Effects of Wood Vinegar as a Coagulant in Rubber Sheet Production: A Sustainable Alternative to Acetic Acid and Formic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Wood Vinegars and Commercial Acid
2.3. Hot-Air Drying Chamber
2.4. Preparation of Raw and Dried Rubber Sheets for Testing
2.5. Properties of Rubber Sheets
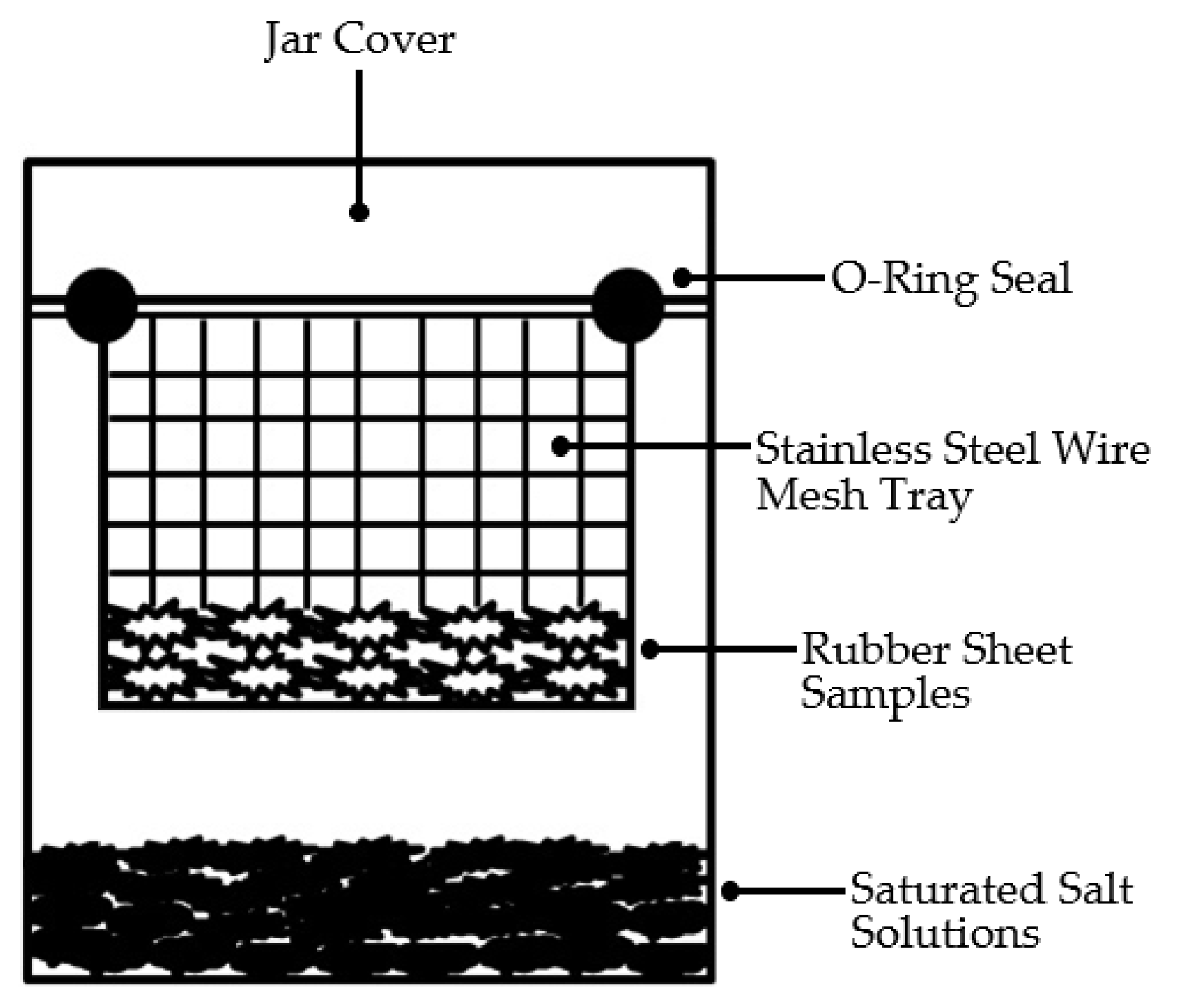
2.6. Determination and Analysis of the Equilibrium Moisture Content of Rubber Sheets
2.7. Determination of Sorption Isotherm Model
2.8. Drying Kinetics Testing
2.9. Thermogravimetric Analysis (TGA)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Wood Vinegar
3.2. Chemical, Physical, and Mechanical Properties of NR Sheets
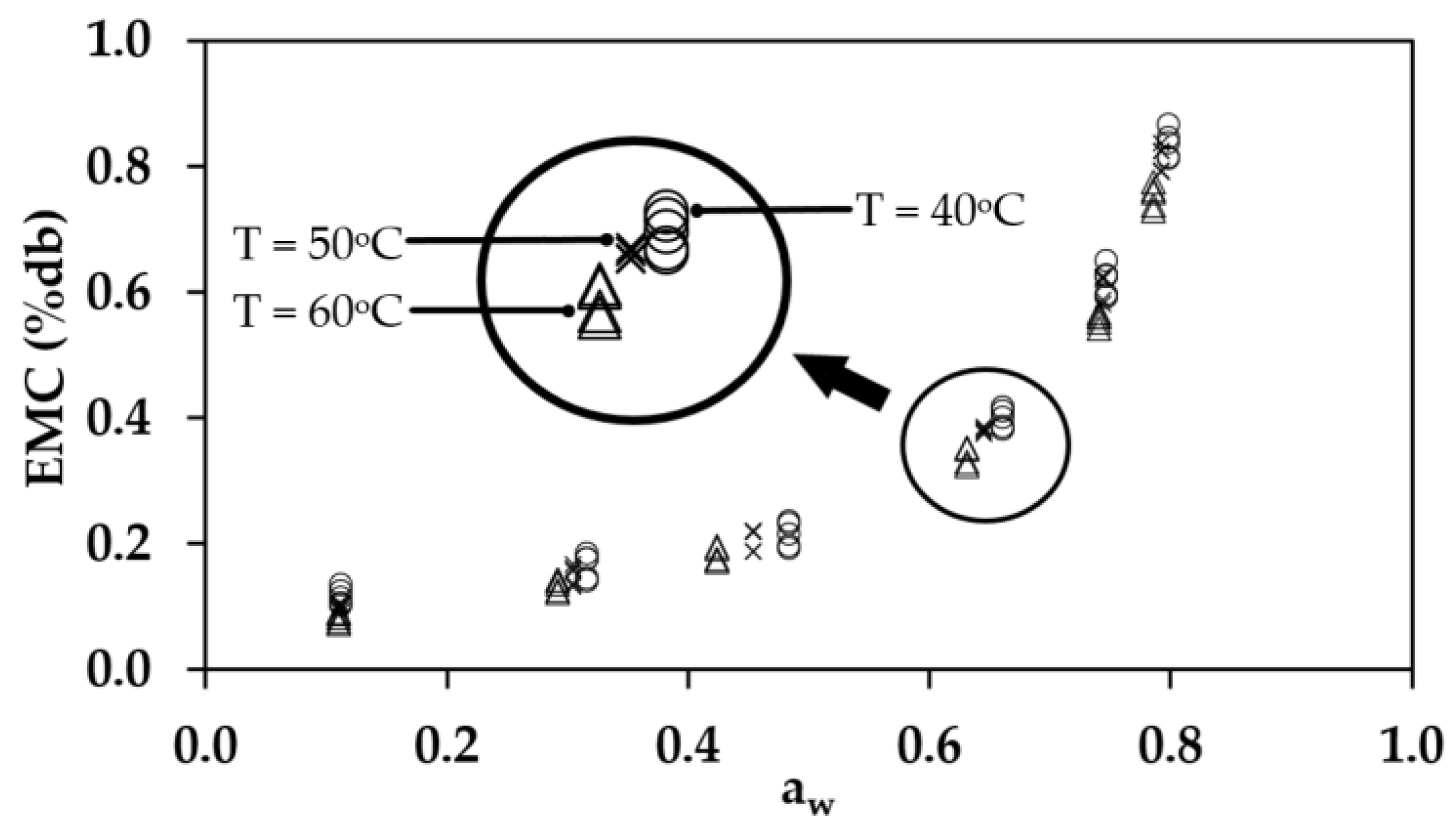
3.3. The Experimental Results for the EMC of Rubber Sheets
3.4. The Results for Sorption Isotherm Model
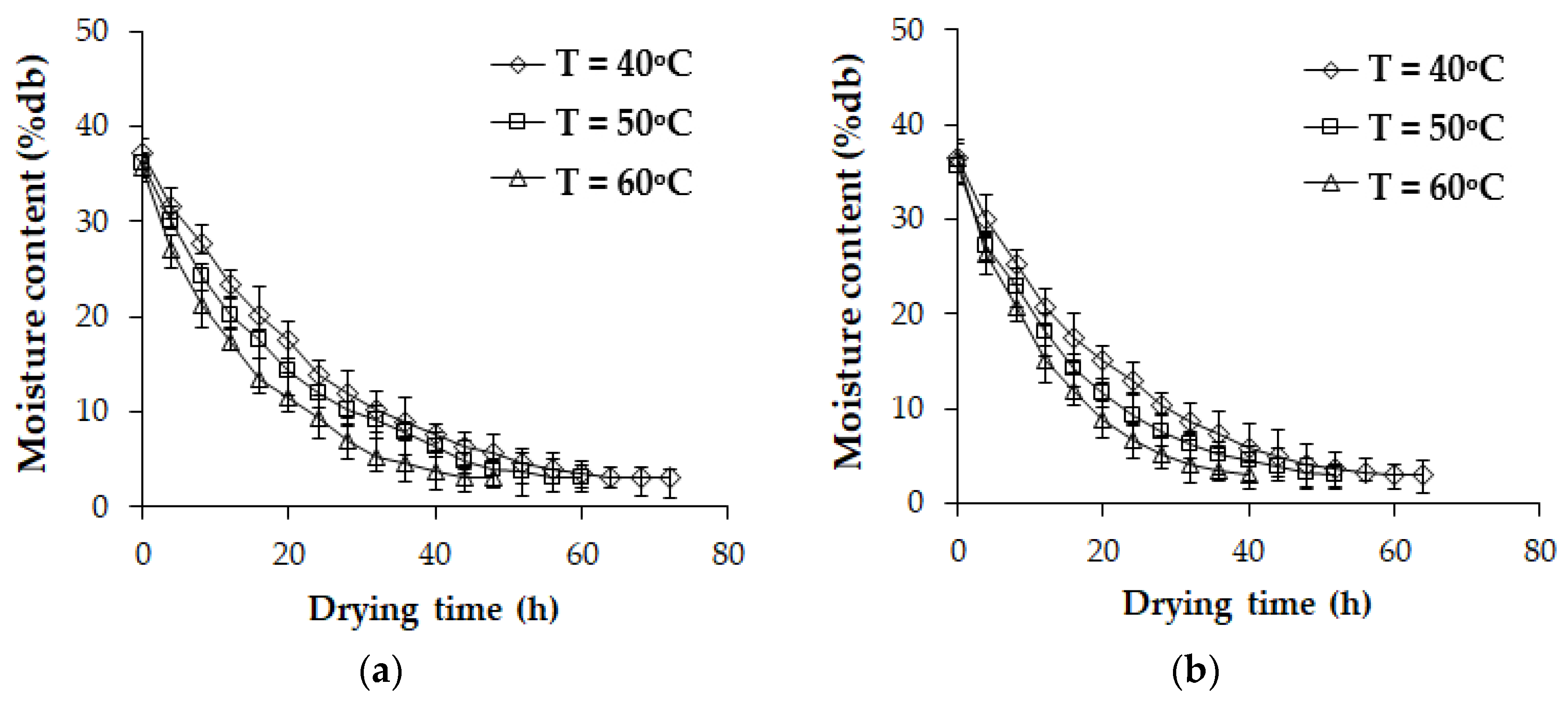
3.5. Drying Kinetics of NR Sheets
3.6. The Results of the Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalasee, W.; Teekapakvisit, C. A Review of Air Pollution and Solutions Way Management Related to Ribbed Smoked Sheets (RSS) Production of Community-Level Rubber Cooperatives in Thailand: Smoke, Soot and PAHs Particles. Pollution 2020, 6, 267–284. [Google Scholar] [CrossRef]
- Choosong, T.; Furuuchi, M.; Tekasakul, P.; Tekasakul, S.; Chomanee, J.; Jinno, T.; Hata, M.; Otani, Y. Working Environment in a Rubber Sheet Smoking Factory Polluted by Smoke from Biomass Fuel Burning and Health Influences to Workers. J. Ecotechnol. Res. 2007, 13, 91–96. [Google Scholar] [CrossRef]
