Incorporation of Protein Hydrolysate into Rapeseed Meal-Based Materials to Improve Flexibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymatic Proteolysis of Rapeseed Meal and Scale-Up
2.3. Polyacrylamide Gel Electrophoresis
2.4. Chromatographic Analysis of Rapeseed Meal Hydrolysates
2.5. Rapeseed Meal-Based Material Preparation
2.6. Thickness
2.7. Mechanical Properties
2.8. Water Absorption Measurements
2.9. Hydrolysate Migration Test
2.10. Thermogravimetric Analysis
2.11. Differential Scanning Calorimetry
2.12. Scanning Electron Microscopy
2.13. Aerobic Biodegradation Measurement
3. Results and Discussion
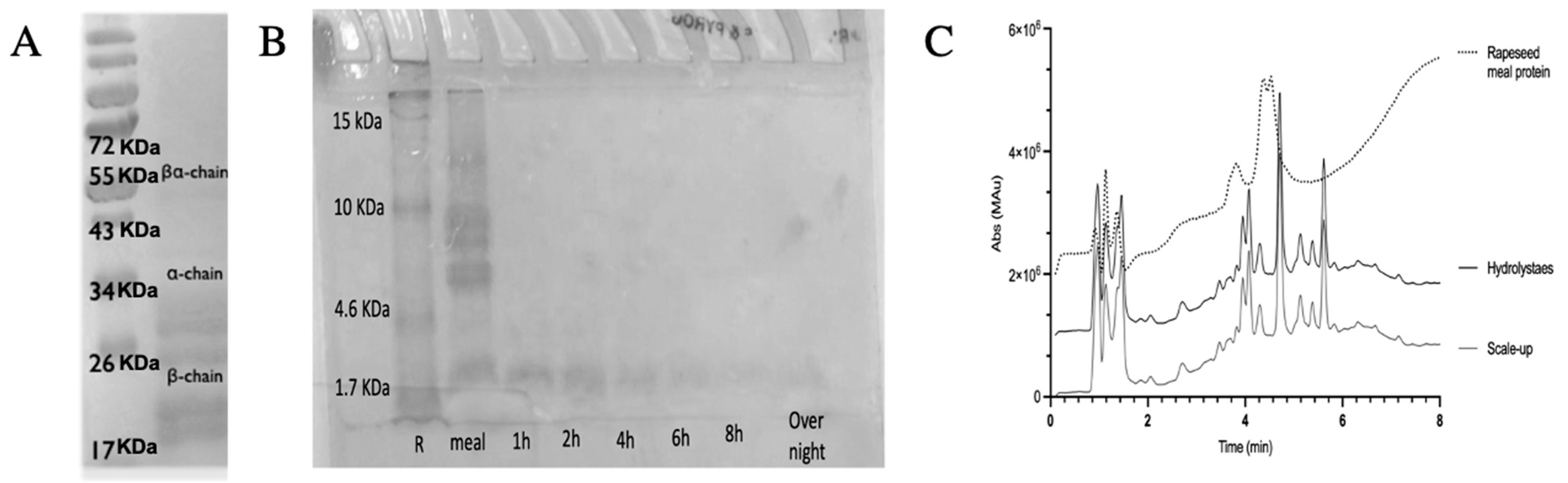
3.1. Rapeseed Meal Protein Hydrolysate Characterization
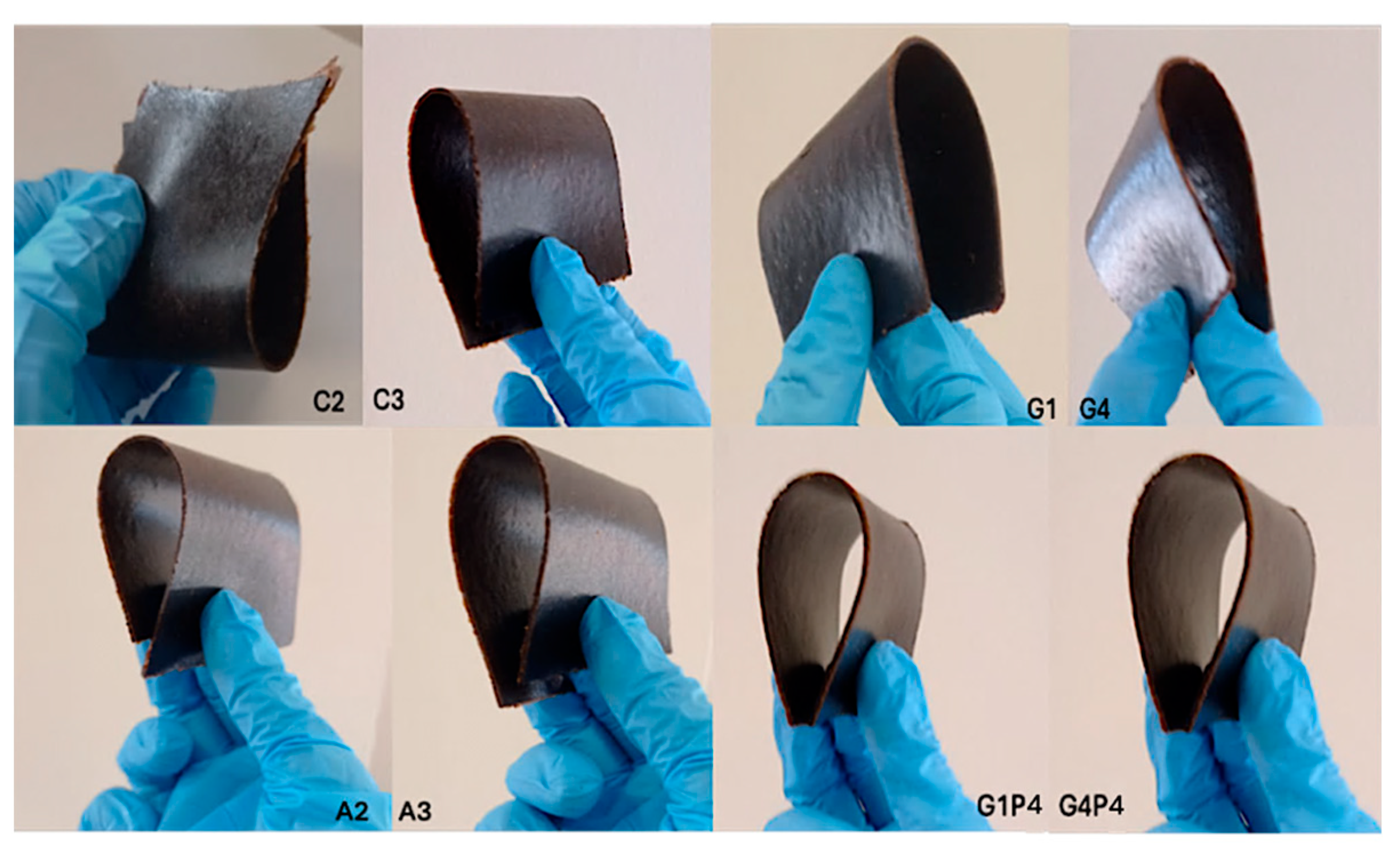
3.2. Rapeseed Meal-Based Materials
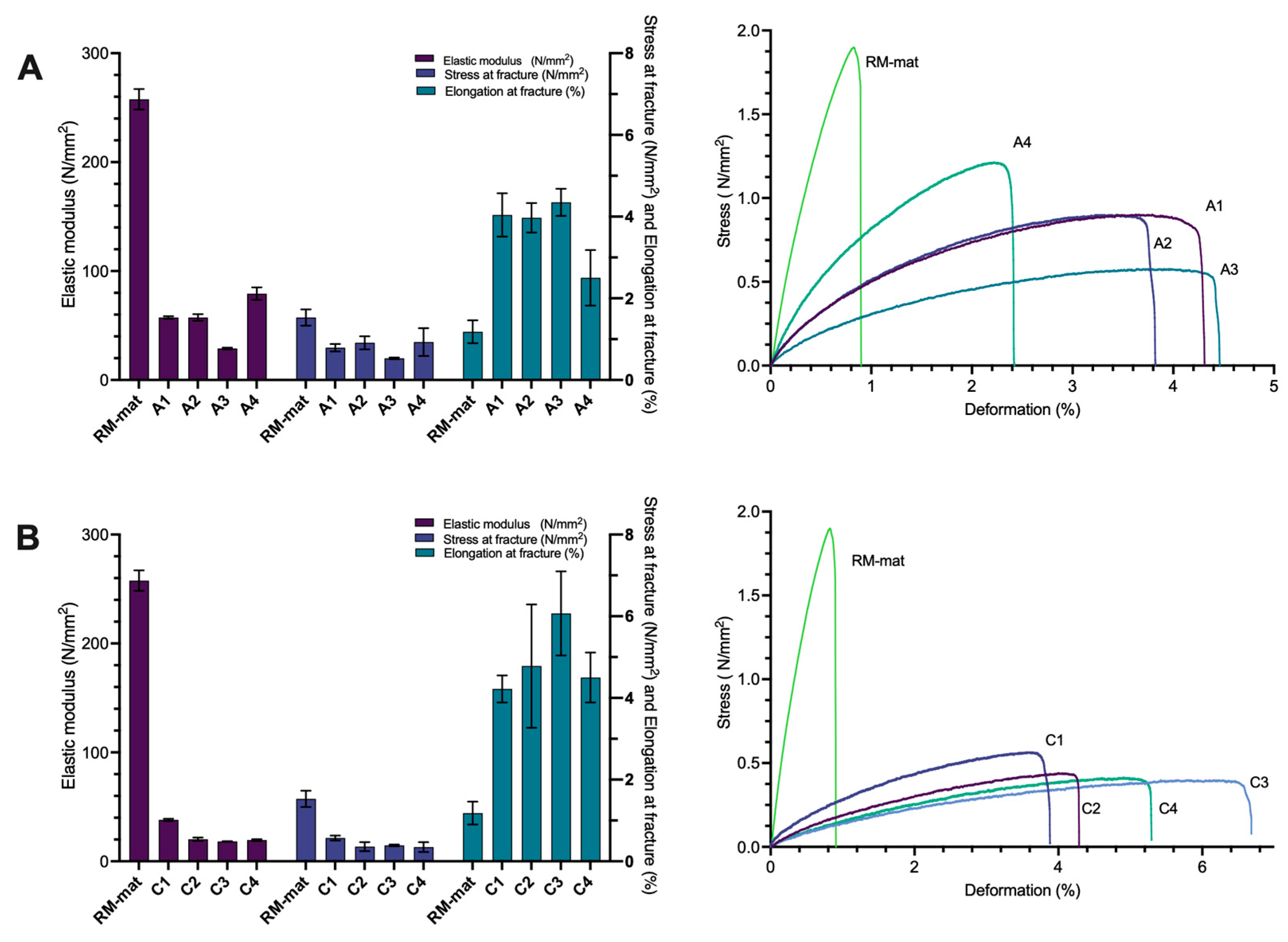
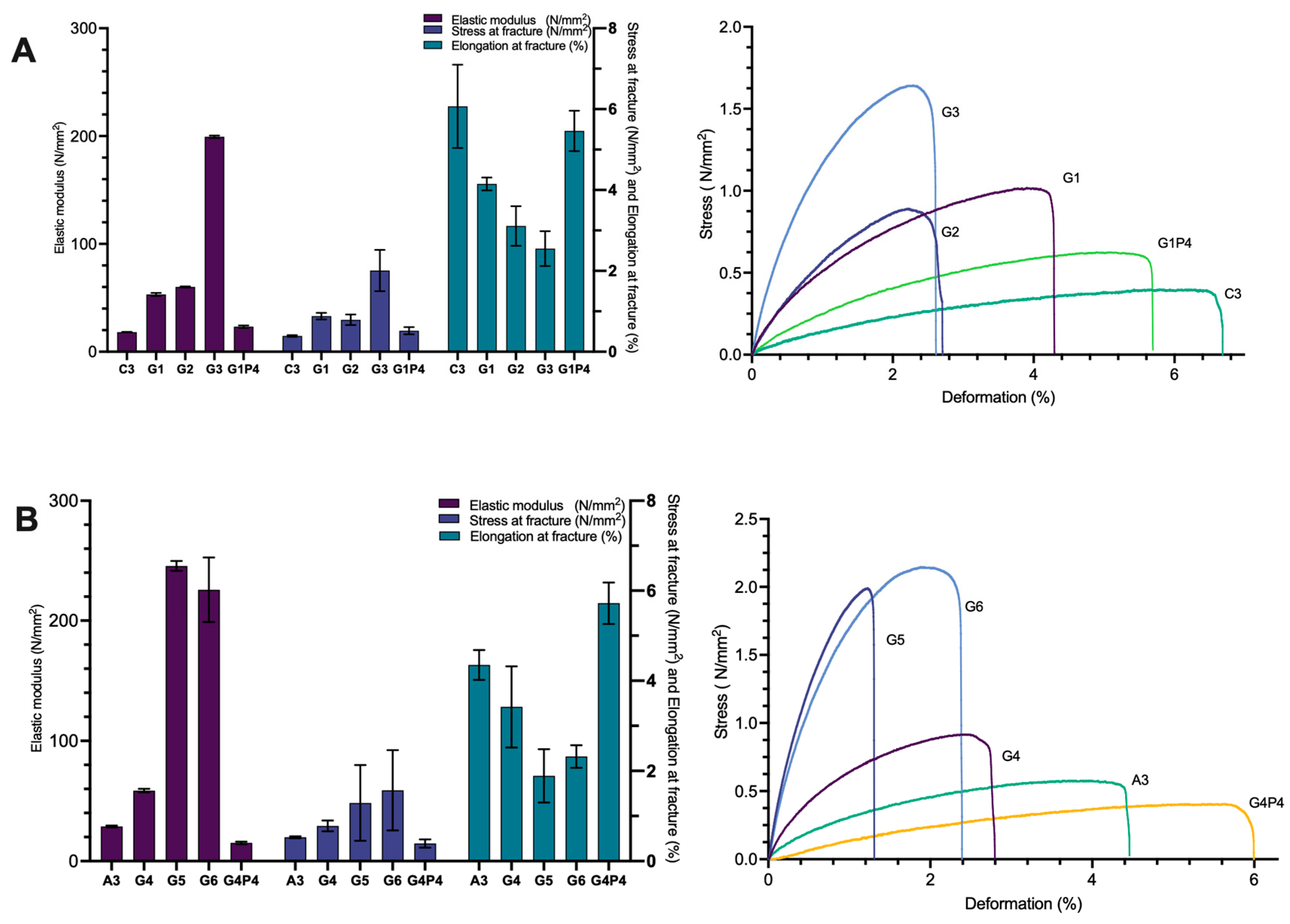
3.3. Mechanical Properties
3.3.1. Use of Collagen and Rapeseed Meal Hydrolysate
3.3.2. Replacement of Glycerol with Proline and Use of Collagen and Rapeseed Meal Hydrolysate
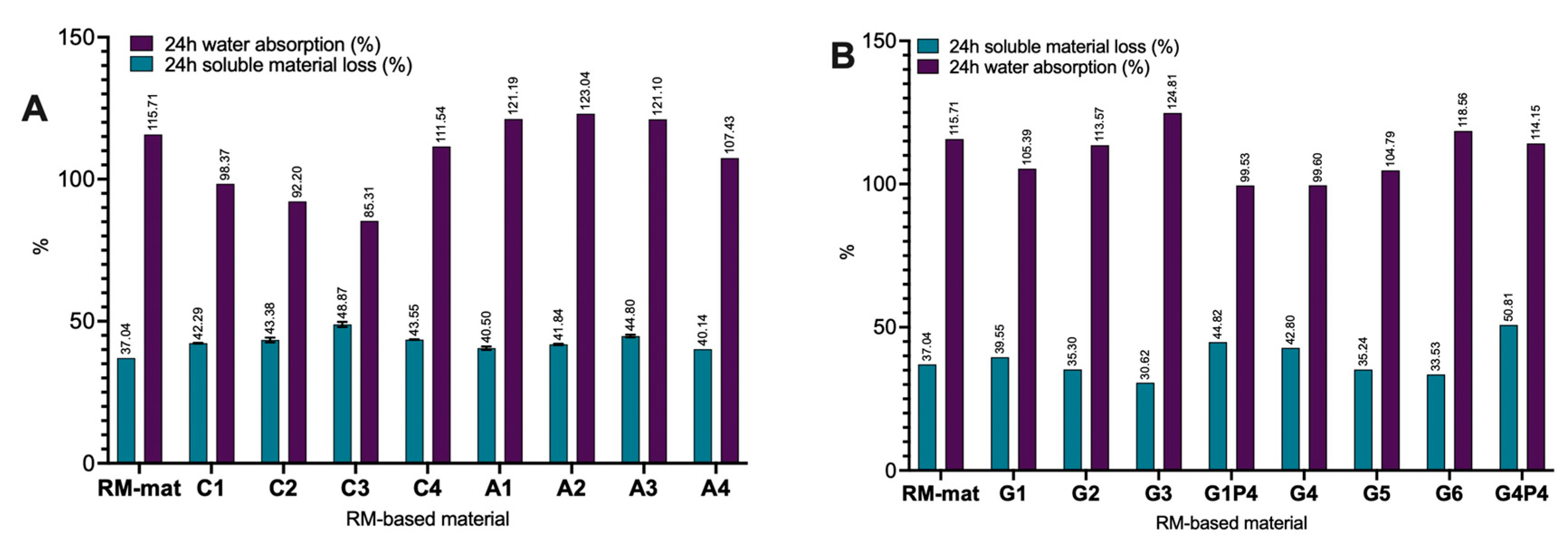
3.4. Water Absorption Measurements
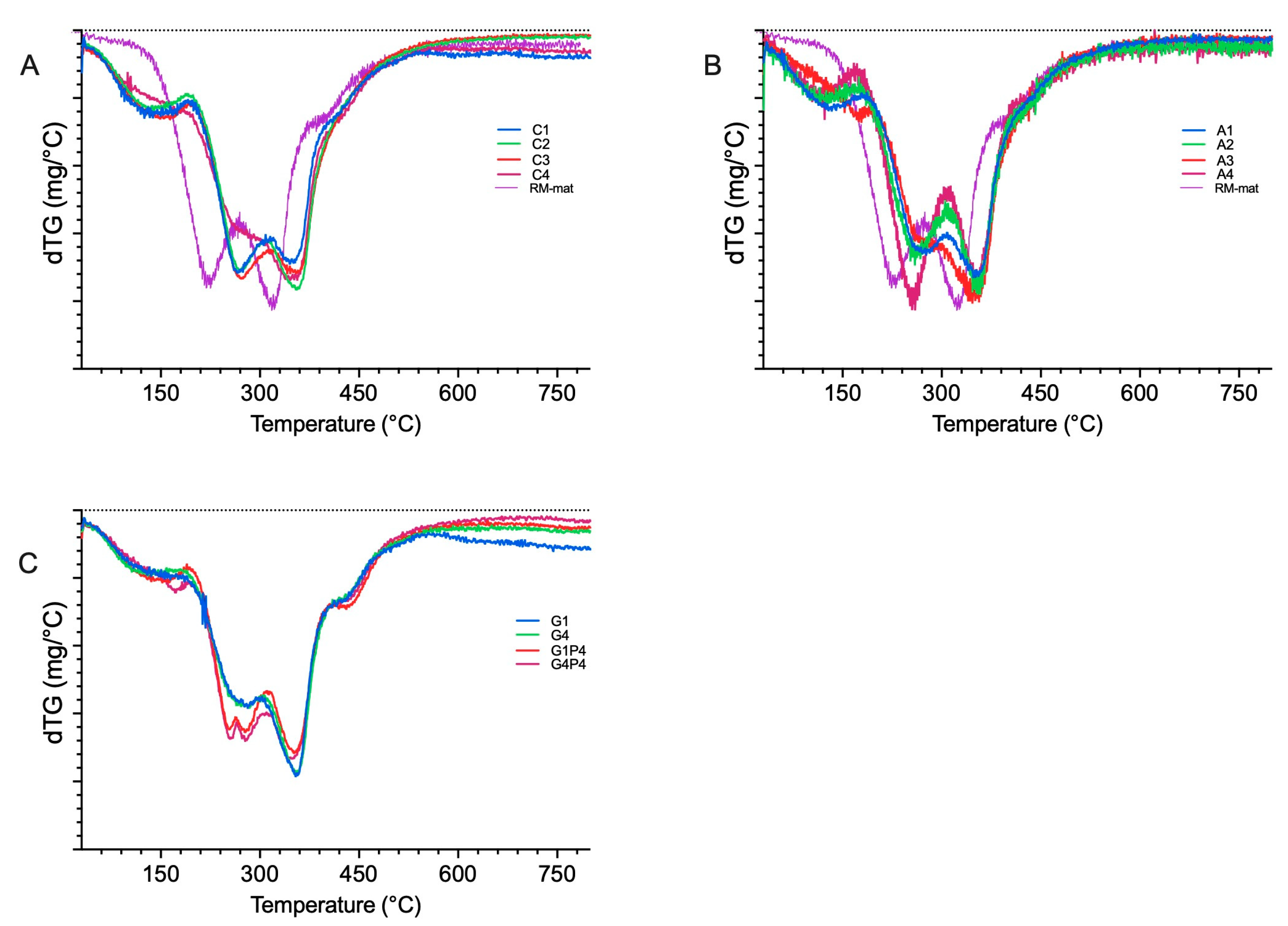
3.5. Thermogravimetric Analysis
3.6. Differential Scanning Calorimetry
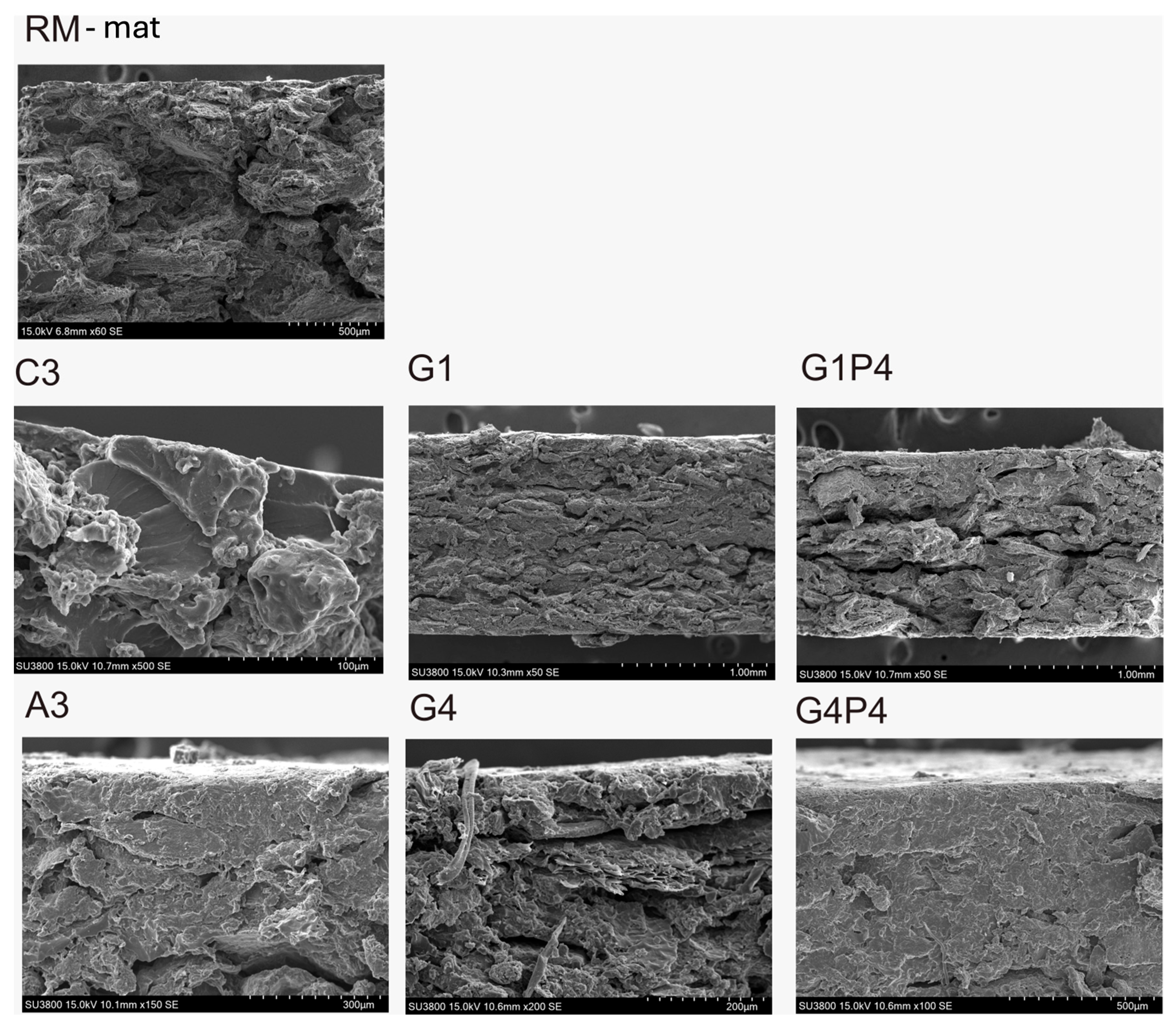
3.7. Scanning Electron Microscopy
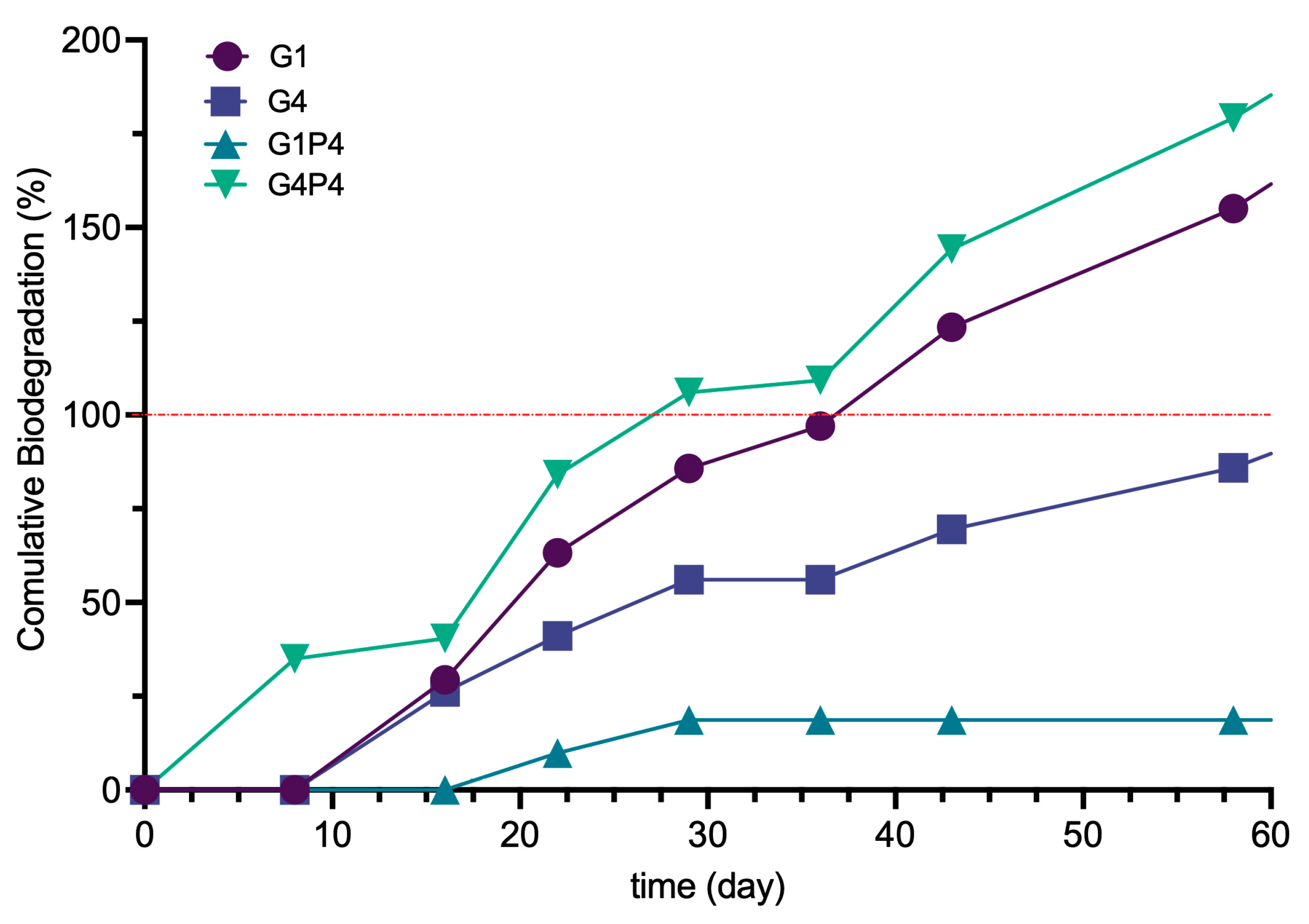
3.8. Aerobic Biodegradation Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | acetonitrile; |
| CH | collagen hydrolysate; |
| ΔH | melting enthalpy; |
| DSC | differential scanning calorimetry; |
| Epoxy-PEG | poly-(ethylene glycol)diglycidyl ether; |
| LDPA | low-density polyethylene; |
| PBSA | Poly-(butylene succinate-co-adipate); |
| PCL | polycaprolactone; |
| PVA | polyvinyl alcohol; |
| PVC | polyvinlylchloride; |
| RM | rapeseed meal; |
| RMH | rapeseed meal hydrolysate; |
| RM-Mat | reference material |
| RP-HPLC | reverse-phase high-performance liquid chromatography; |
| SDS | sodium dodecyl sulfate; |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis; |
| SEM | scanning electron microscopy; |
| TFA | trifluoroacetic acid; |
| TGA | thermogravimetric analysis; |
| Tg | glass transition temperature; |
| Tm | melting temperature; |
