Optimization of the Green Conventional Extraction Method of Sericin from Silkworm
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Conventional Sericin Extraction
2.3. Experimental Design
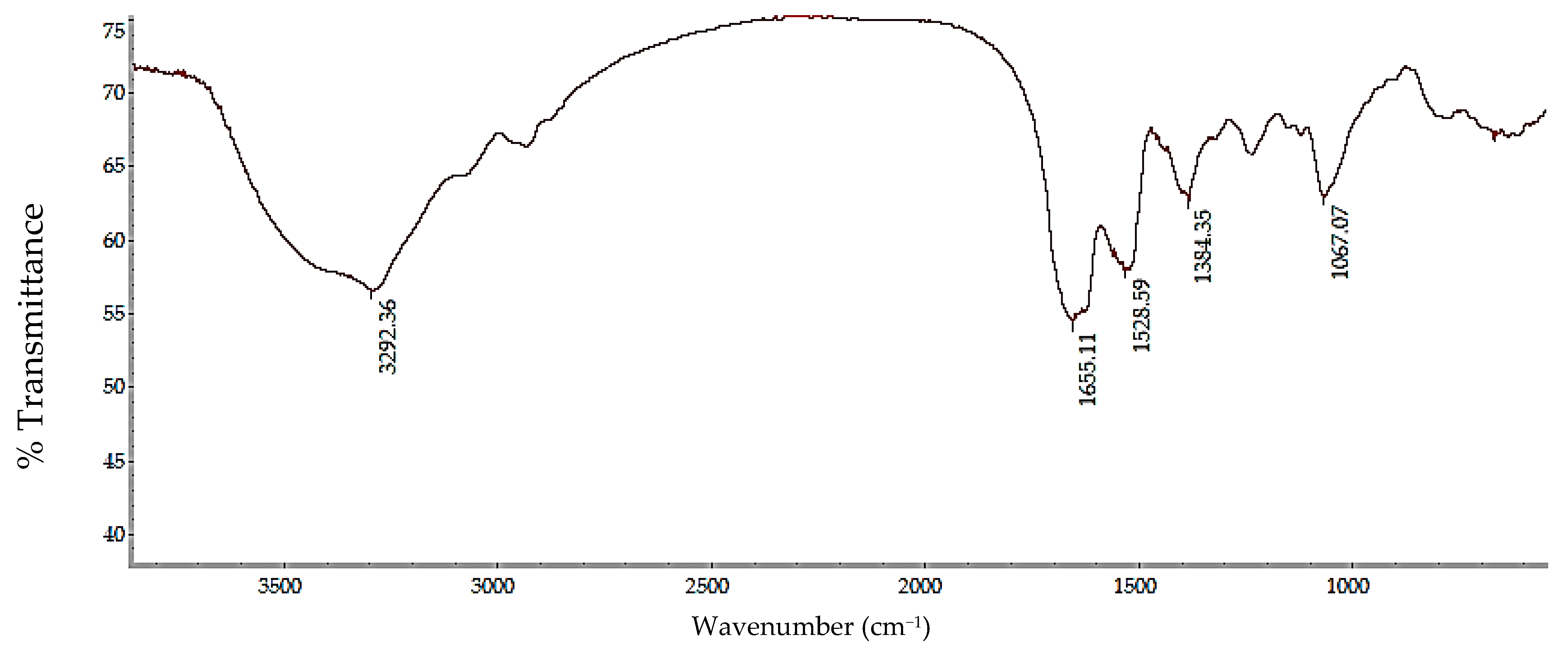
2.4. Structural Analysis by Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Molecular Weight Distribution (SDS-PAGE)
3. Results and Discussion
3.1. Optimization of the Conventional Method Without Chemicals
3.2. Sericin Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saad, M.; El-Samad, L.M.; Gomaa, R.A.; Augustyniak, M.; Hassan, M.A. A comprehensive review of recent advances in silk sericin: Extraction approaches, structure, biochemical characterization, and biomedical applications. Int. J. Biol. Macromol. 2023, 50, 126067. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S. Encyclopedia of Insects. In Chapter 232—Sericulture, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 912–914. [Google Scholar]
- Wang, X.; Wang, M.; Zolotuhin, V.V.; Hirowatari, T.; Wu, S.; Huang, G.H. The fauna of the family Bombycidae sensu lato (Insecta, Lepidoptera, Bombycoidea) from Mainland China, Taiwan and Hainan Islands. Zootaxa 2015, 3989, 1–138. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M.; Aranha, N.; et al. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Barajas-Gamboa, J.A.; Serpa-Guerra, A.M.; Restrepo-Osorio, A.; Alvarez-López, C. Sericin applications: A globular silk protein. Ing. Compet. 2016, 18, 193–206. [Google Scholar] [CrossRef]
- Ritprajak, P.; Sirithanakorn, C.; Nguyen, T.N.; Sereemaspun, A.; Aramwit, P. Biosynthetic sericin 1-like protein skews dendritic cells to tolerogenic-like phenotype. Biotechnol. Appl. Biochem. 2021, 68, 1508–1517. [Google Scholar] [CrossRef]
- Javali, U.C.; Padaki, N.V.; Das, B.; Malali, K.B. Advances in silk science and technology. In 13—Developments in the Use of Silk By-Products and Silk Waste; Basu, A., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 261–270. [Google Scholar]
