Furan–Urethane Monomers for Self-Healing Polyurethanes
Abstract
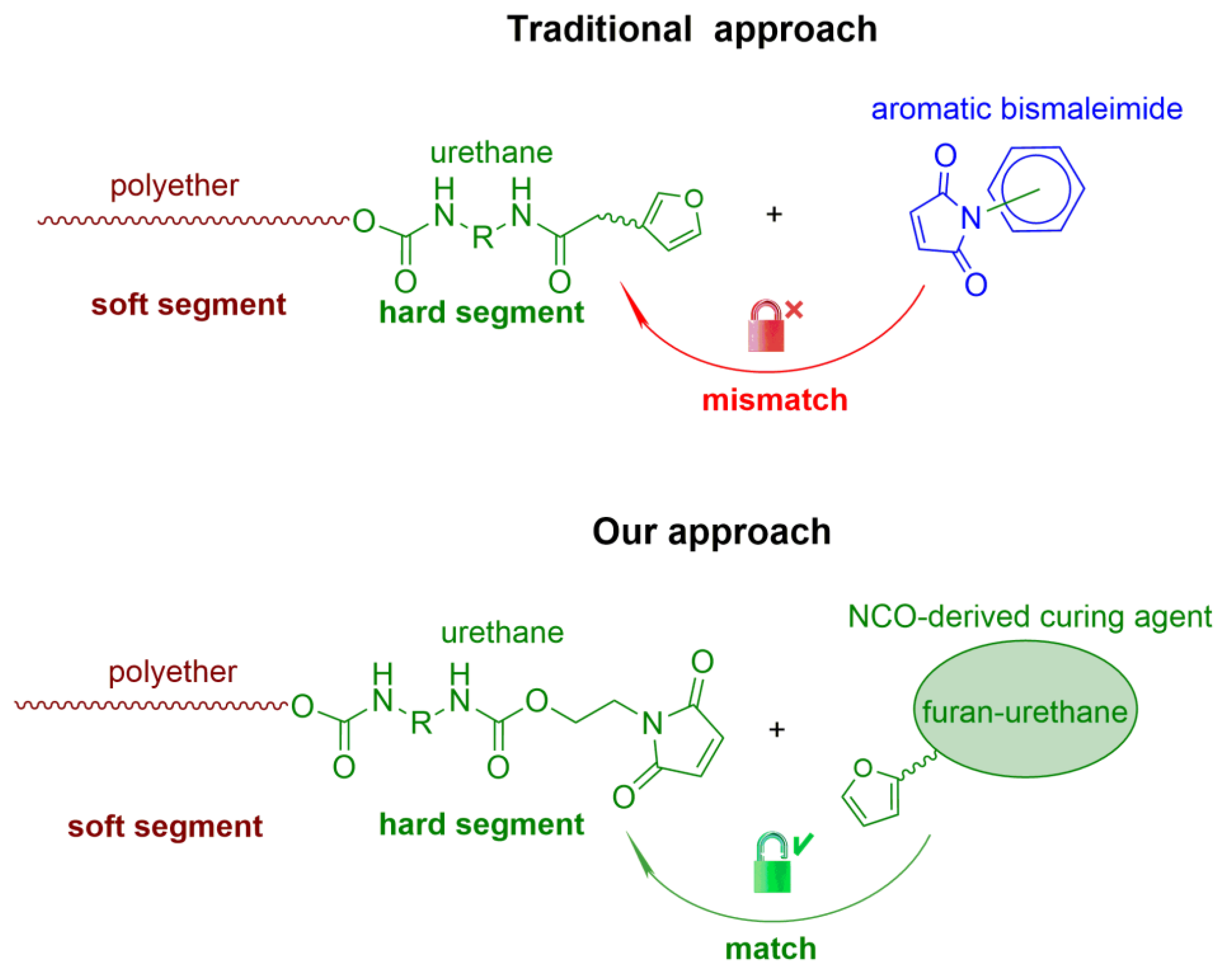
1. Introduction
2. Materials and Methods
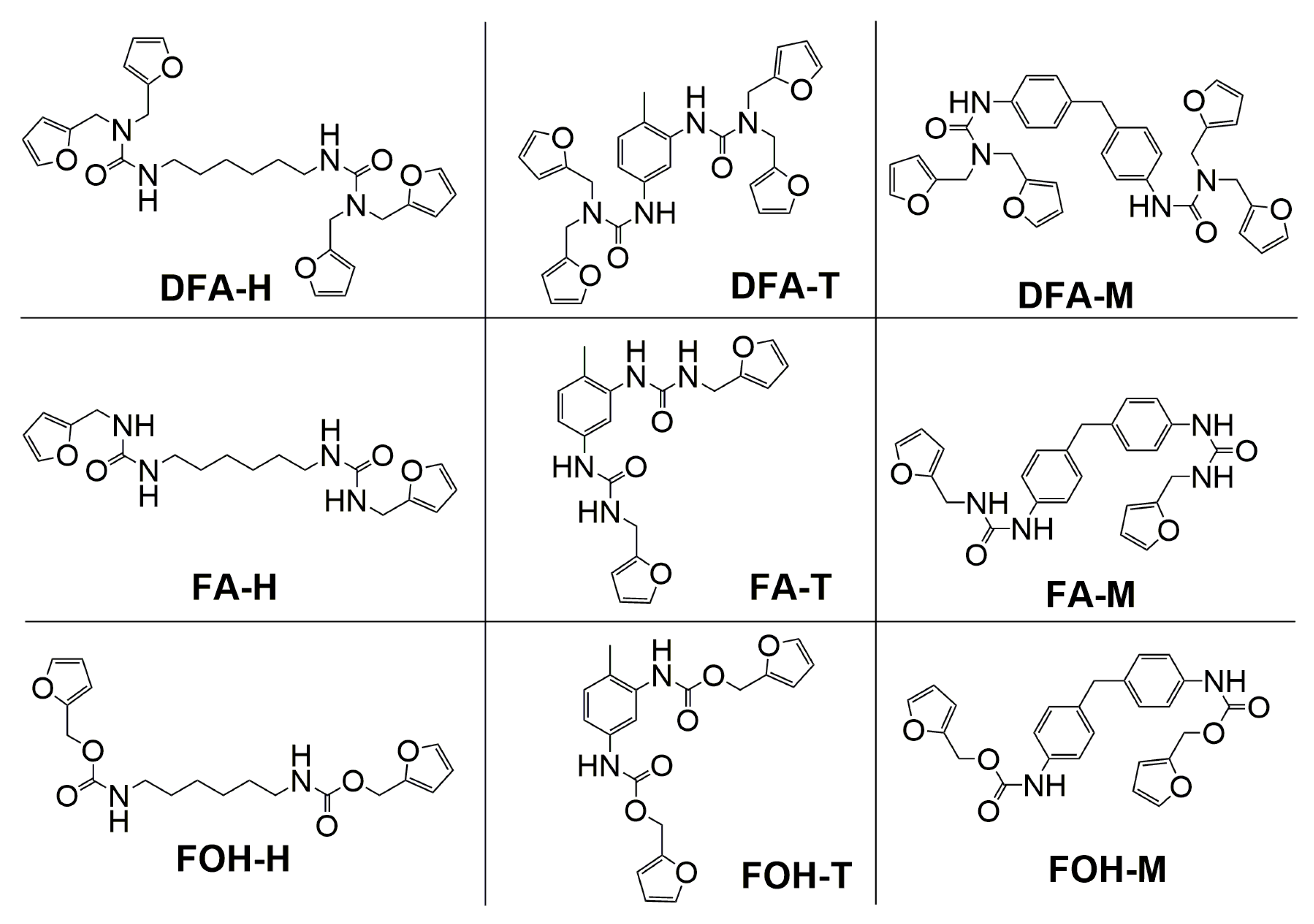
2.1. Materials
2.2. Characterizations of Samples
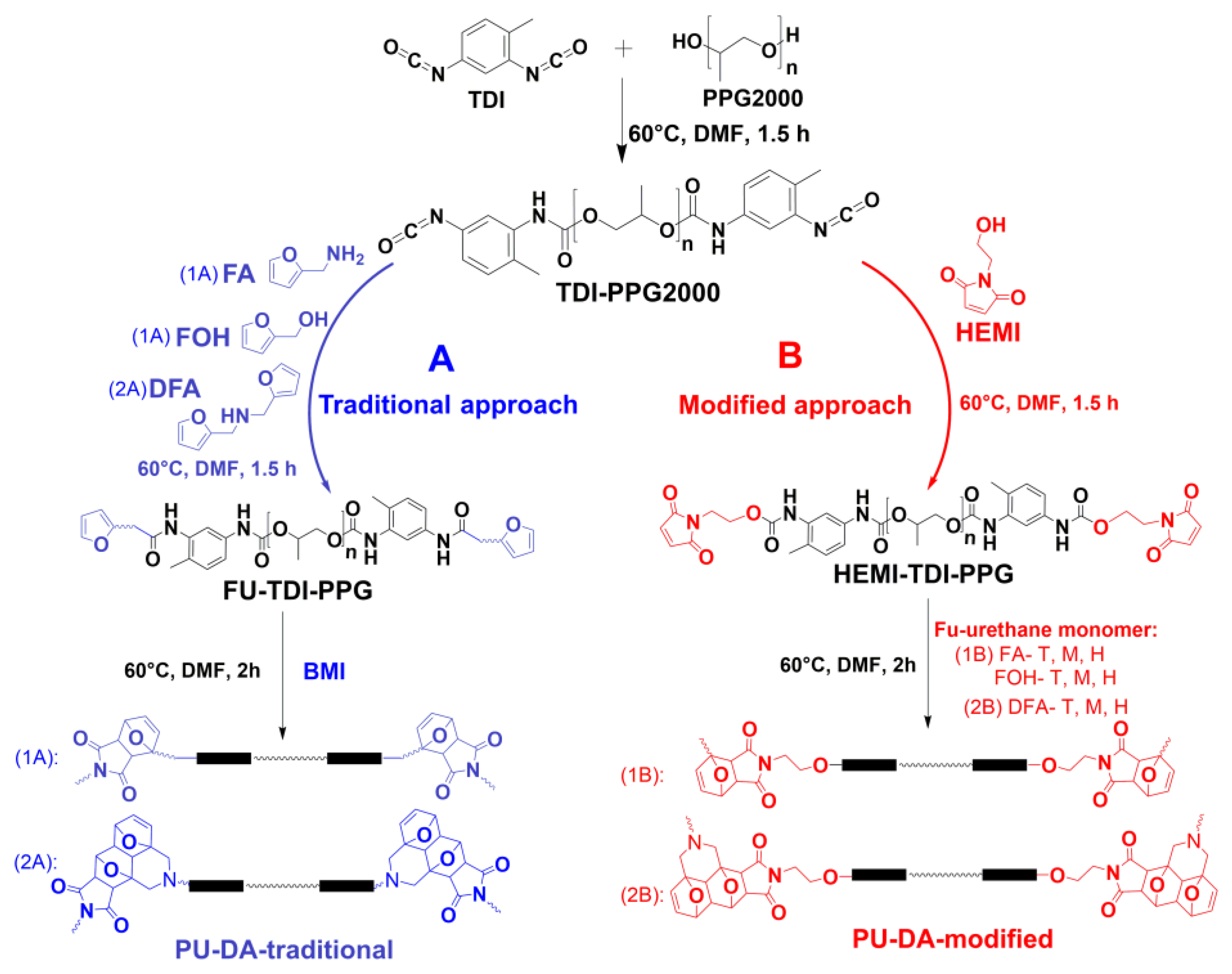
2.3. Preparation Process of HEMI-TDI-PPG Prepolymer
2.4. Synthesis of PU-DA-Modified
2.5. Synthesis of PU-DA-Traditional
2.6. Film Preparation
3. Results and Discussion
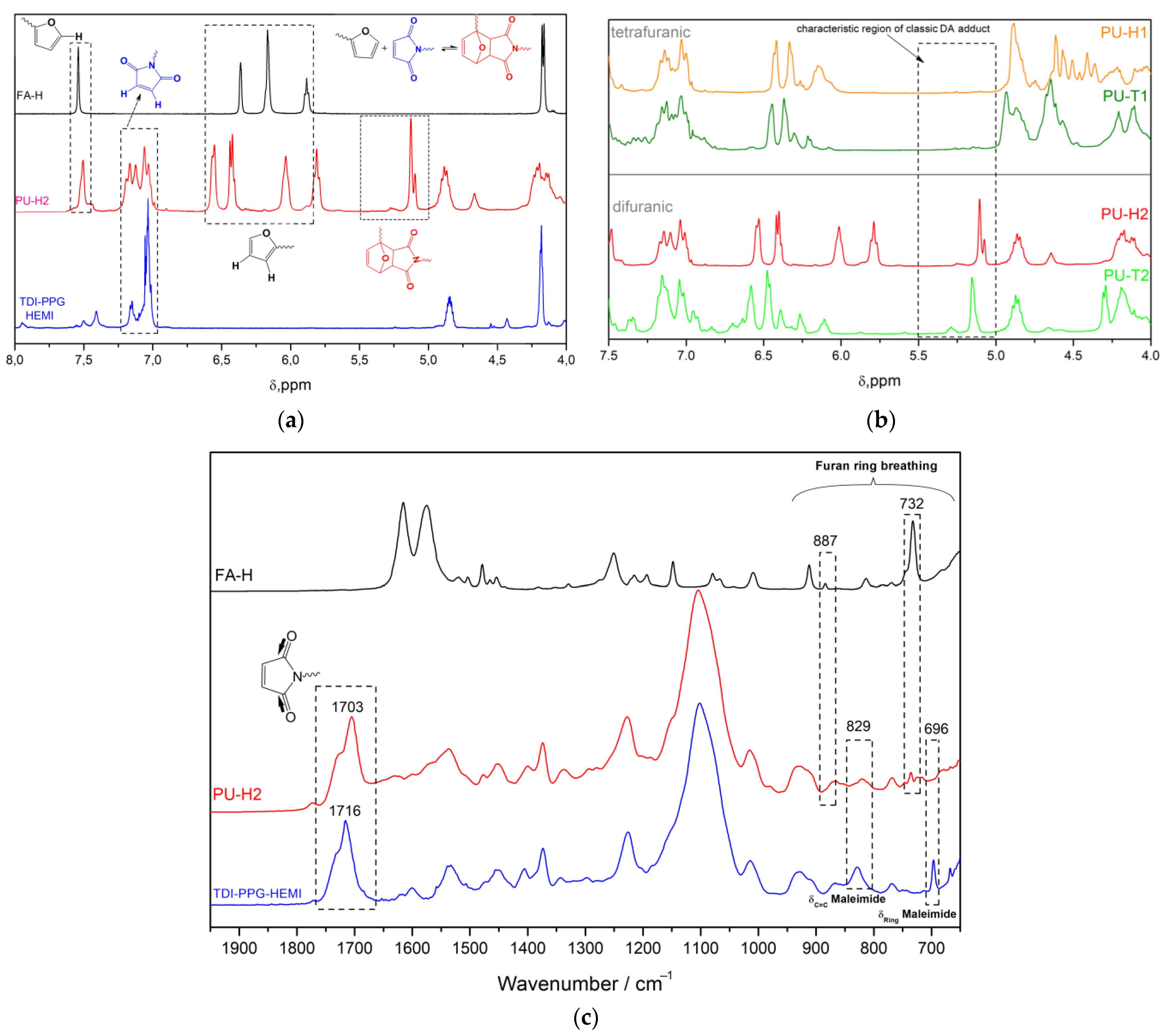
3.1. Synthesis and Characterization of Polyurethanes
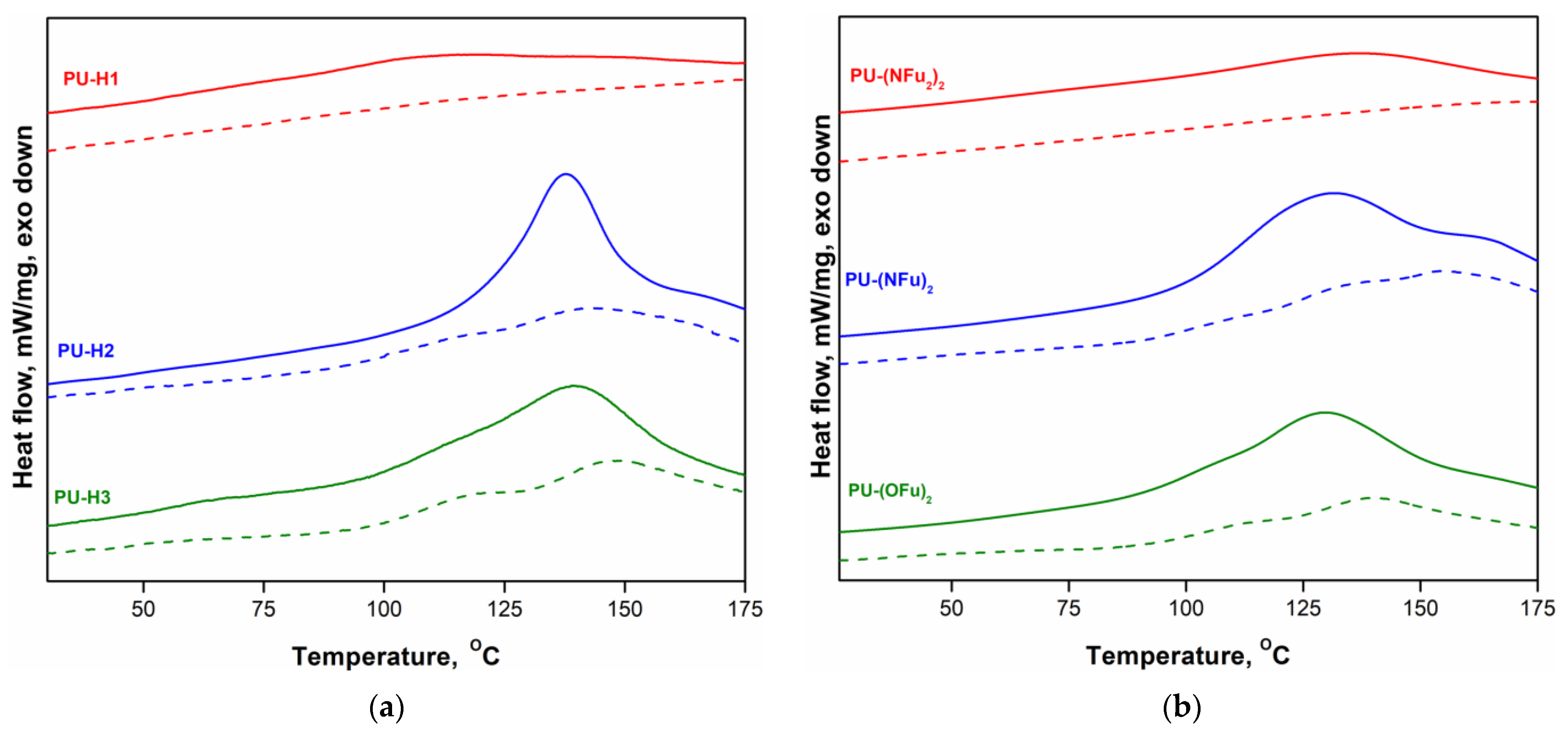
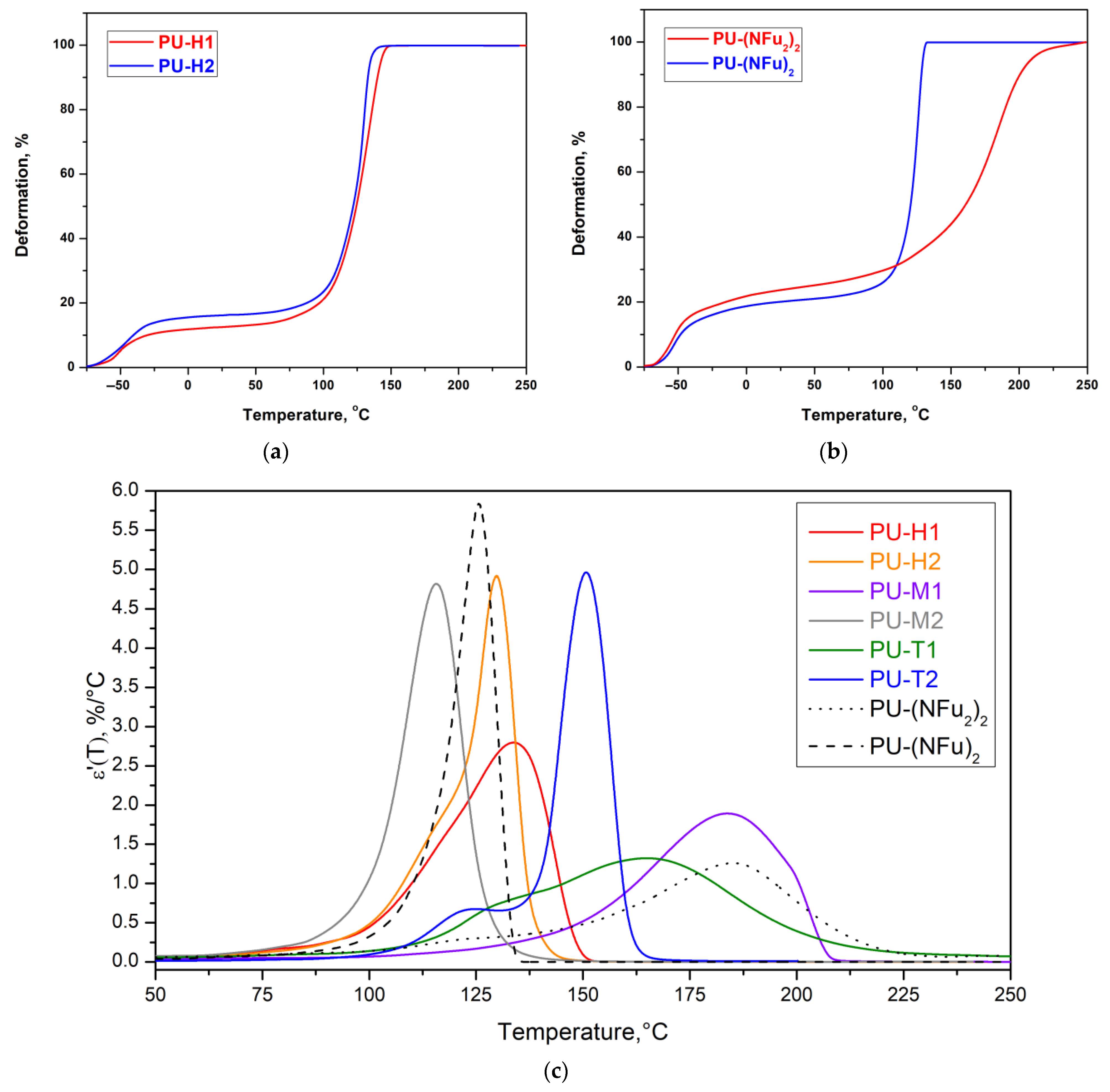
3.2. Thermal Properties
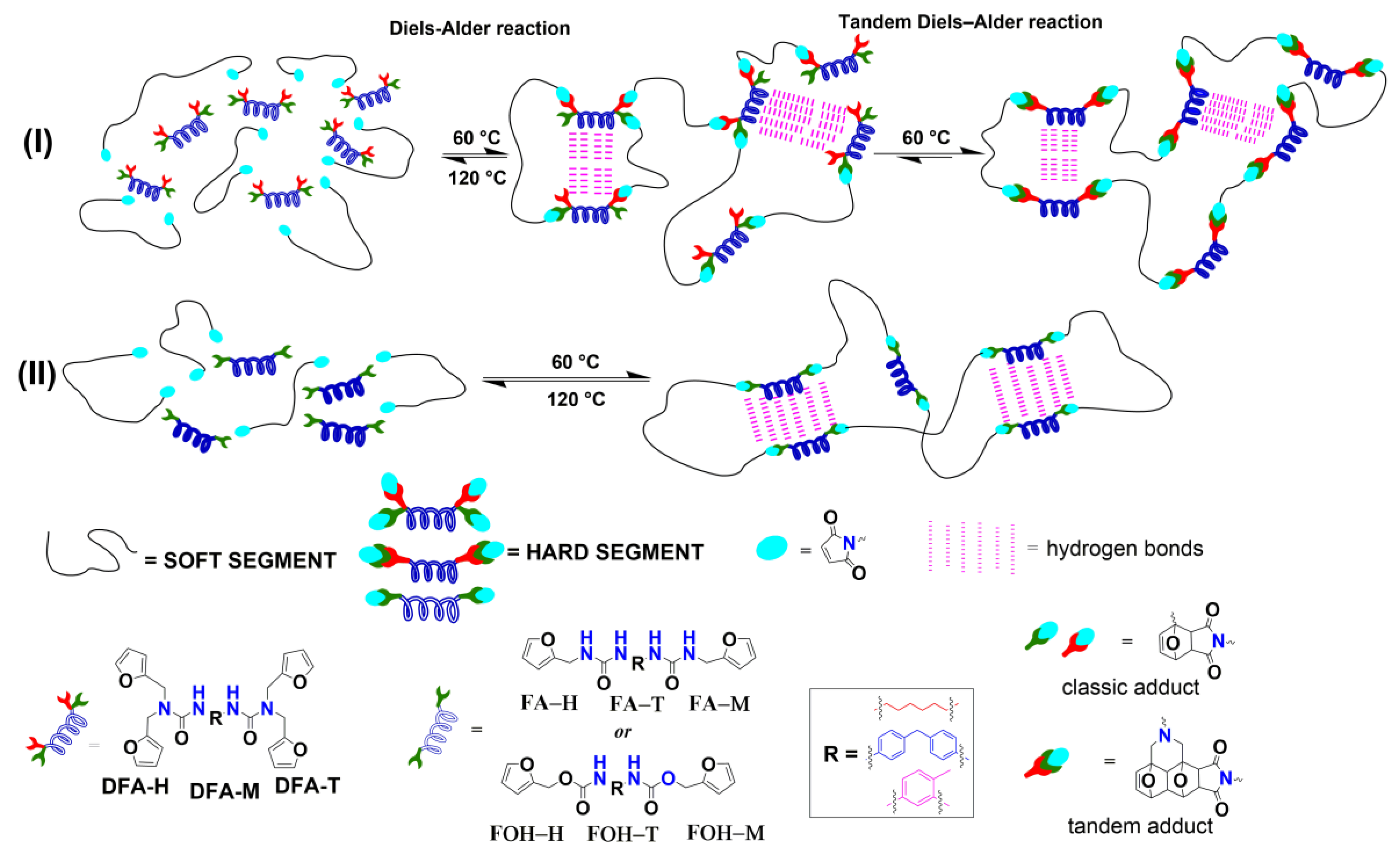
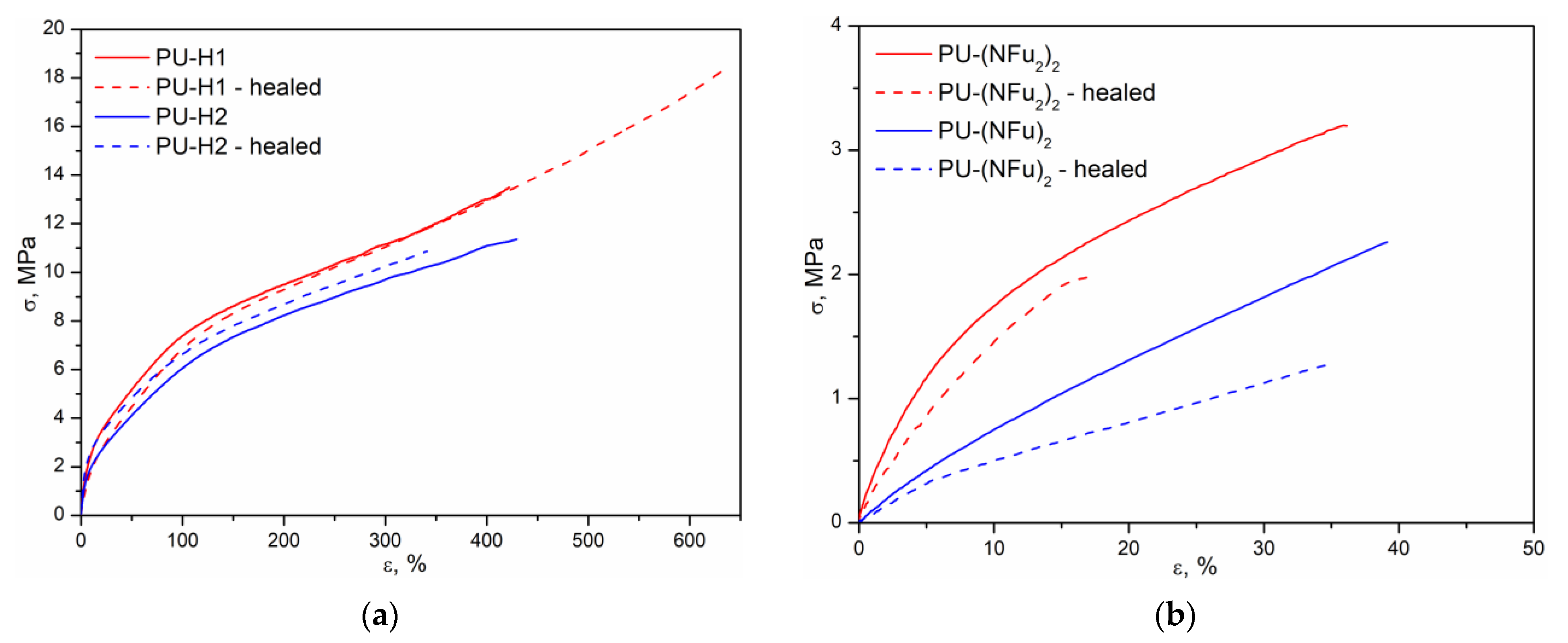
3.3. Mechanical and Self-Healing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Madbouly, S.A. Novel recycling processes for thermoset polyurethane foams. Curr. Opin. Green Sustain. Chem. 2023, 42, 100835. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Salino, R.E.; Catai, R.E. A study of polyurethane waste composite (PUR) and recycled plasterboard sheet cores with polyurethane foam for acoustic absorption. Constr. Build. Mater. 2023, 387, 131201. [Google Scholar] [CrossRef]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Brahmananda Rao, C.V.S.; Sivaramakrishna, A. Recent advances in biodegradable polymers–Properties, applications and future prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Beach, M.; Davey, T.; Subramanian, P.; Such, G. Self-healing organic coatings–Fundamental chemistry to commercial application. Prog. Org. Coat. 2023, 183, 107759. [Google Scholar] [CrossRef]
- Aiswarya, S.; Awasthi, P.; Banerjee, S.S. Self-healing thermoplastic elastomeric materials: Challenges, opportunities and new approaches. Eur. Polym. J. 2022, 181, 111658. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Das, M.; Parathodika, A.R.; Maji, P.; Naskar, K. Dynamic chemistry: The next generation platform for various elastomers and their mechanical properties with self-healing performance. Eur. Polym. J. 2023, 186, 111844. [Google Scholar] [CrossRef]
