Novel Magnetically Charged Grafts for Vascular Repair: Process Optimization, Mechanical Characterization and In Vitro Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning Process
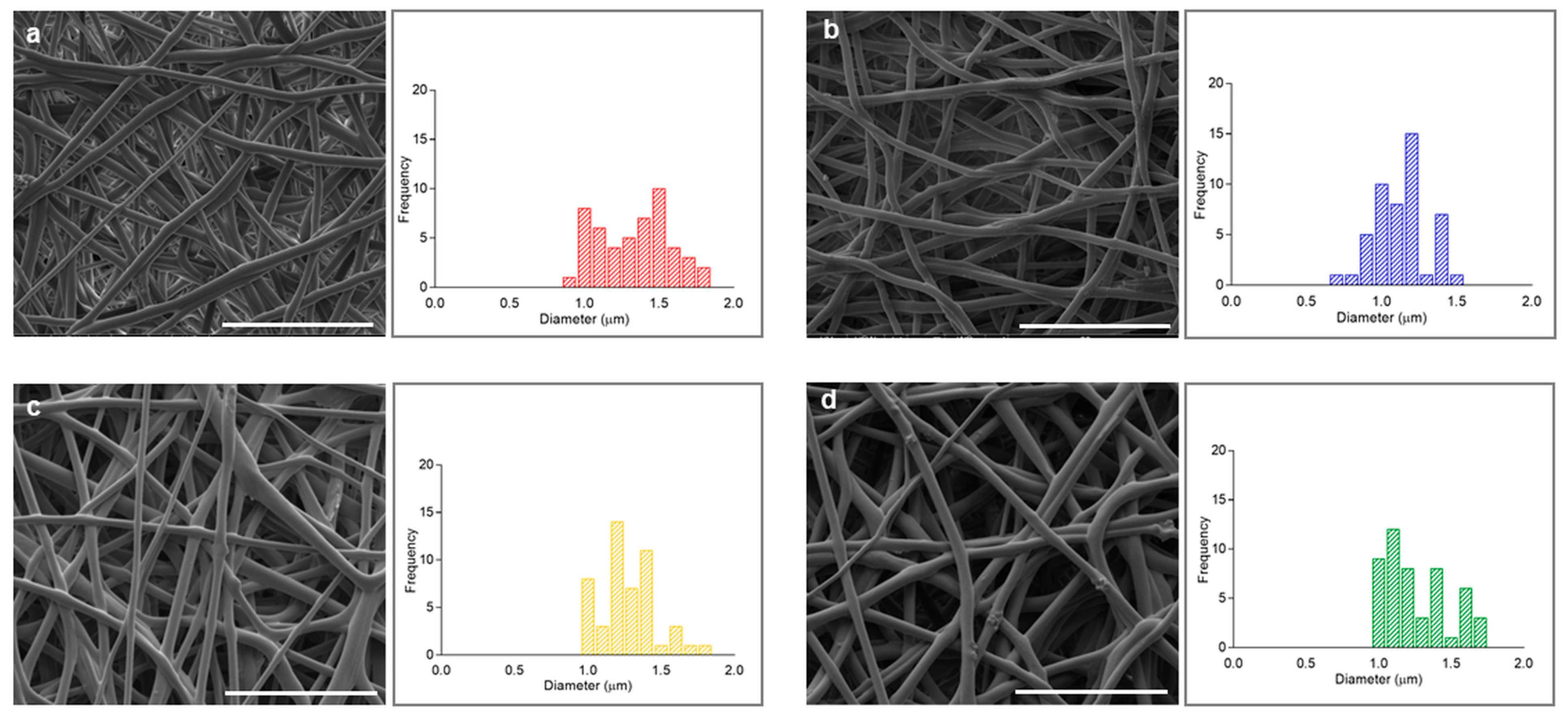
2.3. Morphological Characterization
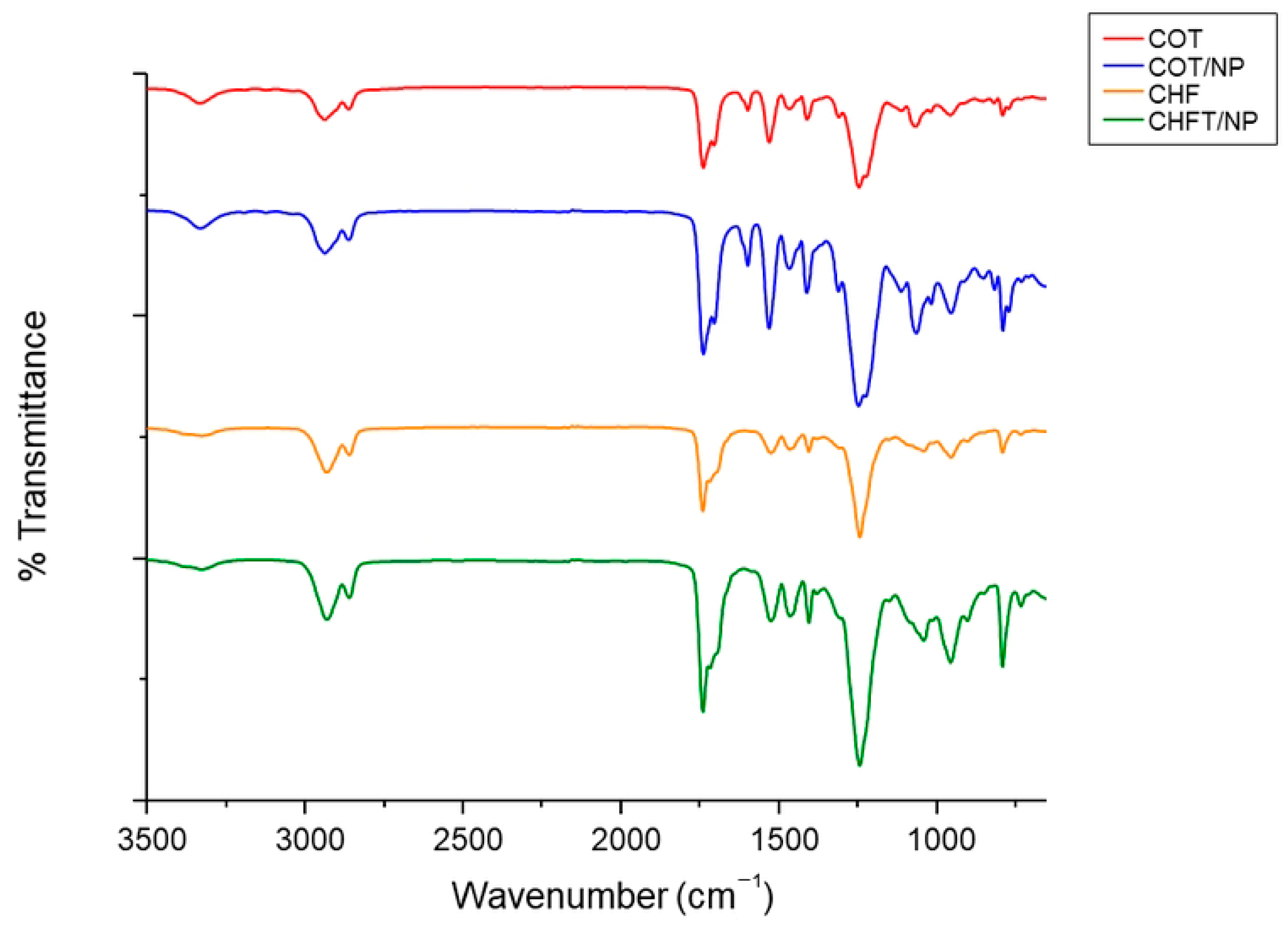
2.4. Physicochemical Analyses
2.5. Thermal Analysis
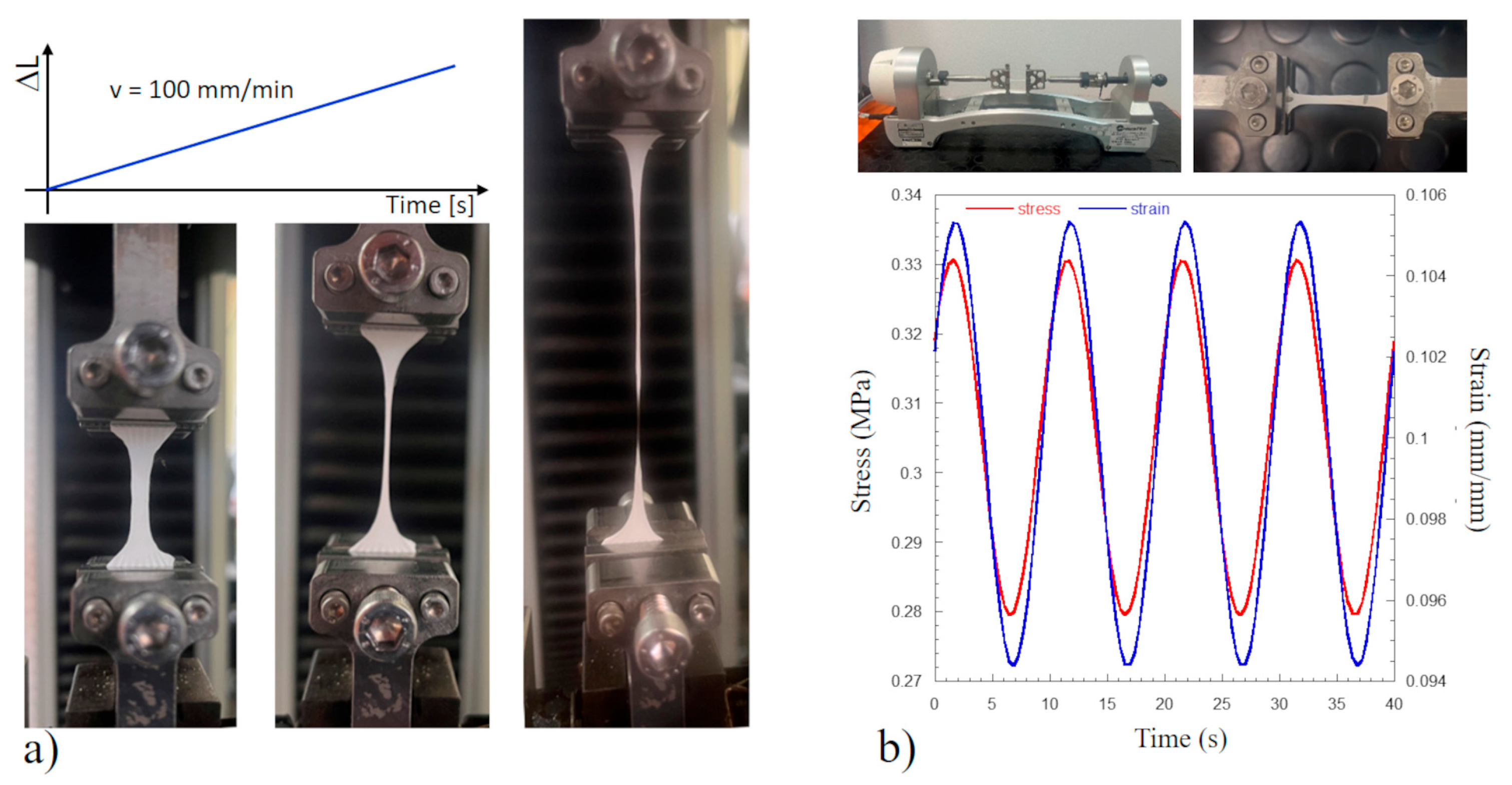
2.6. Mechanical Tests
2.7. In Vitro Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCUs | Polycarbonate urethanes |
| MNPs | Magnetic nanoparticles |
| COT | Corethane A80 |
| CHF | Chronoflex AL80 |
| THF | Tetrahydrofuran |
| DMF | N-N-Dimethylformamide |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| DSC | Differential scanning calorimeter |
| DMA | Dynamic mechanical analysis |
| hBM-MSCs | Human bone marrow mesenchymal stem cells |
| TCP | Tissue culture plate |
| COT/NPs | Corethane A80/nanoparticles |
| CHF/NPs | Chronoflex AL80/nanoparticles |
| ECM | Extracellular matrix |
References
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part. B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, K.M.; Kural, M.H.; Li, Y.; Wang, J.; Hugentobler, E.A.; Niklason, L.E. Bioengineering Human Tissues and the Future of Vascular Replacement. Circ. Res. 2022, 131, 109–126. [Google Scholar] [CrossRef]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef]
- Gupta, P.; Mandal, B.B. Tissue-Engineered Vascular Grafts: Emerging Trends and Technologies. Adv. Funct. Mater. 2021, 31, 2100027. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, D.; Comtois-Bona, M.; Muñoz, M.; Ruel, M.; Suuronen, E.J.; Alarcon, E.I. Manufacturing and Validation of Small-Diameter Vascular Grafts: A Mini Review. iScience 2024, 27, 109845. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Zamani, Y.; Bear, K.L.; Kheradvar, A. Material Characterization and Biocompatibility of Polycarbonate-Based Polyurethane for Biomedical Implant Applications. RSC Adv. 2025, 15, 8839–8850. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of Polyurethane in Biomedical Applications: A Review. Resour. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Jia, L.; Li, M.; Diao, Y.; Han, Z.; Yu, C.; Liu, T. Mechanism Model of Dynamic Polyurethanes with Two Scales of Nodes to Regulate Mechanical Property. Mater. Des. 2025, 250, 113611. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A Brief Discussion on Advances in Polyurethane Applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Zarei, M.; Żwir, M.J.; Michalkiewicz, B.; Gorący, J.; El Fray, M. Template-Assisted Electrospinning and 3D Printing of Multilayered Hierarchical Vascular Grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35525. [Google Scholar] [CrossRef]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun Scaffolds for Tissue Engineering of Vascular Grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Y.; Ma, S.; Wang, L.; Liu, C.; Xu, W.; Shi, J.; Qiao, W.; Yang, H. Small-Diameter Polyurethane Vascular Graft with High Strength and Excellent Compliance. J. Mech. Behav. Biomed. Mater. 2021, 121, 104614. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Chen, M.; Wang, M.; Shao, Z.; Jiang, X.; Wang, K.; Yao, Z.; Yang, S.; Zhang, X.; Gao, W.; et al. Engineering Bioactive M2 Macrophage-Polarized Anti-Inflammatory, Antioxidant, and Antibacterial Scaffolds for Rapid Angiogenesis and Diabetic Wound Repair. Adv. Funct. Mater. 2021, 31, 2100924. [Google Scholar] [CrossRef]
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular Inflammation and Repair: Implications for Re-Endothelialization, Restenosis, and Stent Thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Chen, B.; Li, Y.; Zhu, P.; Wang, P.; Yan, S.; Li, Y.; Yang, F.; Gu, N. Novel Magnetic Silk Fibroin Scaffolds with Delayed Degradation for Potential Long-Distance Vascular Repair. Bioact. Mater. 2022, 7, 126–143. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Tai, G.; Yan, F.; Cai, L.; Xin, C.; Al Islam, S. Graphene Oxide-Loaded Magnetic Nanoparticles within 3D Hydrogel Form High-Performance Scaffolds for Bone Regeneration and Tumour Treatment. Compos. Part. A Appl. Sci. Manuf. 2022, 152, 106672. [Google Scholar] [CrossRef]
- Chen, X.; Ge, X.; Qian, Y.; Tang, H.; Song, J.; Qu, X.; Yue, B.; Yuan, W. Electrospinning Multilayered Scaffolds Loaded with Melatonin and Fe3O4 Magnetic Nanoparticles for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2004537. [Google Scholar] [CrossRef]
