Comparative Evaluation of Xanthan Gum, Guar Gum, and Scleroglucan Solutions for Mobility Control: Rheological Behavior, In-Situ Viscosity, and Injectivity in Porous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Rock Samples
2.2. Fluid Preparations
2.3. Core Sample Preparation
2.4. Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Absolute permeability, mD | |
| Apparent viscosity, mPa·s | |
| Behavior index, dimensionless | |
| Consistency Index, mPa·s | |
| Diameter, cm | |
| Filtration rate, dimensionless | |
| Gas Permeability, mD | |
| Injectivity, cm3/min | |
| Length, cm | |
| Mobility, mPa·s/mD | |
| Overlap concentration, ppm | |
| Porosity, dimensionless | |
| Pressure drops, PSI | |
| Relative injectivity, dimensionless | |
| Residual resistance factor, dimensionless | |
| Resistance factor, dimensionless | |
| Shear rate | |
| , s | |
| Viscosity, mPa·s | |
| Viscosity loss, dimensionless | |
| Absolute permeability, mD | |
| Apparent viscosity, mPa·s | |
| Behavior index, dimensionless | |
| Consistency index, mPa·s | |
| Diameter, cm | |
| Filtration rate, dimensionless |
References
- Akbari, S.; Mahmood, S.M.; Nasr, N.H.; Al-Hajri, S.; Sabet, M. A critical review of concept and methods related to accessible pore volume during polymer-enhanced oil recovery. J. Pet. Sci. Eng. 2019, 182, 106263. [Google Scholar] [CrossRef]
- Al-Shakry, B. Polymer Injectivity: Experimental Studies of Flow in Porous Media for EOR Polymers. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2021. [Google Scholar]
- Hossein Sedaghat, M.; Hossein Ghazanfari, M.; Parvazdavani, M.; Morshedi, S. Experimental Investigation of Microscopic/Macroscopic Efficiency of Polymer Flooding in Fractured Heavy Oil Five-Spot Systems. J. Energy Resour. Technol. 2013, 135, 032901. [Google Scholar] [CrossRef]
- Kang, P.S.; Lim, J.S.; Huh, C. Temperature Dependence of the Shear-Thinning Behavior o Partially Hydrolyzed Polyacrylamide Solution for Enhanced Oil Recovery. J. Energy Resour. Technol. Trans. ASME 2021, 143, 063002. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, M.B.; Al-Shehri, D.; Rahaman, M.M.; Keshavarz, A.; Hossain, S.M.Z. Comparative Study of Green and Synthetic Polymers for Enhanced Oil Recovery. Polymers 2020, 12, 2429. [Google Scholar] [CrossRef]