| UHPLC | ultra-high-performance liquid chromatography; |
| WPI | whey protein isolates. |
References
- North, E.J.; Halden, R.U. Plastics and Environmental Health: The Road Ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning Food Protein Waste into Sustainable Technologies. Chem. Rev. 2023, 123, 2112–2154. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Luzi, F.; Puglia, D. Organic Waste Valorisation towards Circular and Sustainable Biocomposites. Green Chem. 2022, 24, 5429–5459. [Google Scholar] [CrossRef]
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.-N.; Bayer, I.S.; Athanassiou, A. Bioplastics from Vegetable Waste via an Eco-Friendly Water-Based Process. Green Chem. 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Escribano, D.; Romero, A.; Martínez, I. Influence of the Aliphatic Chain Length on the Crosslinking Properties of Aldehydes on Sustainable Bioplastics Obtained from Pea Protein. J. Polym. Environ. 2022, 30, 5163–5172. [Google Scholar] [CrossRef]
- Bagnani, M.; Ehrengruber, S.; Soon, W.L.; Peydayesh, M.; Miserez, A.; Mezzenga, R. Rapeseed Cake Valorization into Bioplastics Based on Protein Amyloid Fibrils. Adv. Mater. Technol. 2023, 8, 2200932–2200945. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from Agri-Food Industrial Biowastes or Co-Products and Their Applications as Green Materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef]
- Tuck, C.S.; Latham, A.; Lee, P.W.; Barone, J.R. Wheat Gluten Plasticized with Its Own Hydrolysate. J. Polym. Environ. 2014, 22, 430–438. [Google Scholar] [CrossRef]
- Di Lena, G.; Sanchez del Pulgar, J.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.-M.; Aguzzi, A.; Ferrari Nicoli, S.; Casini, I.; et al. Valorization Potentials of Rapeseed Meal in a Biorefinery Perspective: Focus on Nutritional and Bioactive Components. Molecules 2021, 26, 6787. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Gallorini, R.; Aquilia, S.; Bello, C.; Ciardelli, F.; Pinna, M.; Papini, A.M.; Rosi, L. Pyrolysis of Spent Rapeseed Meal: A Circular Economy Example for Waste Valorization. J. Anal. Appl. Pyrolysis 2023, 174, 106138–106147. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Pereira, A.G.; Soria-Lopez, A.; Barral, M.; Chamorro, F.; Cao, H.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Chapter 8—Valorization of Food Waste Biomass and Biomaterials from a Circular Economy Approach. In Sustainable Development and Pathways for Food Ecosystems; Accorsi, R., Bhat, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 183–226. [Google Scholar]