- Hata, M.; Chomanee, J.; Thongyen, T.; Bao, L.; Tekasakul, S.; Tekasakul, P.; Otani, Y.; Furuuchi, M. Characteristics of Nanoparticles Emitted from Burning of Biomass Fuels. J. Environ. Sci. 2014, 26, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Kalasee, W.; Dangwilailux, P. Effect of Wood Vinegar Substitutes on Acetic Acid for Coagulating Natural Para Rubber Sheets during the Drying Process. Appl. Sci. 2021, 11, 7891. [Google Scholar] [CrossRef]
- Dejchanchaiwong, R.; Arkasuwan, A.; Kumar, A.; Tekasakul, P. Mathematical Modeling and Performance Investigation of Mixed-Mode and Indirect Solar Dryers for Natural Rubber Sheet Drying. Energy Sustain. Dev. 2016, 34, 44–53. [Google Scholar] [CrossRef]
- Tekasakul, P.; Dejchanchaiwong, R.; Tirawanichakul, Y.; Tirawanichakul, S. Three-Dimension Numerical Modeling of Heat and Moisture Transfer in Natural Rubber Sheet Drying Process. Dry. Technol. 2015, 33, 1124–1137. [Google Scholar] [CrossRef]
- Dejchanchaiwong, R.; Tirawanichakul, Y.; Tirawanichakul, S.; Kumar, A.; Tekasakul, P. Conjugate Heat and Mass Transfer Modeling of a New Rubber Smoking Room and Experimental Validation. Appl. Therm. Eng. 2017, 112, 761–770. [Google Scholar] [CrossRef]
- Kalasee, W.; Dangwilailux, P. Prediction of Size Distribution and Mass Concentration of Smoke Particles on Moisture Content and Combustion Period from Para Rubber Wood Burning. Appl. Sci. 2021, 11, 5649. [Google Scholar] [CrossRef]
- Thailand Rubber Research Institute, Department of Agriculture, Ministry of Agriculture and Cooperative. International Standard of Quality and Packing for Natural Rubber Grades; Thailand Rubber Research Institute, Department of Agriculture, Ministry of Agriculture and Cooperative: Bangkok, Thailand, 2012. [Google Scholar]