- Babu, K.M. (Ed.) Silk. In 9—By-Products of Sericulture and the Silk Industry, 2nd ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 207–233. [Google Scholar]
- Rajput, S.K. Sericin—A unique biomaterial. Int. Organ. Sci. Res. J. Polym. Text. Eng. 2015, 2, 29–35. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Kanokpanont, S.; Srichana, T. Formulation and characterization of silk sericin–PVA scaffold crosslinked with genipin. Int. J. Biol. Macromol. 2010, 47, 668–675. [Google Scholar] [CrossRef]
- Mellado, G.A.M.; Álvarez, C. Extraction and antioxidant activity of sericin, a protein from silk. Braz. J. Food Technol. 2020, 23, e2019058. [Google Scholar] [CrossRef]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; He, X.; Fang, A.; Jiang, R.; Wu, T.; Chen, H.; Cao, X.; Liang, P.; Xia, D.; et al. A sterile self-assembled sericin hydrogel via a simple two-step process. Polym. Test. 2019, 80, 106016. [Google Scholar] [CrossRef]
- Aad, R.; Dragojlov, I.; Vesentini, S. Sericin Protein: Structure, Properties, and Applications. J. Funct. Biomater. 2024, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Bascou, R.; Hardouin, J.; Ben Mlouka, M.A.; Guénin, E.; Nesterenko, A. Detailed investigation on new chemical-free methods for silk sericin extraction. Mater. Today Commun. 2022, 33, 104491. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; McKirdy, N.C. Further development of silk sericin as a biomaterial: Comparative investigation of the procedures for its isolation from Bombyx mori silk cocoons. Prog. Biomater. 2016, 5, 135–145. [Google Scholar] [CrossRef]
- Sothornvit, R.; Chollakup, R.; Suwanruji, P. Extracted sericin from silk waste for film formation. Songklanakarin J. Sci. Technol. 2010, 32, 17–22. [Google Scholar]
- Castrillón, D. Characterization of Colombian Silk Sericin with a View to Being Incorporated as an Ingredient in a Food Matrix. Master’s Thesis, University Pontificia Bolivariana, Medellín, Colombia, 2017. [Google Scholar]