- Clinckspoor, K.J.; De Sousa Ferreira, V.H.; Barros Zanoni Lopes Moreno, R. Bulk rheology characterization of biopolymer solutions and discussions of their potential for enhanced oil recovery applications. CTF-Cienc. Tecnol. Y Futuro. 2021, 11, 123–135. [Google Scholar] [CrossRef]
- Mohsenatabar Firozjaii, A.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Al-Shakry, B.; Skauge, T.; Shiran, B.S.; Skauge, A. Impact of mechanical degradation on polymer injectivity in porous media. Polymers 2018, 10, 742. [Google Scholar] [CrossRef]
- Ding, M.; Han, Y.; Wang, Y.; Liu, Y.; Liu, D.; Qu, Z. Experimental Investigation of the Heterogeneity Limit for the Application of Polymer Flooding in Reservoirs. J. Energy Resour. Technol. 2021, 143, 022901. [Google Scholar] [CrossRef]
- Skauge, A.; Zamani, N.; Gausdal Jacobsen, J.; Shaker Shiran, B.; Al-Shakry, B.; Skauge, T. Polymer Flow in Porous Media: Relevance to Enhanced Oil Recovery. Colloids Interfaces 2018, 2, 27. [Google Scholar] [CrossRef]
- Alfazazi, U.; Thomas, N.C.; Alameri, W.; Al-Shalabi, E.W. Experimental investigation of polymer injectivity and retention under harsh carbonate reservoir conditions. J. Pet. Sci. Eng. 2020, 192, 107262. [Google Scholar] [CrossRef]
- Machale, J.; Majumder, S.K.; Ghosh, P.; Sen, T.K. Role of chemical additives and their rheological properties in enhanced oil recovery. Rev. Chem. Eng. 2020, 36, 789–830. [Google Scholar] [CrossRef]
- Tackie-Otoo, B.N.; Ayoub Mohammed, M.A.; Yekeen, N.; Negash, B.M. Alternative chemical agents for alkalis, surfactants and polymers for enhanced oil recovery: Research trend and prospects. J. Pet. Sci. Eng. 2020, 187, 106828. [Google Scholar] [CrossRef]
- Kaminsky, R.D.; Wattenbarger, R.C.; Szafranski, R.C.; Coutee, A.S. Guidelines for polymer flooding evaluation and development. In IPTC 2007: International Petroleum Technology Conference; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2007; Volume 1, pp. 243–250. [Google Scholar] [CrossRef]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Fournier, R.; Tiehi, J.E.; Zaitoun, A. Laboratory Study of a New EOR-Grade Scleroglucan. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar] [CrossRef]