- Pimsuta, M.; Sosa, N.; Deekamwong, K.; Keawkumay, C.; Thathong, Y.; Rakmae, S.; Junpirom, S.; Prayoonpokarach, S.; Wittayakun, J. Charcoal and Wood Vinegar from Pyrolysis of Lead Tree Wood and Activated Carbon from Physical Activation. J. Sci. Technol. 2018, 25, 177–190. [Google Scholar]
- Kolokolova, O. Biomass Pyrolysis and Optimisation for Bio-Bitumen. Ph.D. Thesis, University of Canterbury, Canterbury, New Zealand, 2013. [Google Scholar]
- Akinola, A.O. Effect of Temperature on Product Yield of Pyrolysis of Seven Selected Wood Species in South West Nigeria. Int. J. Emerg. Res. Manag. Technol. 2016, 5, 176–181. [Google Scholar]
- Yang, J.; Yang, C.; Liang, M.; Gao, Z.; Wu, Y.; Chuang, L. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Ratanapisit, J.; Apiraksakul, S.; Rerngnarong, A.; Chungsiriporn, J.; Bunyakarn, C. Preliminary Evaluation of Production and Characterization of Wood Vinegar from Rubberwood. Songklanakarin J. Sci. Technol. 2009, 31, 343–349. [Google Scholar]
- Nunkaew, T.; Kantachote, D.; Chaiprapat, S.; Nitoda, T.; Kanzaki, H. Use of Wood Vinegar to Enhance 5-Aminolevulinic Acid Production by Selected Rhodopseudomonas palustris in Rubber Sheet Wastewater for Agricultural Use. Saudi J. Biol. Sci. 2018, 25, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Uehara, T.; Furuno, T. Effect of Bamboo Vinegar on Regulation of Germination and Radicle Growth of Seed Plants II: Composition of Moso Bamboo Vinegar at Different Collection Temperature and its Effects. J. Wood Sci. 2004, 50, 470–476. [Google Scholar] [CrossRef]