- Box, G.E.P.; Behnken, D.W. Some New Three-Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Kuehl, R. Design of experiments. In Statistical Principles of Research Design and Analysis, 2nd ed.; Thomson Learning: Stamford, CT, USA, 2000. [Google Scholar]
- Takasu, Y.; Yamada HTsubouchi, K. Isolation of Three Main Sericin Components from the Cocoon of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar] [CrossRef]
- Murphy, A.; Kaplan, D. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef]
- Murillo-Usuga, C.A.; Escobar-Sierra, D.M. Optimization of the fibroin extraction process from the silkworm cocoon Bombyx Mori. ION J. 2022, 35, 33–42. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J. Applied Linear Regression Models, 4th ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Maduna, L.; Patnaik, A.; Nayak, R. The use of the Box–Behnken experimental design to model tensile strength of spunlaced fabrics and evaluating fabrics behaviour in acidic condition. J. Ind. Text. 2022, 51, 837–855. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Jensen, W.A. Response Surface Methodology: Process and Product Optimization Using Designed Experiments 4th edition. J. Qual. Technol. 2017, 49, 186–188. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 6th ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yun, H.; Oh, H.; Kim, M.K.; Kwak, H.W.; Lee, J.Y.; Um, I.C.; Vootla, S.K.; Lee, K.H. Extraction conditions of Antheraea mylitta sericin with high yields and minimum molecular weight degradation. Int. J. Biol. Macromol. 2013, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Teramoto, H.; Miyazawa, M. Molecular orientation behavior of silk sericin film as revealed by ATR infrared spectroscopy. Biomacromolecules 2005, 6, 2049–2057. [Google Scholar] [CrossRef]
- Choudhury, M.; Devi, D. Impact of high temperature and pressure on sericin scouring of muga silk cocoons. Fibre Text. Res. 2016, 41, 93–96. [Google Scholar]
