Interphase Engineering Enabled by Using a Separator with Electrochemically Active Carbazole Polymers for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of PCP
2.2. Separators Coating
2.3. Electrochemical Test
2.4. Fourier Transform Spectroscopy (FT-IR) Characterization
2.5. X-Ray Photoelectron Spectra (XPS) Characterization
2.6. Inductively Coupled Plasma-Mass Spectra (ICP-MS) Characterization
2.7. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.8. Electrolyte Uptake
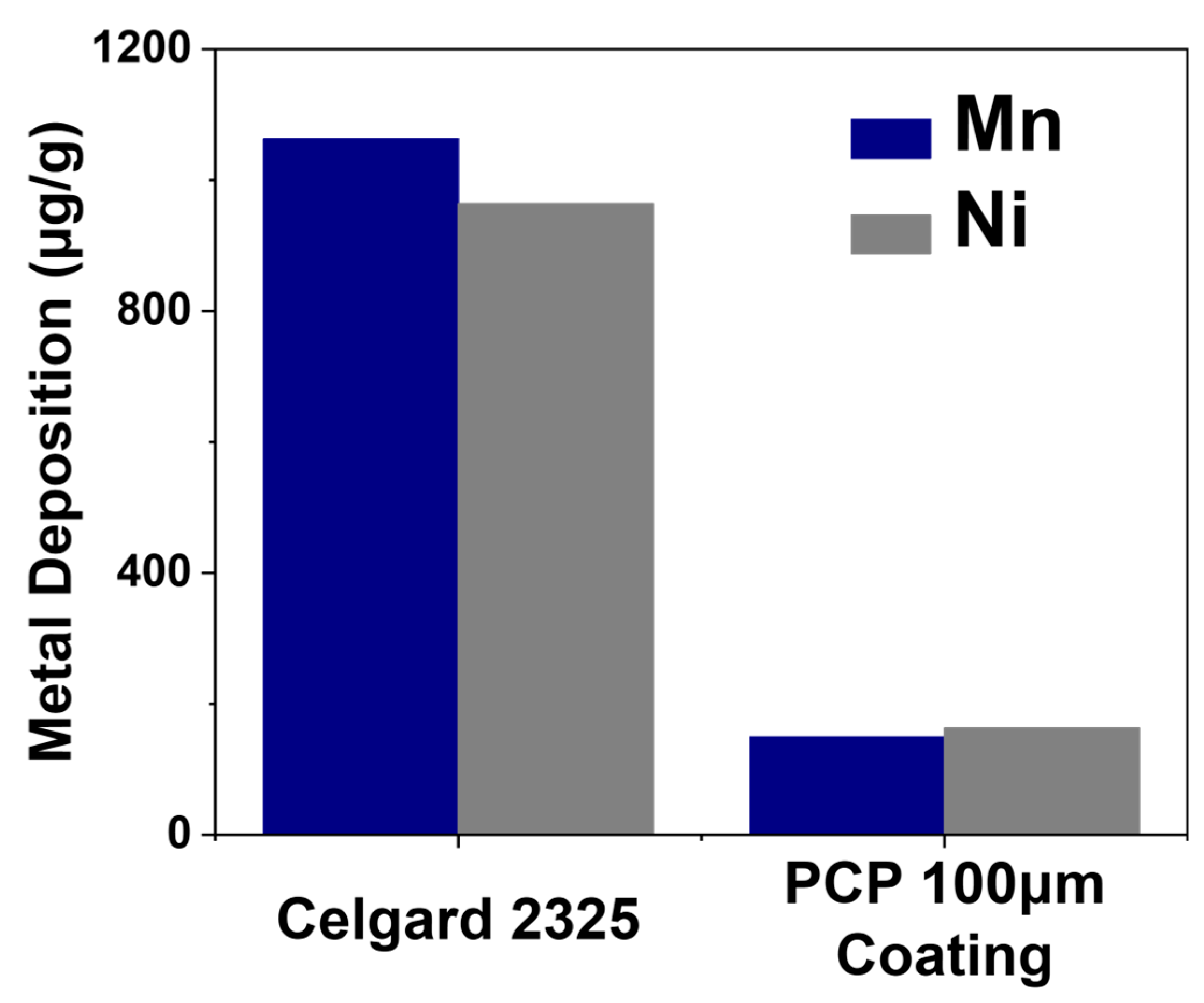
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Zhao, H.; Lam, W.Y.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.; Xu, H.; He, X. Cobalt-Free Cathode Materials: Families and their Prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]
- Voronina, N.; Sun, Y.-K.; Myung, S.-T. Co-Free Layered Cathode Materials for High Energy Density Lithium-Ion Batteries. ACS Energy Lett. 2020, 5, 1814–1824. [Google Scholar] [CrossRef]
- Croy, J.R.; Balasubramanian, M.; Gallagher, K.G.; Burrell, A.K. Review of the U.S. Department of Energy’s “deep dive” effort to understand voltage fade in Li- and Mn-rich cathodes. Acc. Chem. Res. 2015, 48, 2813–2821. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gutierrez, A.; Sultanov, M.A.; Wen, J.; Croy, J.R.; Wang, Y.; Srinivasan, V.; Barai, P. Unveiling Morphology and Crystallinity Dynamics in NixMn1−xCO3 Cathode Precursors through Batch-Mode Coprecipitation. ACS Appl. Energy Mater. 2024, 7, 2167–2177. [Google Scholar] [CrossRef]
- Luo, D.; Zhu, H.; Xia, Y.; Yin, Z.; Qin, Y.; Li, T.; Zhang, Q.; Gu, L.; Peng, Y.; Zhang, J.; et al. A Li-rich layered oxide cathode with negligible voltage decay. Nat. Energy 2023, 8, 1078–1087. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Levi, E.; Kim, S.; Choi, J.W.; Aurbach, D. Al Doping for Mitigating the Capacity Fading and Voltage Decay of Layered Li and Mn-Rich Cathodes for Li-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502398. [Google Scholar] [CrossRef]