- Payamara, J. Usage of Wood Vinegar as New Organic Substance. Int. J. ChemTech Res. 2011, 3, 1658–1662. [Google Scholar]
- Nun-Anan, P.; Suchat, S.; Mahathaninwong, N.; Chueangchayaphan, N.; Karrila, S.; Limhengha, S. Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets. Polymers 2021, 13, 4178. [Google Scholar] [CrossRef]
- Jeentada, W.; Chankrachang, T.; Chooklin, S.; Sirirak, C. Comparison of Mathematical Models Equilibrium Moisture Content for Sheet Rubber. Eng. Appl. Sci. Res. 2012, 39, 11–21. [Google Scholar]
- Tirawanichakul, Y.; Tirawanichakul, S. Mathematical Model of Fixed-Bed Drying and Strategies for Crumb Rubber Producing STR20. Dry. Technol. 2008, 26, 1388–1395. [Google Scholar] [CrossRef]
- Rattanamechaiskul, C.; Junka, N.; Wongs-Aree, C.; Prachayawarakorn, S.; Soponronnarit, S. Influence of Hot Air Fluidized Bed Drying on Quality Changes of Purple Rice. Dry. Technol. 2016, 34, 1462–1470. [Google Scholar] [CrossRef]
- Rattanamechaiskul, C.; Junka, N.; Potichalung, J.; Wingwon, T.; Boontum, W.; Srisang, N. Whiteness Index Prediction of Para Rubber Sheet During Hot Air Drying. Eng. Appl. Sci. Res. 2016, 43, 331–333. [Google Scholar]
- Masa, A.; Jehsoh, N.; Hayeemasae, N. Dye Adsorbent from Natural Rubber Latex Foam: Efficiency and Post-Utilization. Polymers 2025, 17, 106. [Google Scholar] [CrossRef]
- ASTM D1076:1988; Standard Specification for Rubber—Concentrated, Ammonia Stabilized, Creamed, and Centrifuged Natural Latex. ASTM International: West Conshohocken, PA, USA, 2023.
