Nonionic Fast-Penetration System for Diffusion-Driven Degradation of Liquid Plugs
Abstract
1. Introduction
- (1)
- Structural optimization for responsive degradability: Traditional epoxy–anhydride systems offer strong initial sealing performance, yet their high crosslink density imposes rigidity that limits dynamic responsiveness during degradation [12,13,14]. Ding et al. [15] introduced polyester-type degradable monomers to enhance thermal sensitivity and tunability of the degradation window. Similarly, Qin et al. [16] incorporated multifunctional groups into polyester networks to broaden the application window under various downhole conditions. Despite these developments, the degradation response often remains passive and lacks internal dynamic restructuring.
- (2)
- Diffusion-pathway construction and structural response characterization: Diffusion plays a dominant role in controlling degradability. Ren et al. [17] developed a porous plug system that significantly improved the internal diffusion flux of degradation agents. Yao et al. [18] revealed that microcrack-induced primary–secondary diffusion networks could promote a transformation from point-based to area-wide degradation pathways. However, few studies have linked these diffusion pathways directly with the mechanical failure behaviors or structural collapse mechanisms.
- (3)
- Molecular-scale degradation mechanism and reactive interface tracking: Li et al. [19] applied FTIR and SEM to preliminarily correlate ester bond scission with interfacial relaxation. Liu et al. [20] further proposed a combined FTIR mapping–Raman mapping strategy to visualize functional group migration during degradation. Kong et al. [21] used nanoindentation and AFM to establish a correlation between localized elastic