Titanate-Coupled Aluminum as an Interfacial Modifier for Enhanced Thermal and Mechanical Performance in Hybrid Epoxy Composites
Abstract
1. Introduction
2. Experimental
2.1. Materials
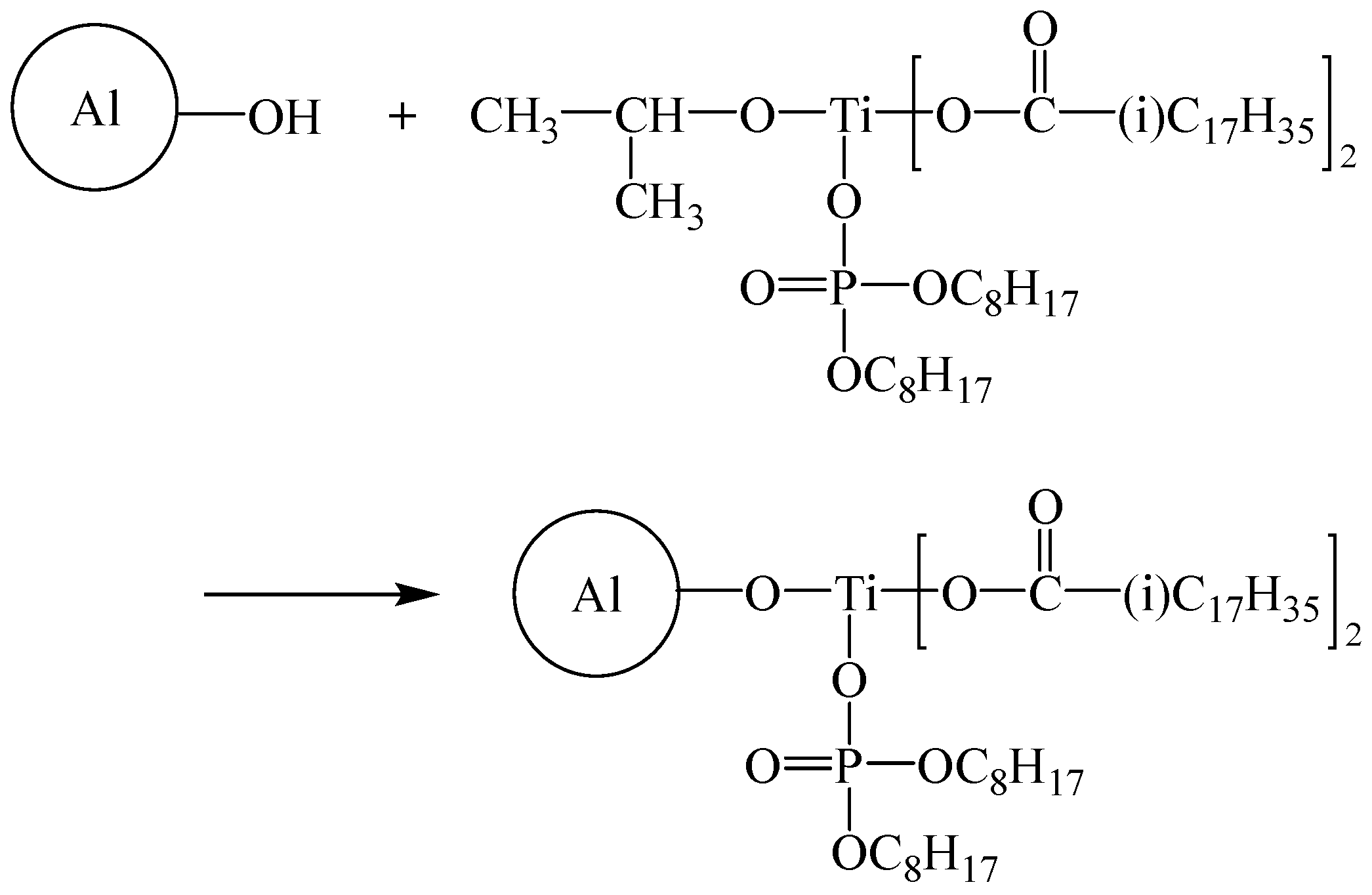
2.2. Surface Modification of Al Powder
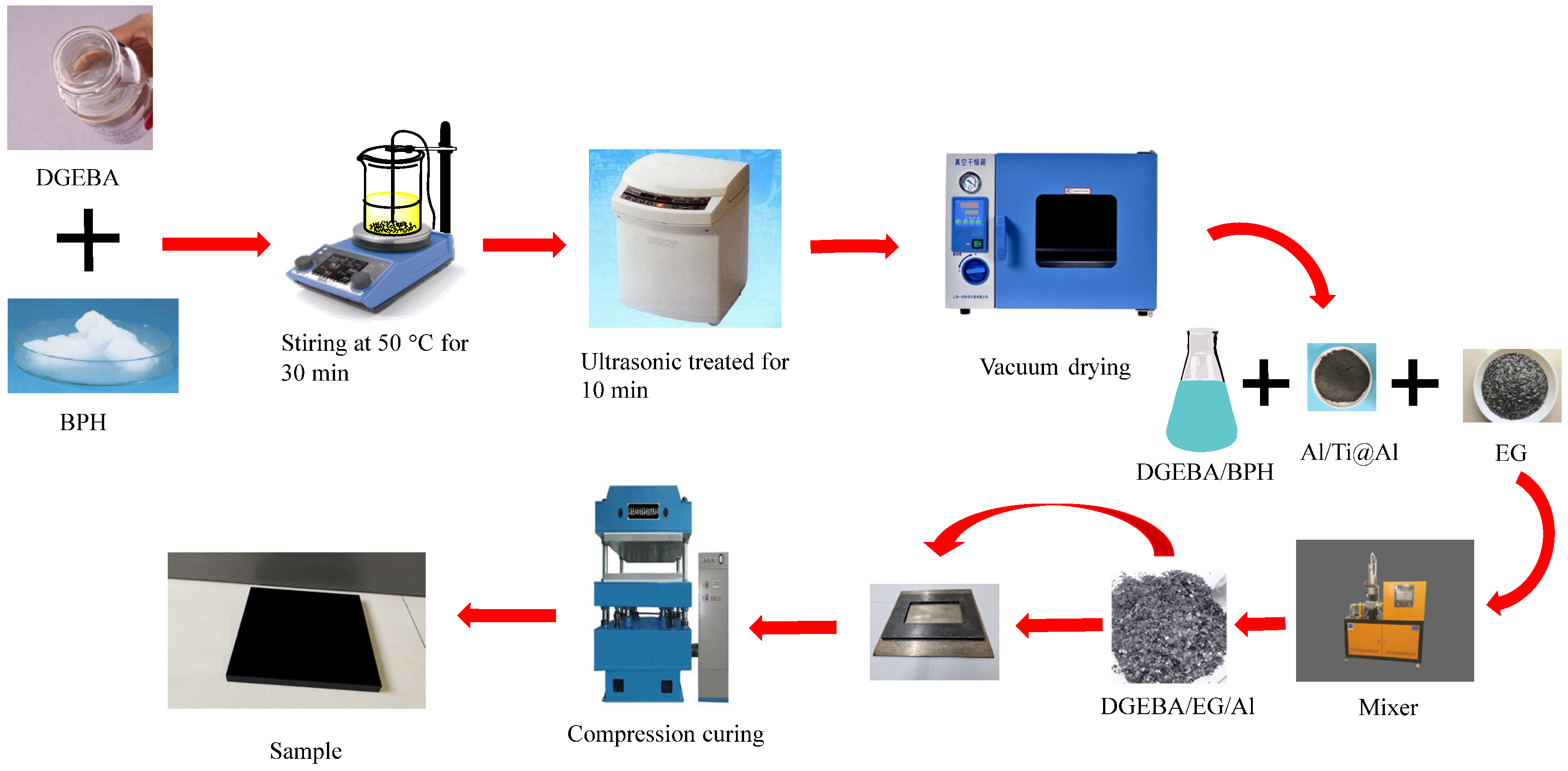
2.3. Preparation of the Composite Samples
2.4. Characterization and Measurements
3. Results and Discussion
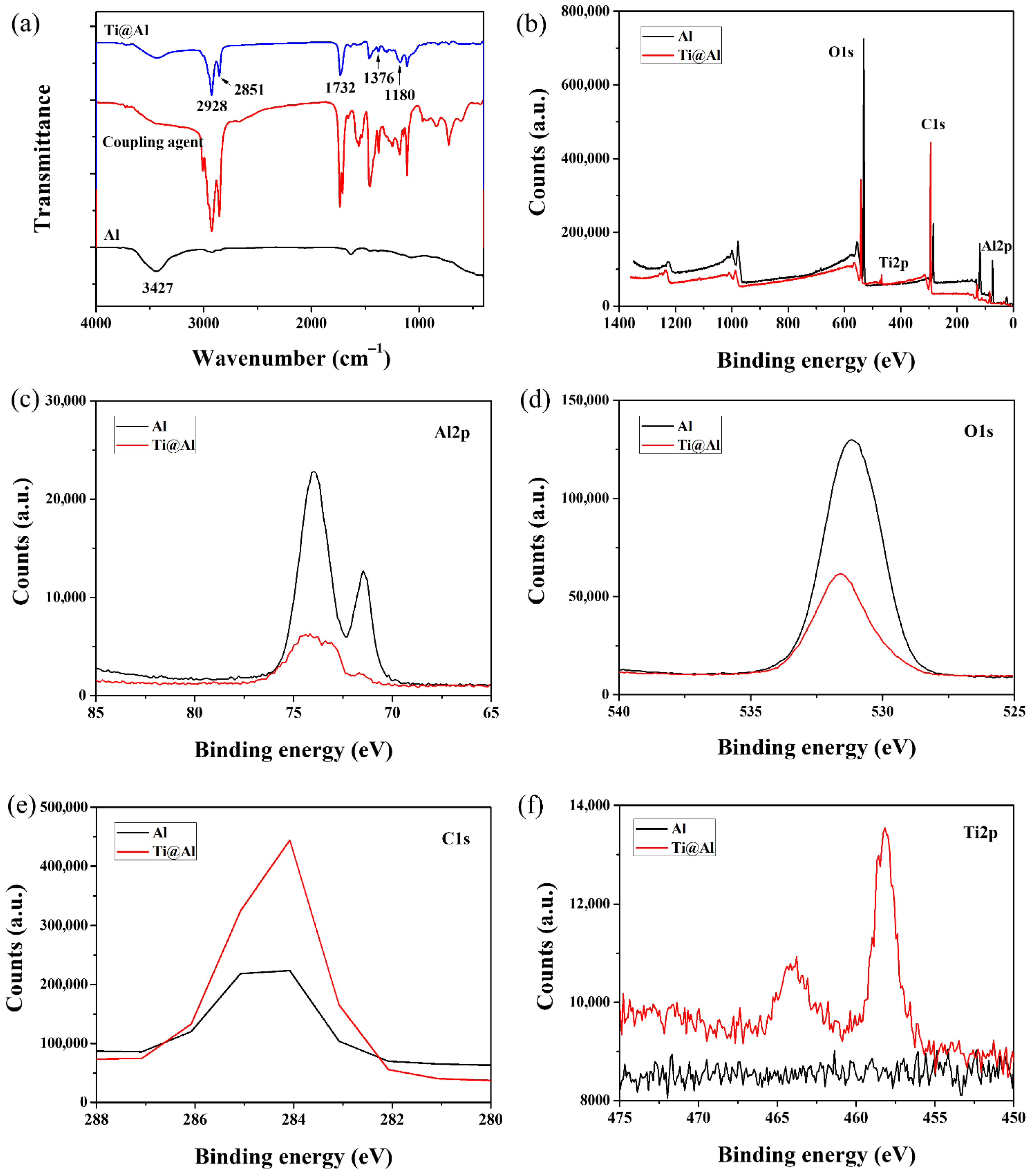
3.1. Characterization of Ti@Al
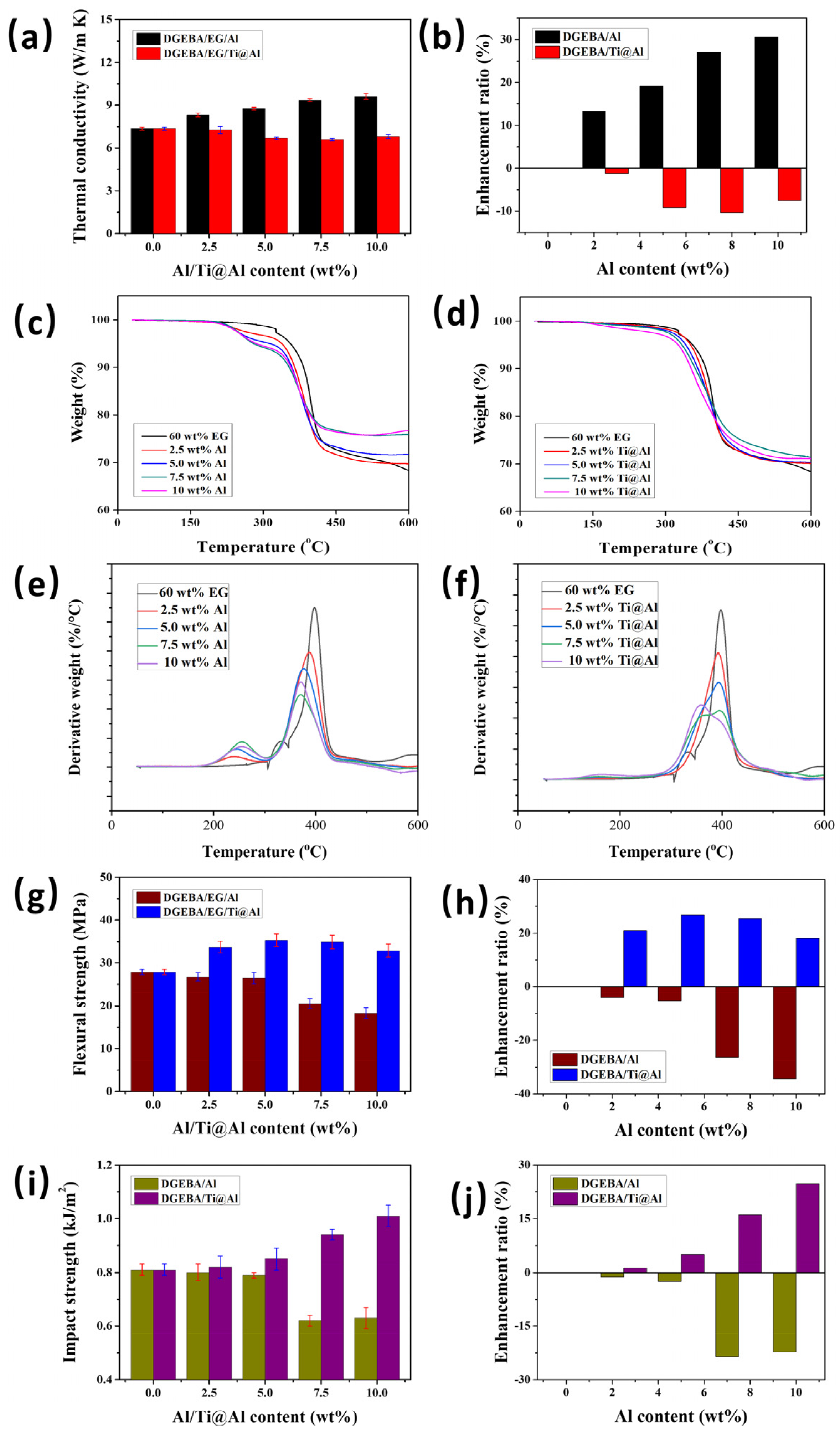
3.2. Thermal Conductivity
3.3. Thermal Stability
3.4. Flexural Strength
3.5. Impact Strength
3.6. Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, P.; Guo, H.; Niu, H.; Li, R.; Yin, G.; Kang, L.; Ren, L.; Lv, R.; Tian, H.; Liu, S.; et al. Core–Shell Engineered Fillers Overcome the Electrical-Thermal Conductance Trade-Off. ACS Nano 2024, 18, 30593–30604. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, W.; Yang, L.; Zhang, C. Construction of alumina framework with a sponge template toward highly thermally conductive epoxy composites. Polym. Eng. Sci. 2024, 64, 1812–1821. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Wu, Y.; Wang, D.; Pan, L. Effect of functionalization on thermal conductivity of hexagonal boron nitride/epoxy composites. Int. J. Heat Mass Transf. 2024, 219, 124844. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Xue, K.; Liu, L.; Li, J.; Huang, Y. Epoxy composites with satisfactory thermal conductivity and electromagnetic shielding yet electrical insulation enabled by Al2O3 platelet-isolated MXene porous microsphere networks. Compos. Sci. Technol. 2024, 248, 110425. [Google Scholar] [CrossRef]
- Guo, F.; Xue, K.; You, T.; Hua, Z.; Liu, L.; Li, J.; Huang, Y. Magnetically assisted construction of Al2O3 platelets dual network and its excellent thermal conductivity in epoxy resin composites. Compos. Part A 2024, 179, 107988. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Luo, M.; Zhang, Y.; Cao, X.; Zhang, Z.; Wang, R.; Zhang, X. Superior thermal transport and electrically insulating properties of epoxy composites with waxberry-like calcined alumina/poly diallyldimethylammonium chloride/diamond. Compos. Sci. Technol. 2024, 248, 110440. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, W.; Mu, M.; Chen, G.; Yu, W.; Liu, X. Highly thermal conductive epoxy composites enabled by 3D graphene/Cu-based dual networks for efficient thermal management. Compos. Commun. 