- Kim, M.; Kwak, H.; Lee, J.; Yun, H.; Kim, M.; Lee, K. Effect of Lyoprotectant on the Solubility and Structure of Silk Sericin. Int. J. Ind. Entomol. 2012, 25, 133–137. [Google Scholar] [CrossRef]
- Quigley, E.; Nilsson, B.L. β-Sheet and β-Hairpin Peptide Nanomaterials. In Peptide Bionanomaterials; Elsawy, M.A., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, G.; Shuai, Y.; Wang, J.; Zhu, L.; Mao, C. Ca2+-induced self-assembly of Bombyx mori silk sericin into a nanofibrous network-like protein matrix for directing controlled nucleation of hydroxylapatite nano-needles. J. Mater. Chem. B 2015, 3, 2455–2462. [Google Scholar] [CrossRef]
- Gulrajani, M.L.; Purwar, R.; Prasad, R.K.; Joshi, M. Studies on structural and functional properties of sericin recovered from silk degumming liquor by membrane technology. J. Appl. Polym. Sci. 2009, 113, 2796–2804. [Google Scholar] [CrossRef]
- Ganapathy, V. Industrial Boilers and Heat Recovery Steam Generators; En CRC Press eBooks: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Ribeiro, V.P.; Reis, R.L. Special Issue: Biopolymer-Based Materials for Biomedical Engineering. Materials 2022, 15, 2942. [Google Scholar] [CrossRef]
- Attard, T.M.; Hunt, A.J.; Matharu, A.S.; Houghton, J.A.; Polikarpov, I. Biomass as a Feedstock. In Introduction to Chemicals from Biomass; Clark, J., Deswarte, F., Eds.; Wiley: Chichester, UK, 2015. [Google Scholar] [CrossRef]


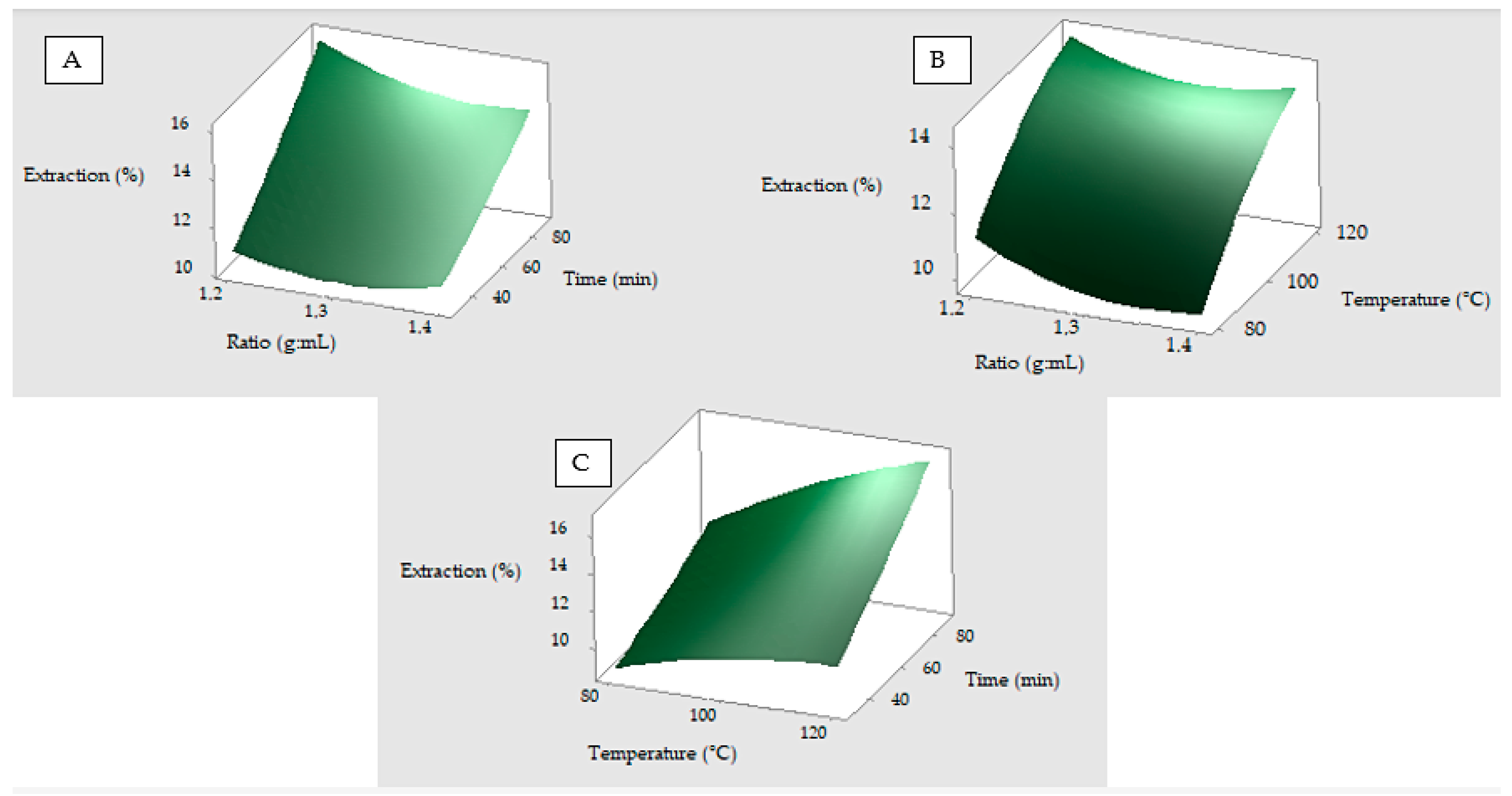
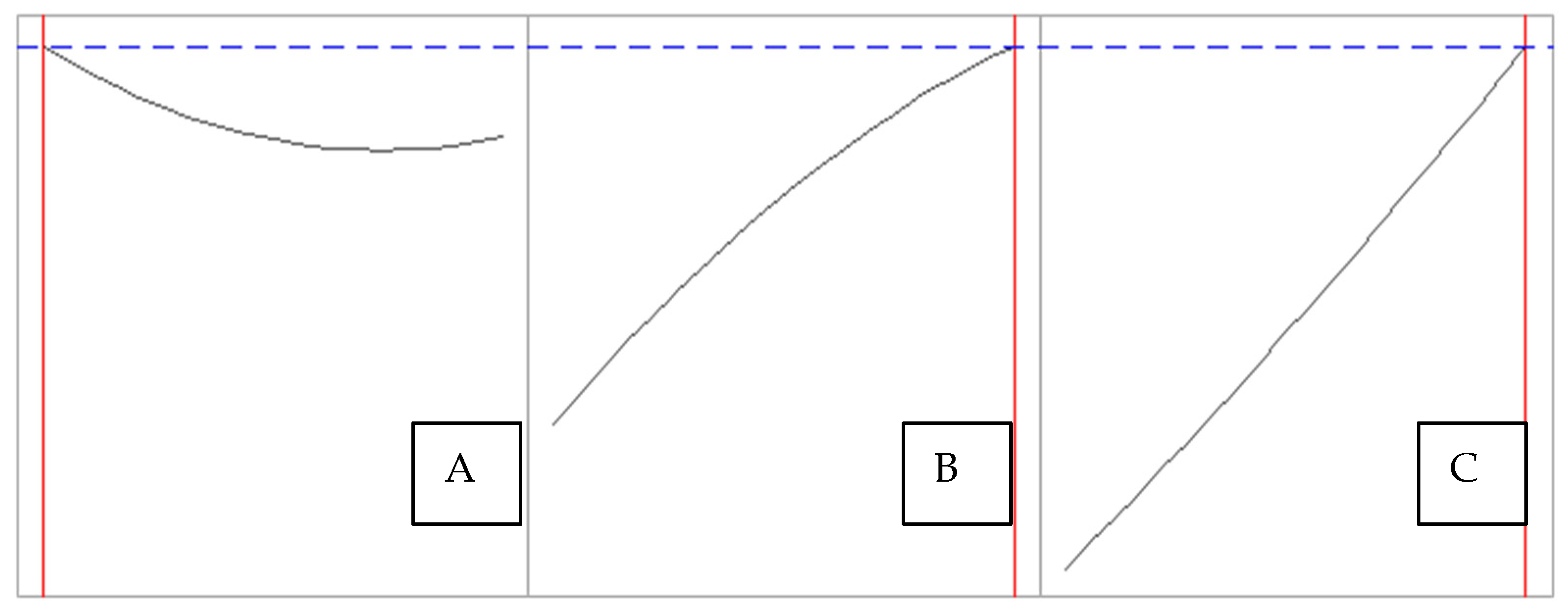

| Ratio (Cocoon:Water) | Temperature (°C) | Time (min) |
|---|---|---|
| 1:20 | 80 | 30 * |
| 1:30 * | 100 | 60 |
| 1:40 | 120 * | 90 |
| Ratio (Cocoon:Water) g:mL | Temperature (°C) | Time (min) | Average Extraction (%) |
|---|---|---|---|
| 1:20 | 100 | 30 | 9.20 VC: 1.49 |
| 1:40 | 100 | 30 | 9.52 VC: 3.33 |
| 1:30 | 80 | 30 | 10,05 VC: 4.74 |
| 1:30 | 120 | 30 | 12.59 VC: 2.53 |
| 1:20 | 80 | 60 | 11.65 VC: 3.24 |
| 1:40 | 80 | 60 | 10.08 VC: 0.20 |
| 1:20 | 120 | 60 | 14.27 VC: 3.60 |
| 1:40 | 120 | 60 | 13.44 VC: 2.88 |
