Preparation of Barium Europium Phosphate and Its Performance in Acrylic Resin Anti-Corrosion Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ba3Eu(PO4)3
2.3. Preparation of Anti-Corrosion Coating
2.4. Characterization
3. Results and Discussion
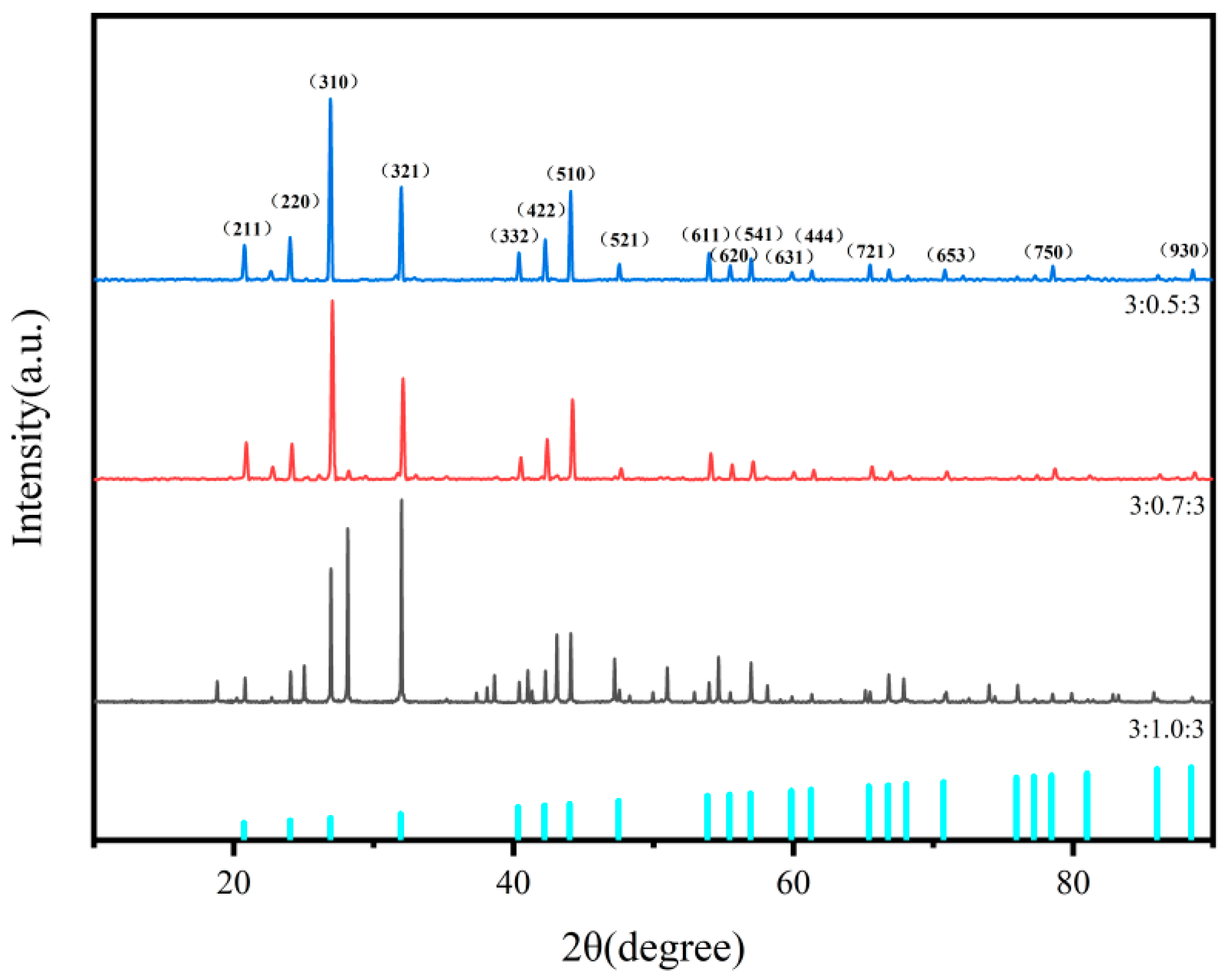
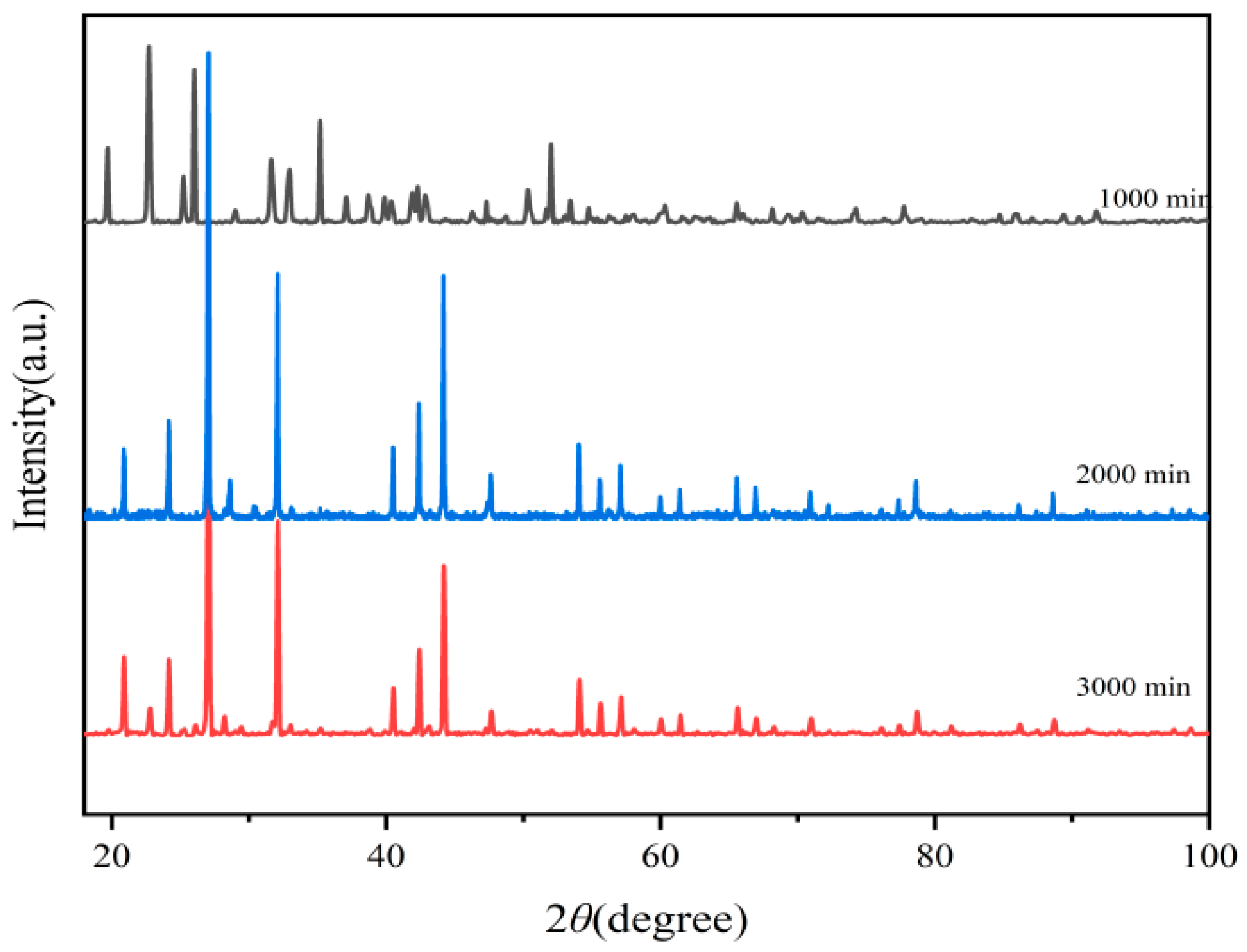
3.1. XRD of Ba3Eu(PO4)3
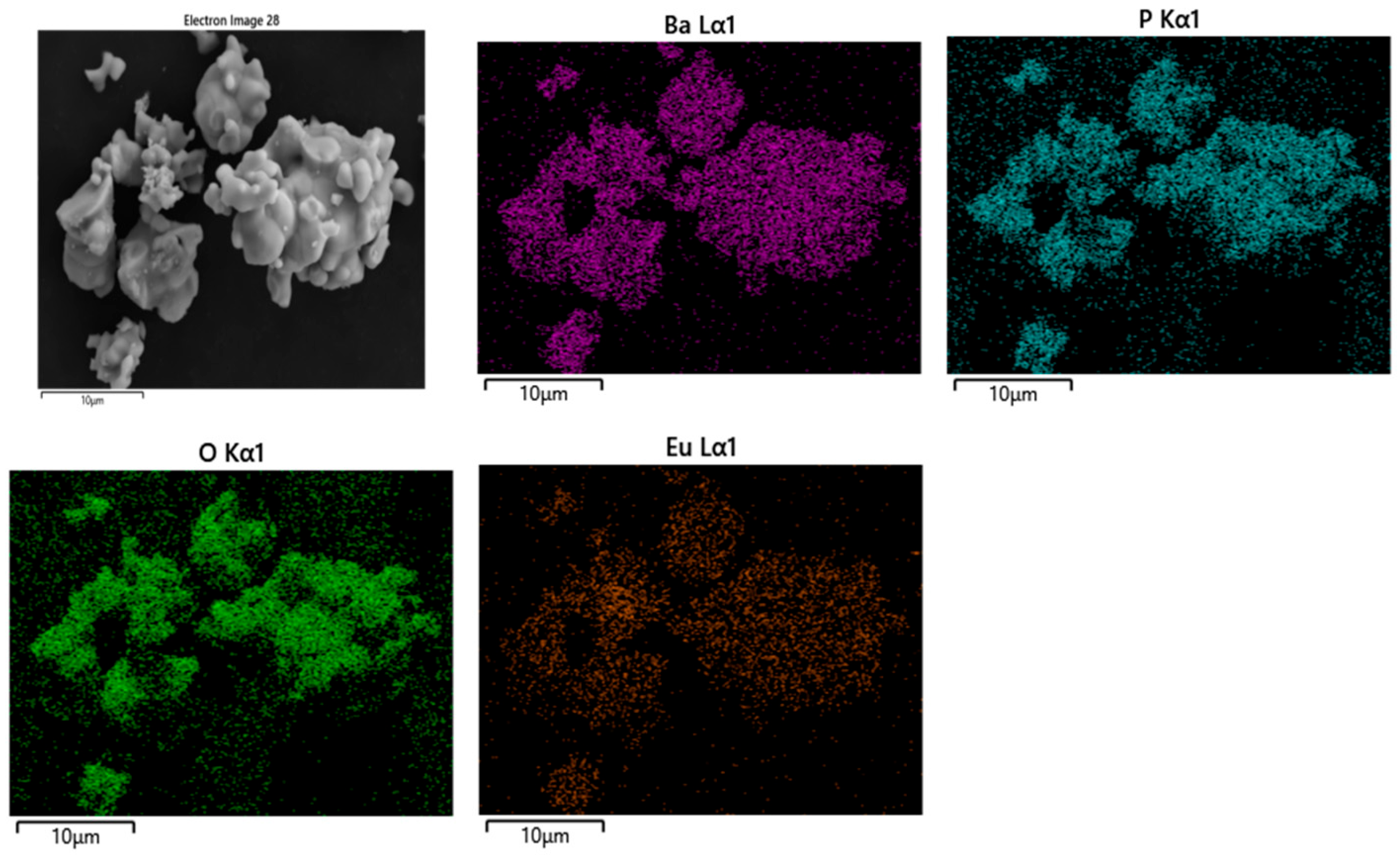
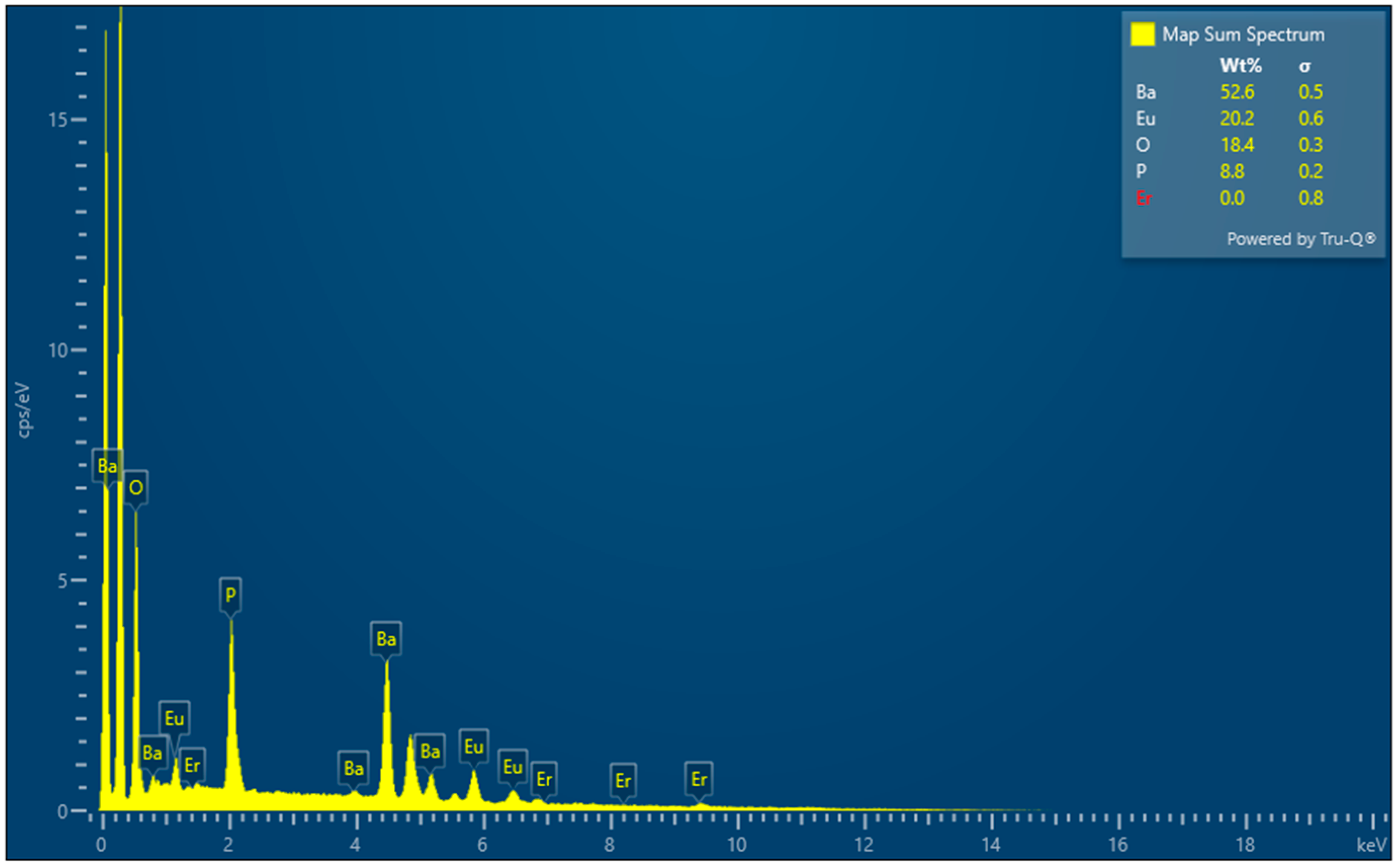
3.2. EDS of Ba3Eu(PO4)3
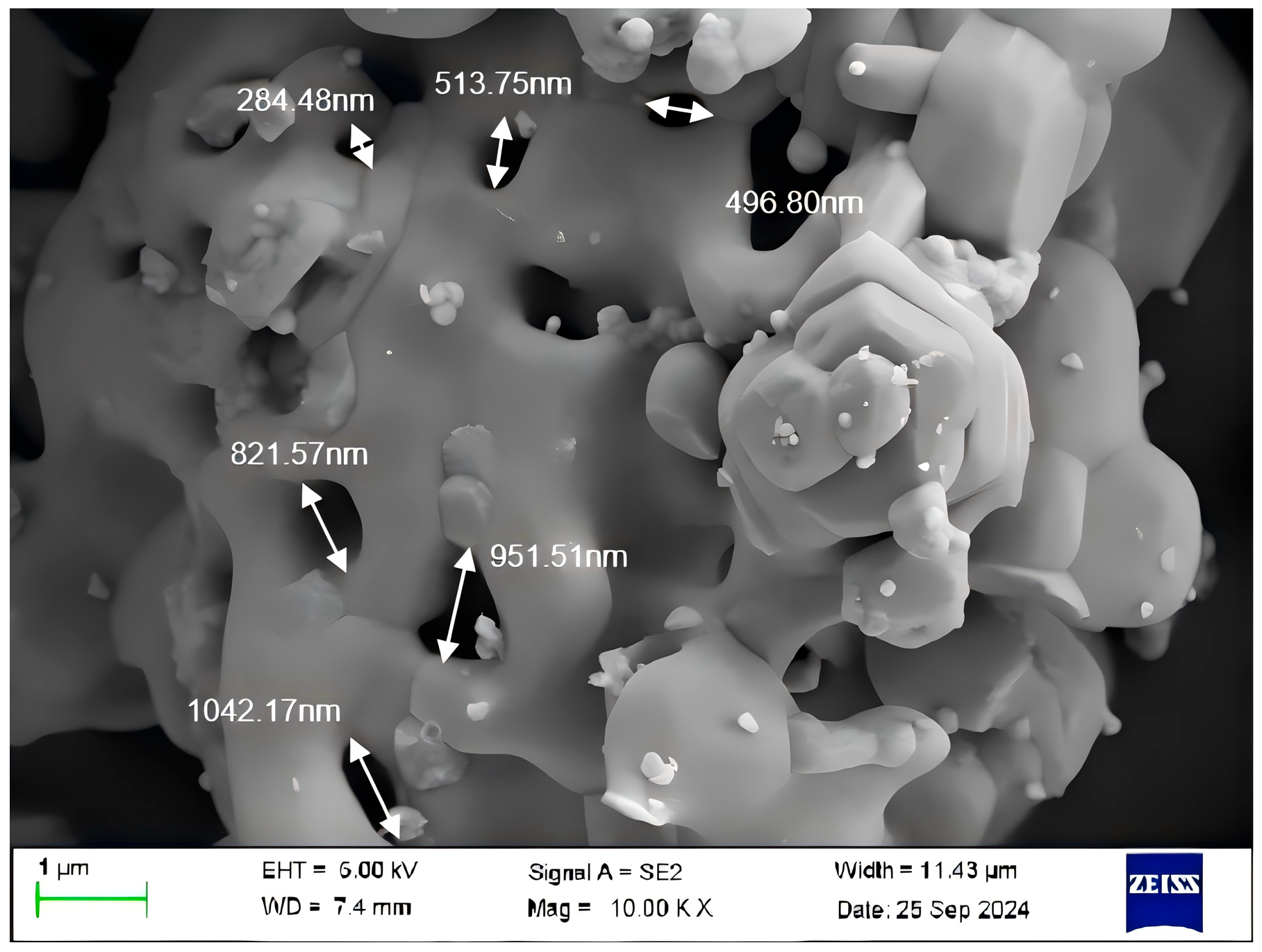
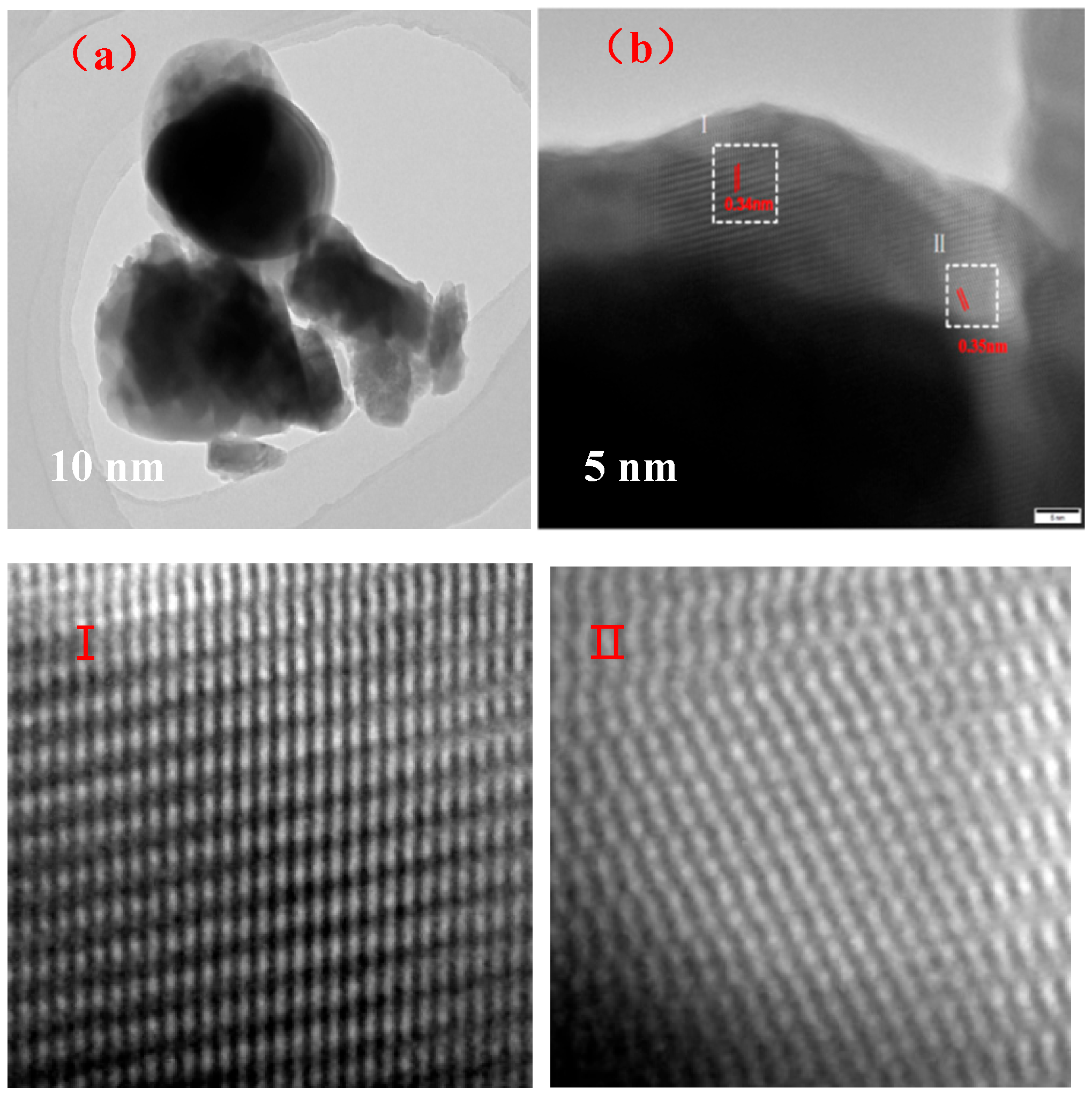
3.3. Morphology of Ba3Eu(PO4)3
3.4. The Anti-Corrosion Performance of Ba3Eu(PO4)3 Coatings
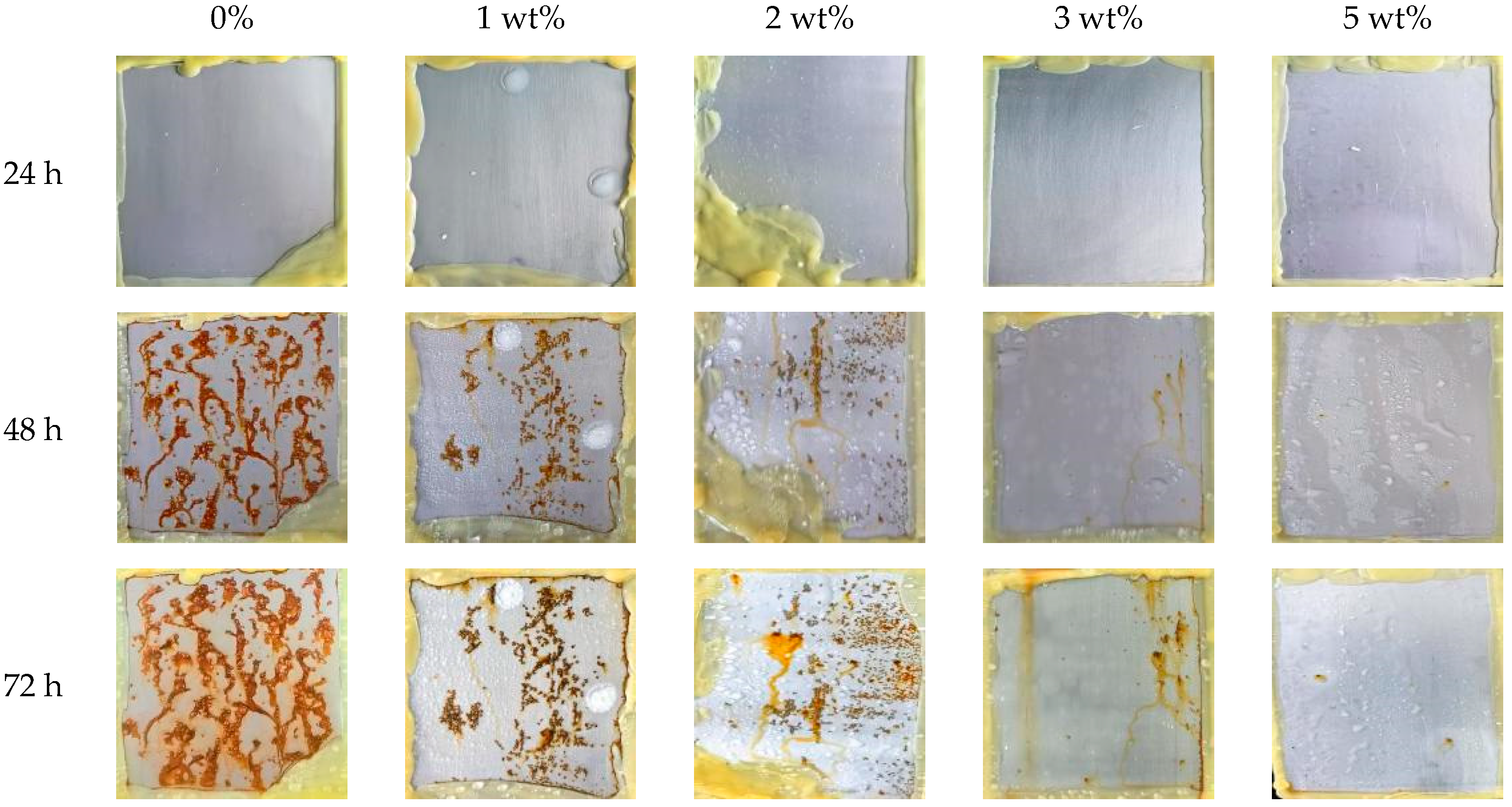
3.4.1. Salt Spray Resistance Test
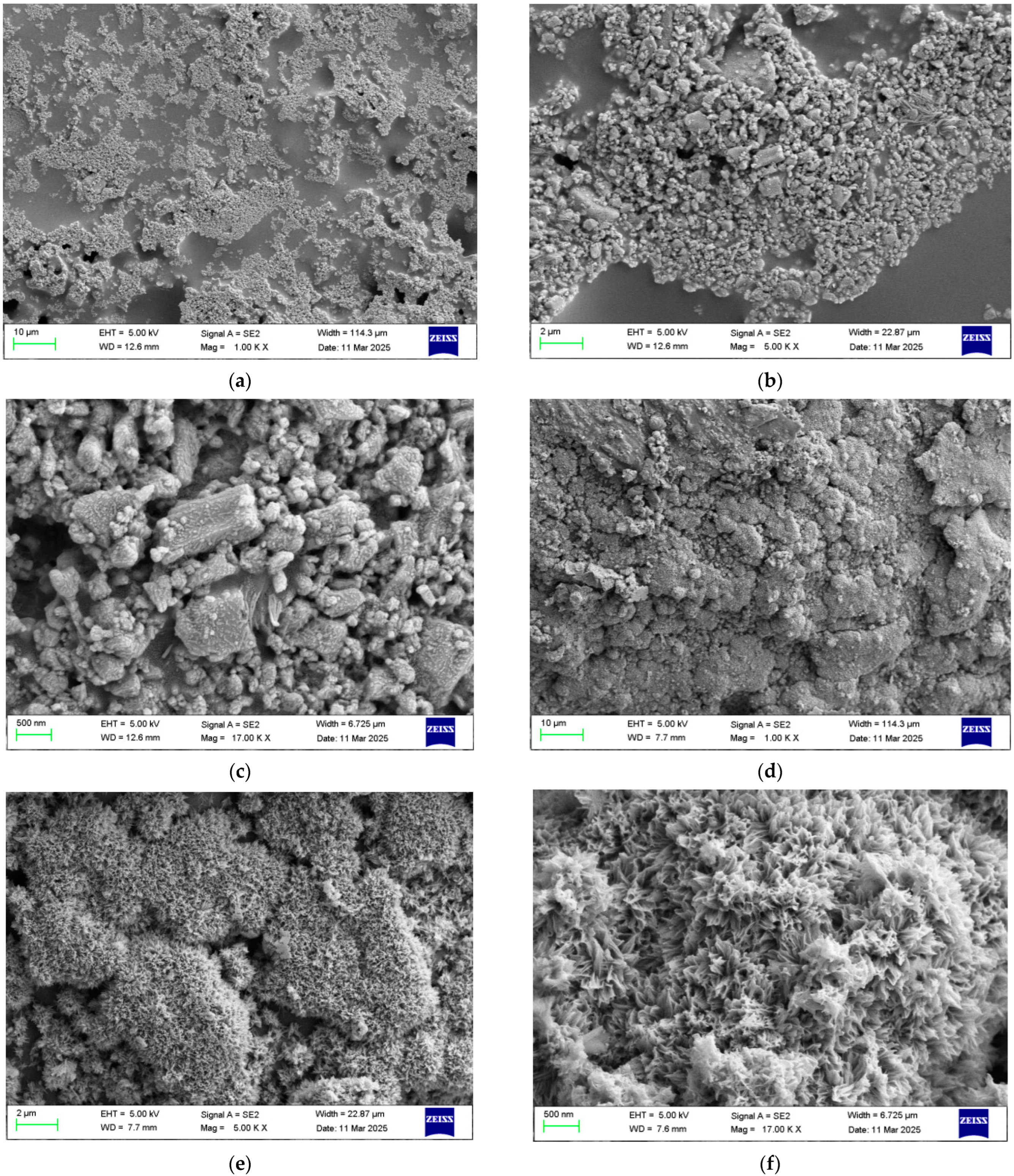
3.4.2. SEM of Corrosion Coatings After Salt Spray Test
3.4.3. Electrochemical Test of Coatings
- (1)
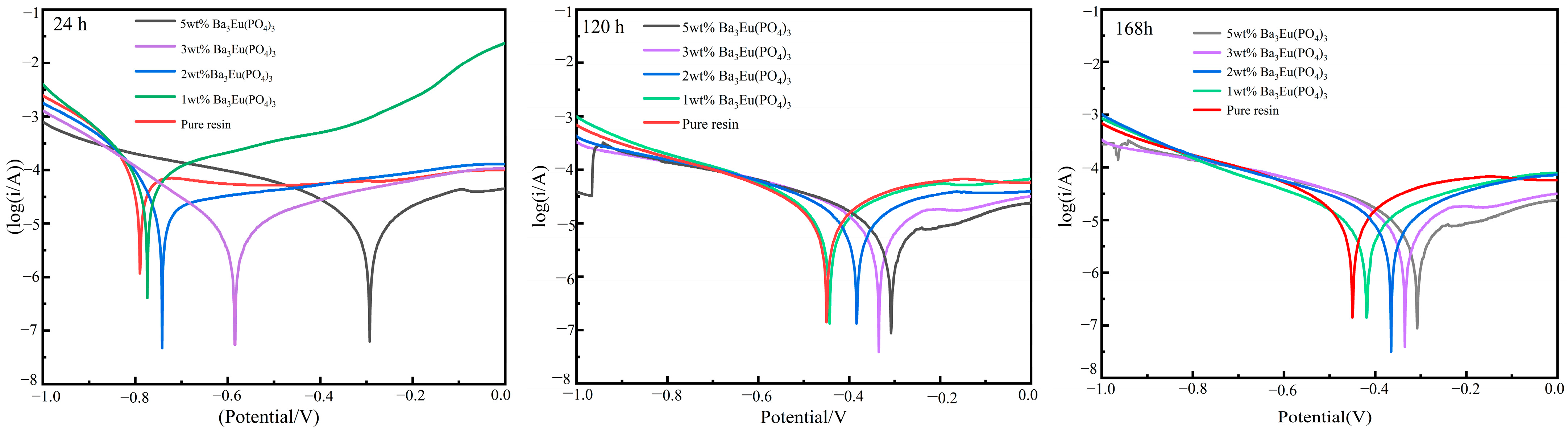
- Tafel Curve
- (2)
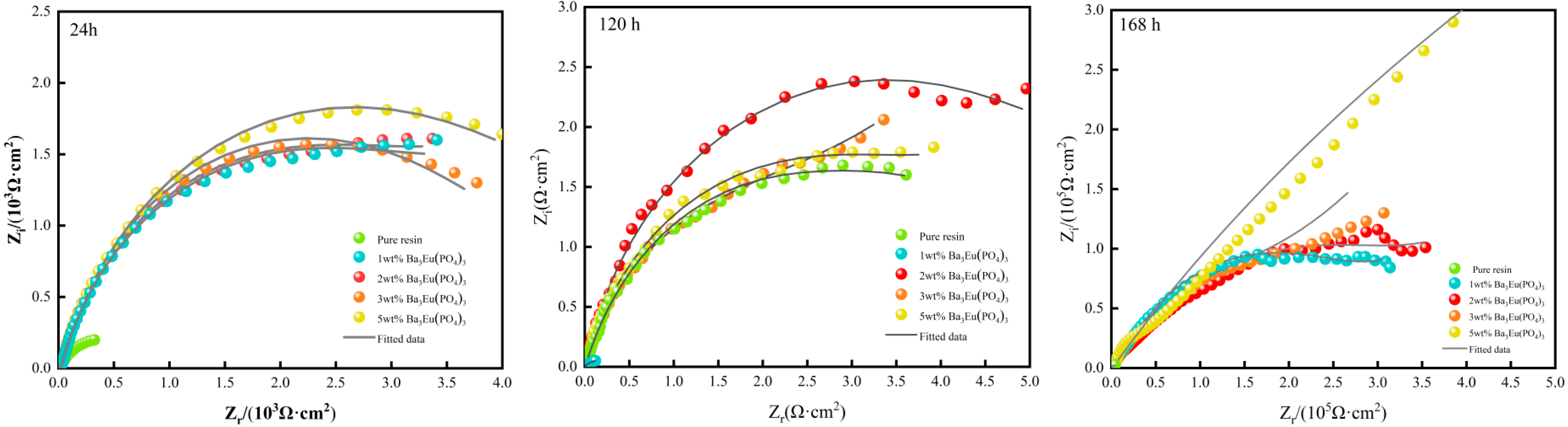
- Nyquist Curve
- (3)
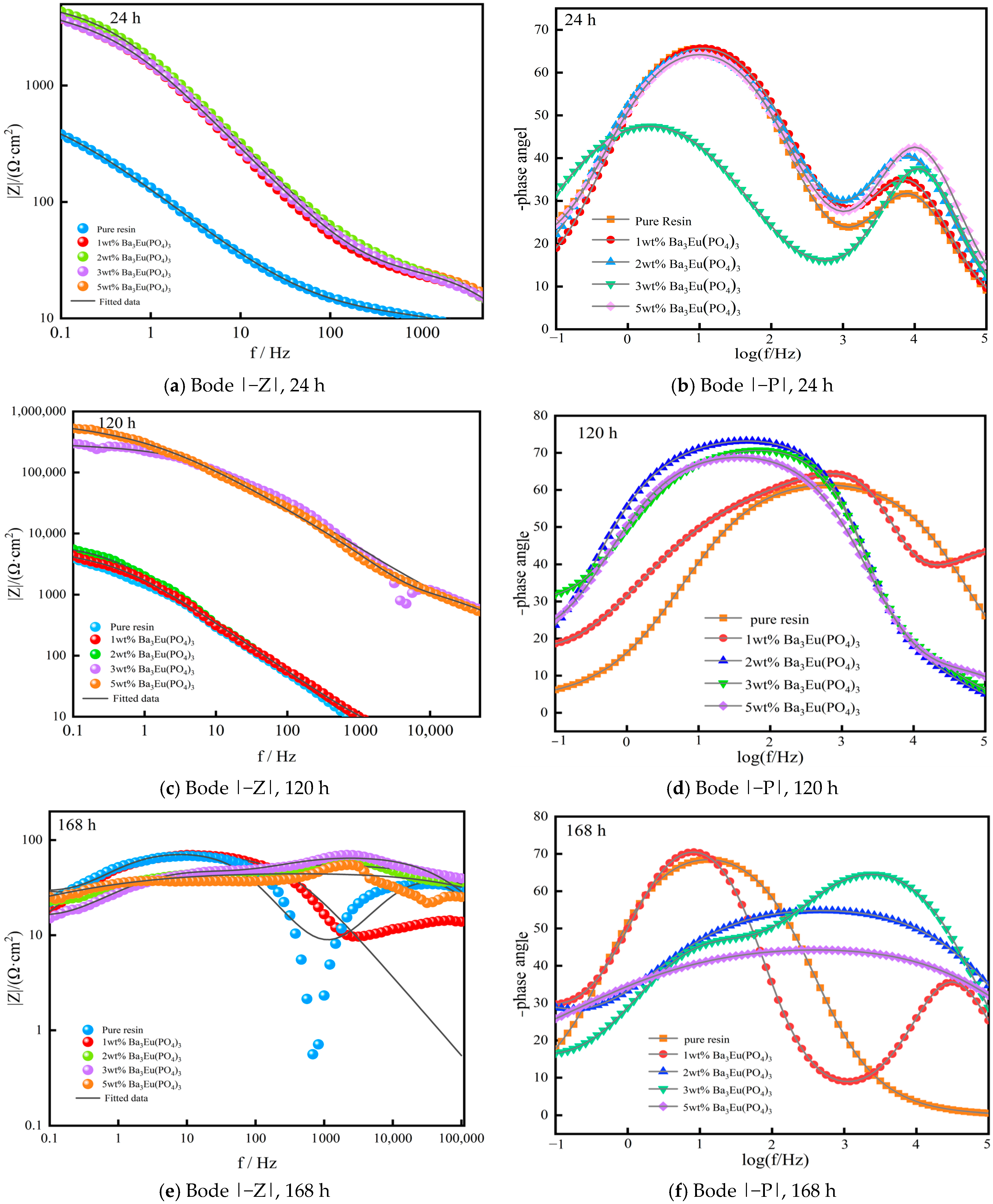
- Bode Curve
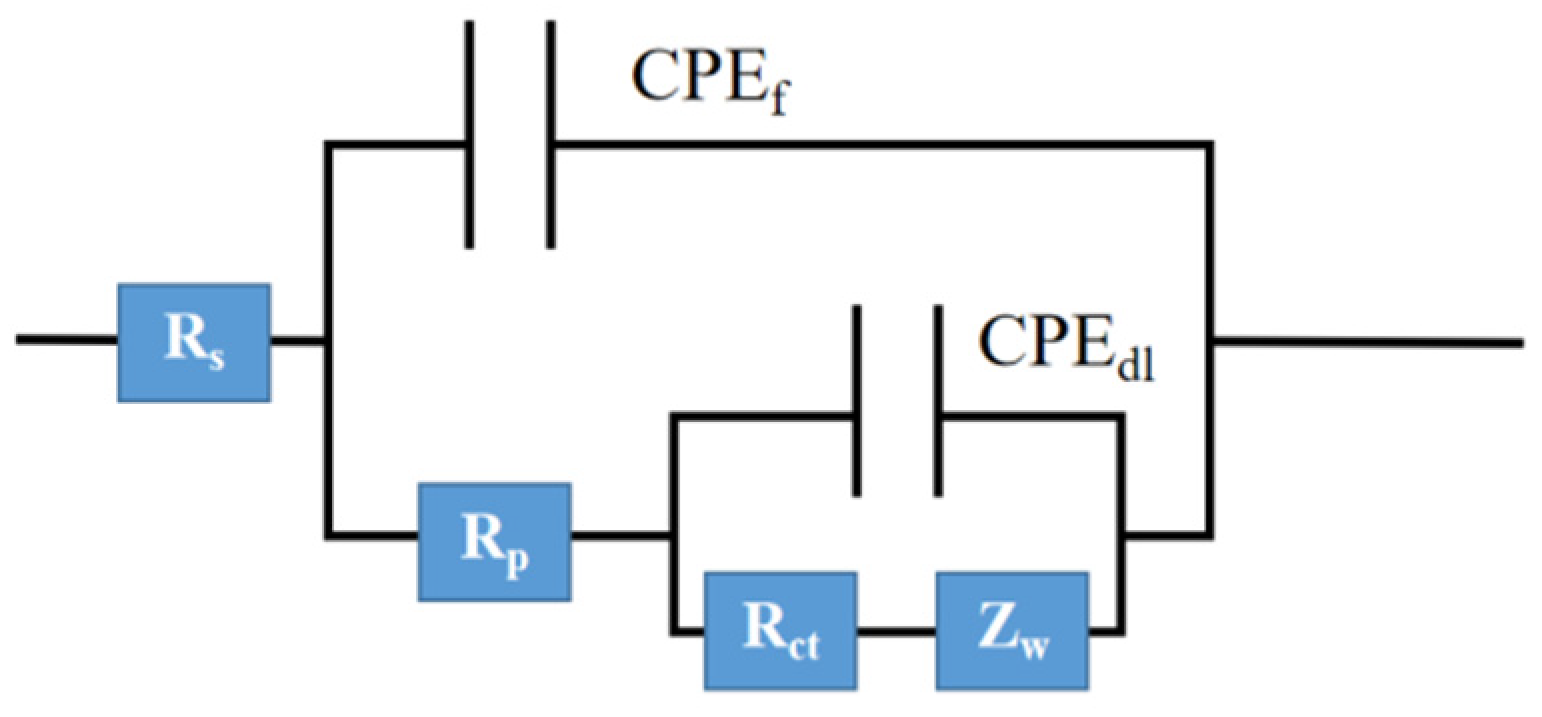
3.4.4. Equivalent Circuit
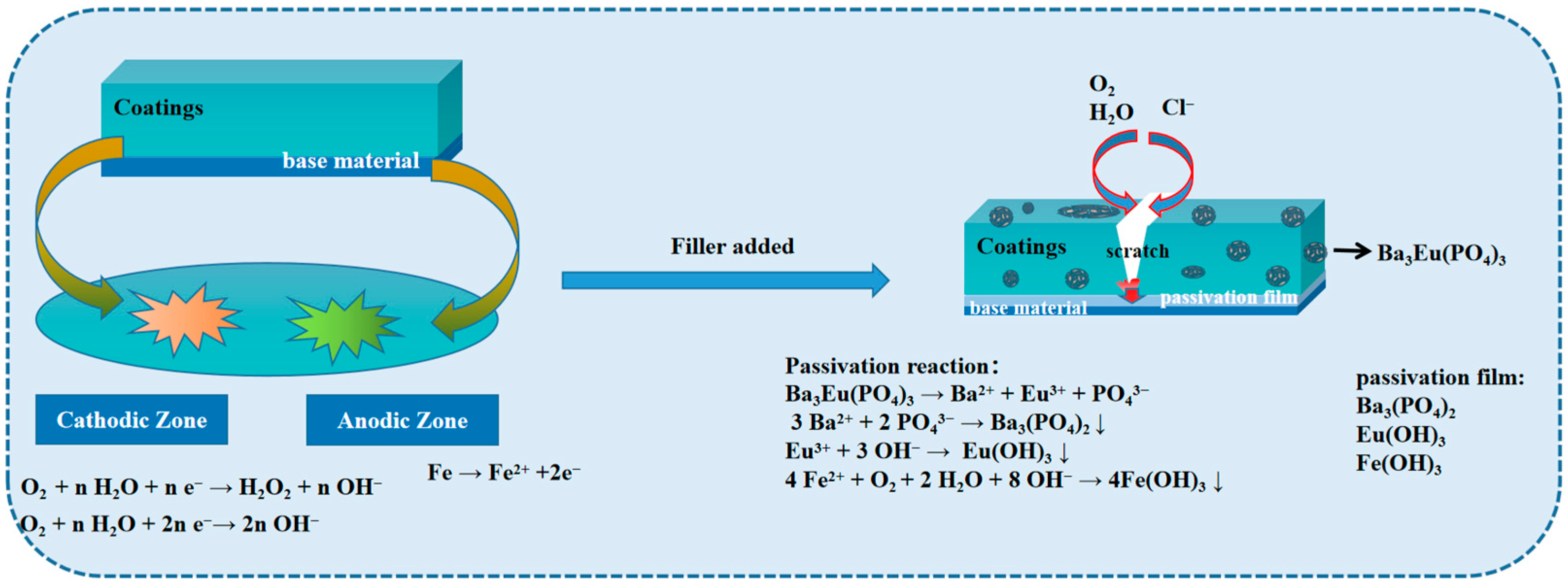
3.5. Anti-Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Wang, S.; Liu, Z.; Lin, S.; Cheng, X.; Wang, H. Fabrication of organic/inorganic hybrid waterborne polyurethane coating based on CeNPs @mTi3C2Tx composites for anti-corrosion applications. Prog. Org. Coat. 2023, 182, 107668. [Google Scholar] [CrossRef]