- Freire Filho, M.E.N.; Moreno, R.B.Z.L. Injection of Xanthan Gum and Guar Gum Through Carbonate Rocks Evaluated by Two-Phase Core Flooding. J. Energy Resour. Technol. Trans. ASME 2022, 144, 113001. [Google Scholar] [CrossRef]
- Jensen, T.; Kadhum, M.; Kozlowicz, B.; Sumner, E.S.; Malsam, J.; Muhammed, F.; Ravikiran, R. Chemical EOR under Harsh conditions: Scleroglucan as a viable commercial solution. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018. [Google Scholar] [CrossRef]
- Glasbergen, G.; Wever, D.; Keijzer, E.; Farajzadeh, R. Injectivity Loss in Polymer Floods: Causes, Preventions and Mitigations. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 11–14 October 2015. [Google Scholar]
- Thomas, A.; Giddins, M.A.; Wilton, R. Why is it so Difficult to Predict Polymer Injectivity in Chemical Oil Recovery Processes? In Proceedings of the IOR 2019–20th European Symposium on Improved Oil Recovery, Pau, France, 8–11 April 2019; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2019; pp. 1–25. [Google Scholar]
- Raniolo, S.; Dovera, L.; Cominelli, A.; Callegaro, C.; Masserano, F. History match and polymer injection optimization in a nature field using the Ensemble Kalman Filter. In Proceedings of the IOR 2013—17th European Symposium on Improved Oil Recovery, Saint Petersburg, Russia, 16–18 April 2013. [Google Scholar] [CrossRef]
- Renouf, G. A survey of polymer flooding in Western Canada. In SPE Improved Oil Recovery Conference; OnePetro: Richardson, TX, USA, 2014; Volume 1, pp. 441–456. [Google Scholar] [CrossRef]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Moe Soe Let, K.P.; Manichand, R.N.; Seright, R.S. Polymer flooding a ∼500-cp oil. In SPE Improved Oil Recovery Conference; OnePetro: Richardson, TX, USA, 2012; Volume 2, pp. 1670–1682. [Google Scholar] [CrossRef]
- Scott, A.J.; Romero-Zerón, L.; Penlidis, A. Evaluation of polymeric materials for chemical enhanced oil recovery. Processes 2020, 8, 361. [Google Scholar] [CrossRef]