- Gutierrez, A.; He, M.; Yonemoto, B.T.; Yang, Z.; Wang, J.; Meyer, H.M.; Thackeray, M.M.; Croy, J.R. Advancing Lithium- and Manganese-Rich Cathodes through a Combined Electrolyte Additive/Surface Treatment Strategy. J. Electrochem. Soc. 2019, 166, A3896–A3907. [Google Scholar] [CrossRef]
- Wang, R.; Weng, B.; Mahadevegowda, A.; Temprano, I.; Wang, H.; He, Z.; Ducati, C.; Xiao, Y.; Grey, C.P.; De Volder, M.F.L. Influence of Carbonate Electrolyte Solvents on Voltage and Capacity Degradation in Li-Rich Cathodes for Li-ion Batteries. Adv. Energy Mater. 2024, 14, 2401097. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Choi, J.; Kim, P.J. A roadmap of battery separator development: Past and future. Curr. Opin. Electrochem. 2022, 31, 100858. [Google Scholar] [CrossRef]
- Takeda, S.; Saito, Y.; Mukai, T.; Senoh, H.; Kaneko, I.; Yoshitake, H. Effect of the Stretching Process of Polyethylene Separators on Rate Capability of Lithium-Ion Batteries. J. Phys. Chem. C 2021, 125, 12496–12503. [Google Scholar] [CrossRef]
- Lin, W.; Wang, F.; Wang, H.; Li, H.; Fan, Y.; Chan, D.; Chen, S.; Tang, Y.; Zhang, Y. Thermal-Stable Separators: Design Principles and Strategies Towards Safe Lithium-Ion Battery Operations. ChemSusChem 2022, 15, e202201464. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, R.; Li, M.; Chee, M.O.L.; Dong, P.; Lu, J. Functionalized separator for next-generation batteries. Mater. Today 2020, 41, 143–155. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Li, S.L.; Xia, L.; Zhang, H.Y.; Ai, X.P.; Yang, H.X.; Cao, Y.L. A poly(3-decyl thiophene)-modified separator with self-actuating overcharge protection mechanism for LiFePO4-based lithium ion battery. J. Power Sources 2011, 196, 7021–7024. [Google Scholar] [CrossRef]
- Li, S.L.; Ai, X.P.; Yang, H.X.; Cao, Y.L. A polytriphenylamine-modified separator with reversible overcharge protection for 3.6V-class lithium-ion battery. J. Power Sources 2009, 189, 771–774. [Google Scholar] [CrossRef]
- Zhang, K.; Xiao, W.; Liu, J.; Yan, C. Advanced poly(vinyl alcohol) porous separator with overcharge protection function for lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 2853–2862. [Google Scholar] [CrossRef]