- Luo, X. Research on Corrosion Factors of Sulfur bearing Gas Wells in the Daniu Underground Paleozoic Based on Grey Relational Analysis. Unconv. Oil Gas 2021, 8, 115–120. [Google Scholar]
- Li, H.Y.; Han, Z.Z.; Bai, F.X.; Guo, X.J.; Wang, L.; Cui, C.C.; Ding, C. Research on Low Surface Treatment Waterborne Acrylic Coating Technology. Coat. Prot. 2024, 45, 37–43. [Google Scholar]
- China Coatings Industry Association. The 14th Five Year Plan for China’s Coatings Industry (III). China Coat. 2021, 36, 1–10. [Google Scholar]
- Li, J.; Chen, L.; Gu, X.M. Research on Corrosion Characteristics and Anti corrosion Technology of Oilfield Gathering and Transportation Pipelines. Chem. Eng. 2024, 2, 38. [Google Scholar]
- Wei, Y.Z.; Zhao, Z.X.; Jiang, T.; Li, W.G.; Gao, Y. Research and application progress of acrylic water-based metal anti-corrosion coatings. Corros. Prot. 2022, 2, 43. [Google Scholar]
- Sun, G.Q.; Yang, J.J.; Chen, C.J.; Cao, Z.-F.; Wu, Q.-Y.; Wu, M.-Y.; Zhang, J.-A.; Liu, J.-Y. Research progress on nano modified waterborne epoxy resin anti-corrosion coatings. Mod. Chem. Ind. 2022, 42, 33–38. [Google Scholar]
- Li, Y.F.; Nan, F. Achieving long term anti-corrosion waterborne epoxy coating by attapulgite loaded octadecylamine/graphene nanocomposite. Polym. Test. 2023, 129, 108290. [Google Scholar] [CrossRef]