- Li, S.; Ciardullo, K.; Donner, E.; Thompson, M.R.; Rempel, C.; Liu, Q. Reactive Processing Preparation of Sustainable Composites from Canola Meal Reinforced by Chemical Modification. Eur. Polym. J. 2018, 102, 187–194. [Google Scholar] [CrossRef]
- Felix, M.; Perez-Puyana, V.; Romero, A.; Guerrero, A. Development of Protein-Based Bioplastics Modified with Different Additives. J. Appl. Polym. Sci. 2017, 134, 45430–45443. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of Seed Oil Cakes as Industrial Co-Streams for Production of Innovative Bioplastics. A Review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Żelaziński, T. Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics. Materials 2021, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Rempel, C. Processing and Characteristics of Canola Protein-Based Biodegradable Packaging: A Review. Crit. Rev. Food Sci. Nutr. 2017, 58, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-A.; Lim, G.-O.; Song, K.B. Preparation and Mechanical Properties of Edible Rapeseed Protein Films. J. Food Sci. 2011, 76, C218–C223. [Google Scholar] [CrossRef]
- Aquilia, S.; Rosi, L.; Pinna, M.; Bianchi, S.; Giurlani, W.; Bonechi, M.; Ciardelli, F.; Papini, A.M.; Bello, C. Study of the Preparation and Properties of Chemically Modified Materials Based on Rapeseed Meal. Biomolecules 2024, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Castillo, E.; Bengoechea, C.; Felix, M.; Guerrero, A. Protein-Based Bioplastics from Biowastes: Sources, Processing, Properties and Applications. In Bioplastics for Sustainable Development; Kuddus, M., Roohi, Eds.; Springer: Singapore, 2021; pp. 137–176. [Google Scholar]
- Mensitieri, G.; Di Maio, E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Iannace, S. Processing and Shelf Life Issues of Selected Food Packaging Materials and Structures from Renewable Resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]