- Sun, L.; Li, B.; Jiang, H.; Li, Y.; Jiao, Y. An injectivity evaluation model of polymer flooding in offshore multilayer reservoir. Energies 2019, 12, 1444. [Google Scholar] [CrossRef]
- Shuler, P.J.; Kuehne, D.L.; Uhl, J.T.; Walkup, G.W. Improving Polymer Injectivity At West Coyote Field, California. SPE Reserv. Eng. Soc. Pet. Eng. 1987, 2, 271–280. [Google Scholar] [CrossRef]
- Sharma, M.M.; Pang, S.; Wennberg, K.E.; Morgenthaler, L. Injectivity decline in water injection wells: An offshore Gulf of Mexico case study. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 2–3 June 1997; p. SPE-38180-MS. [Google Scholar] [CrossRef]
- Olabode, O.; Nnorom, E.; Akinsanyan, O.; Samnuel, A.; Olakunle, O.; Sowunmi, L. Effect of Salt Concentration on Oil Recovery During Polymer Flooding: Simulation Studies on Xanthan Gum and Gum Arabic. Polymers 2023, 15, 4013. [Google Scholar] [CrossRef]
- Beteta, A.; Nurmi, L.; Rosati, L.; Hanski, S.; McIver, K.; Sorbie, K.; Toivonen, S. Polymer Chemical Structure and its Impact on EOR Performance. In Proceedings of the the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Indiana Limestone Institute. Indiana Limestone Handbook, 22nd ed.; Indiana Limestone Institute of America, Inc.: Bloomington, IN, USA, 2007. [Google Scholar]
- Ferreira, V.H.S.; Moreno, R.B.Z.L. Experimental evaluation of low concentration scleroglucan biopolymer solution for enhanced oil recovery in carbonate. Oil Gas Sci. Technol. 2020, 75, 61. [Google Scholar] [CrossRef]
- Rueda, E.; Akarri, S.; Torsæter, O.; Moreno, R.B.Z.L. Experimental Investigation of the Effect of Adding Nanoparticles to Polymer Flooding in Water-Wet Micromodels. Nanomaterials 2020, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Herrera, S. Injectivity of biopolymers solutions through porous media injectivity of biopolymers solutions through porous media. Master’s Thesis, School of Mechanical Engineering, University of Campinas, Campinas, Brazil, 2023. [Google Scholar]