- Wang, B.; Richardson, T.J.; Chen, G. Electroactive Polymer Fiber Separators for Stable and Reversible Overcharge Protection in Rechargeable Lithium Batteries. J. Electrochem. Soc. 2014, 161, A1039–A1044. [Google Scholar] [CrossRef]
- Chen, G.; Richardson, T.J. Overcharge protection for high voltage lithium cells using two electroactive polymers. Electrochem. Solid-State Lett. 2005, 9, A24. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Shilina, Y.; Luski, S.; Aurbach, D.; Halalay, I.C. Acid-Scavenging Separators: A Novel Route for Improving Li-Ion Batteries’ Durability. ACS Energy Lett. 2017, 2, 2388–2393. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, H.; Yang, Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim. Acta 2008, 53, 3539–3546. [Google Scholar] [CrossRef]
- Zheng, J.; Ju, Z.; Zhang, B.; Nai, J.; Liu, T.; Liu, Y.; Xie, Q.; Zhang, W.; Wang, Y.; Tao, X. Lithium ion diffusion mechanism on the inorganic components of the solid–electrolyte interphase. J. Mater. Chem. A 2021, 9, 10251–10259. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Xu, Y.; Cao, X.; Engelhard, M.H.; Matthews, B.E.; Zhong, L.; Wu, H.; Jia, H.; Ren, X.; et al. Advanced Electrolytes for Fast-Charging High-Voltage Lithium-Ion Batteries in Wide-Temperature Range. Adv. Energy Mater. 2020, 10, 2000368. [Google Scholar] [CrossRef]
- Yang, J.; Fonseca Rodrigues, M.T.; Son, S.B.; Garcia, J.C.; Liu, K.; Gim, J.; Iddir, H.; Abraham, D.P.; Zhang, Z.; Liao, C. Dual-Salt Electrolytes to Effectively Reduce Impedance Rise of High-Nickel Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 40502–40512. [Google Scholar] [CrossRef]
- Long, B.R.; Rinaldo, S.G.; Gallagher, K.G.; Dees, D.W.; Trask, S.E.; Polzin, B.J.; Jansen, A.N.; Abraham, D.P.; Bloom, I.; Bareño, J. Enabling high-energy, high-voltage lithium-ion cells: Standardization of coin-cell assembly, electrochemical testing, and evaluation of full cells. J. Electrochem. Soc. 2016, 163, A2999. [Google Scholar] [CrossRef]
