Graphene Oxide-Enriched Polymer: Impact on Dental Pulp Cell Viability and Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. PMMA and PMMA+GO Sample Preparation
2.1.1. PMMA and PMMA+GO Characterization
2.2. Cell-Culture Assays
2.2.1. Cytotoxicity Test
2.2.2. Cell-Proliferation Test
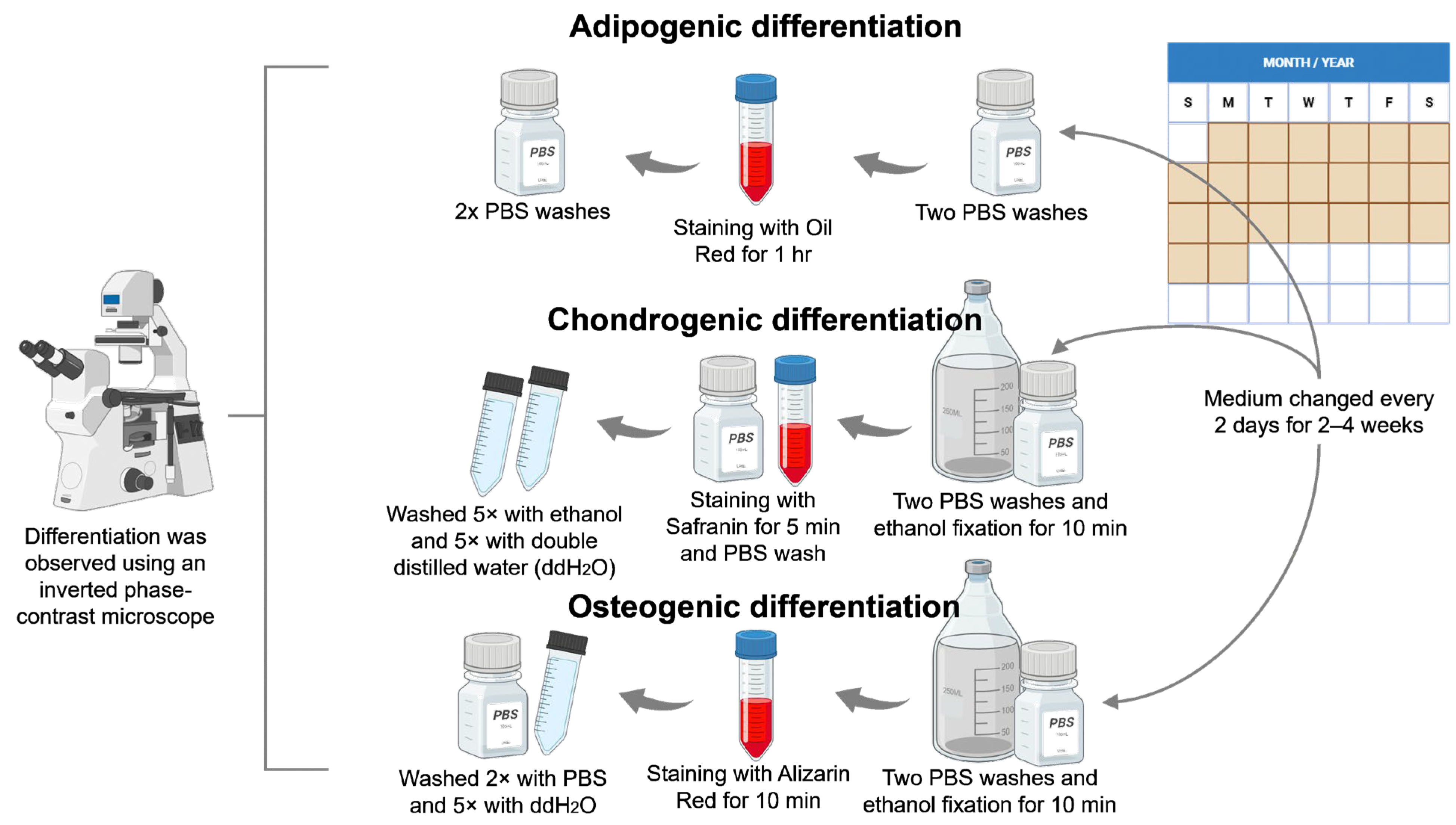
2.2.3. Cell-Differentiation Assay
Adipogenic Differentiation
Chondrogenic Differentiation
Osteogenic Differentiation
2.3. Statistical Analysis
3. Results
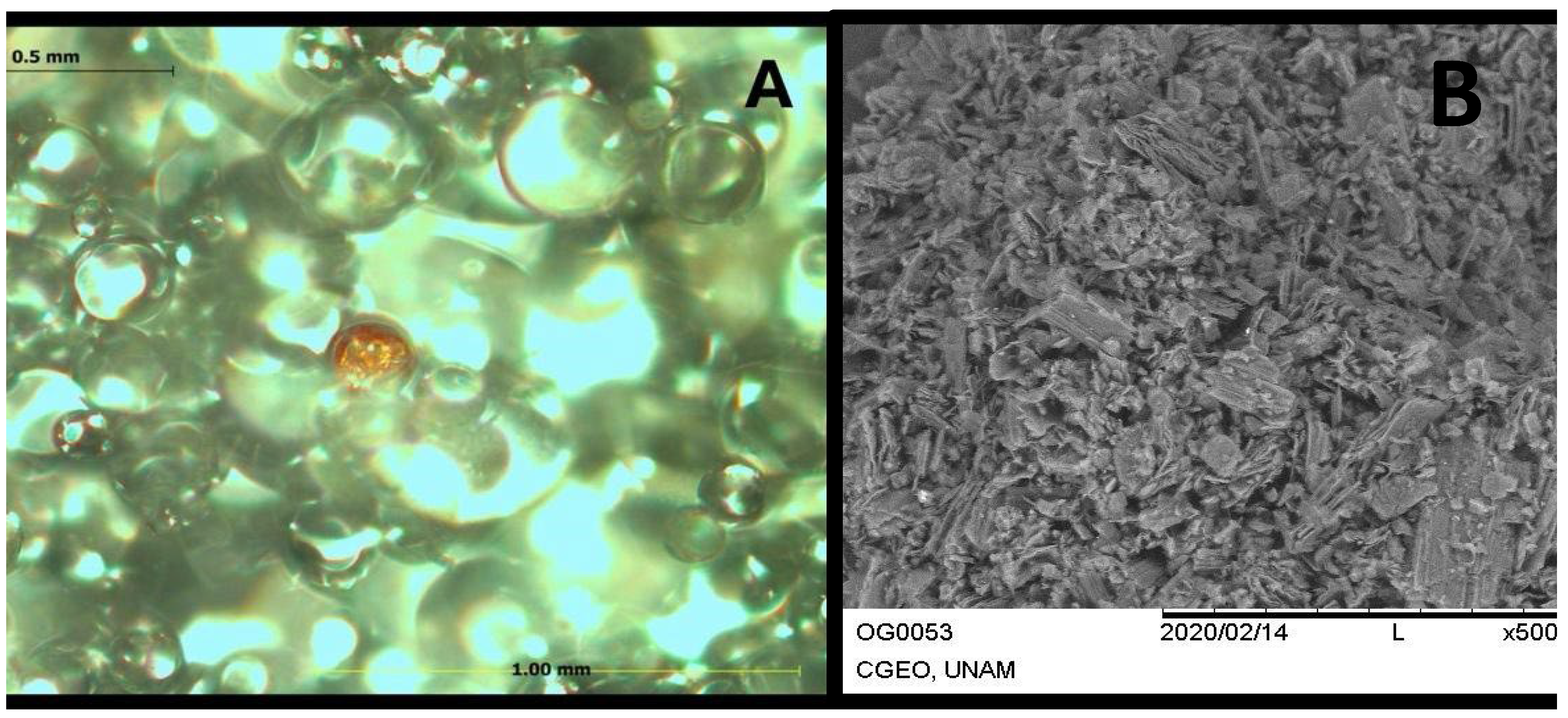
3.1. PMMA+GO Characterization
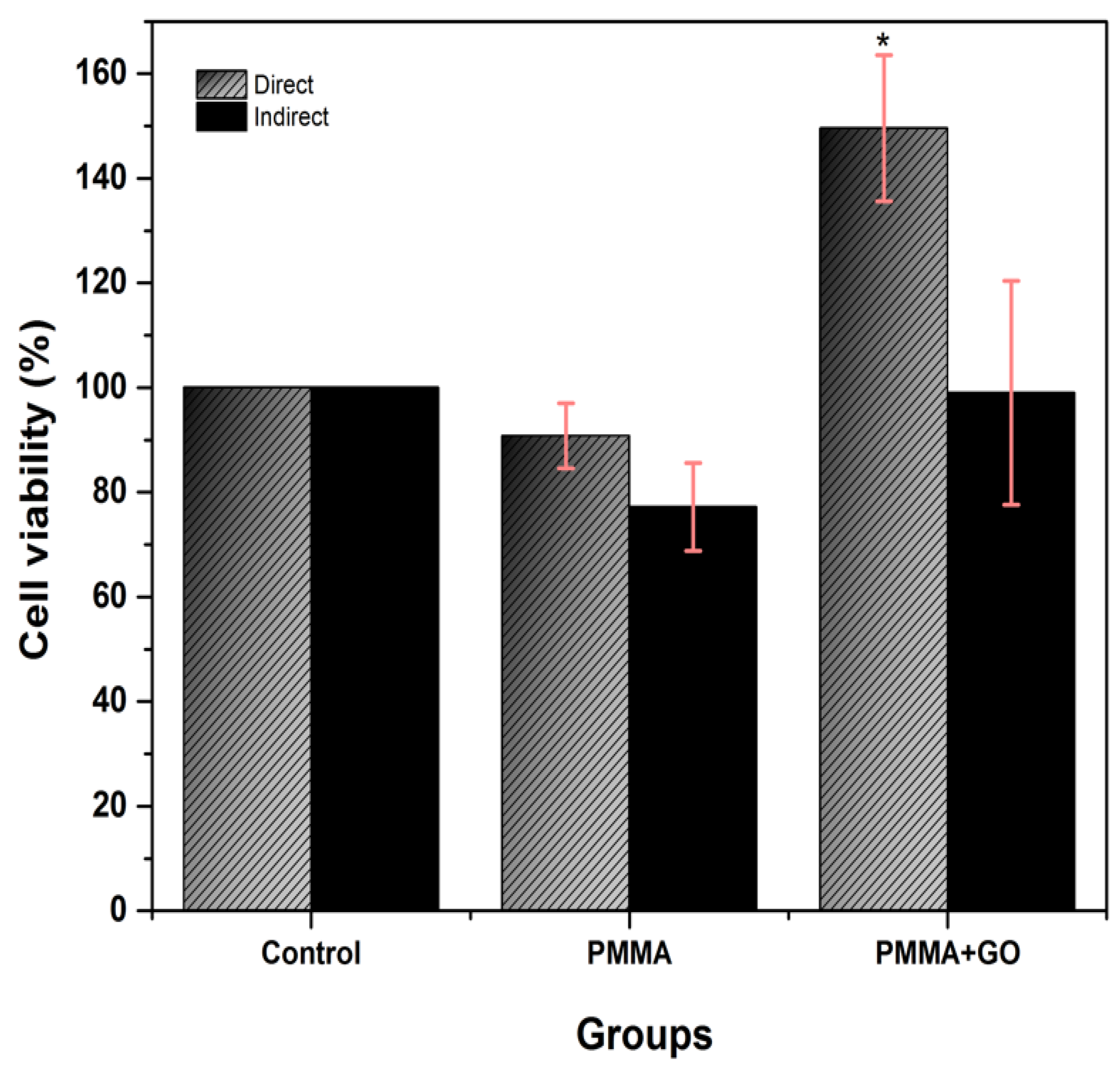
3.2. Cytotoxicity Test
3.3. Cell-Proliferation Assay
3.4. Cell Differentiation
3.4.1. Adipogenic Differentiation
3.4.2. Chondrogenic Differentiation
3.4.3. Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, P.T.; Handorf, A.M.; Jeon, W.B.; Li, W.J. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr. Pharm. Des. 2013, 19, 3429–3445. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, V.R.; Natarajan, P.M.; Swamikannu, B. Regenerative Strategies in Dentistry: Harnessing Stem Cells, Biomaterials and Bioactive Materials for Tissue Repair. Biomolecules 2025, 15, 546. [Google Scholar] [CrossRef]
- Hynds, R.E.; Magin, C.M.; Ikonomou, L.; Aschner, Y.; Beers, M.F.; Burgess, J.K.; Heise, R.L.; Hume, P.S.; Krasnodembskaya, A.D.; Mei, S.H.; et al. Stem cells, cell therapies, and bioengineering in lung biology and diseases 2023. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L327–L340. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.R.; Dehghani, Z.; Pennisi, C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng. Transl. Med. 2022, 8, e10383. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawe, R.A.; Al-Rammahi, H.M.; Cahyanto, A.; Ma’amor, A.; Liew, Y.M.; Sukumaran, P.; Wan Hassan, W.N. Cuttlefish-Bone-Derived Biomaterials in Regenerative Medicine, Dentistry, and Tissue Engineering: A Systematic Review. J. Funct. Biomater. 2024, 15, 219. [Google Scholar] [CrossRef]
- Bansal, R.; Jain, A. Current overview on dental stem cells applications in regenerative dentistry. J. Nat. Sci. Biol. Med. 2015, 6, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, L.; Cordeiro, M.M.; Nör, S.A.; Nör, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene oxide: A promising material for regenerative medicine and tissue engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef]
- Rayannavar, S.; Mv, S.K.; Kamath, V.; Bembalgi, M.; Nayak, N.; Jodalli, P. Osseointegrative and antimicrobial properties of graphene oxide nano coated dental implants: A systematic review. F1000Research 2024, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Graphene-Oxide Peptide-Containing Materials for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 10174. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cataldi, A.; Zara, S.; Gallorini, M. Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials 2022, 15, 2229. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, R.; Guzmán-Juárez, H.; López-Ramos, D.; Alvarez-Gayosso, C. Biological and physico-mechanical properties of poly (methyl methacrylate) enriched with graphene oxide as a potential biomaterial. J. Oral Res. 