- Marovič, N.; Ban, I.; Maver, U.; Maver, T. Magnetic Nanoparticles in 3D-Printed Scaffolds for Biomedical Applications. Nanotechnol. Rev. 2023, 12, 20220570. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Current Investigations into Magnetic Nanoparticles for Biomedical Applications. J. Biomed. Mater. Res. A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu, V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Peluso, V.; Gloria, A.; Oliviero, O.; Rinaldi, L.; Improta, G.; De Santis, R.; D’Antò, V. Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials 2020, 10, 577. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic Field and Nano-Scaffolds with Stem Cells to Enhance Bone Regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Iannotti, V.; Ausanio, G.; Ferretti, A.M.; Babar, Z.U.D.; Guarino, V.; Ambrosio, L.; Lanotte, L. Magnetic Response of Nano/Microparticles into Elastomeric Electrospun Fibers. J. Funct. Biomater. 2023, 14, 78. [Google Scholar] [CrossRef]
- Guarino, V.; Iannotti, V.; Ausanio, G.; Ambrosio, L.; Lanotte, L. Elastomagnetic Nanofiber Wires by Magnetic Field Assisted Electrospinning. Express Polym. Lett. 2019, 13, 419–428. [Google Scholar] [CrossRef]
- Guarino, V.; Ausanio, G.; Iannotti, V.; Ambrosio, L.; Lanotte, L. Electrospun Nanofiber Tubes with Elastomagnetic Properties for Biomedical Use. Express Polym. Lett. 2018, 12, 318–329. [Google Scholar] [CrossRef]
- Zhang, D.; Karki, A.B.; Rutman, D.; Young, D.P.; Wang, A.; Cocke, D.; Ho, T.H.; Guo, Z. Electrospun Polyacrylonitrile Nanocomposite Fibers Reinforced with Fe3O4 Nanoparticles: Fabrication and Property Analysis. Polymer 2009, 50, 4189–4198. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, H.; Liang, C.; Song, P.; Han, Y.; Han, Y.; Gu, J.; Kong, J.; Pan, D.; Guo, Z. Electromagnetic Interference Shielding MWCNT-Fe3O4@Ag/Epoxy Nanocomposites with Satisfactory Thermal Conductivity and High Thermal Stability. Carbon 2019, 141, 506–514. [Google Scholar] [CrossRef]
- Li, Y.; Jing, T.; Xu, G.; Tian, J.; Dong, M.; Shao, Q.; Wang, B.; Wang, Z.; Zheng, Y.; Yang, C.; et al. 3-D Magnetic Graphene Oxide-Magnetite Poly(Vinyl Alcohol) Nanocomposite Substrates for Immobilizing Enzyme. Polymer 2018, 149, 13–22. [Google Scholar] [CrossRef]
- You, G.; Wang, C.; Mei, S.; Yang, B.; Zhou, X. Model and Prediction of Stress Relaxation of Polyurethane Fiber. Mater. Res. Express 2018, 5, 035308. [Google Scholar] [CrossRef]