- Newson, W.R.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Oilseed Meal Based Plastics from Plasticized, Hot Pressed Crambe Abyssinica and Brassica Carinata Residuals. J. Am. Oil Chem. Soc. 2013, 90, 1229–1237. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Oxygen Permeability and Mechanical Properties of Films from Hydrolyzed Whey Protein. J. Agric. Food Chem. 2000, 48, 3913–3916. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in Bio-Based Plastics and Plasticizing Modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Proteins as Raw Materials for Films and Coatings: Definitions, Current Status, and Opportunities. In Protein-Based Films and Coatings; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–42. [Google Scholar]
- Sothornvit, R.; Krochta, J.m. Water Vapor Permeability and Solubility of Films from Hydrolyzed Whey Protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Oh, J.-H.; Wang, B.; Field, P.D.; Aglan, H.A. Characteristics of Edible Films Made from Dairy Proteins and Zein Hydrolysate Cross-Linked with Transglutaminase. Int. J. Food Sci. Technol. 2004, 39, 287–294. [Google Scholar] [CrossRef]
- Nuanmano, S.; Prodpran, T.; Benjakul, S. Potential Use of Gelatin Hydrolysate as Plasticizer in Fish Myofibrillar Protein Film. Food Hydrocoll. 2015, 47, 61–68. [Google Scholar] [CrossRef]
- Bandara, N.; Akbari, A.; Esparza, Y.; Wu, J. Canola Protein: A Promising Protein Source for Delivery, Adhesive, and Material Applications. J. Am. Oil Chem. Soc. 2018, 95, 1075–1090. [Google Scholar] [CrossRef]
- Lamp, A.; Kaltschmitt, M.; Dethloff, J. Options to Improve the Mechanical Properties of Protein-Based Materials. Molecules 2022, 27, 446. [Google Scholar] [CrossRef] [PubMed]
- Van der Sman, R.G.M.; van den Hoek, I.A.F.; Renzetti, S. Sugar Replacement with Zwitterionic Plasticizers like Amino Acids. Food Hydrocoll. 2020, 109, 106113–106124. [Google Scholar] [CrossRef]