- Ali, D.; Arman, Z.; Mohammadreza, M.A. Enhanced barrier properties of epoxy coating containing CaCO3 microparticles modified with cerium nitrate. Prog. Org. Coat. 2020, 144, 105660. [Google Scholar]
- Song, J.J.; Liu, C.W.; Chen, H.J.; Huang, H.W. Research on Modification of Corrosion Resistant Epoxy Powder Coatings. Thermosetting Resin 2007, 22, 24–28. [Google Scholar]
- Li, S.D. A Corrosion-Resistant Coating Material Suitable for Rare Earth Magnesium Silicon Iron Alloy. CN201911014427.6, 11 February 2020. [Google Scholar]
- Mofidabadi, A.H.J.; Bahlakeh, G.; Ramezanzadeh, B. Explorations of the adhesion and anti-corrosion properties of the epoxy coating on the carbon steel surface modified by Eu2O3 nanostructured film. J. Mol. Liq. 2020, 314, 113658. [Google Scholar] [CrossRef]
- ASTM-B117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2011.
- Chandra, B.B.; Ramaraghavulu, R.; Naresh, V.; Thatikayala, D.; Kim, D.; Su Shin, D.; Baker, A.P.; Wang, G.-G.; Park, J. Synthesis and photoluminescent characteristics of Sm3+-doped Ba3(PO4)2 phosphor hierarchical architectures. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2021, 264, 114979. [Google Scholar]
- Osipov, V.V.; Platonov, V.V.; Uimin, M.A.; Podkin, A.V. Laser synthesis of magnetic iron oxide nanopowders. Tech. Phys. 2012, 57, 543–549. [Google Scholar] [CrossRef]