- Marcano, A.; Fatyeyeva, K.; Koun, M.; Dubuis, P.; Grimme, M.; Marais, S. Recent Developments in the Field of Barrier and Permeability Properties of Segmented Polyurethane Elastomers. Rev. Chem. Eng. 2019, 35, 445–474. [Google Scholar] [CrossRef]
- Azarmgin, S.; Torabinejad, B.; Kalantarzadeh, R.; Garcia, H.; Velazquez, C.A.; Lopez, G.; Vazquez, M.; Rosales, G.; Heidari, B.S.; Davachi, S.M. Polyurethanes and Their Biomedical Applications. ACS Biomater. Sci. Eng. 2024, 10, 6828–6859. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Sundarrajan, S.; Hussain, T.; Nazir, A.; Berto, F.; Ramakrishna, S. Electrospun Biomimetic Polymer Nanofibers as Vascular Grafts. Mater. Des. Process. Commun. 2021, 3, e203. [Google Scholar] [CrossRef]
- Asensio, M.; Costa, V.; Nohales, A.; Bianchi, O.; Gómez, C.M. Tunable Structure and Properties of Segmented Thermoplastic Polyurethanes as a Function of Flexible Segment. Polymers 2019, 11, 1910. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Barbalata Mandru, M.; Drobota, M.; Aflori, M.; Butnaru, M.; Spiridon, M.; Doroftei, F.; Aradoaei, M.; Ciobanu, R.C.; Vlad, S. Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of MRI. Polymers 2021, 13, 4316. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Yang, J.; Wang, Y.; Zhang, Z.; Hou, Y.; Lin, F.; Li, Y. Influence of Hard Segments on the Thermal, Phase-Separated Morphology, Mechanical, and Biological Properties of Polycarbonate Urethanes. Appl. Sci. 2017, 7, 306. [Google Scholar] [CrossRef]
- Puszka, A.; Sikora, J.W.; Nurzyńska, A. Influence of the Type of Soft Segment on the Selected Properties of Polyurethane Materials for Biomedical Applications. Materials 2024, 17, 840. [Google Scholar] [CrossRef]
- Kunc, F.; Gallerneault, M.; Kodra, O.; Brinkmann, A.; Lopinski, G.P.; Johnston, L.J. Surface Chemistry of Metal Oxide Nanoparticles: NMR and TGA Quantification. Anal. Bioanal. Chem. 2022, 414, 4409–4425. [Google Scholar] [CrossRef]
- Rami, J.M.; Patel, C.D.; Patel, C.M.; Patel, M.V. Thermogravimetric Analysis (TGA) of Some Synthesized Metal Oxide Nanoparticles. Mater. Today Proc. 2021, 43, 655–659. [Google Scholar] [CrossRef]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.S.; Cameron, R.E. Analysis and Evaluation of a Biomedical Polycarbonate Urethane Tested in an in Vitro Study and an Ovine Arthroplasty Model. Part I: Materials Selection and Evaluation. Biomaterials 2005, 26, 621–631. [Google Scholar] [CrossRef]