- SMR Bulletin No. 7; RRIM Test Methods for Standard Malaysian Rubbers, Part B.2, Dirt. Rubber Research Institute of Malaysia: Kuala Lumpur, Malaysia, 2018.
- SMR Bulletin No. 7; RRIM Test Methods for Standard Malaysian Rubbers, Part B.3, Volatile Matter. Rubber Research Institute of Malaysia: Kuala Lumpur, Malaysia, 2018.
- ASTM D1646:2019; Standard Test Methods for Rubber-Viscosity, Stress Relaxation, and Pre-Vulcanization Characteristics (Mooney Viscometer). ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D3194-04; Standard Test Methods for Natural Rubber—Plasticity Retention Index (PRI). ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 3417–1977; Rubber-Measurement of Vulcanization Characteristics with the Oscillating Disc Cure-Meter. ISO Publishing: Geneve, Switzerland, 1977.
- ISO 37; Type 1, Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress–Strain Properties. ISO Publishing: Geneve, Switzerland, 2017.
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Oswin, C.R. The Kinetics of Package Life. III. The Isotherm. J. Soc. Chem. Ind. 1946, 65, 419–421. [Google Scholar] [CrossRef]
- Smith, S.E. The Sorption of Water Vapor by High Polymers. J. Am. Chem. Soc. 1947, 69, 646–651. [Google Scholar] [CrossRef]
- Henderson, S.M. A Basic Concept of Equilibrium Moisture. Agric. Eng. 1952, 33, 29–32. [Google Scholar]
- Supakarn, S.; Theerakulpisut, S.; Artnaseaw, A. Equilibrium Moisture Content and Thin Layer Drying Model of Shiitake Mushrooms Using a Vacuum Heat-pump Dryer. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Eakvanich, V.; Kalasee, W.; Dangwilailux, P.; Wattana, W.; Taweekun, J. A Review of the Parameters Related to the Rubber Sheets Production: Major Constituents of Rubber Latex, Rubber Particles, Particle Interaction of Rubber Latex, Rubber Porous Structure, Rubber Drying Kinetics and Energy Consumption. J. Adv. Res. Appl. Sci. Eng. Technol. 2023, 29, 159–184. [Google Scholar] [CrossRef]
- Dushkova, M.A.; Simitchiev, A.T.; Kalaydzhiev, H.R.; Ivanova, P.; Menkov, N.D.; Chalova, V.I. Moisture Sorption Behavior of Deproteinized Sunflower Meal and Patterned Food Extrudate. Appl. Sci. 2024, 14, 65. [Google Scholar] [CrossRef]
- Iacomino, G.; Idbella, M.; Staropoli, A.; Nanni, B.; Bertoli, T.; Vinale, F.; Bonanomi, G. Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests. Agronomy 2024, 14, 114. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Tindaon, M.J.; Nurhaida, N.; Diba, F.; Yanti, H. Termicidal Activity and Chemical Components of Wood Vinegar from Nipah Fruit against Coptotermes curvignathus. J. Korean Wood Sci. Technol. 2022, 50, 315–324. [Google Scholar] [CrossRef]
- Samao, H.; Satjaviso, S. Comparison of the Mechanical Properties of Rubber Sheets Coagulated with Different Types of Wood Vinegar. Bachelor Dissertation, King Mongkut’s Institute of Technology Ladkrabang, Chumphon, Thailand, 2017. [Google Scholar]