- Kotov, Y.A.; Azarkevich, E.I.; Medvedev, A.I.; Murzakaev, A.M.; Kuznetsov, V.L.; Samatov, O.M.; Demina, T.M.; Timoshenkova, O.R.; Shtoltz, A.K. Iron oxide nanopowders prepared by the electroexplosion of wire. Inorg. Mater. 2007, 43, 633–637. [Google Scholar] [CrossRef]
- Qian, M. Construction and Absorption Properties of Core-Shell Electromagnetic Composite Nanomaterials. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2019. [Google Scholar]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 3, 63–75. [Google Scholar] [CrossRef]
- Liu, W.; Li, J. Sodium Lignosulfonate-Loaded Halloysite Nanotubes/Epoxy Composites for Corrosion Resistance Coating. ACS Omega 2023, 8, 18425–18434. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.H. Preparation and Performance Study of Amino Polyarylethersulfone/Epoxy Resin Anti Corrosion Coating. Master’s Thesis, Jilin University, Changchun, China, 2024. [Google Scholar]
- Chen, C.; Qiu, S.H.; Cui, M.J.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Kek, M.D.; Zorko, M.; Verpoest, I. Mechanical and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Appl. Surf. Sci. 2014, 292, 432–437. [Google Scholar] [CrossRef]
- Hao, S.S.; Sun, X.F.; Song, W.; Li, Z.M.; Tian, S. The effect of modified graphene on the corrosion resistance of epoxy resin coatings. Coat. Ind. 2018, 48, 34–40. [Google Scholar]