- Lin, C.-L.; Kao, H.-M.; Wu, R.-R.; Kuo, P.-L. Multinuclear Solid-State NMR, DSC, and Conductivity Studies of Solid Polymer Electrolytes Based on Polyurethane/Poly(Dimethylsiloxane) Segmented Copolymers. Macromolecules 2002, 35, 3083–3096. [Google Scholar] [CrossRef]
- Tsen, W.C.; Chuang, F.S. Phase Transition and Domain Morphology of Siloxane-containing Hard-segmented Polyurethane Copolymers. J. Appl. Polym. Sci. 2006, 101, 4242–4252. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Dempsey, D.K.; Cosgriff-Hernandez, E. Electrospun Vascular Grafts with Improved Compliance Matching to Native Vessels. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, M.C.; Farè, S.; Petrini, P. In Vitro Stability of Polyether and Polycarbonate Urethanes. J. Biomater. Appl. 2000, 14, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Casarin, M.; Sandrin, D.; Astolfi, L.; Romanato, F.; Giuggioli, G.; Conte, F.; Gerosa, G.; Fontanella, C.G.; Bagno, A. Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment. Bioengineering 2024, 11, 436. [Google Scholar] [CrossRef]
- Yeganegi, M.; Kandel, R.A.; Santerre, J.P. Characterization of a Biodegradable Electrospun Polyurethane Nanofiber Scaffold: Mechanical Properties and Cytotoxicity. Acta Biomater. 2010, 6, 3847–3855. [Google Scholar] [CrossRef]
- Dwivedi, K.K.; Lakhani, P.; Kumar, S.; Kumar, N. The Effect of Strain Rate on the Stress Relaxation of the Pig Dermis: A Hyper-Viscoelastic Approach. J. Biomech. Eng. 2020, 142. [Google Scholar] [CrossRef]
- Xia, H.; Song, M.; Zhang, Z.; Richardson, M. Microphase Separation, Stress Relaxation, and Creep Behavior of Polyurethane Nanocomposites. J. Appl. Polym. Sci. 2007, 103, 2992–3002. [Google Scholar] [CrossRef]
- Tan, C.J.; Andriyana, A.; Ang, B.C.; Chagnon, G. Inelastic Deformation of Highly Aligned Dry-Spun Thermoplastic Polyurethane Elastomer Microfibres. Mater. Res. Express 2018, 5, 125301. [Google Scholar] [CrossRef]