- Mathew, A.P.; Packirisamy, S.; Thomas, S. Studies on the Thermal Stability of Natural Rubber/Polystyrene Interpenetrating Polymer Networks: Thermogravimetric Analysis. Polym. Degrad. Stab. 2001, 72, 423–439. [Google Scholar] [CrossRef]
- Kalasee, W. Improvement Soot Particles Separation Equipments for Rubber Smoking Chamber. Aerosol Air Qual. Res. 2009, 9, 333–341. [Google Scholar] [CrossRef]





| Salts | Water Activity | ||
|---|---|---|---|
| 40 °C | 50 °C | 60 °C | |
| LiCl | 0.112 | 0.111 | 0.110 |
| MgCl2 | 0.316 | 0.305 | 0.292 |
| Mg(NO3)2 | 0.484 | 0.454 | 0.424 |
| KI | 0.661 | 0.645 | 0.631 |
| NaCl | 0.747 | 0.744 | 0.741 |
| (NH4)2SO4 | 0.799 | 0.792 | 0.786 |
| Model Type I | ||
| Oswin [32] | (2) | |
| Smith [33] | (3) | |
| Henderson [34] | (4) | |
| Model Type II | ||
| Modified Oswin [35] | (5) | |
| Modified Henderson [36] | (6) | |
| Modified Halsey [37] | (7) |
| No. | Compound | Percentage of Total Area | ||
|---|---|---|---|---|
| Para-Rubber | Bamboo | Eucalyptus | ||
| 1 | Acetic acid | 41.34 | 38.19 | 31.25 |
| 2 | Phenol | 8.29 | 7.56 | 7.12 |
| 3 | Phenol, 2,6-dimethoxy (Syringol) | 7.38 | 5.53 | 11.07 |
| 4 | 2-Methoxyphenol (Guaiacol) | 2.81 | 3.27 | 3.89 |
| 5 | p-Cresol | 1.57 | 1.72 | 1.85 |
| Total | 61.39 | 56.27 | 55.18 | |
| Properties of NR Sheets | Types of Coagulating Materials | ||||
|---|---|---|---|---|---|
| Raw Wood Vinegars | |||||
| Para-Rubber | Bamboo | Eucalyptus | Formic Acid | Acetic Acid | |
| Before drying | |||||
| Dirt content (%w/w) | 0.052 ± 0.002 | 0.054 ± 0.003 | 0.049 ± 0.003 | 0.043 ± 0.004 | 0.051 ± 0.002 |
| Volatile content (%w/w) | 0.83 ± 0.03 | 0.85 ± 0.05 | 0.82 ± 0.05 | 0.77 ± 0.02 | 0.83 ± 0.03 |
| Plasticity retention index (PRI) | 99.2 ± 2.5 | 98.7 ± 2.4 | 102.3 ± 1.8 | 108.5 ± 2.9 | 96.8 ± 1.9 |
| Mooney viscosity | 52.1 ± 0.4 | 51.9 ± 0.3 | 53.2 ± 0.3 | 54.7 ± 0.2 | 51.5 ± 0.4 |
| After drying | |||||
| Dirt content (%w/w) | 0.036 ± 0.003 | 0.037 ± 0.002 | 0.034 ± 0.002 | 0.032 ± 0.003 | 0.039 ± 0.003 |
| Volatile content (%w/w) | 0.45 ± 0.03 | 0.47 ± 0.02 | 0.44 ± 0.03 | 0.41 ± 0.03 | 0.49 ± 0.02 |
| Plasticity retention index (PRI) | 93.9 ± 2.2 | 94.1 ± 2.7 | 97.5 ± 2.6 | 102.9 ± 2.5 | 92.7 ± 2.2 |
| Mooney viscosity | 55.9 ± 0.3 | 55.2 ± 0.4 | 56.3 ± 0.4 | 59.4 ± 0.3 | 54.8 ± 0.3 |
| Curing time (min) | 18.3 ± 0.5 | 18.9 ± 0.5 | 17.7 ± 0.8 | 16.2 ± 0.4 | 19.5 ± 0.7 |
| Tensile strength at break (MPa) | 6.2 ± 0.3 | 5.9 ± 0.5 | 4.8 ± 0.5 | 5.1 ± 0.4 | 6.4 ± 0.3 |
| Elongation at break (%) | 671 ± 8 | 664 ± 11 | 625 ± 8 | 631 ± 12 | 678 ± 7 |
| 300% modulus (MPa) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Model | Parameters and Goodness of Fit | Para-Rubber Wood | Bamboo Wood | Eucalyptus Wood | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drying Temperature | Drying Temperature | Drying Temperature | ||||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||