modulus reduction and diffusion front propagation, offering a novel perspective on interfacial softening behavior. Tran and Zhou [22,23] built a microfluidic unsealing simulation platform and mapped thermally driven diffusion-assisted degradation mechanisms to verify process coupling in engineering-relevant scenarios.
- (1)
- (2)
- The causal link between diffusion processes and microstructural failure remains insufficiently resolved in current studies. Existing research has largely focused on evaluating the macroscopic plugging effectiveness or degradation behavior of polymer gels [2,3,4], yet few attempts have been made to systematically correlate the internal diffusion pathway with the initiation and propagation of structural damage.
- (3)
- Interface behavior and reaction pathway coupling in nonionic diffusion systems remain largely unexplored in the existing literature; most related work has focused on nonionic surfactant adsorption kinetics or interface-mediated diffusion behaviors without integrating chemical bond cleavage or structural channel formation into a unified degradation model [25,26,27].
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of the Liquid Plug
2.4. Formulation of the Nonionic Penetrating Degradation Solution
2.5. SEM and EDS Characterization
2.6. Visualization of Penetration Channels
2.7. FTIR Analysis
2.8. FTIR Mapping
2.9. AFM Modulus Mapping
2.10. Nanoindentation
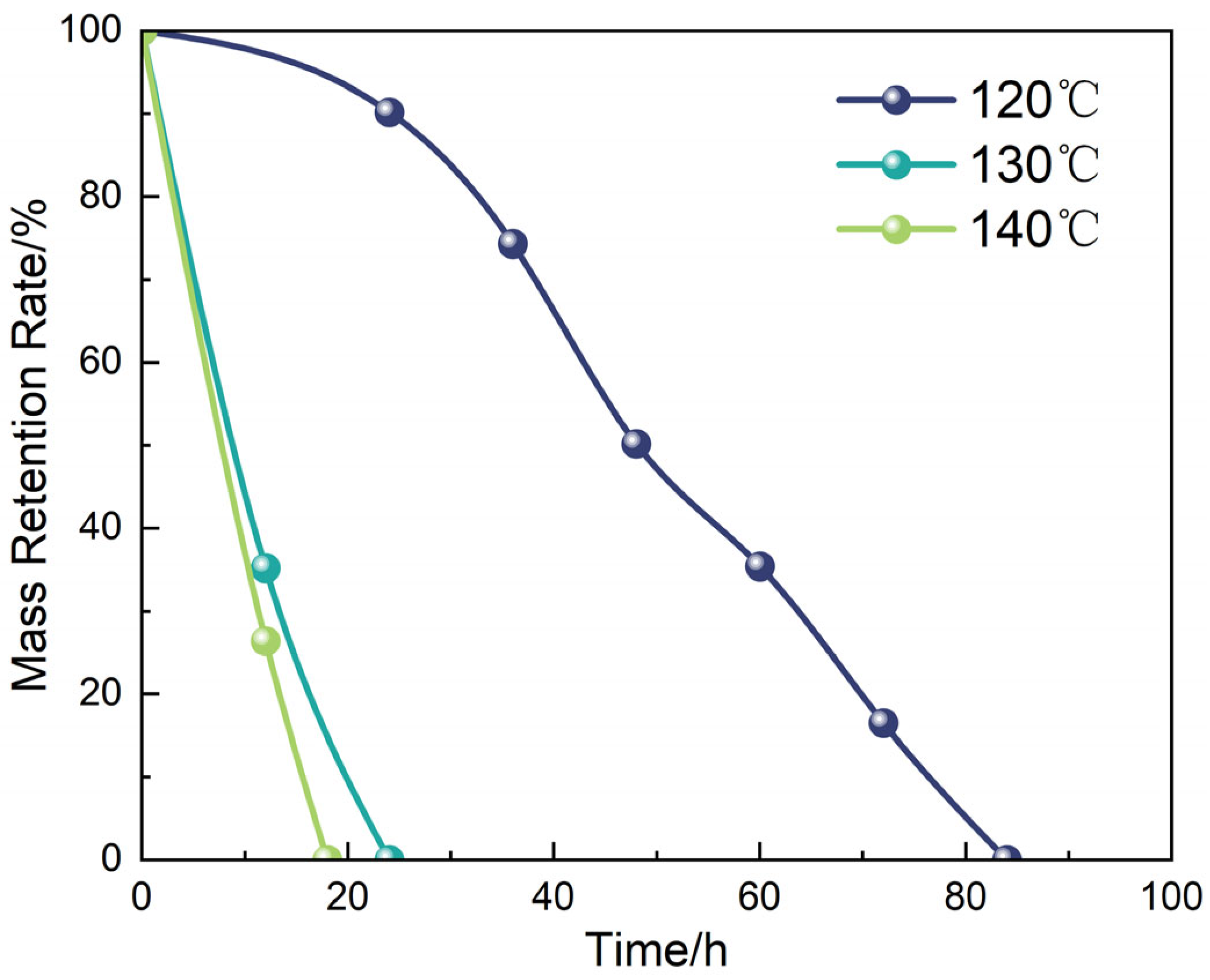
2.11. Evaluation of Degradation Behavior
3. Results and Discussion
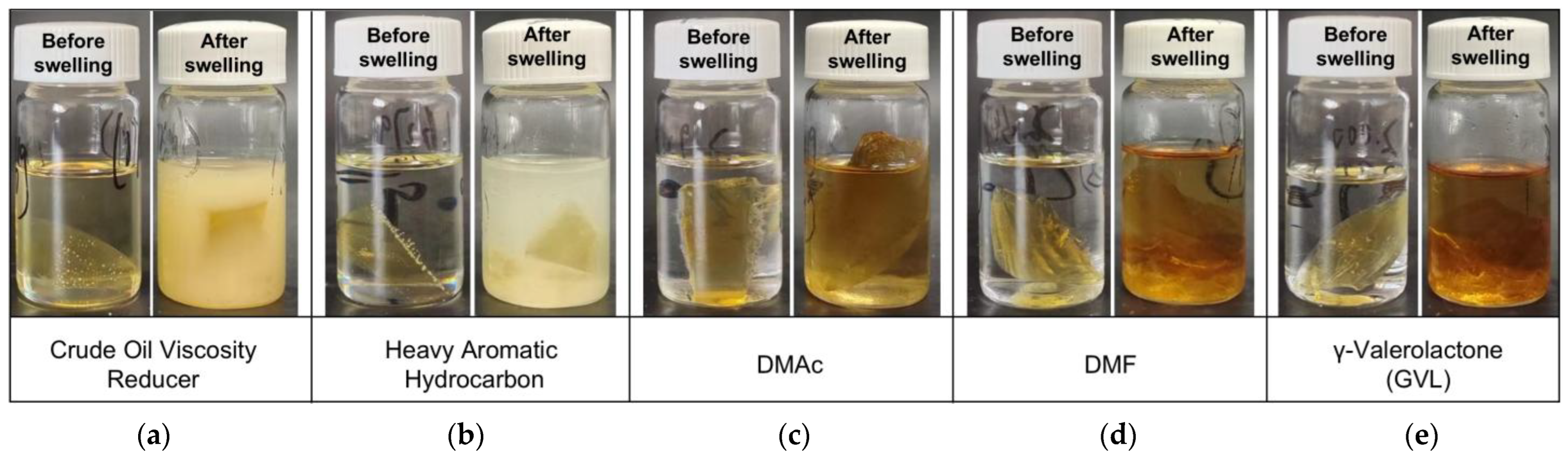
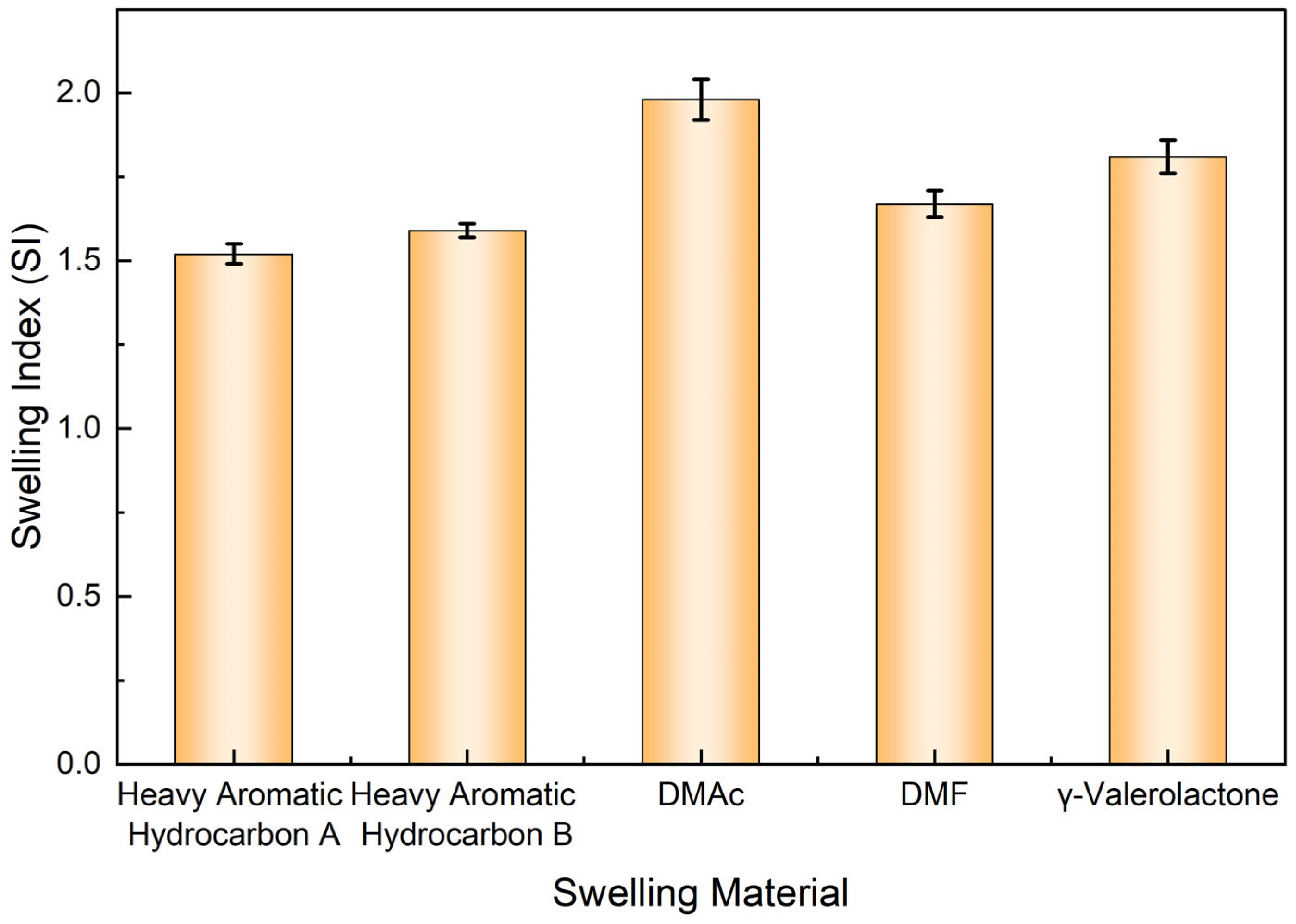
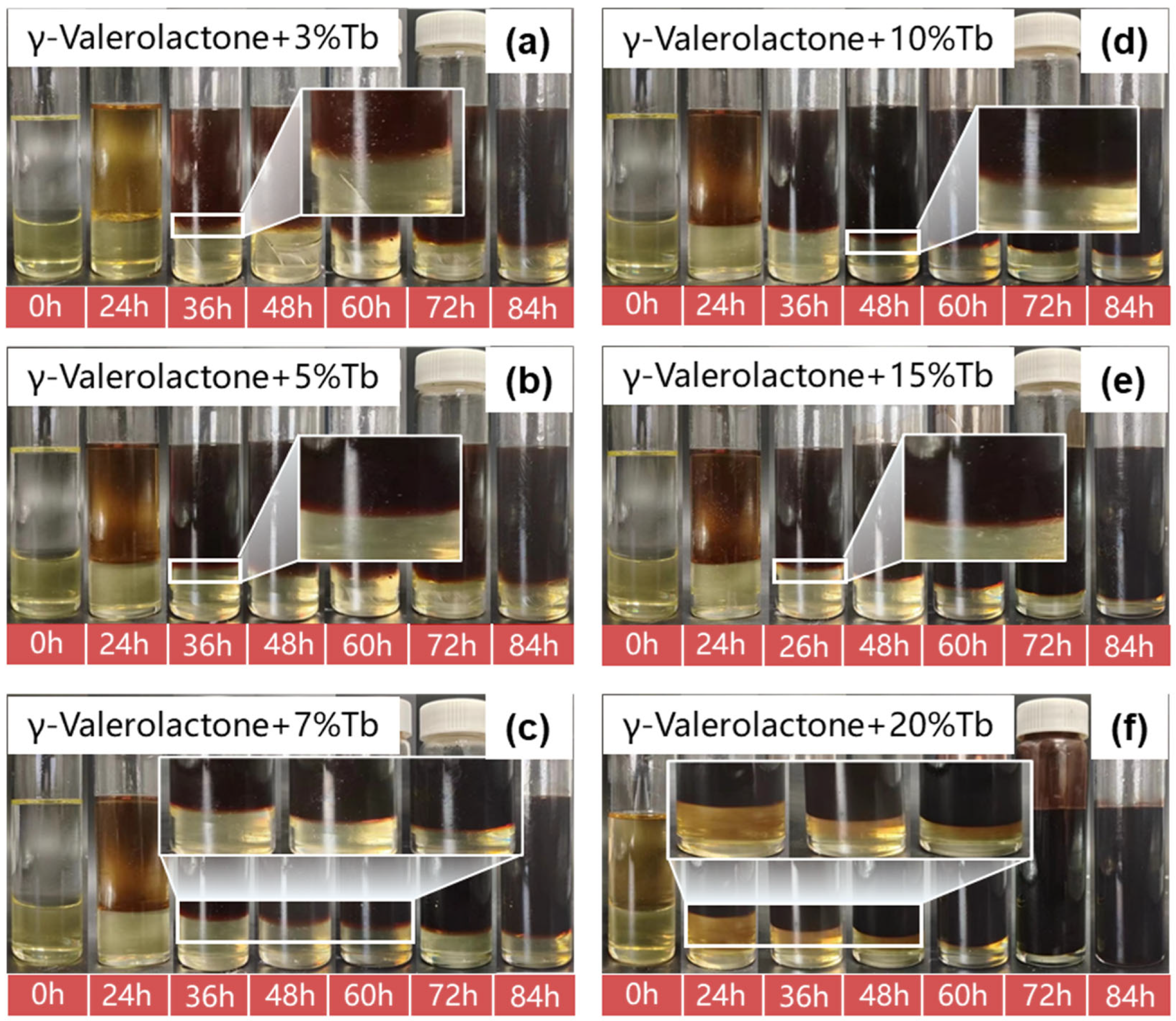
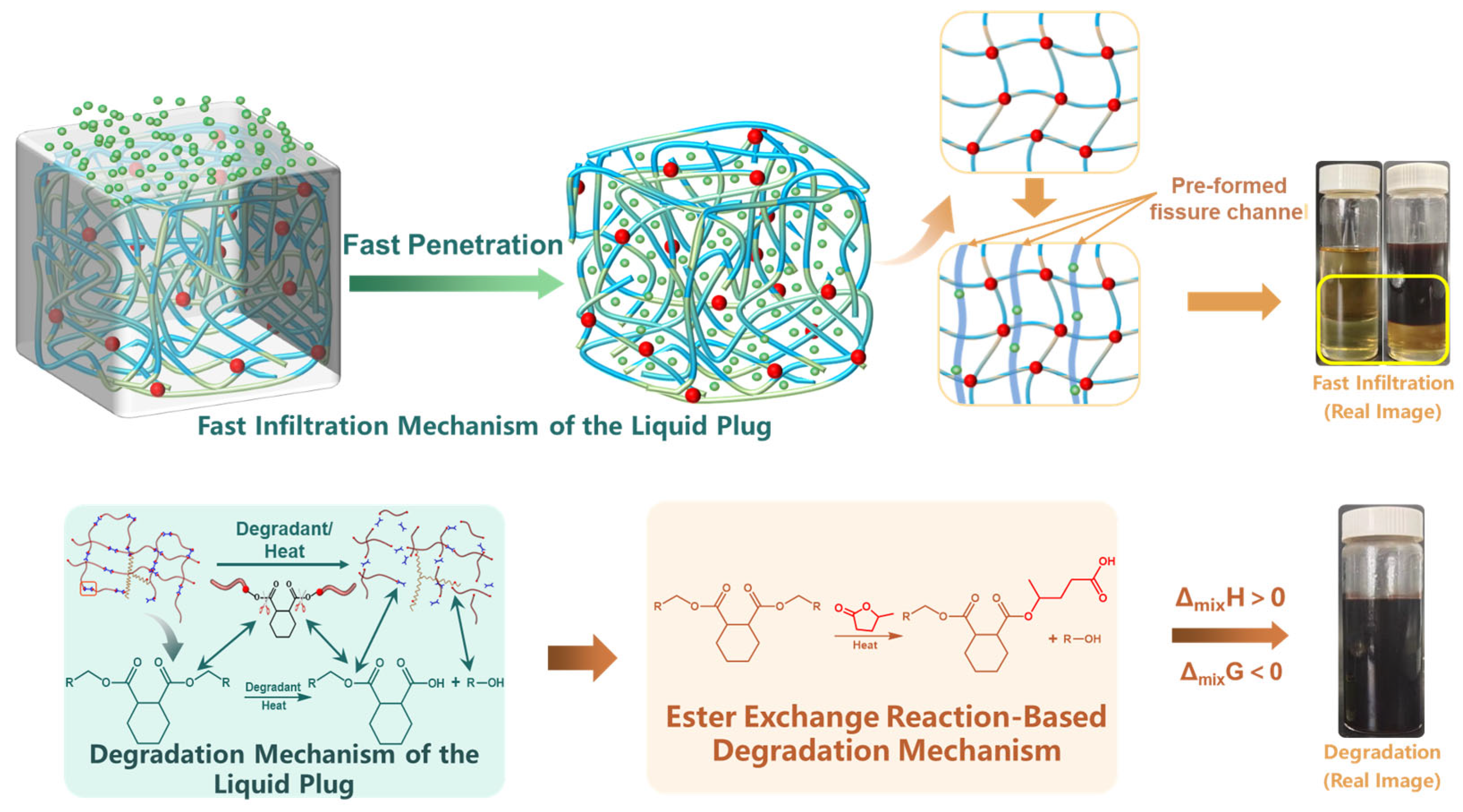
3.1. Diffusion–Swelling Behavior and Structural Response of the Liquid Plug