- Irzhak, V.I.; Uflyand, I.E.; Dzhardimalieva, G.I. Self-Healing of Polymers and Polymer Composites. Polymers 2022, 14, 5404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, R.; He, Y.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Self-Healing Polyurethane Elastomers Based on a Disulfide Bond by Digital Light Processing 3D Printing. ACS Macro. Lett. 2019, 8, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, F.; Shen, X.; He, T.; Qiu, H.; Yin, S.; Stang, P.J. Self-Healing Metallacycle-Cored Supramolecular Polymers Based on a Metal–Salen Complex Constructed by Orthogonal Metal Coordination and Host-Guest Interaction with Amino Acid Sensing. ACS Macro. Lett. 2021, 10, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Sorin, E.S.; Baimuratova, R.K.; Uflyand, I.E.; Perepelitsina, E.O.; Anokhin, D.V.; Ivanov, D.A.; Dzhardimalieva, G.I. New Self-Healing Metallosupramolecular Copolymers with a Complex of Cobalt Acrylate and 4′-Phenyl-2,2′:6′,2″-terpyridine. Polymers 2023, 15, 1472. [Google Scholar] [CrossRef] [PubMed]
- Terryn, S.; Brancart, J.; Roels, E.; Verhelle, R.; Safaei, A.; Cuvellier, A.; Vanderborght, B.; Van Assche, G. Structure–Property Relationships of Self-Healing Polymer Networks Based on Reversible Diels–Alder Chemistry. Macromolecules 2022, 55, 5497–5513. [Google Scholar] [CrossRef]
- Platonova, E.O.; Vlasov, E.; Pavlov, A.A.; Kireynov, A.; Nelyub, V.A.; Polezhaev, A.V. Self-healing polyurethane based on a difuranic monomer from biorenewable source. J. Appl. Polym. Sci. 2019, 136, 47869. [Google Scholar] [CrossRef]