| Oswin | A | 0.6589 | 0.7289 | 0.3257 | 1.0252 | 0.9857 | 0.9312 | 1.0345 | 0.9127 | 1.0526 |
| B | 0.8261 | 0.7891 | 0.9413 | 0.8957 | 0.7241 | 0.8422 | 1.0031 | 1.3042 | 0.8947 | |
| r2 | 0.9772 | 0.9715 | 0.9732 | 0.9722 | 0.9851 | 0.9814 | 0.9927 | 0.9913 | 0.9952 | |
| MRD | 0.0227 | 0.0395 | 0.0412 | 0.0341 | 0.0312 | 0.0435 | 0.0205 | 0.0151 | 0.0177 | |
| Smith | A | 0.1645 | 0.1808 | 0.1082 | 0.1235 | 0.1567 | 0.1051 | 0.2249 | 0.2517 | 0.2135 |
| B | 0.4488 | 0.5044 | 0.2273 | 0.3257 | 0.2891 | 0.3125 | 0.4891 | 0.4622 | 0.3985 | |
| r2 | 0.9497 | 0.9579 | 0.9078 | 0.9141 | 0.9013 | 0.8976 | 0.8827 | 0.9215 | 0.9351 | |
| MRD | 0.0424 | 0.0327 | 0.0359 | 0.0389 | 0.0412 | 0.0422 | 0.0375 | 0.0326 | 0.0394 | |
| Henderson | A | 0.4158 | 0.4297 | 0.3267 | 0.4582 | 0.4131 | 0.3594 | 0.3357 | 0.3882 | 0.3801 |
| B | 0.0541 | 0.0718 | 0.0821 | 0.0725 | 0.0803 | 0.0863 | 0.0543 | 0.0631 | 0.0615 | |
| r2 | 0.9648 | 0.9725 | 0.9528 | 0.9615 | 0.9217 | 0.9516 | 0.9485 | 0.9108 | 0.9253 | |
| MRD | 0.0282 | 0.0325 | 0.0334 | 0.0292 | 0.0304 | 0.0291 | 0.0377 | 0.0364 | 0.0371 | |
| Modified Oswin | A | 0.2887 | 0.3105 | 0.3321 | 0.2951 | 0.3006 | 0.2574 | 0.2886 | 0.3018 | 0.2135 |
| B | 0.0258 | 0.0294 | 0.0315 | 0.0295 | 0.0351 | 0.0312 | 0.0272 | 0.0308 | 0.0367 | |
| C | 5.2194 | 6.0082 | 5.6842 | 4.2219 | 4.3851 | 5.0214 | 5.2197 | 4.8262 | 4.2159 | |
| r2 | 0.9226 | 0.9358 | 0.9412 | 0.9105 | 0.9087 | 0.9521 | 0.9124 | 0.9326 | 0.9572 | |
| MRD | 0.0359 | 0.0397 | 0.0428 | 0.0381 | 0.0395 | 0.0418 | 0.0295 | 0.0316 | 0.0385 | |
| Modified Henderson | A | 0.2658 | 0.2156 | 0.3247 | 0.3189 | 0.4428 | 0.4816 | 0.5562 | 0.5107 | 0.5113 |
| B | 0.6941 | 0.3051 | 0.4518 | 0.4821 | 0.3915 | 0.5237 | 0.8521 | 0.8863 | 0.7642 | |
| C | 0.2319 | 0.3058 | 0.2816 | 0.2943 | 0.3267 | 0.2641 | 0.3025 | 0.3629 | 0.2856 | |
| r2 | 0.9915 | 0.9967 | 0.9928 | 0.9907 | 0.9918 | 0.9952 | 0.9756 | 0.9612 | 0.9705 | |
| MRD | 0.0184 | 0.0201 | 0.0218 | 0.0187 | 0.0176 | 0.0205 | 0.0291 | 0.0316 | 0.0368 | |
| Modified Halsey | A | 15.227 | 11.295 | 19.251 | 10.256 | 9.1275 | 12.354 | 2.6422 | 3.9543 | 2.8162 |
| B | 9.2185 | 7.5421 | 11.265 | 7.5204 | 5.3219 | 6.0058 | 1.0251 | 1.6593 | 1.6945 | |
| C | 1.3684 | 2.3658 | 1.0064 | 2.9642 | 3.0658 | 3.6419 | 6.2289 | 7.2546 | 5.2157 | |
| r2 | 0.8895 | 0.9024 | 0.9117 | 0.8912 | 0.9431 | 0.9357 | 0.9216 | 0.8997 | 0.9176 | |
| MRD | 0.0395 | 0.0375 | 0.0403 | 0.0379 | 0.0357 | 0.0391 | 0.0382 | 0.0408 | 0.0386 | |
| Model | Parameters and Goodness of Fit | Formic Acid | Acetic Acid | ||||
|---|---|---|---|---|---|---|---|
| Drying Temperature | Drying Temperature | ||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||
| Oswin | A | 0.6857 | 0.9561 | 1.0254 | 0.8899 | 0.6795 | 0.2977 |
| B | 0.8624 | 0.8853 | 0.8235 | 0.7955 | 0.7523 | 0.9564 | |
| r2 | 0.9922 | 0.9954 | 0.9938 | 0.9721 | 0.9813 | 0.9754 | |