- Tornheim, A.; O’Hanlon, D.C. What do Coulombic Efficiency and Capacity Retention Truly Measure? A Deep Dive into Cyclable Lithium Inventory, Limitation Type, and Redox Side Reactions. J. Electrochem. Soc. 2020, 167, 110520. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.-Y.; Lin, C.-E.; Zhu, B.-K. Progress in polymeric separators for lithium ion batteries. RSC Adv. 2015, 5, 89848–89860. [Google Scholar] [CrossRef]
- Liao, C.; Wang, W.; Wang, J.; Han, L.; Qiu, S.; Song, L.; Gui, Z.; Kan, Y.; Hu, Y. Magnetron sputtering deposition of silicon nitride on polyimide separator for high-temperature lithium-ion batteries. J. Energy Chem. 2021, 56, 1–10. [Google Scholar] [CrossRef]
- Shen, X.; Li, C.; Shi, C.; Yang, C.; Deng, L.; Zhang, W.; Peng, L.; Dai, J.; Wu, D.; Zhang, P.; et al. Core-shell structured ceramic nonwoven separators by atomic layer deposition for safe lithium-ion batteries. Appl. Surf. Sci. 2018, 441, 165–173. [Google Scholar] [CrossRef]
- Yoo, Y.; Kim, B.G.; Pak, K.; Han, S.J.; Song, H.-S.; Choi, J.W.; Im, S.G. Initiated Chemical Vapor Deposition (iCVD) of Highly Cross-Linked Polymer Films for Advanced Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2015, 7, 18849–18855. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Ye, B.; Pang, P.; Ma, Z.; Chen, H.; Nan, J. A pore-controllable polyamine (PAI) layer-coated polyolefin (PE) separator for pouch lithium-ion batteries with enhanced safety. J. Solid State Electrochem. 2020, 24, 843–853. [Google Scholar] [CrossRef]
- Smith, A.; Burns, J.; Xiong, D.; Dahn, J. Interpreting high precision coulometry results on Li-ion cells. J. Electrochem. Soc. 2011, 158, A1136. [Google Scholar] [CrossRef]
- Read, J.A. In-Situ Studies on the Electrochemical Intercalation of Hexafluorophosphate Anion in Graphite with Selective Cointercalation of Solvent. J. Phys. Chem. C 2015, 119, 8438–8446. [Google Scholar] [CrossRef]