- Platonova, E.; Chechenov, I.; Pavlov, A.; Solodilov, V.; Afanasyev, E.; Shapagin, A.; Polezhaev, A. Thermally Remendable Polyurethane Network Cross-Linked via Reversible Diels–Alder Reaction. Polymers 2021, 13, 1935. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.T.; Thai, S.H.; Nguyen, H.T.; Phung, D.T.T.; Nguyen, L.T.; Pham, H.Q.; Nguyen, L.-T.T. Tailoring the Hard–Soft Interface with Dynamic Diels–Alder Linkages in Polyurethanes: Toward Superior Mechanical Properties and Healability at Mild Temperature. Chem. Mater. 2019, 31, 2347–2357. [Google Scholar] [CrossRef]
- Platonova, E.; Ponomareva, P.; Lokiaeva, Z.; Pavlov, A.; Nelyub, V.; Polezhaev, A. New Building Blocks for Self-Healing Polymers. Polymers 2022, 14, 5394. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.K.; Behera, P.K.; Pal, T.S.; Mondal, P.; Naskar, K.; Singha, N.K. Self-healable hydrophobic polymer material having urethane linkages via a non-isocyanate route and dynamic Diels–Alder ‘click’ reaction. Chem. Commun. 2021, 57, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-Parrado, K.-K.; Avérous, L. Renewable Responsive Systems Based on Original Click and Polyurethane Cross-Linked Architectures with Advanced Properties. ChemSusChem 2020, 13, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, Y.; Du, P.; Wang, X.; Yang, B. Polyurethane hot melt adhesive based on Diels–Alder reaction. Int. J. Adhes. Adhes. 2020, 100, 102597. [Google Scholar] [CrossRef]
- Feng, L.; Bian, Y.; Chai, C.; Qiang, X. Effect of Heat-Treatment on Self-healing and Processing Behavior of Thermally Reversible Polyurethanes. J. Polym. Environ. 2020, 28, 647–656. [Google Scholar] [CrossRef]
- Gol′dfarb, Y.L.; Ostapenko, É.G.; Vinogradova, V.G.; Zverev, A.N.; Polyakov, A.V.; Yanovskii, A.I.; Yufit, D.S.; Struchkov, Y.T. Synthesis, structures, and redox properties of some new dithiocarbamates that include a heteroaromatic ring. Chem. Heterocycl. Compd. 1987, 23, 740–747. [Google Scholar] [CrossRef]