2024, 46, 101845. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Liang, J.; Zhang, T.; Jiang, X.; Liu, P. Synthesis of PVA@S-BNNSs flexible fiber backbone by electrostatic spinning assisting the construction of excellent ductile epoxy thermal interface materials. Compos. Sci. Technol. 2024, 247, 110435. [Google Scholar] [CrossRef]
- Leng, G.; Yan, W.; Tang, Q.; Li, J.; Xu, Z.; Jiang, X.; Han, Y.; Zhang, C.; Li, Z. Exploring epoxy resin sealants for sustained casing pressure mitigation in the wellbore. Constr. Build. Mater. 2024, 413, 134945. [Google Scholar] [CrossRef]
- Yan, Z.-G.; Wang, Z.-P.; Liu, Y.-Y.; Xiao, Y.; Yue, N. Research on Properties of Silicone-Modified Epoxy Resin and 3D Printing Materials. ACS Omega 2023, 8, 23044–23050. [Google Scholar] [CrossRef]
- Yao, D.; Li, Y.; Zhang, H.; Xin, Y.; Fan, W.; Zheng, Y. Preparation and mechanical properties of epoxy resin filled with shaped hybrid nanobrushes. React. Funct. Polym. 2024, 195, 105814. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, X.; Bao, X.; Wu, F.; Han, X.; Wang, J. The Effect of Polyepoxyphenylsilsesquioxane and Diethyl Bis(2-hydroxyethyl)aminomethylphosphonate on the Thermal Stability of Epoxy Resin. ACS Omega 2023, 8, 2077–2084. [Google Scholar] [CrossRef]
- Xie, Y.; Du, X.; Tian, Q.; Dong, Y.; Zhou, Q. Investigation of hydroxyl-terminated polydimethylsiloxane-modified epoxy resin. Mater. Chem. Phys. 2024, 313, 128822. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Wang, Z.; Qin, Q.; Liang, L.; Wang, J.; Jin, P. Effect of epoxy resin/mineralized film composite coating on the corrosion resistance of Mg-3Nd alloy. J. Mater. Res. Technol. 2024, 29, 1650–1663. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Alothman, O.Y.; Fouad, H. Effects of Nanoclay on Mechanical and Dynamic Mechanical Properties of Bamboo/Kenaf Reinforced Epoxy Hybrid Composites. Polymers 2021, 13, 395. [Google Scholar] [CrossRef]
- Je, P.C.; Sultan, M.T.H.; Selvan, C.P.; Irulappasamy, S.; Mustapha, F.; Basri, A.A.; Safri, S.N.A. Manufacturing challenges in self-healing technology for polymer composites—A review. J. Mater. Res. Technol. 2020, 9, 7370–7379. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, J.; Sakai, E.; Feng, H.; Wu, H.; Yamaguchi, H.; Chonan, Y.; Nomura, M. Enhancement of thermal conductivity and mechanical properties of silicone rubber with oriented fillers connected by covalent bonds. Compos. Part A 2025, 198, 109109. [Google Scholar] [CrossRef]