- Qian, Y. Research on Coating Process of Rare Earth Modified Graphene/Waterborne Epoxy Resin Composite Coating. Master’s Thesis, Xi’an University of Technology, Xi’an, China, 2022. [Google Scholar]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Asl, V.Z.; Zhao, J.; Anjum, M.J.; Wei, S.; Wang, W.; Zhao, Z. The effect of cerium cation on the microstructure and anti-corrosion performance of LDH conversion coatings on AZ31 magnesium alloy. J. Alloys Compd. 2020, 821, 32–48. [Google Scholar]
- Wu, H.; Wang, J.H.; Wen, S.G.; Ding, S.N.; Song, J.; Wang, H.P.; Sui, P.C. Preparation of Magnesium Aluminum Hydrotalcite and Study on its Coating Corrosion Resistance. Surf. Technol. 2024, 53, 126–134. [Google Scholar]
- Ma, C.; Wang, W.; Wang, Q.; Sun, N.; Hu, S.Q.; Wei, S.; Feng, H.M.; Hao, X.P.; Li, W.; Kong, D.B.; et al. Facile synthesis of BTA@NiCo2O4 hollow structure for excellent microwave absorption and anticorrosion performance. J. Colloid Interface Sci. 2021, 594, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, S.; Chen, K.; Wang, J.; Wang, G.; Sun, K. Enhanced corrosion resistance of waterborne polyurethane containing sulfonated graphene/zinc phosphate composites. Prog. Org. Coat. 2019, 132, 409–416. [Google Scholar] [CrossRef]
