthening principles). |
| [67] | AM/AMPS Co-Polymer Gels Crosslinked with PEI + Nylon Fiber | Temperature-resistant gel systems: development of robust gels for extreme high-temperature and high-salinity conditions. | Optimized with 1.0% AM/AMPS polymer, 0.1% PEI crosslinker, and 0.5% nylon fiber, these gels achieved H-level strength and excellent thermal stability. Long-term tests showed a syneresis rate < 2.5% (after 120 days at 240,720 mg/L salinity) and stability up to 130 °C. Demonstrated 94% plugging efficiency in sand-filled pipe experiments. | Enhanced oil recovery in harsh (high-temperature/high-salinity) environments. |
| [59] | PEI@Fe3O4@PEI Nanosheets in PAM-PEI Gel Networks | Magnetic nanoparticle-enhanced gel stability: modulating syneresis rate and gel strength through magnetic nanoparticle chelation. | Incorporating PEI@Fe3O4@PEI nanosheets, the gels showed enhanced thermal stability, with decomposition temperature increased to 198.45 °C, and improved weight retention by 25.85% compared to conventional systems. Magnetic responsiveness enabled directional control. | High-performance polymer gels with improved stability and responsiveness. |
| Class | Interaction Type | Mechanism of Enhancement | Comparative Performance vs. Conventional PAM | Experimental Validation |
|---|---|---|---|---|
| Supramolecular (Physical Cross-Links) | Hydrophobic Association and Hydrogen Bonding | Aggregation of nonpolar segments and H-bonds form a robust, energy-dissipating physical network. | Provides high mechanical toughness and thermal stability, whereas conventional gels are brittle and thermally unstable. |
|
| Electrostatic Interactions | Coulombic forces between oppositely charged polymer chains form a dynamic, physically cross-linked network. | Achieves higher viscosity at lower concentrations and exhibits superior shear resilience compared to conventional PAMs, which require higher concentrations and show poorer recovery after shear. | 0.4 wt% supramolecular system achieves viscosity of a 0.6 wt% conventional polymer; retains 73.3% viscosity after shear vs. 53.5% for conventional [83]. | |
| Host–Guest Recognition | Cyclodextrins (hosts) encapsulate hydrophobic guests, forming highly stable, rapidly reversible inclusion complexes. | Imparts exceptional thermal stability and rapid, autonomous self-healing, in contrast to conventional hydrogels which are not self-healing, mechanically weak (G′ ≈ 1–10 kPa), and degrade >90 °C. |
| |
| Dynamic Covalent (Reversible Chemical Bonds) | Dynamic Covalent Bonds | Reversible cleavage and reformation of covalent bonds (e.g., catechol chemistry) in response to a specific stimulus. | Achieves extreme thermal stability and stimulus-gated self-healing, overcoming the irreversible molecular scission and complete loss of function seen in conventional PAMs under high stress. | Mussel-inspired gels maintain robust mechanical performance and self-healing capabilities at temperatures up to 200 °C [88]. Self-healing efficiency of transesterification vitrimers: >80% [91]. |
| Supramolecular Reinforced Chemical Gel | Supramolecular + Covalent Cross-Links | Supramolecular forces provide initial viscosity and shear resistance, followed by chemical crosslinking. | Enables ultra-high temperature resistance at significantly lower polymer and cross-linker concentrations than conventional gels, which require much higher loadings for similar performance. | Final viscosity of 72.35 mPa·s after 2 h at 200 °C using only 0.4 wt% polymer [83]. |
| Characterization Technique | Principle/Measured Property | Key Insight Gained for PAM Systems |
|---|---|---|
| Rheometry | Viscosity, storage/loss moduli (G′/G″), Tan Delta | Fluid injectivity and mobility control; viscoelasticity (elasticity, syneresis prevention). |
| Thermogravimetric Analysis (TGA) | Mass change with temperature | Polymer thermal stability; degradation onset/profiles; compositional analysis. |
| Nuclear Magnetic Resonance (NMR) T2 Spectroscopy | Relaxation time of fluids | Fluid distribution and flow paths; gel/polymer degradation; residual oil mobilization. |
| X-Ray-Computed Tomography (CT Scan) | 3D internal structure, X-ray absorption differences | Gel/NP placement and plugging; 3D fluid/material distribution; magnetic responsiveness. |
| Scanning Electron Microscopy (SEM) | Surface morphology, microstructure | Micro-scale material structure; inter-particle interactions. |
| Transmission Electron Microscopy (TEM) | Nanostructure/morphology (internal) | Nanoparticle morphology; core–shell structures. |
| Dynamic Light Scattering (DLS) | Particle size, hydrodynamic diameter | Dispersion stability; aggregation behavior. |
| X-Ray Diffraction (XRD) | Crystallinity, chemical phases | Material composition; network formation confirmation. |
| Fourier-Transform Infrared (FTIR) Spectroscopy | Functional groups, molecular interactions | Molecular bonds; functional groups; reaction confirmation. |
| Zeta Potential | Surface charge, colloidal stability | Surface charge; colloidal stability; adsorption behavior. |
| Contact Angle Goniometry | Wetting angle | Rock wettability alteration; fluid–surface affinity. |
| Interfacial Tensiometry (IFT Tensiometry) | Interfacial tension (IFT) | IFT reduction efficacy; oil mobilization. |
| UV-Vis Spectrophotometry | Light absorption by chemical species | Chemical concentration quantification. |
| Molecular Dynamics (MD) Simulations | Atomistic-level modeling of interactions | Atomistic-level material properties; interfacial mechanisms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.H.; Mohyaldinn, M.E. Polyacrylamide-Based Solutions: A Comprehensive Review on Nanomaterial Integration, Supramolecular Design, and Sustainable Approaches for Integrated Reservoir Management. Polymers 2025, 17, 2202. https://doi.org/10.3390/polym17162202
Mohamed MH, Mohyaldinn ME. Polyacrylamide-Based Solutions: A Comprehensive Review on Nanomaterial Integration, Supramolecular Design, and Sustainable Approaches for Integrated Reservoir Management. Polymers. 2025; 17(16):2202. https://doi.org/10.3390/polym17162202
Chicago/Turabian StyleMohamed, Moamen Hassan, and Mysara Eissa Mohyaldinn. 2025. "Polyacrylamide-Based Solutions: A Comprehensive Review on Nanomaterial Integration, Supramolecular Design, and Sustainable Approaches for Integrated Reservoir Management" Polymers 17, no. 16: 2202. https://doi.org/10.3390/polym17162202
APA StyleMohamed, M. H., & Mohyaldinn, M. E. (2025). Polyacrylamide-Based Solutions: A Comprehensive Review on Nanomaterial Integration, Supramolecular Design, and Sustainable Approaches for Integrated Reservoir Management. Polymers, 17(16), 2202. https://doi.org/10.3390/polym17162202