- Pušnik Črešnar, K.; Vidal, J. Green Engineering of Bio-Epoxy Resin: Functionalized Iron-Oxide Nanoparticles for Enhanced Thermal, Mechanical, Surface and Magnetic Properties. Polymers 2025, 17, 1819. [Google Scholar] [CrossRef]
- Seon Lee, Y.; Ryeol Kim, N.; Ki Park, S.; Ko, Y.-i.; Shin, Y.; Yang, B.; Yang, C.-M. Effects of high-temperature thermal reduction on thermal conductivity of reduced graphene oxide polymer composites. Appl. Surf. Sci. 2024, 650, 159140. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Tong, X.; Li, Y.; Sun, J.; Qian, L.; Li, H.; Gu, X.; Zhang, S. The fabrication of a boron nitride/ammonium polyphosphate skeleton based on ice template method for thermal conductive and flame retardant epoxy. Polym. Degrad. Stab. 2024, 219, 110606. [Google Scholar] [CrossRef]
- Liang, L.; Feng, Y.; Yang, K.; Wang, Z.; Zhang, Z.; Chen, X.; Chen, Q. High thermal conductivity composite h-BN/EP obtained by pulsed square-wave electric field induction. Polymer 2024, 290, 126491. [Google Scholar] [CrossRef]
- Li, L.; Jiang, H.; Liu, Y.; Zhao, Q.; Tao, J.; Fan, Y.; Liu, Y.; Li, C.; Yi, J. Improvement of thermal conductivity and wear property of Gr/EP composites with CNTs/Cu foam as 3-dimensional reinforcing skeleton. J. Mater. Res. Technol. 2024, 29, 1172–1182. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Singh, P.; Mahajan, R.L. Role of oxygen functional groups for improved performance of graphene-silicone composites as a thermal interface material. Carbon 2019, 145, 131–139. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Fang, Q.; Li, W.; Wei, H.; Zhao, T.; Zhang, Y.; Huang, G.; Cui, C.; Zhang, K. Preparation of nickel nanoparticle-decorated graphene/copper composites with enhanced interfacial bonding and heat dissipation properties. J. Alloys Compd. 2025, 1028, 180696. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H.; Shen, Y.; Yang, P. Single-walled carbon nanotubes to promote thermal conductance at the pillared-graphene/epoxy polymer interface. Eur. Polym. J. 2024, 202, 112632. [Google Scholar] [CrossRef]