- de Moura, M.R.V.; Moreno, R.B.Z.L. Concentration, brine salinity and temperature effects on xanthan gum solutions rheology. Appl. Rheol. 2019, 29, 69–79. [Google Scholar] [CrossRef]
- Javier, E.; Rueda, R. Biopolymer for EOR: Fluid Design, Micromodels and Core Flooding Approach. Master’s Thesis, School of Mechanical Engineering, University of Campinas, Campinas, Brazil, 2020. [Google Scholar]
- American Petroleum Institute. Production Department, Recommended Practices for Evaluation of Polymers Used in Enhanced Oil Recovery Operations. American Petroleum Institute: Harrisburg, PA, USA, 1990; pp. 1–74. [Google Scholar]
- Sheng, J.J. Polymer Flooding-Fundamentals and Field Cases, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123865458. [Google Scholar]
- Wever, D.; Karpan, V.; Glasbergen, G.; Koltsov, I.; Shuster, M.; Volokitin, Y.; Gaillard, N.; Daguerre, F. Polymer Injectivity De-risking for West Salym ASP Pilot. In Proceedings of the IOR Norway 2017-19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017; pp. 1–18. [Google Scholar]
- American Petroleum Institute Recommended Practices for Core Analysis; API Publishing Services: Washington, DC, USA, 1998; pp. 1–220.
- Thomas, A. Polymer Flooding. In Chemical Enhanced Oil Recovery (cEOR)—A Practical Overview; InTech: London, UK, 2016; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Yin, D.; Song, S.; Xu, Q.; Liu, K. Experimental research on the optimization and evaluation of the polymer/chromium-ion deep profile control system for the fractured low-permeability reservoirs. Processes 2020, 8, 1021. [Google Scholar] [CrossRef]
- Kakati, A.; Kumar, G.; Sangwai, J.S. Low Salinity Polymer Flooding: Effect on Polymer Rheology, Injectivity, Retention, and Oil Recovery Efficiency. Energy Fuels 2020, 34, 5715–5732. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, R. Comprehensive Effects of Polymer Flooding on Oil–Water Relative Permeabilities. J. Energy Resour. Technol. Trans. ASME 2022, 144, 033007. [Google Scholar] [CrossRef]
- Sorbie, K.S. Polymer-Improved Oil Recovery, 1st ed.; Springer Dordrecht: Dordrecht, The Netherlands, 1991; ISBN 978-94-010-5354-9. [Google Scholar]