- Praveen, S.; Bahadur, J.; Yadav, R.; Billa, S.; Umasankar Patro, T.; Rath, S.K.; Ratna, D.; Patri, M. Tunable Viscoelastic and Vibration Damping Properties of a Segmented Polyurethane Synergistically Reinforced with Carbon Black and Anisotropic Additives. Appl. Acoust. 2020, 170, 107535. [Google Scholar] [CrossRef]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.S.; Cameron, R.E. Analysis and Evaluation of a Biomedical Polycarbonate Urethane Tested in an in Vitro Study and an Ovine Arthroplasty Model. Part II: In Vivo Investigation. Biomaterials 2005, 26, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, E.; Zaharia, C.; Hudita, A.; Radu, I.-C.; Costache, M.; Galateanu, B. Impact of the Magnetic Field on 3T3-E1 Preosteoblasts inside SMART Silk Fibroin-Based Scaffolds Decorated with Magnetic Nanoparticles. Mater. Sci. Eng. C 2020, 110, 110714. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Z.; Liu, L.; Wang, W.; Leng, J.; Liu, Y. Porous Bone Tissue Scaffold Concept Based on Shape Memory PLA/Fe3O4. Compos. Sci. Technol. 2021, 203, 108563. [Google Scholar] [CrossRef]
- Varalli, L.; Berlet, R.; Abenojar, E.; McDaid, J.; Gascoigne, D.A.; Bailes, J.; Aksenov, D.P. Applications and Efficacy of Iron Oxide Nanoparticles in the Treatment of Brain Tumors. Pharmaceutics 2025, 17, 499. [Google Scholar] [CrossRef]
- Fayol, D.; Le Visage, C.; Ino, J.; Gazeau, F.; Letourneur, D.; Wilhelm, C. Design of Biomimetic Vascular Grafts with Magnetic Endothelial Patterning. Cell Transplant. 2013, 22, 2105–2118. [Google Scholar] [CrossRef]
- Iannotti, V.; Guarino, V.; Cruz-Maya, I.; Babar, Z.U.D.; Lanotte, L.; Ambrosio, L.; Lanotte, L. Exploring the Contraction Actuation of Magnetically Functionalized Electrospun Tubes. Sens. Actuators A Phys. 2024, 371, 115272. [Google Scholar] [CrossRef]
- Ponomareva, S.; Carriere, M.; Hou, Y.; Morel, R.; Dieny, B.; Joisten, H. Microstructured Magnetoelastic Membrane for Magnetic Bioactuators and Soft Artificial Muscles Applications. Adv. Intell. Syst. 2023, 5, 2300022. [Google Scholar] [CrossRef]
- Han, J.-H.; Ko, U.H.; Kim, H.J.; Kim, S.; Jeon, J.S.; Shin, J.H. Electrospun Microvasculature for Rapid Vascular Network Restoration. Tissue Eng. Regen. Med. 2021, 18, 89–97. [Google Scholar] [CrossRef]