- Salewska, N.; Milewska, M.J. Efficient Method for the Synthesis of Functionalized Basic Maleimides. J. Heterocycl. Chem. 2014, 51, 999–1003. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, Y.; Na, Q.; Guo, T.; Zhang, M.; Qi, Z.; Liu, N.; Yang, F.; Luo, Y.; Yang, W. Preparation and characterization of self-healing furan-terminated polybutadiene (FTPB) based on Diels–Alder reaction. RSC Adv. 2021, 11, 32369–32375. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, N.; Mo, H.; Lu, X.; Dou, J.; Tan, B. Synthesis and Properties of Thermally Self-Healing PET Based Linear Polyurethane Containing Diels–Alder Bonds. Polymers 2022, 14, 3334. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.-M.; Vanderstappen, S.; Meyer, F.; Verge, P.; Alexandre, M.; Thomassin, J.-M.; Jérôme, C.; Dubois, P. Design of Cross-Linked Semicrystalline Poly(ε-caprolactone)-Based Networks with One-Way and Two-Way Shape-Memory Properties through Diels–Alder Reactions. Chem–A Eur. J. 2011, 17, 10135–10143. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic study of Diels–Alder reaction involving in maleimide–furan compounds and linear polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Zakharova, D.V.; Aysin, R.R.; Pavlov, A.A.; Khanin, D.A.; Platonova, E.O.; Nelyubina, Y.V.; Polezhaev, A.V. Tandem Diels–Alder reaction overrules entropy: The gate to thermally stable, yet thermally recyclable furan-based polymers. Green Chem. 2025, 27, 2263–2275. [Google Scholar] [CrossRef]
- Averochkin, G.M.; Gordeev, E.G.; Kucherov, F.A.; Ananikov, V.P. Rapid access to molecular complexity from bioderived 5-HMF derivatives via cascade cycloadditions. Green Chemistry 2023, 25, 1045–1055. [Google Scholar] [CrossRef]
- Kvyatkovskaya, E.A.; Epifanova, P.P.; Nikitina, E.V.; Senin, A.A.; Khrustalev, V.N.; Polyanskii, K.B.; Zubkov, F.I. Synthesis and ethylene-promoted metathesis of adducts of tandem [4 + 2]/[4 + 2] cycloaddition between bis-furyl dienes and maleic acid derivatives. New J. Chem. 2021, 45, 3400–3407. [Google Scholar] [CrossRef]
- Engel, T.; Kickelbick, G. Thermoreversible Reactions on Inorganic Nanoparticle Surfaces: Diels–Alder Reactions on Sterically Crowded Surfaces. Chem. Mater. 2013, 25, 149–157. [Google Scholar] [CrossRef]