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Torres-Silva, H.; López-Bonilla, J.L. Aspectos quirales del grafeno. Ingeniare Rev. Chil. Ing. 2011, 19, 67–75. [Google Scholar] [CrossRef]
- Liu, C.; Tan, D.; Chen, X.; Liao, J.; Wu, L. Research on Graphene and Its Derivatives in Oral Disease Treatment. Int. J. Mol. Sci. 2022, 23, 4737. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Lin, Y.; Gou, N.; Rahman, S.M.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Dependence of graphene oxide (GO) toxicity on oxidation level, elemental composition, and size. Int. J. Mol. Sci. 2021, 22, 10578. [Google Scholar] [CrossRef]
- Slimani, M.; Baus, A.; Bich, C.S.; de Rousiers, A.; Duhoux, A.; Brachet, M.; Duhamel, P.; Bey, E. Methylmetacrylate (PMMA) cranioplasty technique: Technical interest of intraoperative modeling and review of the literature. Ann. Chir. Plast. Esthet. 2023, 68, 99–105. [Google Scholar] [CrossRef]
- Leyendecker Junior, A.; Gomes Pinheiro, C.C.; Lazzaretti Fernandes, T.; Franco Bueno, D. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J. Tissue Eng. 2018, 9, 2041731417752766. [Google Scholar] [CrossRef]
- Rüggeberg, F.A. From vulcanite to vinyl, a history of resins in restorative dentistry. J. Prosthet. Dent. 2002, 87, 364–379. [Google Scholar] [CrossRef]
- Majeed, H.F.; Hamad, T.I.; Bairam, L.R. Enhancing 3D-printed denture base resins: A review of material innovations. Sci. Prog. 2024, 107, 368504241263484. [Google Scholar] [CrossRef]