- Ji, H.; Shi, X.; Yang, H. Recent Advances in Polyurethane for Artificial Vascular Application. Polymers 2024, 16, 3528. [Google Scholar] [CrossRef]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.P.; Lopes, É.S.N.; Pereira, K.D.; Huber, S.C.; Jardini, A.L.; Annichino-Bizzacchi, J.M.; Luchessi, A.D.; Gabriel, L.P. Extracellular Matrix-Derived and Low-Cost Proteins to Improve Polyurethane-Based Scaffolds for Vascular Grafts. Sci. Rep. 2022, 12, 5230. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Clements, B.A.; Guarino, V.; Bushman, J.; Kohn, J.; Ambrosio, L. A Comparison of the Performance of Mono- and Bi-Component Electrospun Conduits in a Rat Sciatic Model. Biomaterials 2014, 35, 8970–8982. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Guarino, V.; Ambrosio, L. Design of Bioactive Electrospun Scaffolds for Bone Tissue Engineering. J. Appl. Biomater. Funct. Mater. 2012, 10, 223–228. [Google Scholar] [CrossRef]
- Ratner, B. Vascular Grafts: Technology Success/Technology Failure. BME Front. 2023, 4. [Google Scholar] [CrossRef]
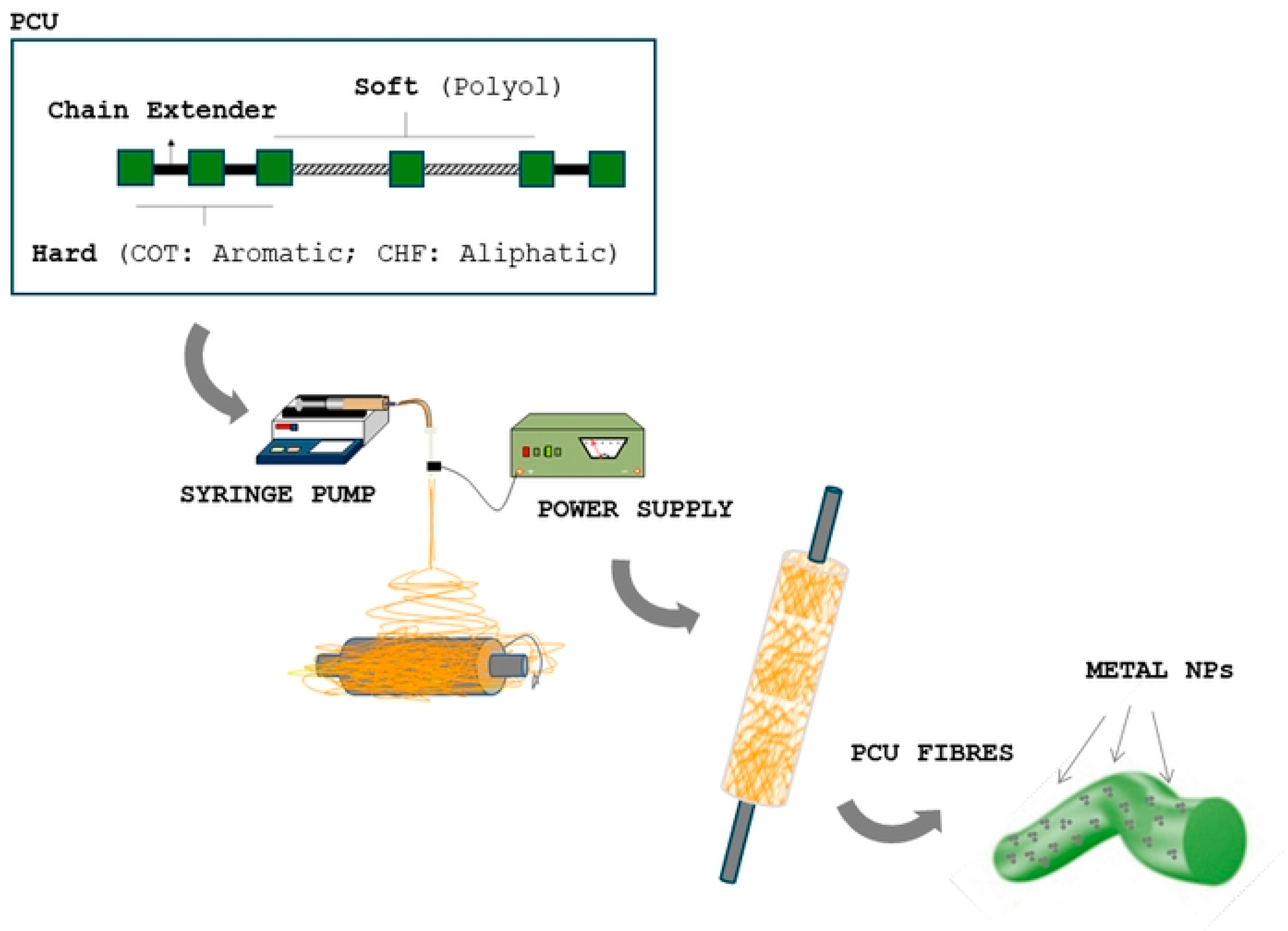
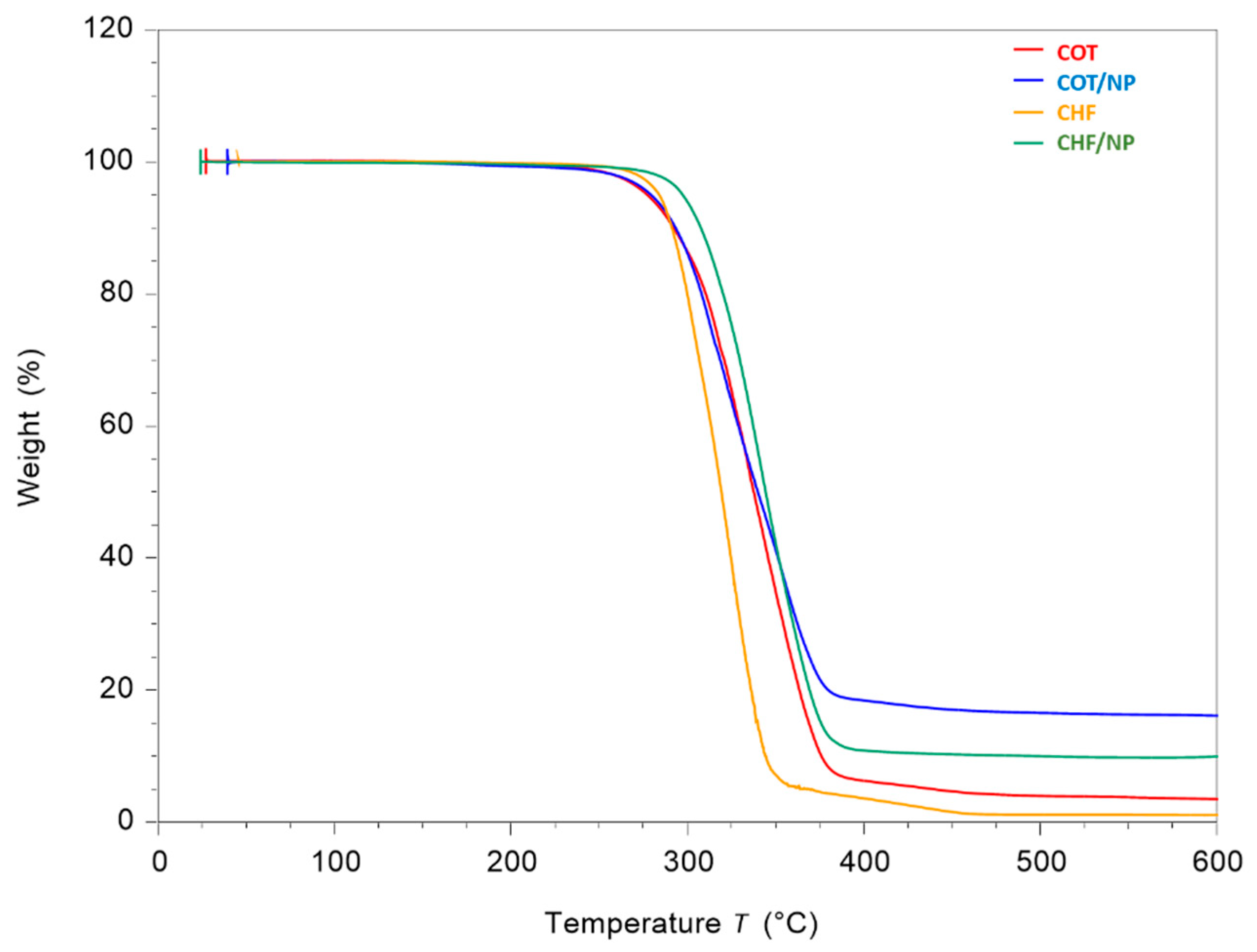
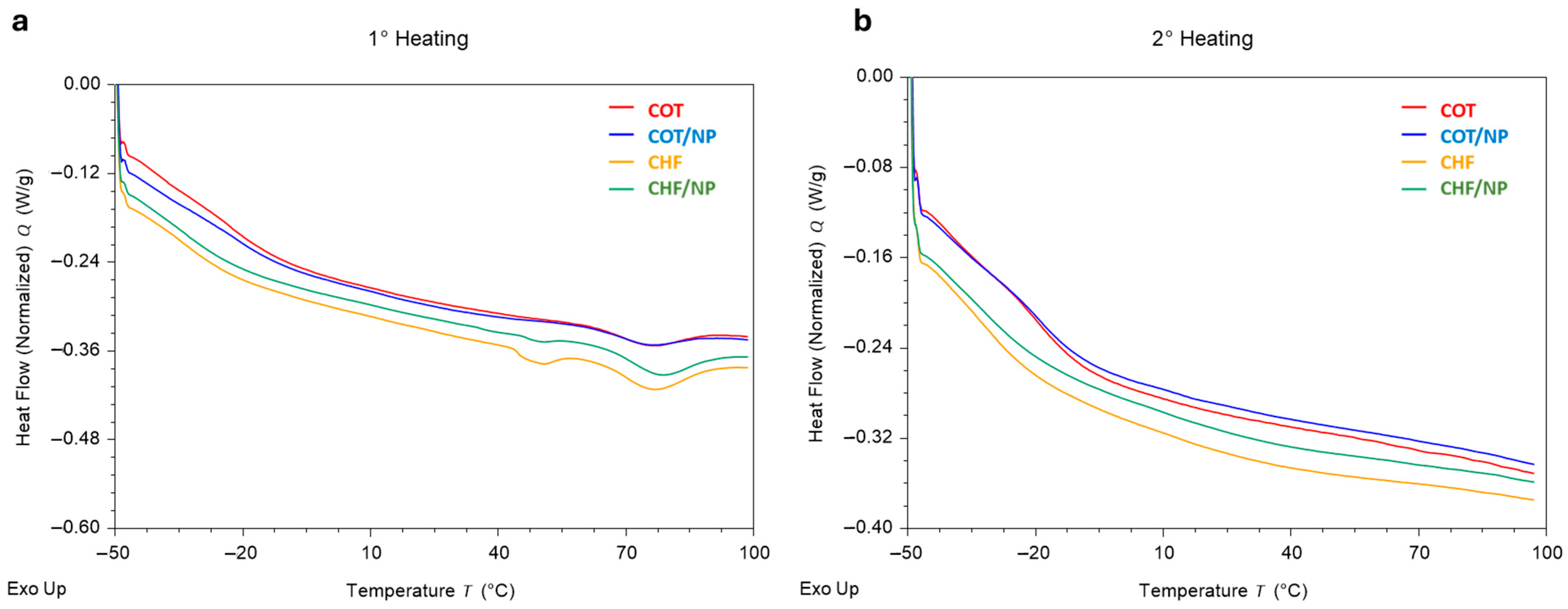
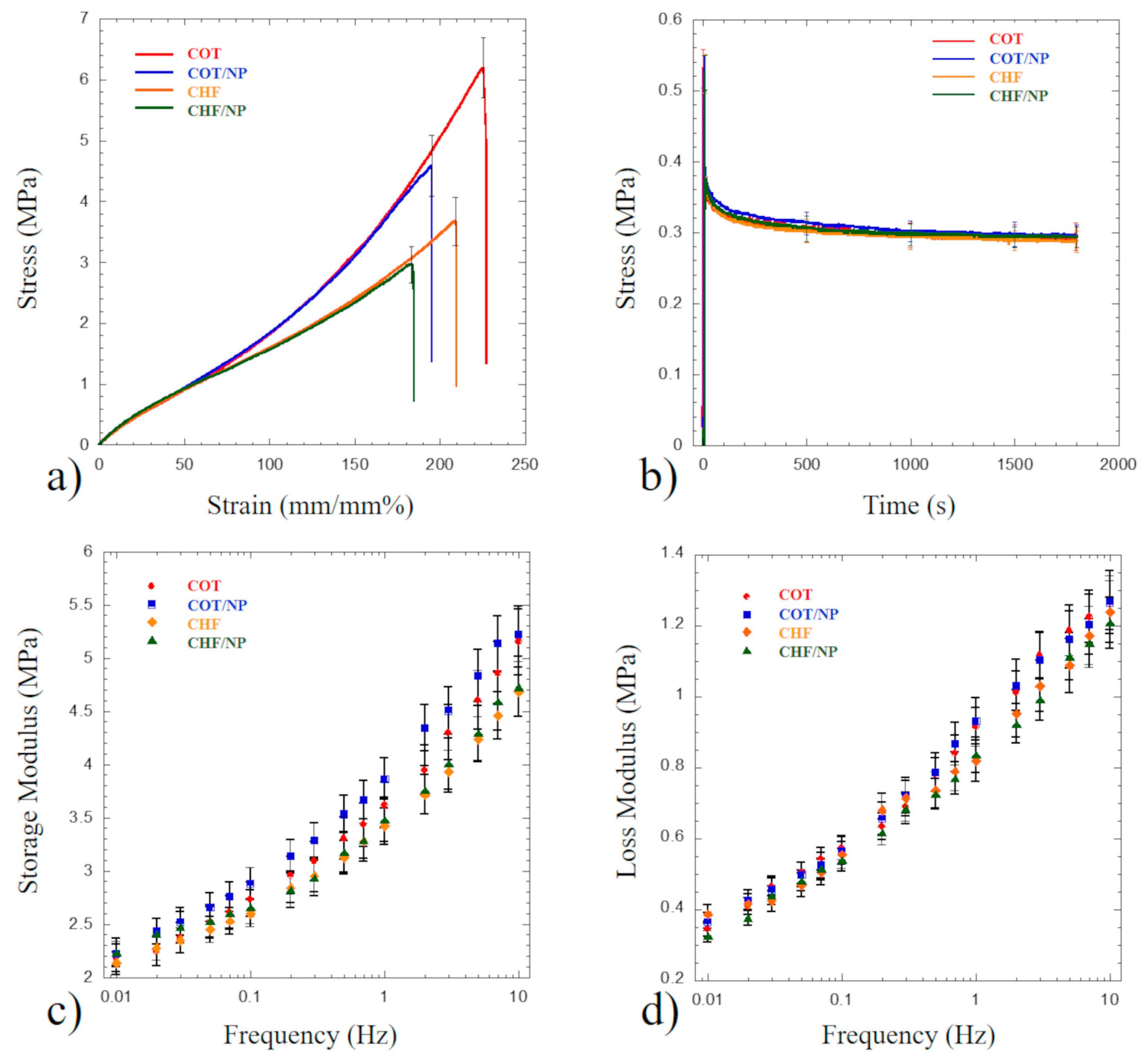
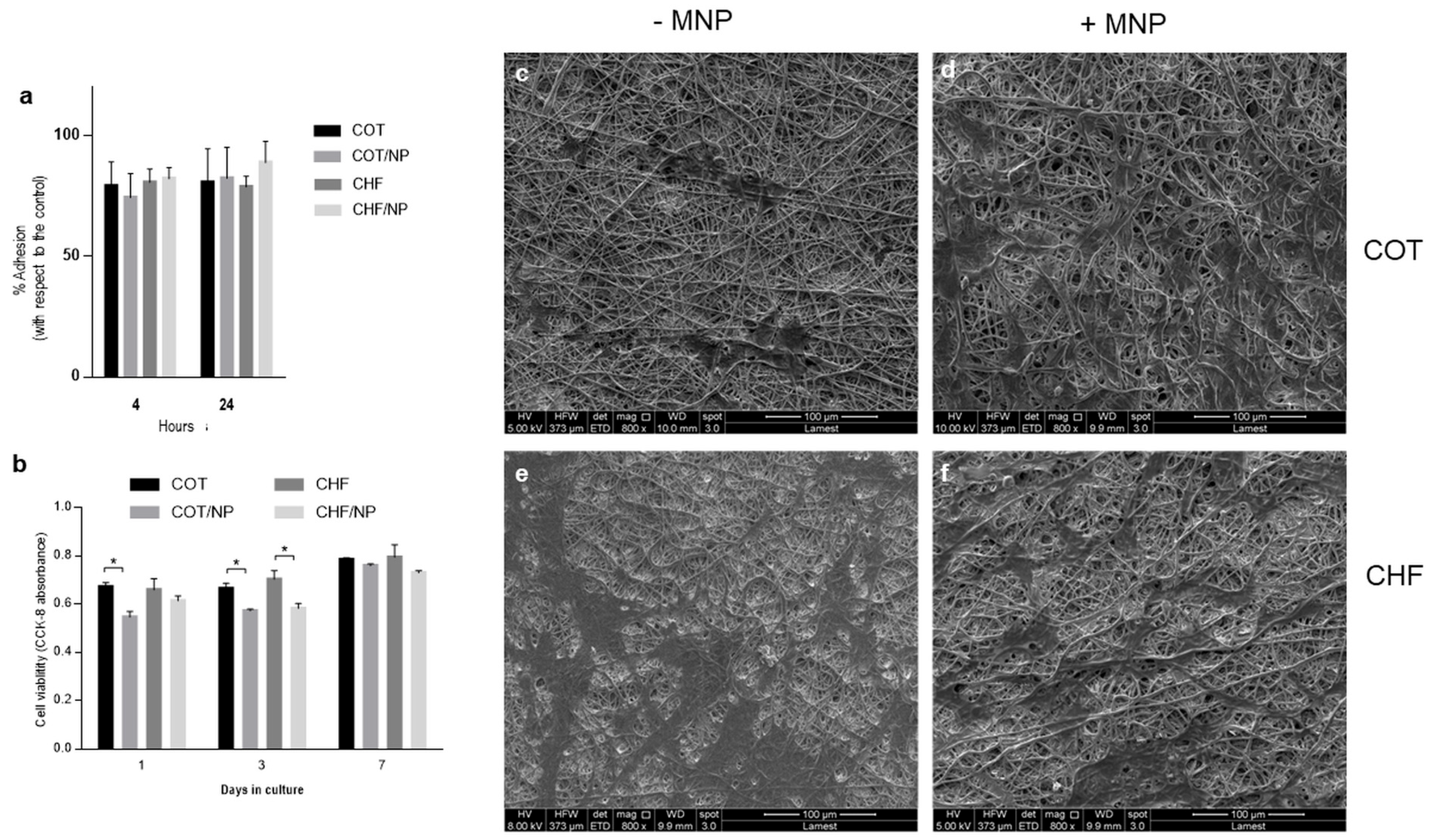
| Polymer | Solvent | MNPs | |
|---|---|---|---|
| COT | Corethane (15% w/w) | THF/DMF 7:3 | |
| COT/NPs | Corethane (15% w/w) | THF/DMF 7:3 | 12% v/v |
| CHF | Chronoflex (15% w/w) | THF/DMF 7:3 | |
| CHF/NPs | Chronoflex (15% w/w) | THF/DMF 7:3 | 12% v/v |
| COT | COT/NPs | CHF | CHF/NPs | |
|---|---|---|---|---|
| First temperature scan | ||||
| Tg (°C) | −17.4 ± 0.4 a | −16.3 ± 0.3 b | −21.3 ± 0.4 c | −20.8 ± 0.2 d |
| ΔH1 (J/g) | - | - | 0.82 ± 0.03 a | 0.19 ± 0.02 b |
| T1 (°C) | - | - | 50.2 ± 0.2 a | 50.8 ± 0.2 b |
| ΔH2 (J/g) | 1.73 ± 0.15 a | 1.23 ± 0.16 b | 2.10 ± 0.19 c | 2.67 ± 0.15 d |
| T2 (°C) | 75.1 ± 0.2 a | 75.5 ± 0.2 b | 76.7 ± 0.3 c | 78.9 ± 0.2 d |
| Second temperature scan | ||||
| Tg (°C) | −12.4 ± 0.4 a | −13.8 ± 0.3 b | −23.4 ± 0.3 c | −24.8 ± 0.4 d |
| COT | COT/NPs | CHF | CHF/NPs | |
|---|---|---|---|---|
| Young’s Modulus (MPa) | 2.92 ± 0.15 a | 2.93 ± 0.17 a | 2.86 ± 0.14 a | 2.87 ± 0.15 a |
| Maximum Stress (MPa) | 6.16 ± 0.33 a | 4.55 ± 0.25 b | 3.67 ± 0.18 c | 2.97 ± 0.22 d |
| Maximum Strain (mm/mm %) | 225 ± 21 a | 194 ± 20 a,b | 209 ± 20 a,b | 183 ± 19 b |
| COT | COT/NPs | CHF | CHF/NPs | |