3.1.1. Macroscopic Volume Expansion and Morphological Evolution Under Solvent Action
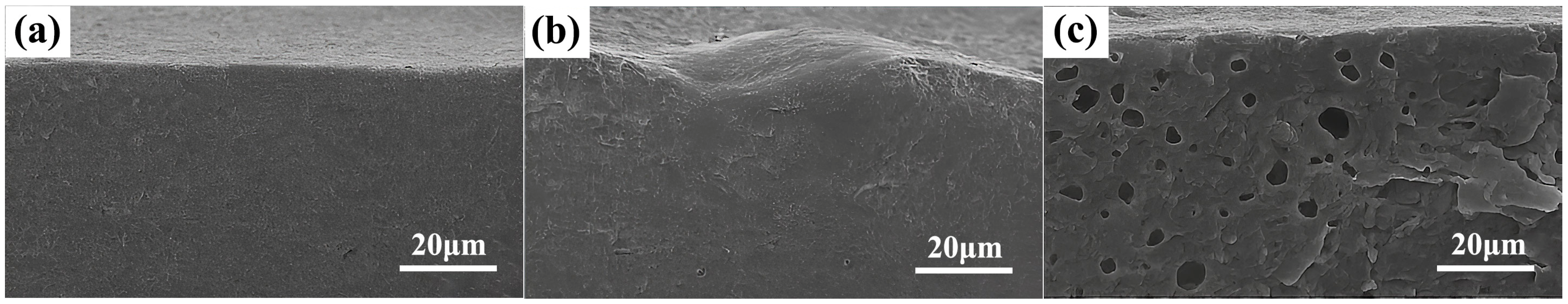
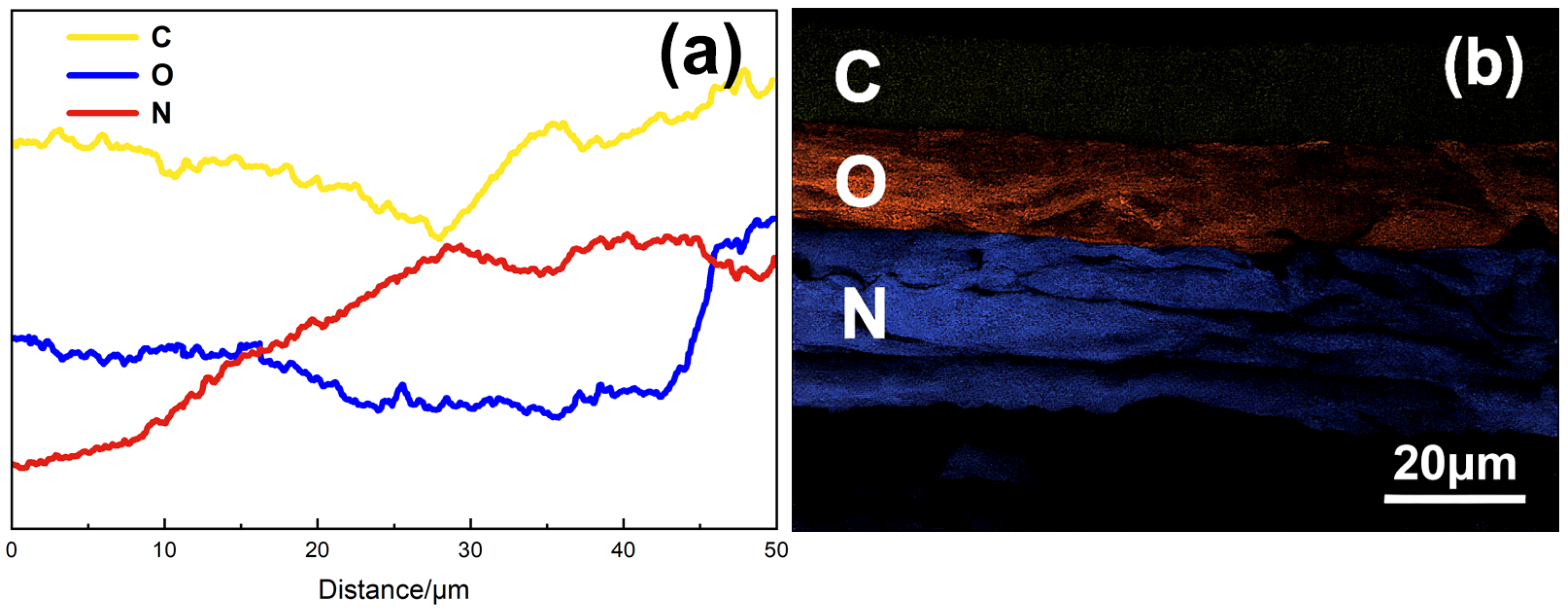
3.1.2. Microstructural Evolution Analysis
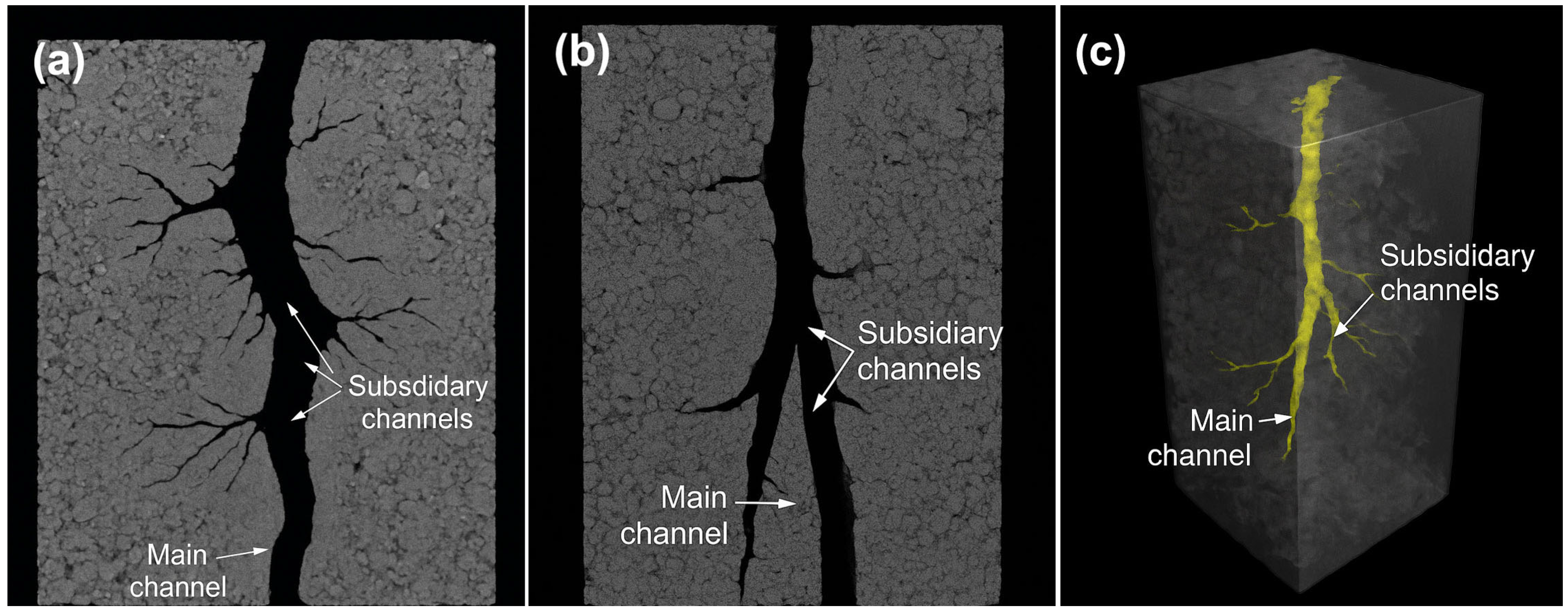
3.1.3. Analysis of Degradation Channel Formation
3.2. Mechanistic Insights into Diffusion-Driven Degradation Behavior Under Variable Penetrant Concentration
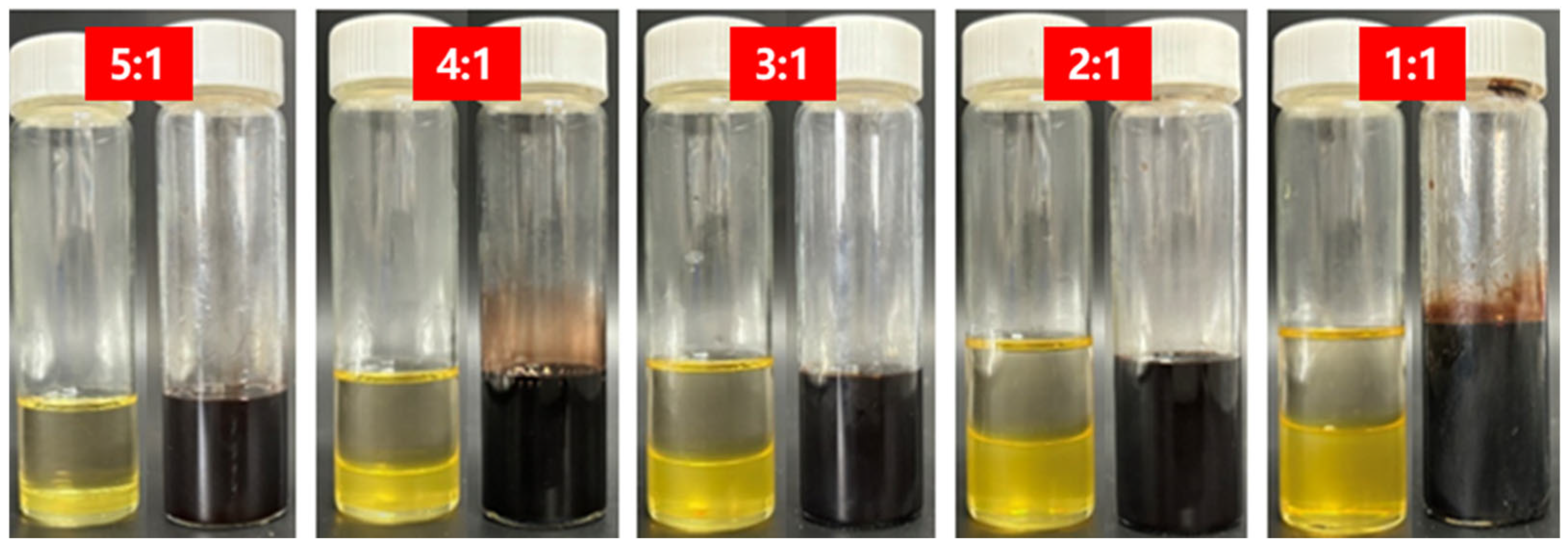
3.2.1. Coupled Effects of Penetrant Concentration on Diffusion Rate and Degradation Kinetics
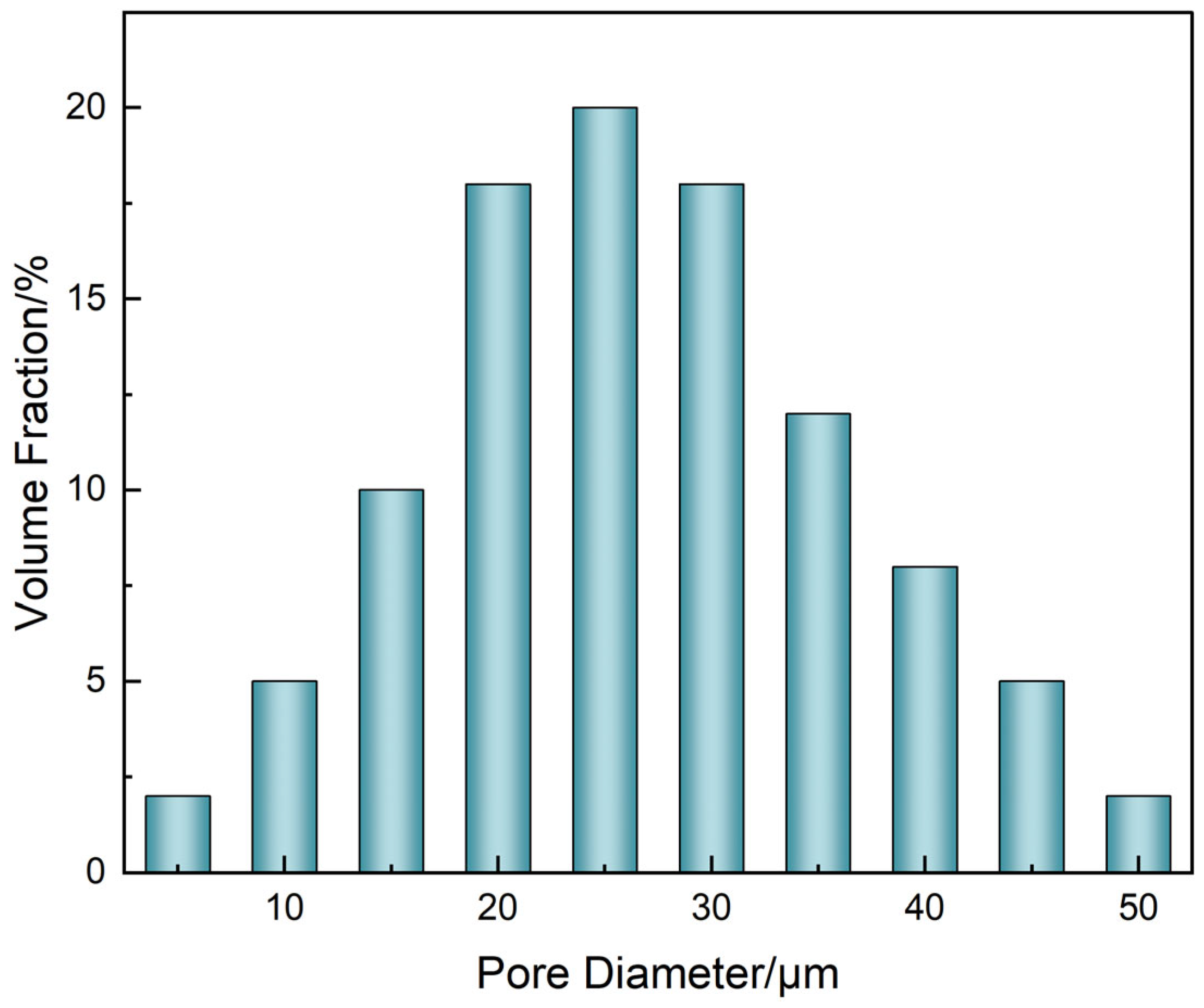
3.2.2. Structural Evolution and Channel Formation During Diffusion–Degradation Coupling
- (1)
- Channel diameters were extracted using ImageJ (v1.54) with the BoneJ plugin, employing the “Thickness” function based on sphere-fitting geometry across over 800 slices, from which the diameter histogram (Figure 10) was constructed.