- Song, Z.; Miao, L.; Duan, H.; Ruhlmann, L.; Lv, Y.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Anionic Co-insertion Charge Storage in Dinitrobenzene Cathodes for High-Performance Aqueous Zinc-Organic Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202208821. [Google Scholar] [CrossRef]
- Russo, R.; Murugesan, R.; Jamali, A.; Chotard, J.N.; Toussaint, G.; Frayret, C.; Stevens, P.; Becuwe, M. N-Substituted Carbazole Derivate Salts as Stable Organic Electrodes for Anion Insertion. ChemNanoMat 2022, 8, e202200248. [Google Scholar] [CrossRef]
- Rajesh, M.; Dolhem, F.; Davoisne, C.; Becuwe, M. Reversible Anion Insertion in Molecular Phenothiazine-Based Redox-Active Positive Material for Organic Ion Batteries. ChemSusChem 2020, 13, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, I.A.; Bommier, C.; Fuller, D.D.; Leonard, D.P.; Williams, A.G.; Ji, X. Toward Higher Capacities of Hydrocarbon Cathodes in Dual-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 43311–43315. [Google Scholar] [CrossRef] [PubMed]
- Seel, J.; Dahn, J. Electrochemical intercalation of PF 6 into graphite. J. Electrochem. Soc. 2000, 147, 892. [Google Scholar] [CrossRef]
- Wang, B.; Garcia, J.C.; Chen, J.; Son, S.-B.; Trask, S.E.; Qin, Y.; Iddir, H.H.; Liao, C. Unexpected Exchange Reactions between LiPF6 and Additives: Enhancing Thermal Stability and Mitigating Transition-Metal Dissolution. ACS Appl. Eng. Mater. 2024, 2, 2910–2918. [Google Scholar] [CrossRef]
- Winter, M. The solid electrolyte interphase–the most important and the least understood solid electrolyte in rechargeable Li batteries. Z. Für Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Edström, K.; Herstedt, M.; Abraham, D.P. A new look at the solid electrolyte interphase on graphite anodes in Li-ion batteries. J. Power Sources 2006, 153, 380–384. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Huang, X.; He, X. Unveiling the mystery of LiF within solid electrolyte interphase in lithium batteries. Small 2024, 20, 2305429. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, X.; Zhang, B.; Huang, F.; Wang, Y.; Ma, Z.; Liu, J. Regulating Steric Hindrance of Porous Organic Polymers in Composite Solid-State Electrolytes to Induce the Formation of LiF-Rich SEI in Li-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202308738. [Google Scholar] [CrossRef]