- Di, Y.L.; Li, L.K.; Jiao, Y.; Huang, H.Y.; He, X.; Luo, Q.; Zhang, W.M. Research progress and application of rare earth conversion coatings in metal corrosion prevention. Mater. Prot. 2022, 55, 156–166. [Google Scholar]
- Fu, X.Q.; Duan, S.L.; Lin, J.R.; Shen, M.Q.; Wang, Q.Q. The effect of nano rare earth CeO2 doping on the properties of Ni-Fe-Co-P alloy coatings. Rare Met. Mater. Eng. 2020, 49, 2095–2103. [Google Scholar]
| Raw Material | wt/% |
|---|---|
| Waterborne acrylic lotion | 50.0–60.0 |
| Defoamer C-15 | 0.4–0.6 |
| Deionized water | 15.0–8.0 |
| pH regulator DMAE | 0.2–0.4 |
| Wetting agent G-033 | 0.5–1.0 |
| Leveling agent HTK-3020 | 0.5–0.8 |
| Film-forming aid alcohol ester 12 | 2.4–3.0 |
| Anti-flash-rust agent NaNO2 | 1.0–2.0 |
| Thickener WT-105A | appropriate amount |
| Dispersant 5040 | 0.4–0.8 |
| Ba3Eu(PO4)3 | 0–5.0 |
| Total | 100 |
| Samples | Time (h) | Ecorr (V) | Icorr (μA/cm2) | Rp (Ω) | ba | −bc |
|---|---|---|---|---|---|---|
| 0 wt% | 24 | −1.068 | 135.90 | 372 | 0.817 | 7.785 |
| 120 | −1.127 | 505.20 | 107 | 1.309 | 6.754 | |
| 168 | −1.082 | 495.70 | 122 | 0.076 | 7.104 | |
| 1 wt% | 24 | −1.036 | 80.62 | 447 | 3.441 | 8.617 |
| 120 | −1.028 | 76.92 | 567 | 1.476 | 8.497 | |
| 168 | −1.026 | 70.65 | 3419 | 3.156 | 5.108 | |
| 2 wt% | 24 | −1.015 | 24.03 | 1232 | 1.736 | 8.866 |