- Stein, T.M.; Gordon, S.H.; Greene, R.V. Amino Acids as Plasticizers: II. Use of Quantitative Structure-Property Relationships to Predict the Behavior of Monoammoniummonocarboxylate Plasticizers in Starch–Glycerol Blends. Carbohydr. Polym. 1999, 39, 7–16. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kang, K.; Bond, T.; Karunakaran, C.; Dalai, A.K.; Kozinski, J.A. Effects of Bio-Additives on the Physicochemical Properties and Mechanical Behavior of Canola Hull Fuel Pellets. Renew. Energy 2019, 132, 296–307. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, Y.; Fu, L.; Pan, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Potential Use of Gluten Hydrolysate as a Plasticizer in High-Moisture Soy Protein–Gluten Extrudates. J. Food Eng. 2023, 354, 111565–111576. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ibresam, O.T.; Al-Hatim, R.R.; Al-Ali, R.M.; Maslekar, N.; Yao, Y.; Agarwal, V. Development of Chitosan/Whey Protein Hydrolysate Composite Films for Food Packaging Application. J. Compos. Sci. 2023, 7, 94. [Google Scholar] [CrossRef]
- Oh, L.; Nj, R.; Al, F.; Rj, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Chabanon, G.; Chevalot, I.; Framboisier, X.; Chenu, S.; Marc, I. Hydrolysis of Rapeseed Protein Isolates: Kinetics, Characterization and Functional Properties of Hydrolysates. Process Biochem. 2007, 42, 1419–1428. [Google Scholar] [CrossRef]
- ASTM D638-91; Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Material ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D570-81; Standard Test Method for Water Absorption of Plastics. American Society for Testing and Material ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D3418−21; Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. American Society for Testing and Material ASTM: West Conshohocken, PA, USA, 2022.
- Ferri, M.; Graen-Heedfeld, J.; Bretz, K.; Guillon, F.; Michelini, E.; Calabretta, M.M.; Lamborghini, M.; Gruarin, N.; Roda, A.; Kraft, A.; et al. Peptide Fractions Obtained from Rice By-Products by Means of an Environment-Friendly Process Show In Vitro Health-Related Bioactivities. PLoS ONE 2017, 12, e0170954. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic Production of Bioactive Peptides from Scotta, an Exhausted by-Product of Ricotta Cheese Processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef]
- Larré, C.; Mulder, W.; Sánchez-Vioque, R.; Lazko, J.; Bérot, S.; Guéguen, J.; Popineau, Y. Characterisation and Foaming Properties of Hydrolysates Derived from Rapeseed Isolate. Colloids Surf. B Biointerfaces 2006, 49, 40–48. [Google Scholar] [CrossRef]