- Alvarado, J.; Schroeder, M.A.; Pollard, T.P.; Wang, X.; Lee, J.Z.; Zhang, M.; Wynn, T.; Ding, M.; Borodin, O.; Meng, Y.S.; et al. Bisalt ether electrolytes: A pathway towards lithium metal batteries with Ni-rich cathodes. Energy Environ. Sci. 2019, 12, 780–794. [Google Scholar] [CrossRef]
- Qin, M.; Zeng, Z.; Liu, X.; Wu, Y.; He, R.; Zhong, W.; Cheng, S.; Xie, J. Revealing Surfactant Effect of Trifluoromethylbenzene in Medium-Concentrated PC Electrolyte for Advanced Lithium-Ion Batteries. Adv. Sci. 2023, 10, e2206648. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Shkrob, I.A.; Abraham, D.P. Transition Metal Dissolution, Ion Migration, Electrocatalytic Reduction and Capacity Loss in Lithium-Ion Full Cells. J. Electrochem. Soc. 2017, 164, A389–A399. [Google Scholar] [CrossRef]
- Wang, C.; Xing, L.; Vatamanu, J.; Chen, Z.; Lan, G.; Li, W.; Xu, K. Overlooked electrolyte destabilization by manganese (II) in lithium-ion batteries. Nat. Commun. 2019, 10, 3423. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Zhu, G.-N.; Hou, H.; Xia, Y.-Y.; Liu, T. Electrospun polyimide nanofiber-based nonwoven separators for lithium-ion batteries. J. Power Sources 2013, 226, 82–86. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, S.H.; Kim, D.-W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Zhu, C.; Nagaishi, T.; Shi, J.; Lee, H.; Wong, P.Y.; Sui, J.; Hyodo, K.; Kim, I.S. Enhanced Wettability and Thermal Stability of a Novel Polyethylene Terephthalate-Based Poly(Vinylidene Fluoride) Nanofiber Hybrid Membrane for the Separator of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 26400–26406. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, J.; Li, C. Nanostructured Carbon Nitride Polymer-Reinforced Electrolyte to Enable Dendrite-Suppressed Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2017, 9, 11615–11625. [Google Scholar] [CrossRef]