- Hugo de Sousa Ferreira, V. Key Parameters on the Flow of Polymer Solutions Through Porous Medium. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, July 2019. [Google Scholar]
- Seright, R.S.; Seheult, M.; Talashek, T. Injectivity characteristics of EOR polymers. Proc.-SPE Annu. Tech. Conf. Exhib. 2008, 2, 767–780. [Google Scholar] [CrossRef]

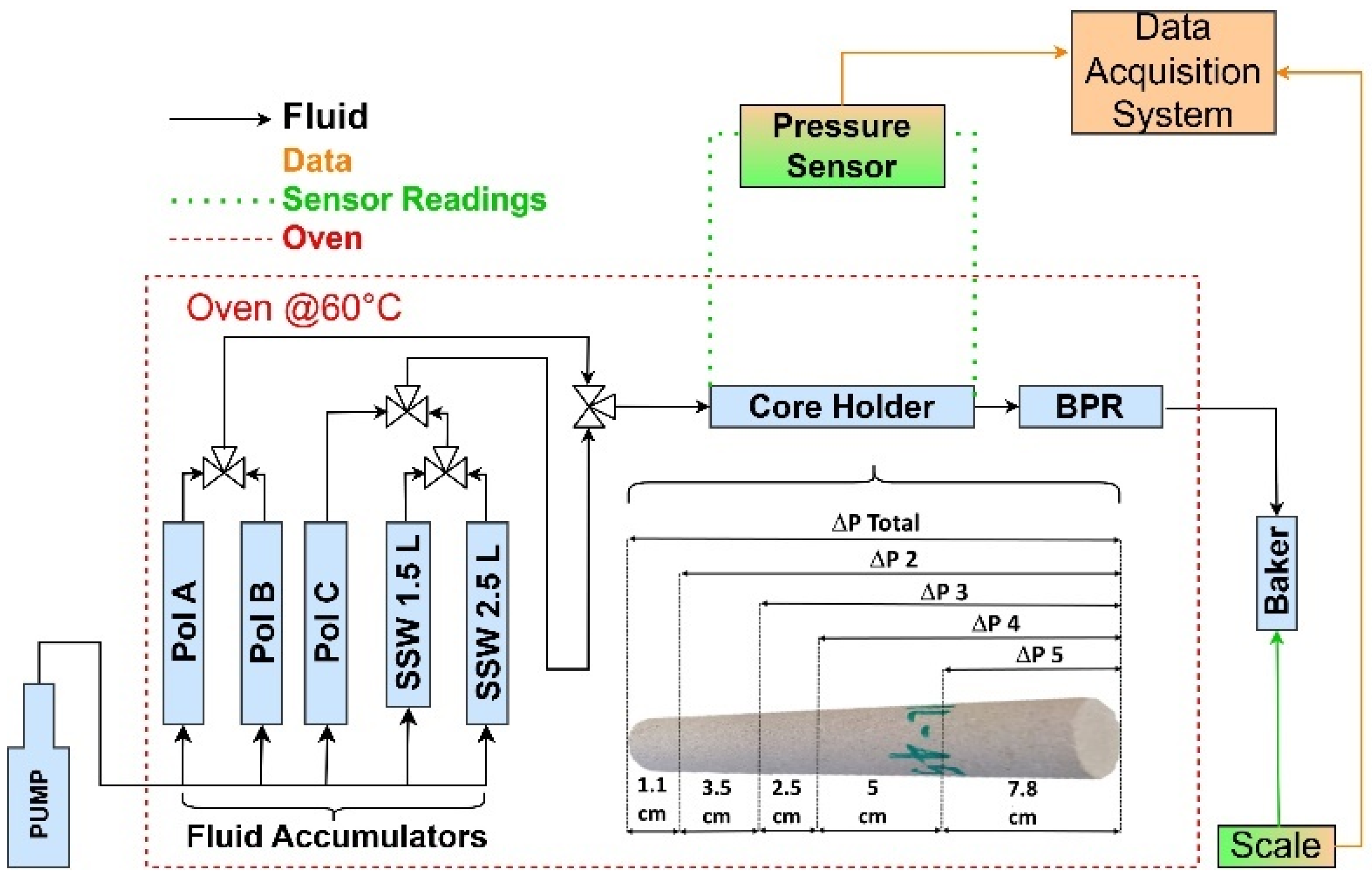


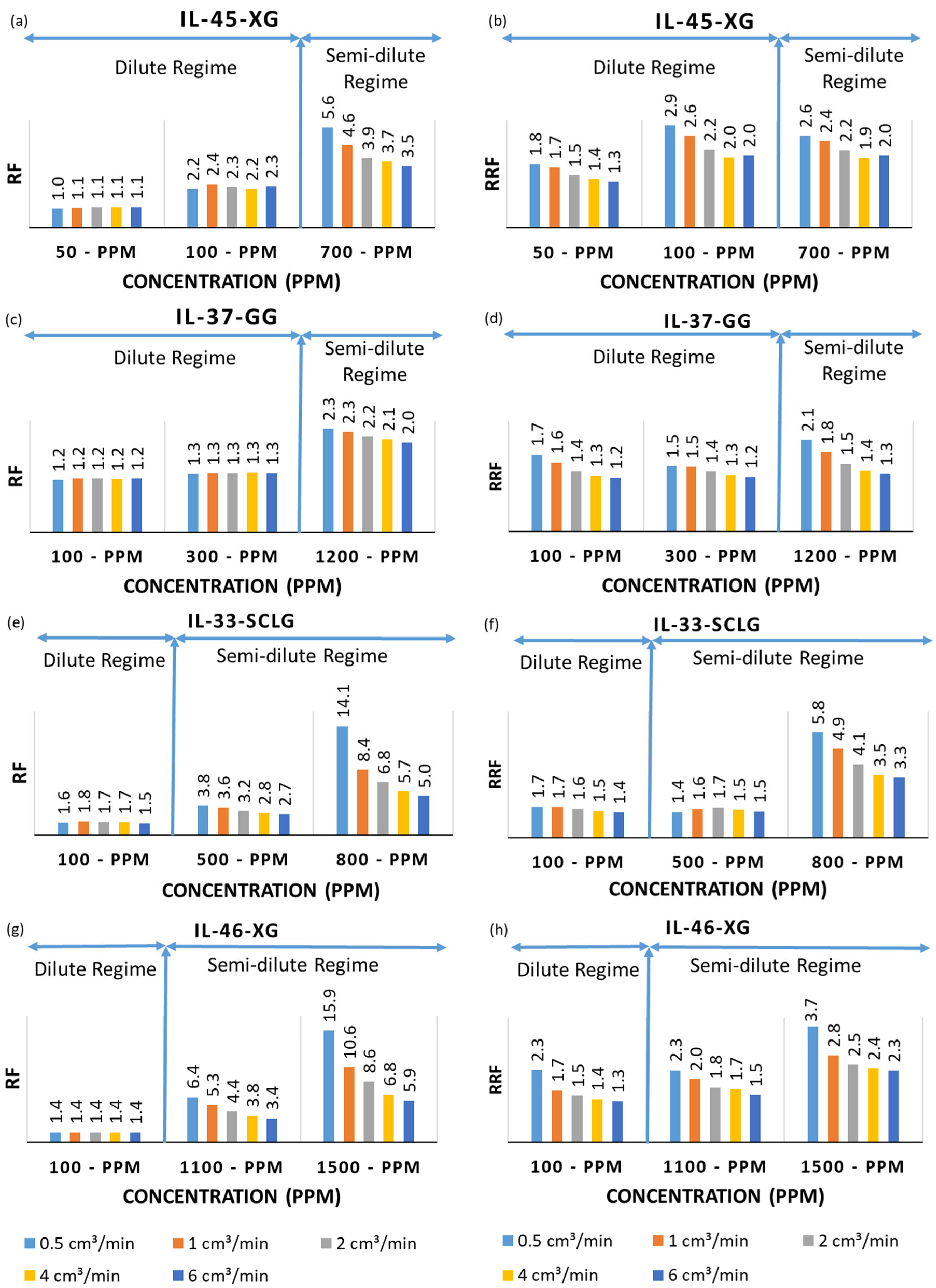

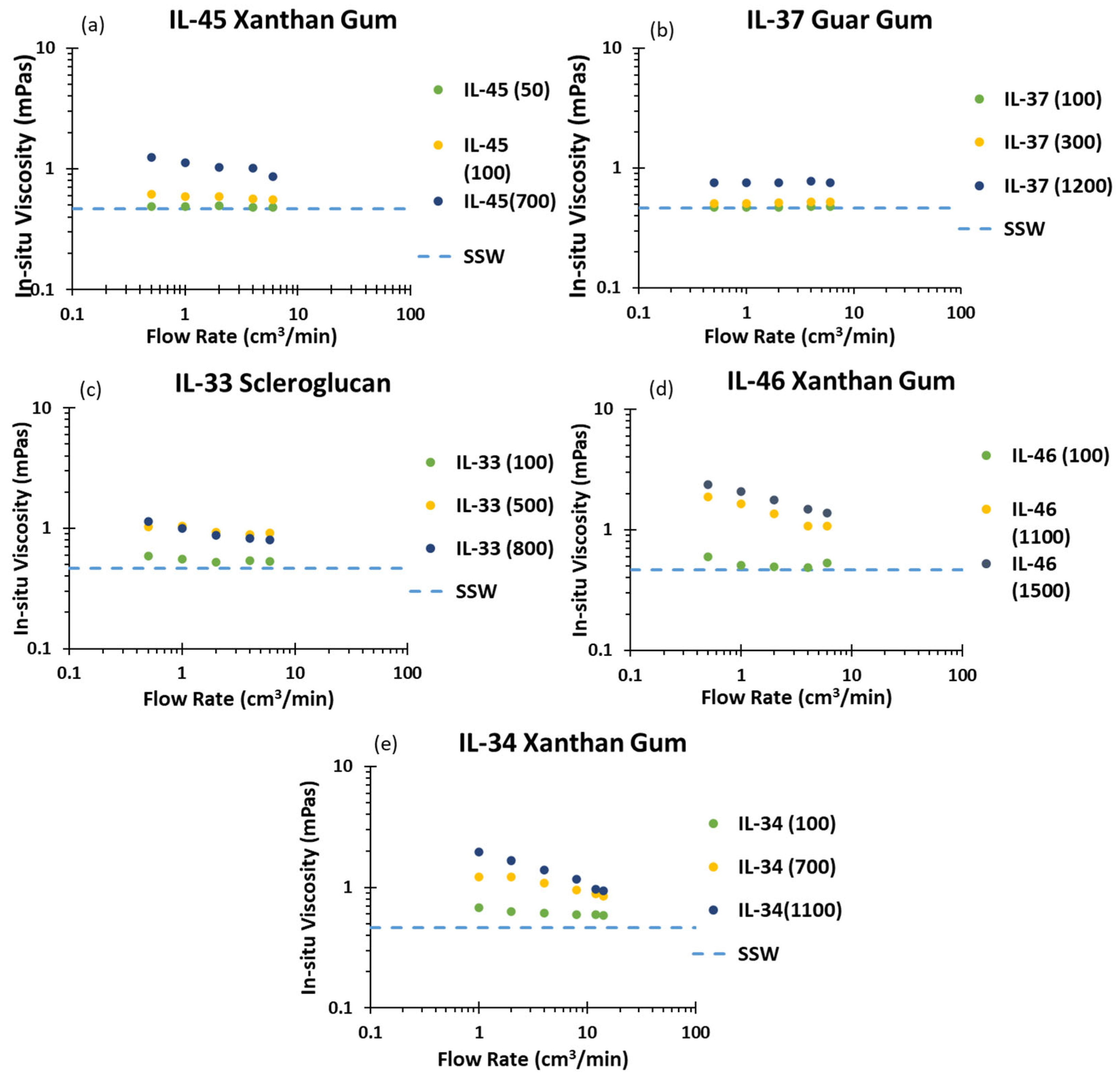

| Rock/Polymer Code | Gas Porosity (%) | Gas Permeability (mD) | Length (cm) | Diameter (cm) | Mass (g) |
|---|---|---|---|---|---|
| IL-45-XG | 17.59 | 173.3 | 19.9 | 3.78 | 499.9 |
| IL-37-GG | 17.95 | 171.2 | 19.9 | 3.78 | 500.2 |
| IL-33-SCLG | 17.59 | 142.6 | 19.9 | 3.78 | 502.9 |
| IL-46-XG | 16.50 | 160.1 | 19.9 | 3.78 | 501.9 |
| IL-34-XG | 18.38 | 639.7 | 20.0 | 3.78 | 501.5 |
| Composition | Chemical Formula | Concentration 100% (ppm) |
|---|---|---|
| Potassium Chloride | KCl | 749.3 |
| Calcium Chloride Dihydrate | CaCl2 2H2O | 484.2 |
| Magnesium Chloride Hydrate | MgCl2 H2O | 1271.3 |
| Strontium Chloride Hexahydrate | SrCl2 6H2O | 5.2 |
| Barium Chloride Hexahydrate | BaCl2 2H2O | 2.0 |
| Lithium Chloride | LiCl | 1.2 |
| Sodium Bromide | NaBr | 82.4 |
| Sodium Sulfate | Na2SO4 | 57.7 |
| Sodium Chloride | NaCl | 28,252.2 |
| Total TDS | 30,905.5 | |
| Parameter\Code | IL-45 | IL-46 | IL-34 | IL-33 | IL-37 |
|---|---|---|---|---|---|
| Polymer | Xanthan Gum | Xanthan Gum | Xanthan Gum | Scleroglucan | Guar Gum |
| Overlap Concentration C * (ppm) | 285 | 285 | 285 | 175 | 950 |
| Biocide | Yes | Yes | Yes | Yes | Yes |
| Agitation | 1 day | 1 day | 1 day | 7 days | 1 day |
| Dissolution Before Aging (ppm) | 1000 | 2000 | 2000 | 1000 | 3000 |
| Aging Time | 1 day | 1 day | 1 day | 1 day | 3 h |
| Filtration Mesh | 8 µm 1.2 µm | 8 µm 1.2 µm | 8 µm 1.2 µm | 8 µm 1.2 µm | 55 µm * + 20 µm * + 12 µm * + 8 µm + 1.2 µm |
| Filtration Ratio at 1.2 Mesh | 1.02 | 1.03 | 1.04 | 1.05 | 1.02 |
| Concentration Test (ppm) | 50 | 100 | 100 | 100 | 100 |
| 100 | 1100 | 700 | 500 | 300 | |
| 700 | 1500 | 1100 | 800 | 1200 | |
| Viscosity (cP) at 10 s−1 | 0.56 | 0.66 | 0.66 | 0.73 | 0.49 |