- (2)
- Connectivity was defined as the ratio of connected pore voxels to total pore voxels within the segmented region, calculated using Bruker CTAn software (version 1.16) based on a 26-voxel neighborhood rule.
- (3)
- Effective permeability was estimated using Avizo XLab Hydro(version 2020.2) via steady-state Navier–Stokes simulation under a pressure gradient of 100 Pa. The segmented μCT domain (resolution 0.7 μm/voxel) was used directly as the simulation input.
3.2.3. Molecular Degradation Pathways and Functional Group Migration Analysis
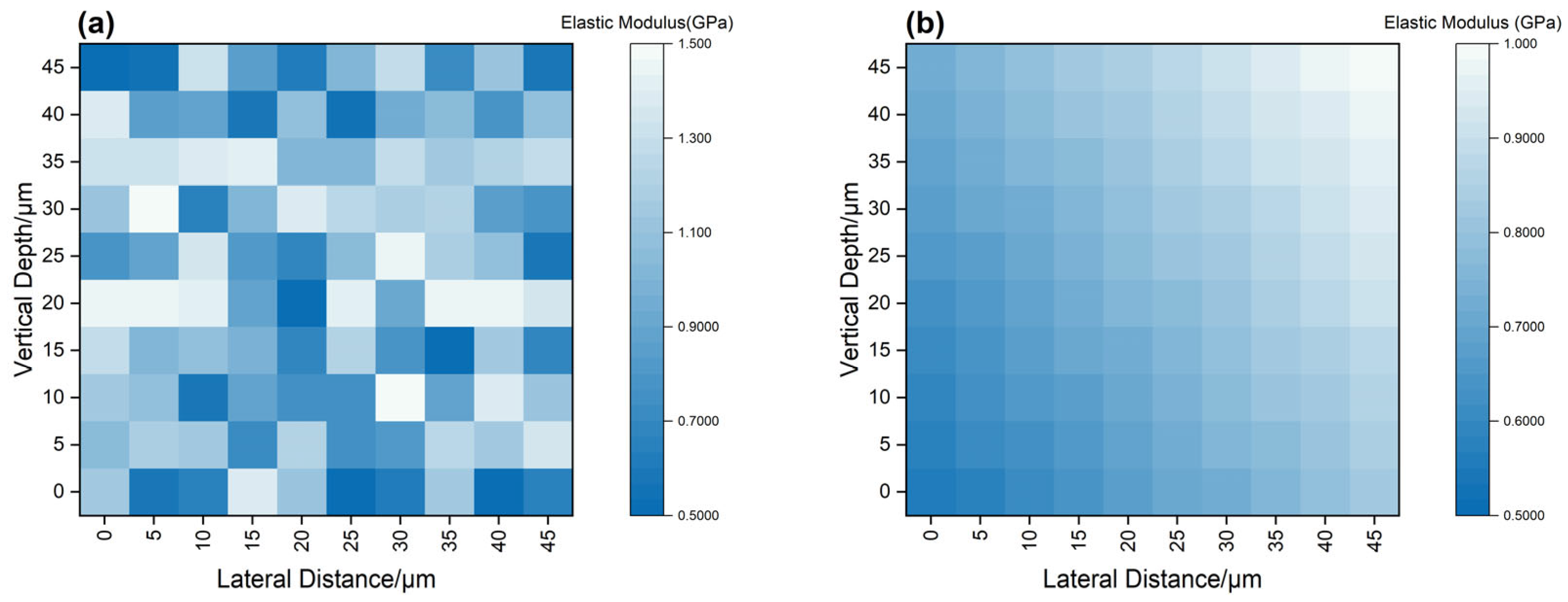
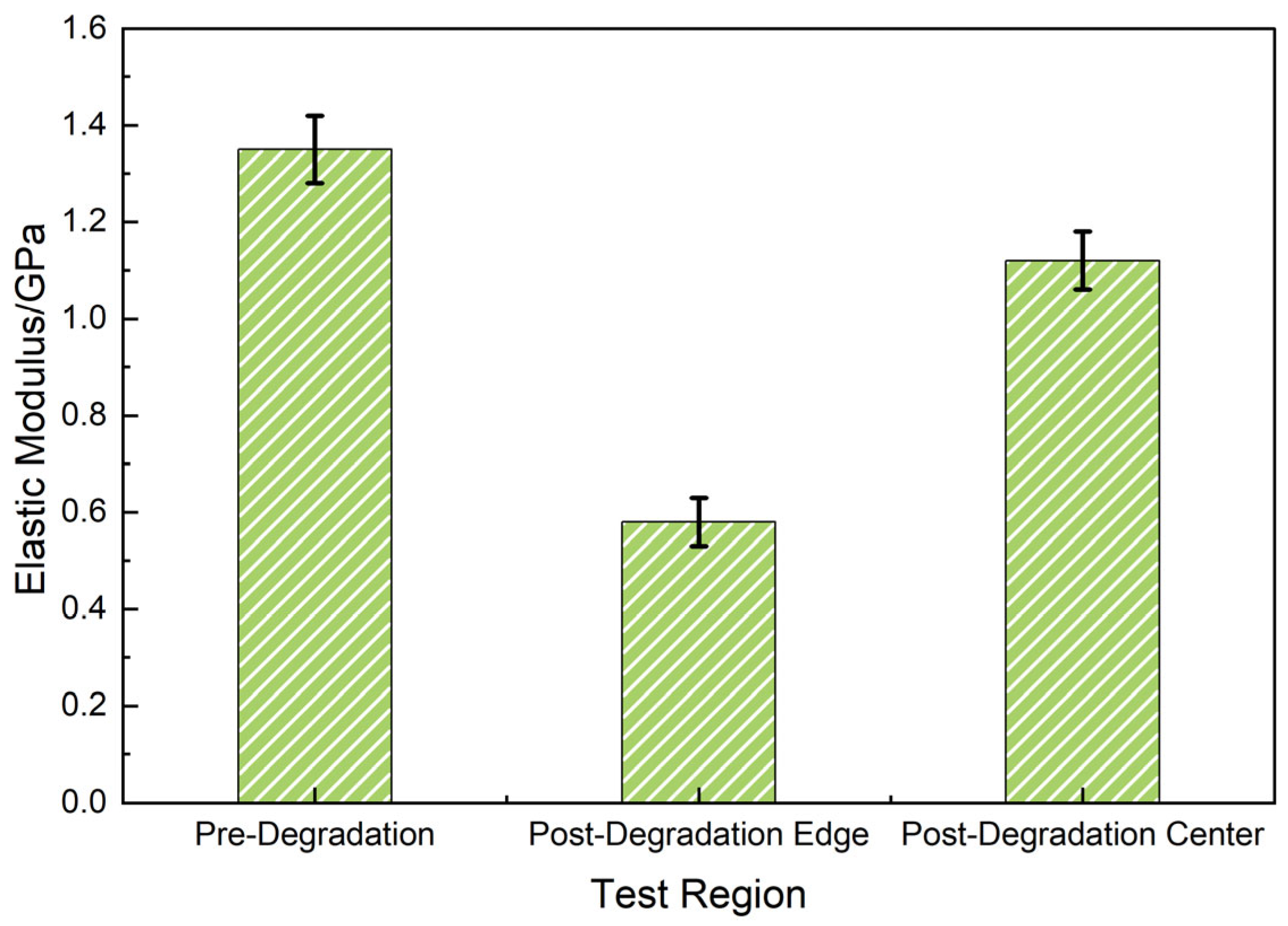
3.2.4. Local Mechanical Softening and Interface Weakening Mechanism
3.3. Construction of a Synergistic Penetration–Degradation Mechanism Map and Analysis of Interfacial Regulation Behavior
3.3.1. Mechanistic Imaging and Structural Evolution Under Synergistic Penetration–Degradation Conditions
3.3.2. Regulation of Interfacial Response Behavior by Temperature and Degradation Liquid Ratio
4. Conclusions
- (1)
- The incorporation of octadecyltrichlorosilane (OTS) as a bifunctional interfacial modifier significantly enhanced the interfacial bonding performance between the liquid plug and steel substrate. Among the tested variants, OTS-18, featuring an 18-carbon alkyl chain, demonstrated the most pronounced strengthening effect at a concentration of 0.25 wt%, increasing bonding strength by 445% and shear strength by 73.8% compared with the unmodified interface. This study systematically revealed the regulatory role of OTS chain length on plug–steel interfacial coupling, providing molecular design parameters for sealing material modification.
- (2)
- Analysis of the interfacial microstructural evolution mechanism showed that OTS molecules constructed a flexible interlayer and root-like interlocking units on the steel surface via a synergistic “anchoring–entanglement–buffering” mechanism. The penetration depth of the interfacial layer increased from 39.6 nm to 391.6 nm, effectively enhancing stress dispersion and crack propagation resistance. This multiscale synergistic pathway validated the OTS-induced interfacial enhancement mechanism of “wetting–penetration–locking,” offering a mechanistic solution to interfacial failure in liquid plug systems.
- (3)
- A structure–property coupling model was established integrating the contact angle, interfacial free energy, penetration depth, and mechanical metrics (shear and bonding strength). This model enabled, for the first time, a full-pathway interpretation from molecular-scale interfacial activation to macroscopic mechanical performance improvement. The findings not only propose an interfacial design methodology suitable for liquid plug systems, but also provide theoretical and practical guidance for the development of high-reliability wellbore sealing materials and interfacial modification strategies in complex downhole environments.
- (4)
- The degradation-induced formation of internal channels was confirmed to be an irreversible process, involving permanent structural disruption and elastic modulus reduction. This supports the unidirectional nature of the penetrant–material interaction and ensures a robust functional transition from sealing to unblocking, thereby establishing a time-controlled degradation pathway for practical field deployment.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ying, X.; Yuan, X.; Yadong, Z.; Ziyi, F. Study of gel plug for temporary blocking and well-killing technology in low-pressure, leakage-prone gas well. SPE Prod. Oper. 2021, 36, 234–244. [Google Scholar] [CrossRef]