- Feng, L.; Yu, Z.; Bian, Y.; Lu, J.; Shi, X.; Chai, C. Self-healing behavior of polyurethanes based on dual actions of thermo-reversible Diels–Alder reaction and thermal movement of molecular chains. Polymer 2017, 124, 48–59. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, C.; Zhang, Z.; Xu, Q.; Hu, H.; Wei, Y. Preparation and properties of self-healing polyurethane without external stimulation. Polym. Bull. 2022, 79, 10723–10739. [Google Scholar] [CrossRef]
- Chen, T.K.; Chui, J.Y.; Shieh, T.S. Glass Transition Behaviors of a Polyurethane Hard Segment based on 4,4’-Diisocyanatodiphenylmethane and 1,4-Butanediol and the Calculation of Microdomain Composition. Macromolecules 1997, 30, 5068–5074. [Google Scholar] [CrossRef]
- Kojio, K.; Nozaki, S.; Takahara, A.; Yamasaki, S. Influence of chemical structure of hard segments on physical properties of polyurethane elastomers: A review. J. Polym. Res. 2020, 27, 140. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, K.; Huang, X.; Ma, X.; Wei, Y. Preparation and properties of self-healing polyether amines based on Diels–Alder reversible covalent bonds. High Perform. Polym. 2018, 31, 51–62. [Google Scholar] [CrossRef]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels-Alder and retro-Diels-Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Zenitova, I.N.B.D.A.R.F.G.A. Thermomechanical analysis of polyurethanes based on secondary raw materials. Polym. Sci. Ser. B 1998, 40, 1666–1670. [Google Scholar]
- Yu, S.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P. Bio-Inspired High-Performance and Recyclable Cross-Linked Polymers. Adv. Mater. 2013, 25, 4912–4917. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, C.; Jia, H.; Shen, Z.; Zhao, R.; Su, T.; Xiang, B.; Wang, X.; Boukhvalov, D.W.; Luo, Z.; et al. Novel Molecular-Level Insight into the Self-Healing Behavior and Mechanism of Polyurethane-Urea Elastomer Based on a Noncovalent Strategy. Macromolecules 2022, 55, 4776–4789. [Google Scholar] [CrossRef]