- Zong, Y.; Gui, D.; Niu, K. Enhanced thermal and electrical conductivity in epoxy nanocomposites via liquid crystal-driven alignment of graphene. Mater. Today Commun. 2024, 38, 108067. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Du, J.; Hu, S. Effect of N–Ni coordination bond on the electrical and thermal conductivity of epoxy resin/nickel-coated graphite. J. Appl. Polym. Sci. 2024, 141, e55090. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, H.; Wen, J.; Chen, J.; Mao, Z.; Zhu, P.; Sun, R.; Wu, W.; Peng, J. In-situ growth of diamond/Ag as hybrid filler for enhancing thermal conductivity of liquid crystal epoxy. Diamond Relat. Mater. 2024, 141, 110659. [Google Scholar] [CrossRef]
- Li, S.; Wu, W.; Drummer, D.; Wang, Y.; Lu, Z.; Zhao, X. Copper and graphene work together to construct a three-dimensional skeleton thermal conductivity network to improve the thermal conductivity of the epoxy resin. Polym. Compos. 2023, 44, 5369–5380. [Google Scholar] [CrossRef]
- Arockia Jaswin, M.; Florence, A.; Thirumal Azhagan, M.; Mathialagan, S. Influence of graphene oxide nano particle and aluminum powder on mechanical and thermal behavior of carbon fiber epoxy composite. Mater. Today Proc. 2023, 72, 2358–2368. [Google Scholar] [CrossRef]
- Kim, H.; Yeo, S.; Lee, Y.-k. Graphite-boron composites for enhanced thermal conductivity and irradiation shielding for use in spent nuclear fuel dry casks. J. Alloys Compd. 2025, 1036, 181515. [Google Scholar] [CrossRef]
- Wu, L.; Xiang, D. Novel in-situ constructing approach for vertically aligned AlN skeleton and its thermal conductivity enhancement effect on epoxy. Ceram. Int. 2023, 49, 5707–5719. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, Y.; Shen, M.; Yu, S.; Zhu, Y.; Xu, Y.; Liu, H.; Fu, R. 3D printing of a SiO2@BN TPMS structure: Efficient heat transfer strategy for BN/epoxy composites. Ceram. Int. 2024, 50, 3820–3828. [Google Scholar] [CrossRef]
- Hanif, Z.; Khoe, D.D.; Choi, K.-I.; Jung, J.-H.; Pornea, A.G.M.; Yanar, N.; Kwak, C.; Kim, J. Synergistic effect on dispersion, thermal conductivity and mechanical performance of pyrene modified boron nitride nanotubes with Al2O3/epoxy composites. Compos. Sci. Technol. 2024, 247, 110419. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Zhang, D.; Peng, Y.; Wan, D.; Peng, T.; Lv, K. Multifunctional Graphite Nanosheet–Hydrophilic Epoxy Anticorrosion Coatings via Size Confinement of Exfoliated Graphite. Polymers 2025, 17, 1803. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Yin, H.; Ding, Y.; Li, C. Convenient preparation of expanded graphite and graphite nanosheets as well as improvement of electrical conductivity of polyurethane by filling graphite nanosheets. Mater. Sci. Eng. B 2024, 300, 117061. [Google Scholar] [CrossRef]