- Nisar, U.; Muralidharan, N.; Essehli, R.; Amin, R.; Belharouak, I. Valuation of Surface Coatings in High-Energy Density Lithium-ion Battery Cathode Materials. Energy Storage Mater. 2021, 38, 309–328. [Google Scholar] [CrossRef]
- Yang, R.; Yu, G.; Wu, Z.; Lu, T.; Hu, T.; Liu, F.; Zhao, H. Aging of lithium-ion battery separators during battery cycling. J. Energy Storage 2023, 63, 107107. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Shi, Y.; Yang, Y.; Hou, Y.; Wu, Y. A Composite Gel Polymer Electrolyte with High Performance Based on Poly(Vinylidene Fluoride) and Polyborate for Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300647. [Google Scholar] [CrossRef]
- Wang, B.; Gim, J.; Son, S.-B.; Shkrob, I.A.; Abraham, D.P.; Trask, S.E.; Qin, Y.; Kahvecioglu, O.; Jansen, A.N.; Liao, C. Electrolyte Study for High-Nickel LiNi0.9Mn0.05Co0.05O2 Cathodes. J. Electrochem. Soc. 2023, 170, 020505. [Google Scholar] [CrossRef]
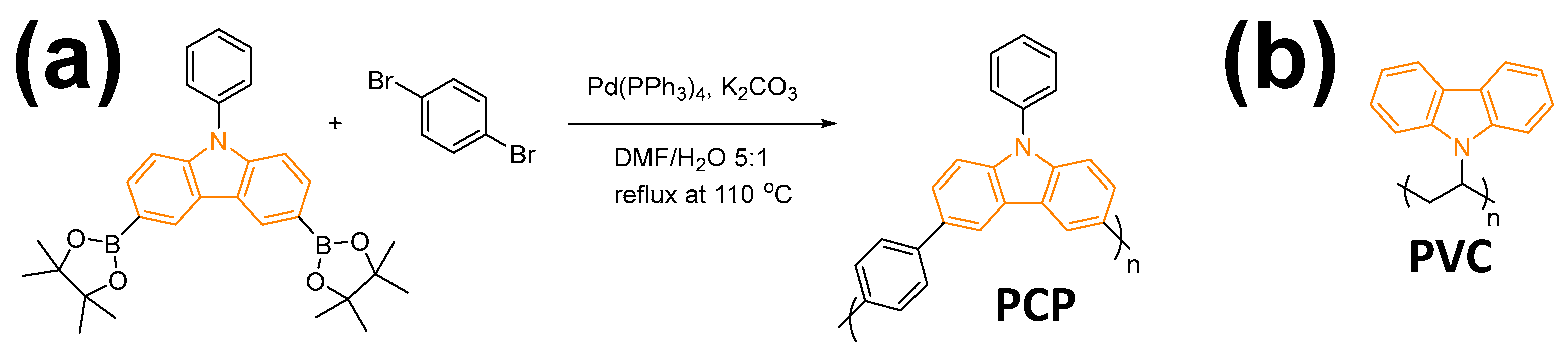
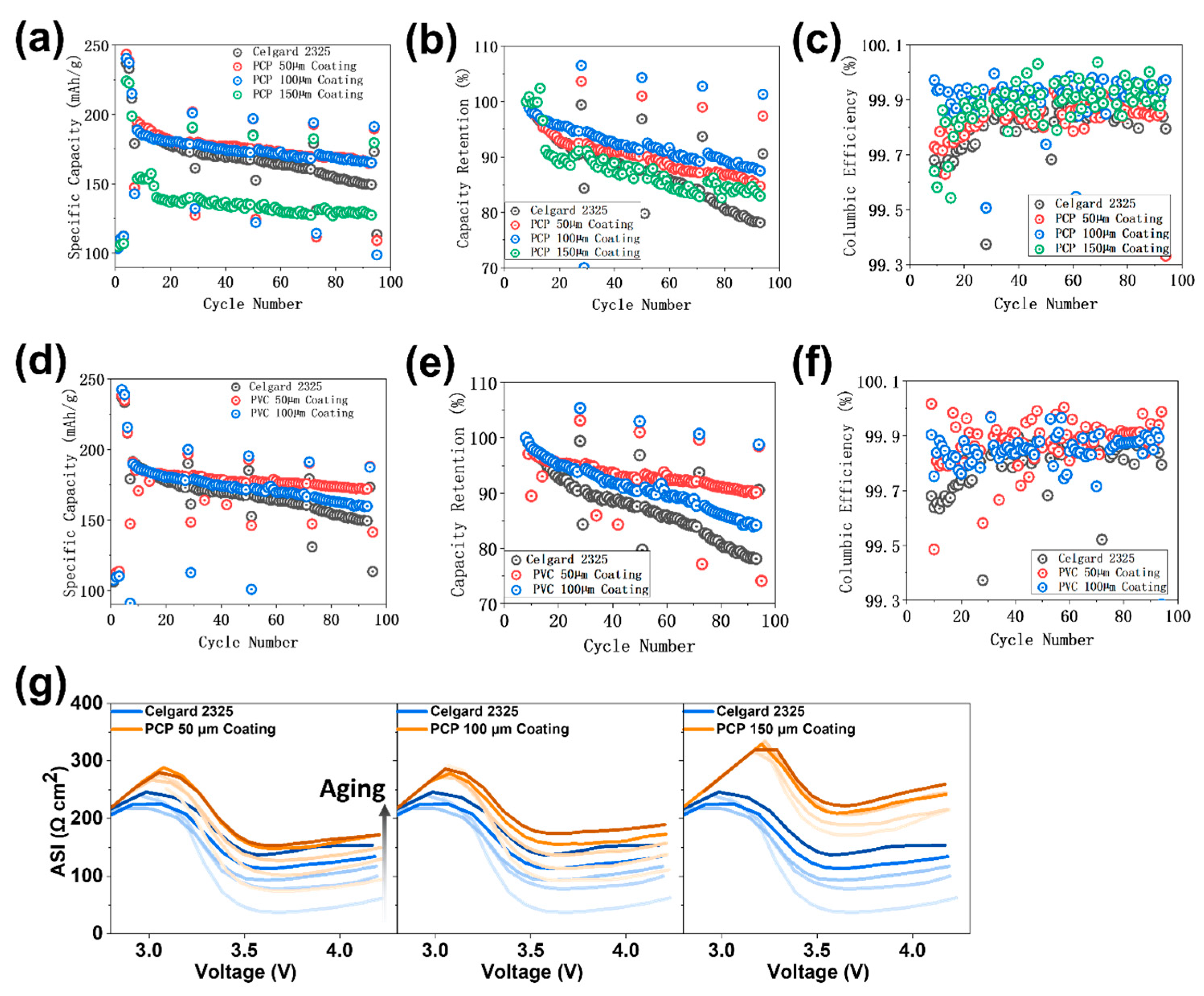
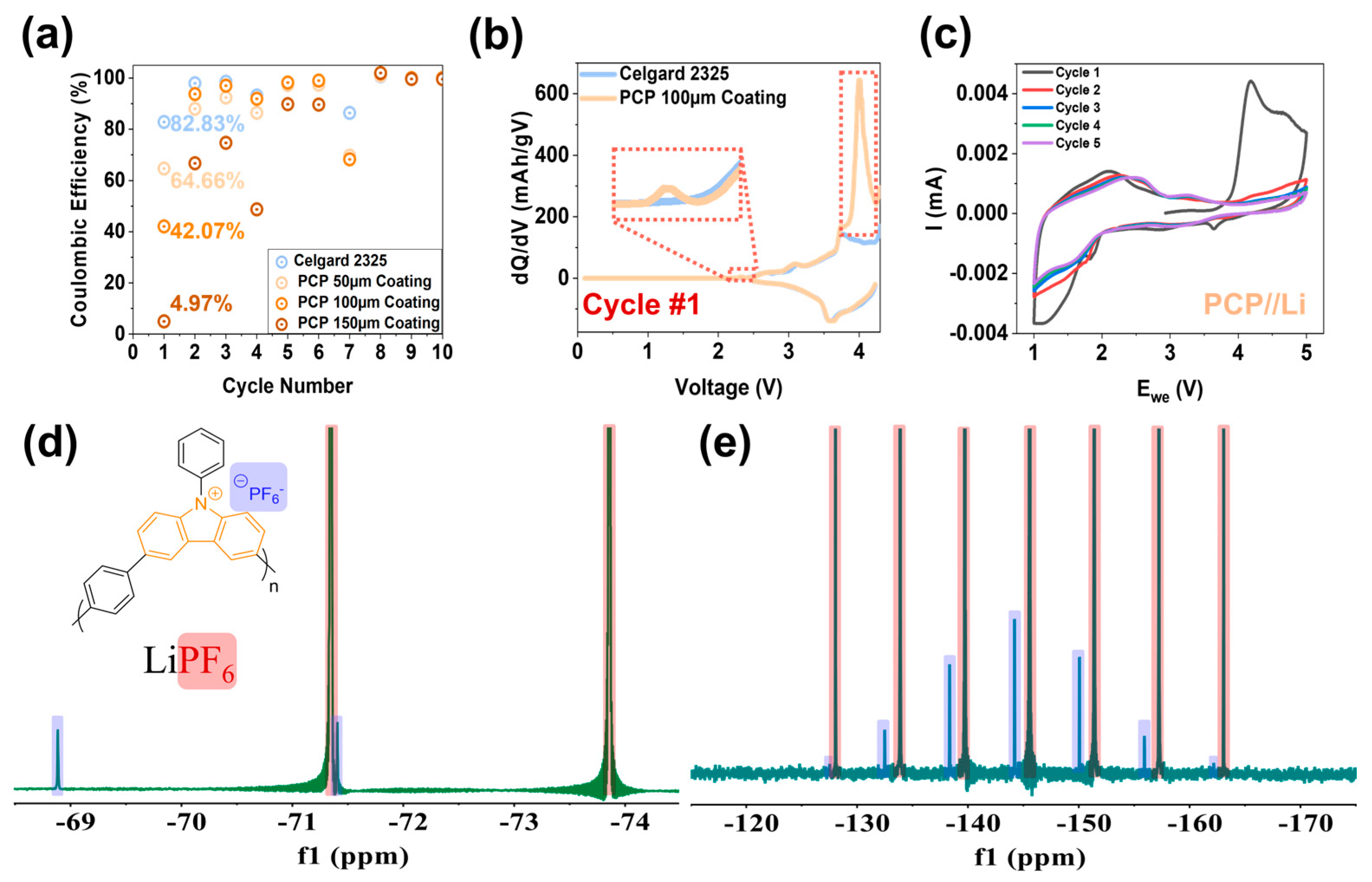
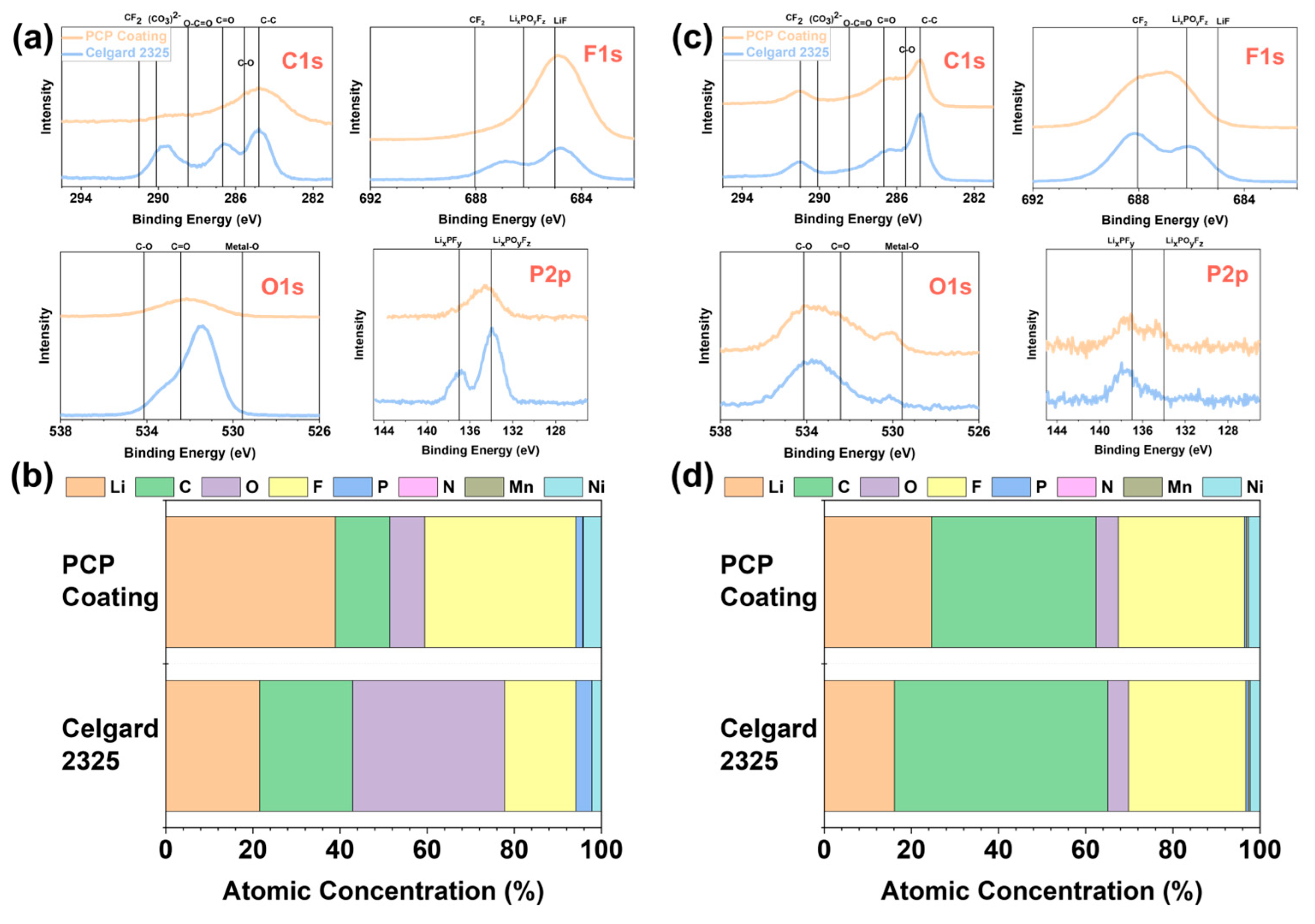

| Separator Used | First Cycle Discharge Specific Capacity (mAh g−1) | Eighth Cycle Coulombic Efficiency (%) | Final Cycle Discharge Specific Capacity (mAh g−1) | Initial ASI (Ω∙cm2) | Final ASI (Ω∙cm2) |
|---|---|---|---|---|---|
| Celgard 2325 | 106 | 99.03 | 113 | 37.63 | 137.38 |
| PCP 50 μm coating | 103.9 | 99.07 | 109 | 74.37 | 153.5 |
| PCP 100 μm coating | 103.4 | 98.8 | 98.7 | 92.84 | 174.23 |
| PVC 50 μm coating | 111.3 | 97.1 | 113.4 | 52.53 | 76.36 |
| PVC 100 μm coating | 107.4 | 99.2 | 141.4 | 80.87 | 143.66 |
| Properties | Mechanisms | Future Directions | References |
|---|---|---|---|
| Thermal Stability | Improved thermal stability reduces risk of thermal runaway | High-performance thermally stable separators (e.g., ceramic-coated) increase cost | [53,54,55] |
| Mechanical Strength | Enhanced mechanical strength prevents internal short circuits | Thick or reinforced separators can reduce ionic conductivity, lowering power density | [56] |