- Ghoroghchian, F.; Bayat, Y.; Abrishami, F. Preparation of the polyurethane elastomer based on polypropylene glycol-glycidyl azide polymer-polypropylene glycol. J. Polym. Res. 2022, 29, 472. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Cai, S.-Y.; Chuang, F.-S.; Rwei, S.-P. A self-healing waterborne poly(urethane-urea) on reversible covalent interaction for textile breathable coating. J. Appl. Polym. Sci. 2022, 139, e52773. [Google Scholar] [CrossRef]
- Hu, S.; Shou, T.; Guo, M.; Wang, R.; Wang, J.; Tian, H.; Qin, X.; Zhao, X.; Zhang, L. Fabrication of New Thermoplastic Polyurethane Elastomers with High Heat Resistance for 3D Printing Derived from 3,3-Dimethyl-4,4′-diphenyl Diisocyanate. Ind. Eng. Chem. Res. 2020, 59, 10476–10482. [Google Scholar] [CrossRef]
- Yi, P.; Chen, J.; Chang, J.; Wang, J.; Lei, Y.; Jing, R.; Liu, X.; Sun, A.; Wei, L.; Li, Y. Self-Healable, Strong, and Tough Polyurethane Elastomer Enabled by Carbamate-Containing Chain Extenders Derived from Ethyl Carbonate. Polymers 2022, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Cao, W.; Duan, X.; Xie, T.; Li, Z.; Gao, W.; Luo, F. Synthesis of Diels–Alder bond and hydrogen bond synergistic shape memory self-healing polyurethane elastomers. J. Appl. Polym. Sci. 2024, 141, e55070. [Google Scholar] [CrossRef]
- Jourdain, A.; Asbai, R.; Anaya, O.; Chehimi, M.M.; Drockenmuller, E.; Montarnal, D. Rheological Properties of Covalent Adaptable Networks with 1,2,3-Triazolium Cross-Links: The Missing Link between Vitrimers and Dissociative Networks. Macromolecules 2020, 53, 1884–1900. [Google Scholar] [CrossRef]
- Yang, R.; Hu, J.; Li, W.; Zhang, X. A super strong polyurethane elastomer with efficient self-healing performance enabled by partially mixing reversible hard domains. Eur. Polym. J. 2024, 213, 113123. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and self-healing polyurethanes using dynamic chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]