|---|---|---|---|---|
| Peak stress (MPa) | 0.512 ± 0.029 a | 0.514 ± 0.027 a | 0.513 ± 0.026 a | 0.515 ± 0.027 a |
| Plateau stress (MPa) | 0.287 ± 0.017 a | 0.292 ± 0.018 a | 0.293 ± 0.018 a | 0.297 ± 0.019 a |
| Stress decay (%) | 43.9 | 43.2 | 42.9 | 42.3 |
| τ1 (s) | 697.3 | 701.6 | 706.4 | 705.0 |
| τ2 (s) | 54.1 | 52.2 | 52.4 | 52.8 |
| τ3 (s) | 6.8 | 7.1 | 6.9 | 6.5 |
| τ4 (s) | 0.9 | 0.9 | 0.8 | 0.9 |
| COT | COT/NPs | CHF | CHF/NPs | |
|---|---|---|---|---|
| E′ (MPa) @ 0.01 Hz | 2.18 ± 0.13 a | 2.22 ± 0.16 a | 2.13 ± 0.15 a | 2.23 ± 0.17 a |
| E″ (MPa) @ 0.01 Hz | 0.37 ± 0.03 a | 0.34 ± 0.02 a | 0.34 ± 0.02 a | 0.36 ± 0.02 a |
| E′ (MPa) @ 0.1 Hz | 2.73 ± 0.16 a | 2.88 ± 0.19 a | 2.60 ± 0.15 a | 2.66 ± 0.18 a |
| E″ (MPa) @ 0.1 Hz | 0.55 ± 0.03 a | 0.53 ± 0.03 a | 0.57 ± 0.04 a | 0.56 ± 0.03 a |
| E′ (MPa) @ 1 Hz | 3.62 ± 0.23 a | 3.86 ± 0.27 a | 3.42 ± 0.21 a | 3.48 ± 0.24 a |
| E″ (MPa) @ 1 Hz | 0.82 ± 0.05 a | 0.84 ± 0.05 a | 0.91 ± 0.06 a | 0.93 ± 0.06 a |
| E′ (MPa) @ 10 Hz | 5.15 ± 0.31 a | 5.22 ± 0.33 a | 4.68 ± 0.28 a | 4.72 ± 0.30 a |
| E″ (MPa) @ 10 Hz | 1.23 ± 0.07 a | 1.21 ± 0.07 a | 1.26 ± 0.08 a | 1.27 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Maya, I.; De Santis, R.; Lanotte, L.; Guarino, V. Novel Magnetically Charged Grafts for Vascular Repair: Process Optimization, Mechanical Characterization and In Vitro Validation. Polymers 2025, 17, 1877. https://doi.org/10.3390/polym17131877
Cruz-Maya I, De Santis R, Lanotte L, Guarino V. Novel Magnetically Charged Grafts for Vascular Repair: Process Optimization, Mechanical Characterization and In Vitro Validation. Polymers. 2025; 17(13):1877. https://doi.org/10.3390/polym17131877
Chicago/Turabian StyleCruz-Maya, Iriczalli, Roberto De Santis, Luciano Lanotte, and Vincenzo Guarino. 2025. "Novel Magnetically Charged Grafts for Vascular Repair: Process Optimization, Mechanical Characterization and In Vitro Validation" Polymers 17, no. 13: 1877. https://doi.org/10.3390/polym17131877
APA StyleCruz-Maya, I., De Santis, R., Lanotte, L., & Guarino, V. (2025). Novel Magnetically Charged Grafts for Vascular Repair: Process Optimization, Mechanical Characterization and In Vitro Validation. Polymers, 17(13), 1877. https://doi.org/10.3390/polym17131877