- Navin, M.; Ramakrishnan, T.; Balaji, D.; Bhuvaneswari, V. CRITIC–EDAS Approach for Evaluating Mechanical Properties of Flax/Vetiver/MFF Hybrid Composites. Polymers 2025, 17, 1790. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Z.; Guo, X.; Yang, S.; Jia, H.; Tao, Z.; Liu, J.; Yan, X.; Liu, Z.; Li, J. Expanded graphite/graphene composites for high through-plane thermal conductivity. Diamond Relat. Mater. 2024, 143, 110865. [Google Scholar] [CrossRef]
- Sun, X.; Liu, D.; Zhang, Y.; Zhang, G.; Wang, F.; Wang, X. Methane hydrate formation enhanced by thermally expanded graphite with multi-sized pores. Chem. Eng. J. 2024, 480, 148280. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hsiao, C.-H.; Ya, J.-Y.; Hsieh, P.-Y. Preparation of expanded graphite with (NH4)2S2O8 and H2SO4 by using microwave irradiation. J. Taiwan Inst. Chem. Eng. 2024, 154, 105026. [Google Scholar] [CrossRef]
- Khoramian, R.; Issakhov, M.; Pourafshary, P.; Gabdullin, M.; Sharipova, A. Surface modification of nanoparticles for enhanced applicability of nanofluids in harsh reservoir conditions: A comprehensive review for improved oil recovery. Adv. Colloid Interface Sci. 2024, 333, 103296. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, C.; Hou, A.; Qiu, J.; Wang, S. One-pot in situ synthesis of expandable graphite-encapsulated paraffin composites for thermal energy storage. Chem. Eng. J. 2024, 481, 148541. [Google Scholar] [CrossRef]
- Harichandran, R.; Kumar, R.V.; Venkateswaran, M. Experimental and numerical evaluation of thermal conductivity of graphene nanoplatelets reinforced aluminium composites produced by powder metallurgy and hot extrusion technique. J. Alloys Compd. 2022, 900, 163401. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Y.; Liu, J.; Chen, Q. Copper coated graphene reinforced aluminum composites with enhanced mechanical strength and conductivity. Vacuum 2023, 218, 112610. [Google Scholar] [CrossRef]
- Miller, K.K.; Shancita, I.; Bhattacharia, S.K.; Pantoya, M.L. Surface modifications of plasma treated aluminum particles and direct evidence for altered reactivity. Mater. Des. 2021, 210, 110119. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Chu, N.; Jin, F.-L.; Park, S.-J. Role of Copper Nanoparticles in the Thermal and Mechanical Properties of Expanded Graphite-Reinforced Epoxy Hybrids. ACS Omega 2024, 9, 17533–17540. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, L.; Verma, R. Optimising thermal conductivity of the epoxy using copper as filler particles for improved performance of cryosorption pumps: An experimental investigation. Fusion Eng. Des. 2024, 205, 114529. [Google Scholar] [CrossRef]