| PU System | Prepolymer | Curing Agent |
|---|---|---|
| PU-H1 | HEMI-TDI-PPG | DFA-H |
| PU-H2 | FA-H | |
| PU-H3 | FOH-H | |
| PU-M1 | DFA-M | |
| PU-M2 | FA-M | |
| PU-M3 | FOH-M | |
| PU-T1 | DFA-T | |
| PU-T2 | FA-T | |
| PU-T3 | FOH-T | |
| PU-(NFu2)2 | DFA-TDI-PPG | BMI |
| PU-(NFu)2 | FA-TDI-PPG | |
| PU-(OFu)2 | FOH-TDI-PPG |
| Sample | Mn (Da) | Mw (Da) | Mw/Mn |
|---|---|---|---|
| PU-H1 | 3100 | 10,300 | 3 |
| PU-H2 | 3000 | 9900 | 3 |
| PU-H3 | 3700 | 11,300 | 3 |
| PU-M1 | 4700 | 27,000 | 6 |
| PU-M2 | 13,500 | 93,300 | 7 |
| PU-M3 | 4800 | 26,000 | 6 |
| PU-T1 | 3100 | 9900 | 3 |
| PU-T2 | 2800 | 9800 | 4 |
| PU-T3 | 5600 | 38,400 | 7 |
| PU-(NFu2)2 | 2700 | 28,000 | 10 |
| PU-(NFu)2 | 6200 | 112,400 | 18 |
| PU-(OFu)2 | 2700 | 23,000 | 9 |
| Sample | T5%, °C | T10%, °C | Tmax, °C | Char Residue, % |
|---|---|---|---|---|
| PU-H1 | 278 | 304 | 371 | 9 |
| PU-H2 | 239 | 283 | 373 | 4 |
| PU-H3 | 236 | 278 | 377 | 15 |
| PU-M1 | 268 | 287 | 383 | 10 |
| PU-M2 | 241 | 253 | 269 | 23 |
| PU-M3 | 270 | 301 | 382 | 17 |
| PU-T1 | 256 | 284 | 376 | 9 |
| PU-T2 | 233 | 266 | 378 | 10 |
| PU-T3 | 207 | 215 | 226 | 26 |
| PU-(NFu2)2 | 275 | 297 | 380 | 17 |
| PU-(NFu)2 | 247 | 286 | 386 | 17 |
| PU-(OFu)2 | 278 | 314 | 387 | 18 |
| Sample | Tgss, °C | Tghs,°C | TrDA(classic),°C | TrDA(tandem),°C |
|---|---|---|---|---|
| PU-H1 | −48 | −2 | 114 | 230 |
| PU-H2 | −50 | −4 | 137 | - |
| PU-H3 | −44 | −4 | 139 | - |
| PU-M1 | −50 | 7 | 116 | 220 |
| PU-M2 | −44 | 7 | 142 | - |
| PU-M3 | −38 | 4 | 139 | - |
| PU-T1 | −50 | 7 | 106, 155 | 226 |
| PU-T2 | −47 | 7 | 114, 141 | - |
| PU-T3 | −40 | 6 | 110, 143 | - |
| PU-(NFu2)2 | −55 | −4 | 137 | 226 |
| PU-(NFu)2 | −54 | −7 | 136 | - |
| PU-(OFu)2 | −51 | - | 113 | - |
| Sample | Tgss, °C | TrDA_H, °C |
|---|---|---|
| PU-H1 | −51 | 134 |