- Pozdnyakov, N.; Shilov, S.; Lukin, A.; Bolshakov, M.; Sogorin, E. Investigation of Enzymatic Hydrolysis Kinetics of Soy Protein Isolate: Laboratory and Semi-Industrial Scale. Bioresour. Bioprocess. 2022, 9, 37–55. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Rodrigues, J.M.; Valadares, A.C.F.; de Almeida, A.B.; de Lima, T.M.; Takeuchi, K.P.; Alves, C.C.F.; Sousa, H.A.d.F.; da Silva, E.R.; Dyszy, F.H.; et al. Active Food Packaging: Alginate Films with Cottonseed Protein Hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The Preparation and Physiochemical Characterization of Rapeseed Protein Hydrolysate-Chitosan Composite Films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef]
- Seggiani, M.; Gigante, V.; Cinelli, P.; Coltelli, M.-B.; Sandroni, M.; Anguillesi, I.; Lazzeri, A. Processing and Mechanical Performances of Poly(Butylene Succinate–Co–Adipate) (PBSA) and Raw Hydrolyzed Collagen (HC) Thermoplastic Blends. Polym. Test. 2019, 77, 105900–105910. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Rubio-Valle, J.F.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Comparison between Pea and Soy Protein-Based Bioplastics Obtained by Injection Molding. J. Appl. Polym. Sci. 2021, 138, 50412–50422. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; dos Santos, N.Z.; May, I.C.; Pollo, L.D.; Tessaro, I.C. Impact of Acid Type and Glutaraldehyde Crosslinking in the Physicochemical and Mechanical Properties and Biodegradability of Chitosan Films. Polym. Bull. 2021, 78, 981–1000. [Google Scholar] [CrossRef]
- Ullah, A.; Wu, J. Feather Fiber-Based Thermoplastics: Effects of Different Plasticizers on Material Properties. Macromol. Mater. Eng. 2013, 298, 153–162. [Google Scholar] [CrossRef]
- Li, S.; Donner, E.; Thompson, M.; Zhang, Y.; Rempel, C.; Liu, Q. Preparation and Characterization of Cross-Linked Canola Protein Isolate Films. Eur. Polym. J. 2017, 89, 419–430. [Google Scholar] [CrossRef]