- Mumtaz, N.; Li, Y.; Artiaga, R.; Farooq, Z.; Mumtaz, A.; Guo, Q.; Nisa, F.-U. Fillers and methods to improve the effective (out-plane) thermal conductivity of polymeric thermal interface materials: A review. Heliyon 2024, 10, e25381. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, G.; Yang, F.; Zhang, S.; Wu, Q.; Xu, L.; Li, Y.; Tan, L.; Meng, X.; Yu, J.; et al. Multi-scale-filler reinforcement strategy enabled stretchable silicone elastomer with synergistically enhanced thermal conductivity and mechanical strength. Compos. Part A 2023, 175, 107784. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, K.; Chen, J.; Li, P.; Luo, S.; Yu, S. Isopropyl Trioleyl Titanate Modified AlN for Preparing High-Thermal-Conductivity and Low-Dielectric-Loss Epoxy Electronic Packaging Films. In Proceedings of the 2024 25th International Conference on Electronic Packaging Technology (ICEPT), Tianjin, China, 7–9 August 2024; pp. 1–5. [Google Scholar]
- Wang, J.; Li, W.; Ji, Y.; Li, S.; Yu, Y.; Xiao, R.; Lu, D.; Long, F. Effect of surface modification of boron nitride nanosheets by titanate coupling agent on properties of epoxy resin. Surf. Interfaces 2024, 54, 105270. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, H.; Yang, X.; Tan, Q.; He, C. Study on the Properties of Titanate Coupling Agent, Modified Heavy Calcium Carbonate, and SBS Composite Modified Asphalt. J. Mater. Civ. Eng. 2024, 36, 04024136. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tian, Y.; Qiang, W.; Luo, M.; Wang, H.; Wang, J.; Ma, S. Reinforced polyurethane acrylic resin coating on liquid-crystalline polyester substrates. Mater. Chem. Phys. 2025, 337, 130550. [Google Scholar] [CrossRef]
- Jin, F.-L.; Chu, N.; Yao, S.-S.; Park, S.-J. Thermal and electrical conductivity improvement in epoxy resin with expanded graphite and silver plating. Korean J. Chem. Eng. 2022, 39, 2182–2191. [Google Scholar] [CrossRef]
- GB/T 10294-2008; Thermal Insulation. Determination of Steady-State Thermal Resistance and Related Properties. Guarded Hot Plate Apparatus. The Standards Press of China: Beijing, China, 2008.