| Electrolyte Wettability | Improved wettability ensures better electrolyte uptake, enhancing ionic conductivity | Some coatings to improve wettability may degrade over cycling or cause side reactions | [55,57] |
| Chemical Stability | Stability against high-voltage cathodes (>4.3 V) reduces side reactions and capacity fade | Some separators degrade under high voltage or react with electrolyte additives | [58] |
| Ionic Conductivity | Thin separators with porous structures enable high ionic conductivity | Too thin separators may have lower puncture resistance, risking safety | [59] |
| Electrochemical and interfacial stability | Electroactive carbazole polymers demonstrate first cycling lithium inventory improvement and interfacial stability | Polymer may need engineering to get a thin layer | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Gao, L.; Yang, Z.; Wu, X.; Zhu, Q.; Liu, Q.; Dogan, F.; Qin, Y.; Liao, C. Interphase Engineering Enabled by Using a Separator with Electrochemically Active Carbazole Polymers for Lithium-Ion Batteries. Polymers 2025, 17, 1815. https://doi.org/10.3390/polym17131815
Wang B, Gao L, Yang Z, Wu X, Zhu Q, Liu Q, Dogan F, Qin Y, Liao C. Interphase Engineering Enabled by Using a Separator with Electrochemically Active Carbazole Polymers for Lithium-Ion Batteries. Polymers. 2025; 17(13):1815. https://doi.org/10.3390/polym17131815
Chicago/Turabian StyleWang, Bingning, Lihong Gao, Zhenzhen Yang, Xianyang Wu, Qijia Zhu, Qian Liu, Fulya Dogan, Yang Qin, and Chen Liao. 2025. "Interphase Engineering Enabled by Using a Separator with Electrochemically Active Carbazole Polymers for Lithium-Ion Batteries" Polymers 17, no. 13: 1815. https://doi.org/10.3390/polym17131815
APA StyleWang, B., Gao, L., Yang, Z., Wu, X., Zhu, Q., Liu, Q., Dogan, F., Qin, Y., & Liao, C. (2025). Interphase Engineering Enabled by Using a Separator with Electrochemically Active Carbazole Polymers for Lithium-Ion Batteries. Polymers, 17(13), 1815. https://doi.org/10.3390/polym17131815