- Bandara, N.; Wu, J. Chemically Modified Canola Protein–Nanomaterial Hybrid Adhesive Shows Improved Adhesion and Water Resistance. ACS Sustain. Chem. Eng. 2018, 6, 1152–1161. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and Anaerobic Biodegradability of Polymer Films and Physico-Chemical Characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Versino, F.; Urriza, M.; García, M.A. Cassava-based biocomposites as fertilizer controlled-release systems for plant growth improvement. Ind. Crops Prod. 2020, 144, 112062–112068. [Google Scholar] [CrossRef]
| Formulation | ||||||
|---|---|---|---|---|---|---|
| Sample | RM/Casein | Glycerol (%) | Water Solution (%) | Epoxy-Peg | CH (%) | RMH (%) |
| RM-mat | 04:01 | 27 | 14 | 2 × mol Lys. | - | - |
| C1 | 04:01 | 27 | 14 | 2 × mol Lys | 0.25 | - |
| C2 | 04:01 | 27 | 14 | 2 × mol Lys | 0.50 | - |
| C3 | 04:01 | 27 | 14 | 2 × mol Lys | 0.75 | - |
| C4 | 04:01 | 27 | 14 | 2 × mol Lys | 1.00 | - |
| A1 | 04:01 | 27 | 14 | 2 × mol Lys | - | 0.25 |
| A2 | 04:01 | 27 | 14 | 2 × mol Lys | - | 0.50 |
| A3 | 04:01 | 27 | 14 | 2 × mol Lys | - | 0.75 |
| A4 | 04:01 | 27 | 14 | 2 × mol Lys | - | 1.00 |
| Formulation | |||||||
|---|---|---|---|---|---|---|---|
| Sample | RM/Casein | Glycerol (%) | Water Solution (%) | Epoxy-Peg | CH (%) | RMH (%) | Pro (%) |
| RM-mat | 04:01 | 27 | 14 | 2 × mol Lys | - | - | - |
| G1 | 04:01 | 20 | 14 | 2 × mol Lys | 0.75 | - | - |
| G2 | 04:01 | 15 | 14 | 2 × mol Lys | 0.75 | - | - |
| G3 | 04:01 | 10 | 14 | 2 × mol Lys | 0.75 | - | - |
| G4 | 04:01 | 20 | 14 | 2 × mol Lys | - | 0.75 | - |
| G5 | 04:01 | 15 | 14 | 2 × mol Lys | - | 0.75 | - |
| G6 | 04:01 | 10 | 14 | 2 × mol Lys | - | 0.75 | - |
| G1P4 | 04:01 | 20 | 14 | 2 × mol Lys | 0.75 | - | 7 |
| G4P4 | 04:01 | 20 | 14 | 2 × mol Lys | - | 0.75 | 7 |
| Mechanical Properties | ||||
|---|---|---|---|---|
| Sample | CH | Stress at Fracture (N/mm2) | Elastic Modulus (N/mm2) | Elongation at Fracture (%) |
| RM-mat | / | 0.20 | 9.40 | 0.28 |
| C1 | 0.25% | 0.06 | 1.04 | 0.33 |
| C2 | 0.50% | 0.11 | 1.55 | 1.51 |
| C3 | 0.75% | 0.02 | 0.21 | 1.03 |
| C4 | 1.00% | 0.12 | 0.89 | 0.61 |
| Mechanical Properties | ||||
|---|---|---|---|---|
| Sample | RMH | Stress at Fracture (N/mm2) | Elastic Modulus (N/mm2) | Elongation at Fracture (%) |
| RM-mat | / | 0.20 | 9.40 | 0.28 |
| A1 | 0.25% | 0.09 | 1.15 | 0.53 |
| A2 | 0.50% | 0.16 | 2.99 | 0.36 |