| PU-H2 | −44 | 130 |
| PU-M1 | −50 | 184 |
| PU-M2 | 6 | 116 |
| PU-T1 | −62 | 165 |
| PU-T2 | −50 | 151 |
| PU-(NFu2)2 | −54 | 185 |
| PU-(NFu)2 | −53 | 126 |
| PU-H1 | −51 | 134 |
| Samples | Eo (MPa) | Eh (MPa) | ηE (%) | σo (MPa) | σh (MPa) | ησ (%) |
|---|---|---|---|---|---|---|
| PU-H1 | 42 ± 4 | 27 ± 4 | 64 | 14 ± 1 | 18 ± 2 | 129 |
| PU-H2 | 54 ± 4 | 54 ± 12 | 100 | 11 ±1 | 9 ± 2 | 81 |
| PU-M1 | 95 ± 7 | 42 ± 4 | 44 | 20 ± 3 | 12 ± 3 | 60 |
| PU-M2 | 23 ± 2 | 17 ± 3 | 74 | 11 ± 1 | 7 ± 2 | 64 |
| PU-T1 | 110 ± 5 | 84 ± 17 | 76 | 8 ± 1 | 9 ± 1 | 113 |
| PU-T2 | 55 ± 3 | 51 ± 8 | 93 | 10 ± 1 | 9 ± 1 | 90 |
| PU-(NFu2)2 | 40 ± 4 | 27 ± 7 | 68 | 3.2 ± 0.3 | 2.1 ± 0.3 | 67 |
| PU-(NFu)2 | 14 ± 4 | 7 ± 1 | 50 | 2.4 ± 0.3 | 1.2 ± 0.1 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponomareva, P.; Lokiaeva, Z.; Zakharova, D.; Tretyakov, I.; Platonova, E.; Shapagin, A.; Alexeeva, O.; Antoshkina, E.; Solodilov, V.; Yurkov, G.; et al. Furan–Urethane Monomers for Self-Healing Polyurethanes. Polymers 2025, 17, 1951. https://doi.org/10.3390/polym17141951
Ponomareva P, Lokiaeva Z, Zakharova D, Tretyakov I, Platonova E, Shapagin A, Alexeeva O, Antoshkina E, Solodilov V, Yurkov G, et al. Furan–Urethane Monomers for Self-Healing Polyurethanes. Polymers. 2025; 17(14):1951. https://doi.org/10.3390/polym17141951
Chicago/Turabian StylePonomareva, Polina, Zalina Lokiaeva, Daria Zakharova, Ilya Tretyakov, Elena Platonova, Aleksey Shapagin, Olga Alexeeva, Evgenia Antoshkina, Vitaliy Solodilov, Gleb Yurkov, and et al. 2025. "Furan–Urethane Monomers for Self-Healing Polyurethanes" Polymers 17, no. 14: 1951. https://doi.org/10.3390/polym17141951
APA StylePonomareva, P., Lokiaeva, Z., Zakharova, D., Tretyakov, I., Platonova, E., Shapagin, A., Alexeeva, O., Antoshkina, E., Solodilov, V., Yurkov, G., & Berlin, A. (2025). Furan–Urethane Monomers for Self-Healing Polyurethanes. Polymers, 17(14), 1951. https://doi.org/10.3390/polym17141951