| 0.67 | 10.71 | 3.73 | 4.46 | 0.62 | |
| 4.40 | 20.51 | 8.51 | 9.81 | 2.30 | |
| Kw (mD) | 127.9 | 156.7 | 476.4 | 147.1 | 156.4 |
| Test | Concentration | K | n | R2 |
|---|---|---|---|---|
| IL-45 | XG-50 | 0.489 | 0.995 | 0.216 |
| IL-45 | XG-100 | 0.597 | 0.962 | 0.932 |
| IL-45 | XG-700 | 1.142 | 0.868 | 0.934 |
| IL-37 | GG-100 | 0.509 | 1.015 | 0.951 |
| IL-37 | GG-300 | 0.473 | 1.004 | 0.459 |
| IL-37 | GG-1200 | 0.760 | 1.003 | 0.149 |
| IL-33 | SCLG-100 | 0.560 | 0.996 | 0.715 |
| IL-33 | SClLG-500 | 1.001 | 0.933 | 0.806 |
| IL-33 | SCLG-800 | 1.007 | 0.859 | 0.959 |
| IL-46 | XG-100 | 0.542 | 0.948 | 0.435 |
| IL-46 | XG-1100 | 1.599 | 0.757 | 0.983 |
| IL-46 | XG-1500 | 2.057 | 0.776 | 0.995 |
| IL-34 | XG-100 | 0.666 | 0.952 | 0.936 |
| IL-34 | XG-700 | 1.293 | 0.851 | 0.928 |
| IL-34 | XG-1100 | 2.010 | 0.718 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera Saravia, J.M.; Barros Zanoni Lopes Moreno, R. Comparative Evaluation of Xanthan Gum, Guar Gum, and Scleroglucan Solutions for Mobility Control: Rheological Behavior, In-Situ Viscosity, and Injectivity in Porous Media. Polymers 2025, 17, 1742. https://doi.org/10.3390/polym17131742
Herrera Saravia JM, Barros Zanoni Lopes Moreno R. Comparative Evaluation of Xanthan Gum, Guar Gum, and Scleroglucan Solutions for Mobility Control: Rheological Behavior, In-Situ Viscosity, and Injectivity in Porous Media. Polymers. 2025; 17(13):1742. https://doi.org/10.3390/polym17131742
Chicago/Turabian StyleHerrera Saravia, Jose Maria, and Rosangela Barros Zanoni Lopes Moreno. 2025. "Comparative Evaluation of Xanthan Gum, Guar Gum, and Scleroglucan Solutions for Mobility Control: Rheological Behavior, In-Situ Viscosity, and Injectivity in Porous Media" Polymers 17, no. 13: 1742. https://doi.org/10.3390/polym17131742
APA StyleHerrera Saravia, J. M., & Barros Zanoni Lopes Moreno, R. (2025). Comparative Evaluation of Xanthan Gum, Guar Gum, and Scleroglucan Solutions for Mobility Control: Rheological Behavior, In-Situ Viscosity, and Injectivity in Porous Media. Polymers, 17(13), 1742. https://doi.org/10.3390/polym17131742