- Wu, H.; Ge, J.; Yang, L.; Yang, Y.; Zhang, T.; Guo, H. Developments of polymer gel plug for temporary blocking in SAGD wells. J. Pet. Sci. Eng. 2022, 208, 109650. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, X.-G.; Du, K.; Liu, H.-K. Development of a new high-temperature and high-strength polymer gel for plugging fractured reservoirs. Upstream Oil Gas Technol. 2020, 5, 100014. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Liao, R.; Zhang, G.; Zhang, M.; Li, X. Study of adhesive self-degrading gel for wellbore sealing. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 651, 129567. [Google Scholar] [CrossRef]
- Jia, H.; Kang, Z.; Zhu, J.; Ren, L.; Cai, M.; Wang, T.; Xu, Y.; Li, Z. High density bromide-based nanocomposite gel for temporary plugging in fractured reservoirs with multi-pressure systems. J. Pet. Sci. Eng. 2021, 205, 108778. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Sun, J.; Lv, K. Use of a polymer gel for killing a high-temperature and high-pressure gas well. SPE J. 2022, 27, 3297–3313. [Google Scholar] [CrossRef]
- Jia, H.; Kang, Z.; Li, S.; Li, Y.; Ge, J.; Feng, D. Thermal degradation behavior of seawater based temporary plugging gel crosslinked by polyethyleneimine for fluid loss control in gas well: Kinetics study and degradation prediction. J. Dispers. Sci. Technol. 2021, 42, 1299–1310. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, S.; Wang, C.; Cui, X.; Zhao, T.; Yuan, L.; Qi, Y.; Hou, X.; Jin, H.; Deng, T. Efficient catalytic degradation of anhydride-cured epoxy resin by amphiphilic molecule catalysts. Green Chem. 2022, 24, 7395–7402. [Google Scholar] [CrossRef]
- Fang, J.; Sun, J.; Feng, X.; Pan, L.; Bai, Y.; Yang, J. Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties. Gels 2025, 11, 350. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Peng, Y.; Zhang, D.; Ye, J.; Jiang, Y. Preparation of Low-Viscosity Epoxy Resin Sealing Agent and Evaluation of Injection, Plugging, and Degradation Properties. ACS Omega 2024, 9, 19992–20002. [Google Scholar] [CrossRef]
- Al-Yami, A.; Al-Jubran, M.; Aswad, H.; Looni, Q. Innovative Epoxy Resin Formulation for Downhole Casing Repair Applications. In Proceedings of the International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 12–15 November 2018. D021S043R003. [Google Scholar]
- Rashid, M.A.; Islam, A.; Hasan, N.; Anu, M.N.N.; Ikbal, H. A critical review of dynamic bonds containing curing agents for epoxy resin: Synthesis, challenges, and emerging applications. Polym. Degrad. Stab. 2024, 229, 110980. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Li, K.; Lin, H.; Wang, M.; Zheng, L.; Wu, C.; Zhang, X. Recycling of epoxy resins with degradable structures or dynamic cross-linking networks: A review. Ind. Eng. Chem. Res. 2024, 63, 5005–5027. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Yu, R.; Yuan, J.; Yang, K.; Liu, R.; He, Y.; Feng, W.; Tian, W. Hyperbranched dynamic crosslinking networks enable degradable, reconfigurable, and multifunctional epoxy vitrimer. Adv. Sci. 2024, 11, 2306350. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, C.; Shi, B.; Wang, Y.; Tang, P.; Liu, C.; Fan, J.; Wang, Z.; Jiang, F. Sustainable ultra-strong polyesteramide elastomers with rapid degradation and high resilience. Eur. Polym. J. 2024, 210, 112901. [Google Scholar] [CrossRef]
- Qin, J.; Liu, J.; Wang, D.; Liang, L.; Yang, C.; Lyu, M. High performance, self-healable and recyclable epoxy thermosets by integrating dynamic hindered urea bonds. Polymer 2024, 299, 126994. [Google Scholar] [CrossRef]