| MRD | 0.0202 | 0.0175 | 0.0194 | 0.0292 | 0.0351 | 0.0372 | |
| Smith | A | 0.1925 | 0.2037 | 0.2185 | 0.0321 | 0.0112 | 0.0437 |
| B | 0.3812 | 0.4105 | 0.3992 | 0.0139 | 0.0614 | 0.0842 | |
| r2 | 0.9021 | 0.9106 | 0.8973 | 0.9622 | 0.9741 | 0.8579 | |
| MRD | 0.0419 | 0.0367 | 0.0374 | 0.0341 | 0.0253 | 0.0282 | |
| Henderson | A | 0.4005 | 0.3991 | 0.3462 | 0.3725 | 0.3107 | 0.2975 |
| B | 0.0522 | 0.0634 | 0.0593 | 0.0219 | 0.0261 | 0.0189 | |
| r2 | 0.9027 | 0.9564 | 0.9629 | 0.9753 | 0.9618 | 0.9827 | |
| MRD | 0.0355 | 0.0311 | 0.0395 | 0.0317 | 0.0398 | 0.0356 | |
| Modified Oswin | A | 0.2612 | 0.2591 | 0.2982 | 0.1776 | 0.2188 | 0.2745 |
| B | 0.0259 | 0.0286 | 0.0317 | 0.0086 | 0.0067 | 0.0132 | |
| C | 5.0068 | 5.8125 | 5.2953 | 4.0841 | 0.4188 | 8.6156 | |
| r2 | 0.9615 | 0.9214 | 0.9362 | 0.9695 | 0.9525 | 0.9462 | |
| MRD | 0.0327 | 0.0371 | 0.0405 | 0.0271 | 0.0243 | 0.0315 | |
| Modified Henderson | A | 0.4921 | 0.4527 | 0.4118 | 0.0513 | 0.0193 | 0.2298 |
| B | 0.9216 | 0.8611 | 0.9028 | 0.6705 | 0.4438 | 0.2007 | |
| C | 0.2785 | 0.3192 | 0.3109 | 0.4043 | 0.1469 | 0.0482 | |
| r2 | 0.9837 | 0.9724 | 0.9778 | 0.9902 | 0.9918 | 0.9965 | |
| MRD | 0.0352 | 0.0271 | 0.0344 | 0.0152 | 0.0211 | 0.0193 | |
| Modified Halsey | A | 7.2158 | 4.3269 | 6.3251 | 1.3382 | 4.7023 | 28.761 |
| B | 6.3142 | 7.2261 | 8.1165 | 0.7547 | 6.4395 | 13.707 | |
| C | 1.9967 | 2.3154 | 1.2635 | 7.1962 | 1.0211 | 0.6212 | |
| r2 | 0.8722 | 0.8753 | 0.8931 | 0.9066 | 0.9242 | 0.8748 | |
| MRD | 0.0425 | 0.0379 | 0.0418 | 0.0271 | 0.0415 | 0.0432 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eakvanich, V.; Lakachaiworakun, P.; Rachsiriwatcharabul, N.; Wattana, W.; Kalasee, W.; Dangwilailux, P. Effects of Wood Vinegar as a Coagulant in Rubber Sheet Production: A Sustainable Alternative to Acetic Acid and Formic Acid. Polymers 2025, 17, 1718. https://doi.org/10.3390/polym17131718
Eakvanich V, Lakachaiworakun P, Rachsiriwatcharabul N, Wattana W, Kalasee W, Dangwilailux P. Effects of Wood Vinegar as a Coagulant in Rubber Sheet Production: A Sustainable Alternative to Acetic Acid and Formic Acid. Polymers. 2025; 17(13):1718. https://doi.org/10.3390/polym17131718
Chicago/Turabian StyleEakvanich, Visit, Putipong Lakachaiworakun, Natworapol Rachsiriwatcharabul, Wassachol Wattana, Wachara Kalasee, and Panya Dangwilailux. 2025. "Effects of Wood Vinegar as a Coagulant in Rubber Sheet Production: A Sustainable Alternative to Acetic Acid and Formic Acid" Polymers 17, no. 13: 1718. https://doi.org/10.3390/polym17131718
APA StyleEakvanich, V., Lakachaiworakun, P., Rachsiriwatcharabul, N., Wattana, W., Kalasee, W., & Dangwilailux, P. (2025). Effects of Wood Vinegar as a Coagulant in Rubber Sheet Production: A Sustainable Alternative to Acetic Acid and Formic Acid. Polymers, 17(13), 1718. https://doi.org/10.3390/polym17131718