| 1:30 | 100 | 60 | 12.28 VC: 2.36 |
| 1:30 | 100 | 60 | 12.35 VC: 2.78 |
| 1:30 | 100 | 60 | 12.13 VC: 3.44 |
| 1:30 | 120 | 90 | 15.43 VC: 4.76 |
| 1:30 | 80 | 90 | 9.69 VC: 4.54 |
| 1:20 | 100 | 90 | 17.32 VC:4.04 |
| 1:40 | 100 | 90 | 16.20 VC:1.73 |
| Variable | Sum of Squares | Freedom Degrees | Value F | Value p | VIF |
|---|---|---|---|---|---|
| Block | 0.30 | 2 | 0.08 | 0.93 | - |
| Ratio | 3.86 | 1 | 1.96 | 0.17 | 1.00 |
| Temperature | 76.40 | 1 | 38.84 | 0.00 | 1.00 |
| Time | 111.63 | 1 | 56.75 | 0.00 | 1.00 |
| Rate2 | 4.15 | 1 | 2.11 | 0.16 | 1.01 |
| Temperature2 | 2.84 | 1 | 1.44 | 0.24 | 1.01 |
| Time2 | 0.41 | 1 | 0.21 | 0.65 | 1.01 |
| Ratio × Temperature | 0.41 | 1 | 0.21 | 0.65 | 1.00 |
| Ratio × Time | 1.54 | 1 | 0.78 | 0.38 | 1.00 |
| Temperature × Time | 7.66 | 1 | 3.89 | 0.06 | 1.00 |
| Model | 209.76 | 11 | 9.69 | 0.00 | - |
| Missing adjustment | 64.11 | 27 | 17.83 | 0.00 | - |
| Error | 0.80 | 6 | - | - | - |
| Total | 274.67 | 44 | - | - | - |
| R2 | 76.37% | ||||
| R2-Adjusted | 68.49% | ||||
| R2-Predictive | 52.73% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos Gomez, D.S.; Rada-Mendoza, M.; Chito-Trujillo, D.M. Optimization of the Green Conventional Extraction Method of Sericin from Silkworm. Polymers 2025, 17, 1823. https://doi.org/10.3390/polym17131823
Burgos Gomez DS, Rada-Mendoza M, Chito-Trujillo DM. Optimization of the Green Conventional Extraction Method of Sericin from Silkworm. Polymers. 2025; 17(13):1823. https://doi.org/10.3390/polym17131823
Chicago/Turabian StyleBurgos Gomez, Daniel Stiven, Maite Rada-Mendoza, and Diana M. Chito-Trujillo. 2025. "Optimization of the Green Conventional Extraction Method of Sericin from Silkworm" Polymers 17, no. 13: 1823. https://doi.org/10.3390/polym17131823
APA StyleBurgos Gomez, D. S., Rada-Mendoza, M., & Chito-Trujillo, D. M. (2025). Optimization of the Green Conventional Extraction Method of Sericin from Silkworm. Polymers, 17(13), 1823. https://doi.org/10.3390/polym17131823