- Ren, Q.; Zhu, X.; Li, W.; Wu, M.; Cui, S.; Ling, Y.; Ma, X.; Wang, G.; Wang, L.; Zheng, W. Fabrication of super-hydrophilic and highly open-porous poly (lactic acid) scaffolds using supercritical carbon dioxide foaming. Int. J. Biol. Macromol. 2022, 205, 740–748. [Google Scholar] [CrossRef]
- Yao, J.; Song, W.; Wang, D.; Sun, H.; Li, Y. Multi-scale pore network modelling of fluid mass transfer in nano-micro porous media. Int. J. Heat Mass Transf. 2019, 141, 156–167. [Google Scholar] [CrossRef]
- Li, H.; Xu, S.; Teng, J.; Jiang, X.; Zhang, H.; Qin, Y.; He, Y.; Fan, L. Deep learning assisted ATR-FTIR and Raman spectroscopy fusion technology for microplastic identification. Microchem. J. 2025, 212, 113224. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Lin, L.; Yang, B.; Huang, M.; Chang, M.; Huang, X.; Dai, Z.; Sun, S.; Ren, L.; et al. Overcoming the fluorescent interference during Raman spectroscopy detection of microplastics. Sci. Total Environ. 2023, 897, 165333. [Google Scholar] [CrossRef]
- Kong, L.; Hadavimoghaddam, F.; Li, C.; Liu, K.; Liu, B.; Semnani, A.; Ostadhassan, M. AFM vs. Nanoindentation: Nanomechanical properties of organic-rich Shale. Mar. Pet. Geol. 2021, 132, 105229. [Google Scholar] [CrossRef]
- Tran, T.V. Investigation of Plugging in Orifice, Slot, and Pore-Throat by Fine Particles; The University of Oklahoma: Oklahoma, SC, USA, 2010. [Google Scholar]
- Zhou, B.; Drusch, S.; Hogan, S.A. Effects of temperature and pH on interfacial viscoelasticity, bulk rheology and tribological properties of whey protein isolate-stabilized high internal phase emulsions. Food Hydrocoll. 2023, 142, 108756. [Google Scholar] [CrossRef]
- Gao, W.; Wang, M.; Lian, S.; Bai, Y.; Yang, J. Preparation and Evaluation of Physical and Chemical Properties of Resin Plugging System Suitable for Formation Plugging of Malignant Lost Circulation. Gels 2024, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Borges, V.F.; Monteiro, M.K.S.; da Silva Filho, E.D.; da Silva, D.C.; Fonseca, J.L.C.; Neto, A.O.W.; Braga, T.P. Adsorption of Nonionic Surfactants (Nonylphenols) on Sandstone Rock via Alcoholic Micellar Solution. Langmuir 2024, 40, 19430–19440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Y.; Wang, D. Tracking of nanoparticle diffusion at a liquid–liquid interface adsorbed by nonionic surfactants. Langmuir 2021, 37, 12118–12127. [Google Scholar] [CrossRef]
- Markale, I.; Velásquez-Parra, A.; Alcolea, A.; Jiménez-Martínez, J. Mixing controlled adsorption at the liquid-solid interfaces in unsaturated porous media. Transp. Porous Media 2023, 146, 159–175. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Liu, Y.; Dong, H.; Liu, X.; Huang, J. Nonionic Fast-Penetration System for Diffusion-Driven Degradation of Liquid Plugs. Polymers 2025, 17, 1757. https://doi.org/10.3390/polym17131757
Tian Y, Liu Y, Dong H, Liu X, Huang J. Nonionic Fast-Penetration System for Diffusion-Driven Degradation of Liquid Plugs. Polymers. 2025; 17(13):1757. https://doi.org/10.3390/polym17131757
Chicago/Turabian StyleTian, Yuexin, Yintao Liu, Haifeng Dong, Xiangjun Liu, and Jinjun Huang. 2025. "Nonionic Fast-Penetration System for Diffusion-Driven Degradation of Liquid Plugs" Polymers 17, no. 13: 1757. https://doi.org/10.3390/polym17131757
APA StyleTian, Y., Liu, Y., Dong, H., Liu, X., & Huang, J. (2025). Nonionic Fast-Penetration System for Diffusion-Driven Degradation of Liquid Plugs. Polymers, 17(13), 1757. https://doi.org/10.3390/polym17131757