| A3 | 0.75% | 0.02 | 0.78 | 0.33 |
| A4 | 1.00% | 0.34 | 5.79 | 0.68 |
| Mechanical Properties | ||||
|---|---|---|---|---|
| Sample | Glycerol | Stress at Fracture (N/mm2) | Elastic Modulus (N/mm2) | Elongation at Fracture (%) |
| C3 | 27% | 0.02 | 0.21 | 1.03 |
| G1 | 20% | 0.80 | 1.41 | 0.16 |
| G2 | 15% | 0.08 | 0.50 | 0.49 |
| G3 | 10% | 0.51 | 1.14 | 0.43 |
| G1P4 | 20% | 0.09 | 1.12 | 0.50 |
| Mechanical Properties | ||||
|---|---|---|---|---|
| Sample | Glycerol | Stress at Fracture (N/mm2) | Elastic Modulus (N/mm2) | Elongation at Fracture (%) |
| A3 | 27% | 0.02 | 0.78 | 0.33 |
| G4 | 20% | 0.12 | 1.44 | 0.90 |
| G5 | 15% | 0.84 | 4.12 | 0.59 |
| G6 | 10% | 0.89 | 26.89 | 0.25 |
| G4P4 | 20% | 0.90 | 1.12 | 0.46 |
| Thermal Properties | |||
|---|---|---|---|
| Sample | Tg (°C) | Tm (°C) | ΔH (J/g) |
| RM-mat | 163.55 | 186.33 | 182.48 |
| A3 | -------- | <160 | -------- |
| C3 | 163.38 | 185.20 | 224.17 |
| G1 | 163.43 | 186.63 | 235.86 |
| G4 | 167.98 | 176.73 | 222.74 |
| G1P4 | 162.97 | 186.05 | 228.30 |
| G4P4 | -------- | <160 | -------- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquilia, S.; Bello, C.; Pinna, M.; Bianchi, S.; Giurlani, W.; Ciardelli, F.; Rosi, L.; Papini, A.M. Incorporation of Protein Hydrolysate into Rapeseed Meal-Based Materials to Improve Flexibility. Polymers 2025, 17, 1740. https://doi.org/10.3390/polym17131740
Aquilia S, Bello C, Pinna M, Bianchi S, Giurlani W, Ciardelli F, Rosi L, Papini AM. Incorporation of Protein Hydrolysate into Rapeseed Meal-Based Materials to Improve Flexibility. Polymers. 2025; 17(13):1740. https://doi.org/10.3390/polym17131740
Chicago/Turabian StyleAquilia, Sara, Claudia Bello, Michele Pinna, Sabrina Bianchi, Walter Giurlani, Francesco Ciardelli, Luca Rosi, and Anna Maria Papini. 2025. "Incorporation of Protein Hydrolysate into Rapeseed Meal-Based Materials to Improve Flexibility" Polymers 17, no. 13: 1740. https://doi.org/10.3390/polym17131740
APA StyleAquilia, S., Bello, C., Pinna, M., Bianchi, S., Giurlani, W., Ciardelli, F., Rosi, L., & Papini, A. M. (2025). Incorporation of Protein Hydrolysate into Rapeseed Meal-Based Materials to Improve Flexibility. Polymers, 17(13), 1740. https://doi.org/10.3390/polym17131740