| 120 | −0.888 | 14.70 | 2977 | 4.652 | 5.285 | |
| 168 | −0.784 | 12.92 | 3416 | 4.357 | 5.493 | |
| 3 wt% | 24 | −0.676 | 7.793 | 5202 | 4.321 | 6.404 |
| 120 | −0.916 | 15.39 | 3419 | 3.156 | 5.108 | |
| 168 | −0.868 | 13.33 | 3300 | 4.604 | 5.281 | |
| 5 wt% | 24 | −0.736 | 8.82 | 4591 | 4.504 | 6.232 |
| 120 | −0.794 | 10.96 | 3734 | 5.521 | 5.107 | |
| 168 | −0.838 | 9.616 | 5023 | 3.714 | 5.288 |
| Time (h) | Additive Amount (wt%) | Rs (Ω·cm2) | CPEf (Ω−1·cm−2·s−n) | Rp (Ω·cm 2) | CPEdl (Ω−1·cm−2·s−n) | Rct (Ω·cm2) | Zw (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| 24 | 0 | 6.27 | 7.18 × 10−6 | 193.70 | 1.14 × 10−4 | 3499 | 0.002 |
| 1 | 4.96 | 5.05 × 10−6 | 22.31 | 1.1 × 10−4 | 3840 | 0.030 | |
| 2 | 4.49 | 6.34 × 10−6 | 23.50 | 1.1 × 10−4 | 4766 | 0.002 | |
| 3 | 3.79 | 4.95 × 10−6 | 22.07 | 1.2 × 10−4 | 3654 | 0.002 | |
| 5 | 5.45 | 7.58 × 10−6 | 20.64 | 9.85 × 10−5 | 4112 | 0.030 | |
| 120 | 0 | 0.01 | 3.59 × 10−6 | 341.30 | 2.46 × 10−7 | 1.58 × 109 | 2.90 × 10−7 |
| 1 | 36.75 | 6.08 × 10−7 | 2058 | 1.38 × 10−8 | 5.37 × 105 | 9.66 × 10−6 | |
| 2 | 2.70 | 8.62 × 10−5 | 3.03 | 1.04 × 10−4 | 7.83 × 106 | 1.31 × 10−3 | |
| 3 | 2.51 | 1.08 × 10−4 | 3.42 | 8.41 × 10−6 | 3160 | 5.99 × 10−4 | |
| 5 | 2.90 | 4.72 × 10−5 | 2.10 | 6.02 × 10−6 | 4154 | 1.05 × 10−3 | |
| 168 | 0 | 17.56 | 8.64 × 10−5 | 5012 | 3.50 × 10−3 | 533 | 3.69 × 107 |