- Kausar, A. Poly (methyl methacrylate) nanocomposite reinforced with graphene, graphene oxide, and graphite: A review. Polym.-Plast. Technol. Mater. 2019, 58, 821–842. [Google Scholar] [CrossRef]
- Shah, P.; Aghazadeh, M.; Rajasingh, S.; Dixon, D.; Jain, V.; Rajasingh, J. Stem cells in regenerative dentistry: Current understanding and future directions. J. Oral Biosci. 2024, 66, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; An, S.S.; Hulme, J. Current applications of graphene oxide in nanomedicine. Int. J. Nanomed. 2015, 10, 9–24. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.; Shi, H.; Tang, L.A.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Mirza, E.H.; Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Fareedi, F.; Khan, M.M.; Alfayez, M.; Al-Fotawi, R.; Vallittu, P.K.; et al. Characterization of osteogenic cells grown over modified graphene-oxide-biostable polymers. Biomed. Mater. 2019, 14, 065004. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Kasiri-Asgarani, M.; Omidi, M.; Razzaghi, M.; Berto, F. CNT and rGO reinforced PMMA based bone cement for fixation of load bearing implants: Mechanical property and biological response. J. Mech. Behav. Biomed. Mater. 2021, 116, 104320. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl methacrylate-based bone cements containing carbon nanotubes and graphene oxide: An overview of physical, mechanical, and biological properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Putzer, D.; Stuendl, N.; Lohberger, B.; Awaja, F. Enhanced osteogenic differentiation of human primary mesenchymal stem and progenitor cultures on graphene oxide/poly (methyl methacrylate) composite scaffolds. Materials 2020, 13, 2991. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Quiroz, M.; Reyes-Maciel, A.; Lopez-Ayuso, C.A.; Jurado, C.A.; Guzman-Juarez, H.; Alvarez-Gayosso, C.A.; Aranda-Herrera, B.; Alshabib, A.; Garcia-Contreras, R. Graphene Oxide-Enriched Polymer: Impact on Dental Pulp Cell Viability and Differentiation. Polymers 2025, 17, 1768. https://doi.org/10.3390/polym17131768
Vega-Quiroz M, Reyes-Maciel A, Lopez-Ayuso CA, Jurado CA, Guzman-Juarez H, Alvarez-Gayosso CA, Aranda-Herrera B, Alshabib A, Garcia-Contreras R. Graphene Oxide-Enriched Polymer: Impact on Dental Pulp Cell Viability and Differentiation. Polymers. 2025; 17(13):1768. https://doi.org/10.3390/polym17131768
Chicago/Turabian StyleVega-Quiroz, Magdalena, Agustin Reyes-Maciel, Christian Andrea Lopez-Ayuso, Carlos A. Jurado, Hector Guzman-Juarez, Carlos Andres Alvarez-Gayosso, Benjamin Aranda-Herrera, Abdulrahman Alshabib, and Rene Garcia-Contreras. 2025. "Graphene Oxide-Enriched Polymer: Impact on Dental Pulp Cell Viability and Differentiation" Polymers 17, no. 13: 1768. https://doi.org/10.3390/polym17131768
APA StyleVega-Quiroz, M., Reyes-Maciel, A., Lopez-Ayuso, C. A., Jurado, C. A., Guzman-Juarez, H., Alvarez-Gayosso, C. A., Aranda-Herrera, B., Alshabib, A., & Garcia-Contreras, R. (2025). Graphene Oxide-Enriched Polymer: Impact on Dental Pulp Cell Viability and Differentiation. Polymers, 17(13), 1768. https://doi.org/10.3390/polym17131768