- GB/T 9341-2008; Plastics—Determination of Flexural Properties. The Standards Press of China: Beijing, China, 2008.
- GB/T 1843-2008; Plastics—Determination of Izod Impact Strength. The Standards Press of China: Beijing, China, 2008.
- Liu, D.; Liu, Y.; Sui, G. Synthesis and properties of sandwiched films of epoxy resin and graphene/cellulose nanowhiskers paper. Compos. Part A 2016, 84, 87–95. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, Y.; Han, D.; Zheng, J.; Wu, X.; Wang, Z.; Li, X.; Ye, X.; Zhang, L. New designed coupling agents for silica used in green tires with low VOCs and low rolling resistance. Appl. Surf. Sci. 2021, 558, 149819. [Google Scholar] [CrossRef]
- de Paula Santos, L.F.; Monticeli, F.M.; Ribeiro, B.; Costa, M.L.; Alderliesten, R.; Botelho, E.C. Effect of carbon nanotube buckypapers on interlaminar fracture toughness of thermoplastic composites subjected to fatigue tests. Int. J. Fatigue 2025, 195, 108868. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, C.; Zhang, N.; Chen, C.; Di, X.; Zhang, Y. Surface modification of silica micro-powder by titanate coupling agent and its utilization in PVC based composite. Constr. Build. Mater. 2021, 307, 124933. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Zhang, H.; Jin, F.-L.; Park, S.-J. Enhancement of impact strength of poly(lactic acid)/silicon carbide nanocomposites through surface modification with titanate-coupling agents. Bull. Mater. Sci. 2019, 43, 6. [Google Scholar] [CrossRef]
- Ozen, M.; Demircan, G.; Kisa, M.; Acikgoz, A.; Ceyhan, G.; Işıker, Y. Thermal properties of surface-modified nano-Al2O3/Kevlar fiber/epoxy composites. Mater. Chem. Phys. 2022, 278, 125689. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, T.; Fu, M.; Jiang, S.; Zhang, G. Effects of combinative surface modification on the stability and conductivity of the copper particles. J. Alloys Compd. 2014, 585, 277–281. [Google Scholar] [CrossRef]
- Chipara, M.; Lozano, K.; Hernandez, A.; Chipara, M. TGA analysis of polypropylene–carbon nanofibers composites. Polym. Degrad. Stab. 2008, 93, 871–876. [Google Scholar] [CrossRef]
- Golebiewski, J.; Galeski, A. Thermal stability of nanoclay polypropylene composites by simultaneous DSC and TGA. Compos. Sci. Technol. 2007, 67, 3442–3447. [Google Scholar] [CrossRef]
- Kumar, R.; Mohanty, S.; Nayak, S.K. Study on epoxy resin-based thermal adhesive filled with hybrid expanded graphite and graphene nanoplatelet. SN Appl. Sci. 2019, 1, 180. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, K.; Liu, Q.; Wang, C.; Huang, Y.; Wan, W.; Feng, W.; Yao, P. Synergistic reinforcement of copper matrix composites with MoSi2 and graphene: Microstructure, mechanical properties, and tribological performance. Compos. Struct. 2025, 369, 119353. [Google Scholar] [CrossRef]
- Ge, M.; Liang, G.; Gu, A. Structural design strategy of biobased propargyl ether-functionalized epoxy monomer to access low viscosity resin with excellent heat resistance and mechanical properties for advanced composite via VARTM. Chem. Eng. J. 2023, 475, 146269. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Chen, S.; Zhang, J.; Cheng, J.; Miao, M.; Zhang, D. Preparation of Epoxy Resins with Excellent Comprehensive Performance by Thiol-Epoxy Click Reaction. Prog. Org. Coat. 2020, 139, 105436. [Google Scholar] [CrossRef]
- Wang, H.; Yao, S.-S.; Guan, Z.; Jin, F.-L.; Park, S.-J. Electrical property improvement of phenolic formaldehyde resin with graphene and ionic liquid. Korean J. Chem. Eng. 2021, 38, 2332–2340. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Wang, H.; Shin, H. Theoretical understanding of fracture toughness improvement in carbon nanotube-coated fiber/epoxy composites: A multiscale study. Eng. Fract. Mech. 2025, 325, 111276. [Google Scholar] [CrossRef]