| 1 | 11.36 | 7.95 × 10−5 | 42.77 | 5.73 × 10−5 | 3071 | 4.77 × 10−4 | |
| 2 | 122.40 | 1.16 × 10−6 | 23,600 | 8.42 × 10−8 | 1.79 × 103 | 6.76 × 10−6 | |
| 3 | 183.80 | 1.70 × 10−7 | 3180 | 7.11 × 10−7 | 2.73 × 103 | 1.63 × 10−6 | |
| 5 | 9192 | 4.38 × 10−11 | 6567 | 3.28 × 10−8 | 4.03 × 107 | 3.81 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Wang, J.; Wen, S.; Zhao, J.; Zhang, X.; Zhao, Y.; Deng, Z. Preparation of Barium Europium Phosphate and Its Performance in Acrylic Resin Anti-Corrosion Coating. Polymers 2025, 17, 1966. https://doi.org/10.3390/polym17141966
Deng X, Wang J, Wen S, Zhao J, Zhang X, Zhao Y, Deng Z. Preparation of Barium Europium Phosphate and Its Performance in Acrylic Resin Anti-Corrosion Coating. Polymers. 2025; 17(14):1966. https://doi.org/10.3390/polym17141966
Chicago/Turabian StyleDeng, Xuying, Jihu Wang, Shaoguo Wen, Jiale Zhao, Xue Zhang, Yicheng Zhao, and Zhiying Deng. 2025. "Preparation of Barium Europium Phosphate and Its Performance in Acrylic Resin Anti-Corrosion Coating" Polymers 17, no. 14: 1966. https://doi.org/10.3390/polym17141966
APA StyleDeng, X., Wang, J., Wen, S., Zhao, J., Zhang, X., Zhao, Y., & Deng, Z. (2025). Preparation of Barium Europium Phosphate and Its Performance in Acrylic Resin Anti-Corrosion Coating. Polymers, 17(14), 1966. https://doi.org/10.3390/polym17141966