- Gfrerrer, M.; Koss, V.; Wiener, J.; Schuecker, C.; Brunner, A.J.; Pinter, G. Comparing Mode I, Mode II and Mixed-Mode I/II interlaminar fracture toughness of glass and carbon fiber reinforced polymer laminates with the same epoxy matrix system. Eng. Fract. Mech. 2025, 320, 111009. [Google Scholar] [CrossRef]
- Quan, H.; Li, W.; Huang, D.; Gao, W.; Sui, S.; Zhang, Y.; Liu, H.; Gu, Z.; Fan, Z.; Liu, J.; et al. Enhanced ablation resistance and mechanical properties of CMP/C-HfC composites contributed by dual-skeleton reinforcement and coating protection. Compos. Part B 2025, 305, 112723. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, G.; Xu, Q.; Liu, G.; Li, S. Fracture behavior and microcracking mechanism of epoxy resin-grouted fractured granite under different loading conditions. Constr. Build. Mater. 2024, 449, 138545. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Zhao, Q.; Li, Z.; Xu, X. Mophology-modulations of TiO2 nanostructures for enhanced photocatalytic performance. Appl. Surf. Sci. 2015, 332, 224–228. [Google Scholar] [CrossRef]





| Al Content (wt%) | Ti@Al Content (wt%) | Thermal Conductivity (W/(m⋅K)) |
|---|---|---|
| 0 | 0 | 7.35 ± 0.12 |
| 2.5 | 0 | 8.33 ± 0.13 |
| 5 | 0 | 8.76 ± 0.08 |
| 7.5 | 0 | 9.34 ± 0.10 |
| 10 | 0 | 9.60 ± 0.21 |
| 0 | 2.5 | 7.26 ± 0.26 |
| 0 | 5 | 6.68 ± 0.09 |
| 0 | 7.5 | 6.59 ± 0.08 |
| 0 | 10 | 6.80 ± 0.14 |
| Al Content (wt%) | Ti@Al Content (wt%) | T5% (°C) a | Amount of Char Formation at 600 °C (%) a |
|---|---|---|---|
| 0 | 0 | 355.7 | 68.3 |
| 2.5 | 0 | 338.2 | 70.1 |
| 5 | 0 | 313.7 | 71.7 |
| 7.5 | 0 | 279.9 | 75.9 |
| 10 | 0 | 285.7 | 76.7 |
| 0 | 2.5 | 351.4 | 69.7 |
| 0 | 5 | 341.4 | 70.3 |
| 0 | 7.5 | 333.5 | 71.1 |
| 0 | 10 | 325.8 | 71.4 |
| Al Content (wt%) | Ti@Al Content (wt%) | Flexural Strength (MPa) |
|---|---|---|
| 0 | 0 | 27.88 ± 0.61 |
| 2.5 | 0 | 26.74 ± 0.98 |
| 5 | 0 | 26.42 ± 1.36 |
| 7.5 | 0 | 20.51 ± 1.17 |
| 10 | 0 | 18.29 ± 1.27 |
| 0 | 2.5 | 33.71 ± 1.37 |
| 0 | 5 | 35.31 ± 1.46 |
| 0 | 7.5 | 34.91 ± 1.61 |
| 0 | 10 | 32.88 ± 1.51 |
| Al Content (wt%) | Ti@Al Content (wt%) | Impact Strength (kJ/m2) |
|---|---|---|
| 0 | 0 | 0.81 ± 0.02 |
| 2.5 | 0 | 0.80 ± 0.03 |
| 5 | 0 | 0.79 ± 0.01 |
| 7.5 | 0 | 0.62 ± 0.02 |
| 10 | 0 | 0.63 ± 0.04 |
| 0 | 2.5 | 0.82 ± 0.04 |
| 0 | 5 | 0.85 ± 0.04 |
| 0 | 7.5 | 0.94 ± 0.02 |
| 0 | 10 | 1.01 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-L.; Lee, S.-Y.; Chu, N.; Lee, S.-Y.; Jin, F.-L.; Park, S.-J. Titanate-Coupled Aluminum as an Interfacial Modifier for Enhanced Thermal and Mechanical Performance in Hybrid Epoxy Composites. Polymers 2025, 17, 1922. https://doi.org/10.3390/polym17141922
Cheng H-L, Lee S-Y, Chu N, Lee S-Y, Jin F-L, Park S-J. Titanate-Coupled Aluminum as an Interfacial Modifier for Enhanced Thermal and Mechanical Performance in Hybrid Epoxy Composites. Polymers. 2025; 17(14):1922. https://doi.org/10.3390/polym17141922
Chicago/Turabian StyleCheng, Hai-Long, Seul-Yi Lee, Na Chu, Se-Yeol Lee, Fan-Long Jin, and Soo-Jin Park. 2025. "Titanate-Coupled Aluminum as an Interfacial Modifier for Enhanced Thermal and Mechanical Performance in Hybrid Epoxy Composites" Polymers 17, no. 14: 1922. https://doi.org/10.3390/polym17141922
APA StyleCheng, H.-L., Lee, S.-Y., Chu, N., Lee, S.-Y., Jin, F.-L., & Park, S.-J. (2025). Titanate-Coupled Aluminum as an Interfacial Modifier for Enhanced Thermal and Mechanical Performance in Hybrid Epoxy Composites. Polymers, 17(14), 1922. https://doi.org/10.3390/polym17141922







