Core–Shell CoS2/FeS2 Heterojunction Encapsulated in N-Doped Carbon Nanocubes Derived from Coordination Polymers for Electrocatalytic Alkaline Water/Seawater Splitting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
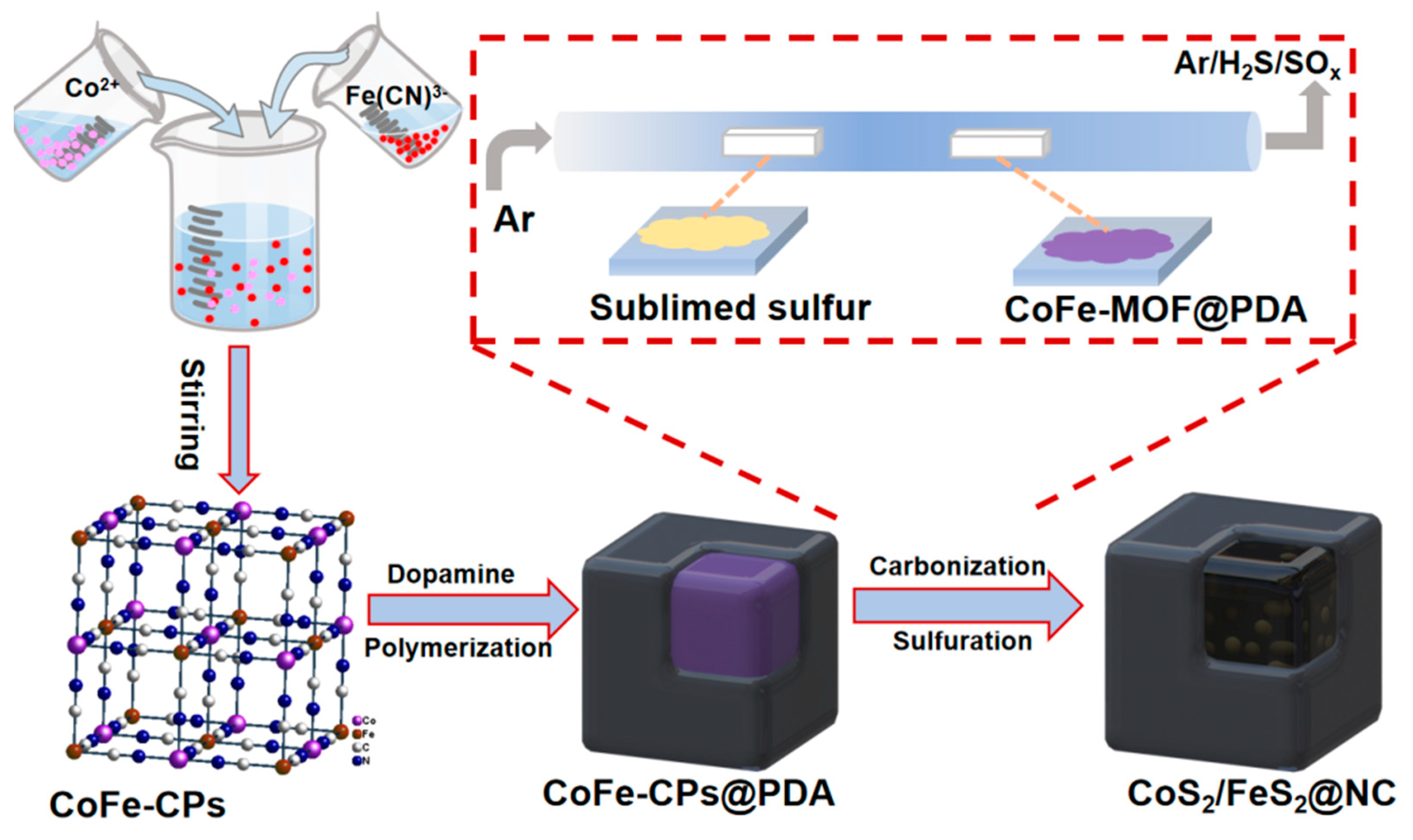
2.2. Synthesis of Co-Fe Hybrid Coordination Polymer (CoFe-CPs)
2.3. Synthesis of Polydopamine Coated CoNi-CPs Hollow Cubes (CoFe-CPs@PDA)
2.4. Synthesis of CoS2/FeS2@NC Nanocubes
2.5. Characterization
2.6. Electrocatalytic Measurements
3. Results and Discussion
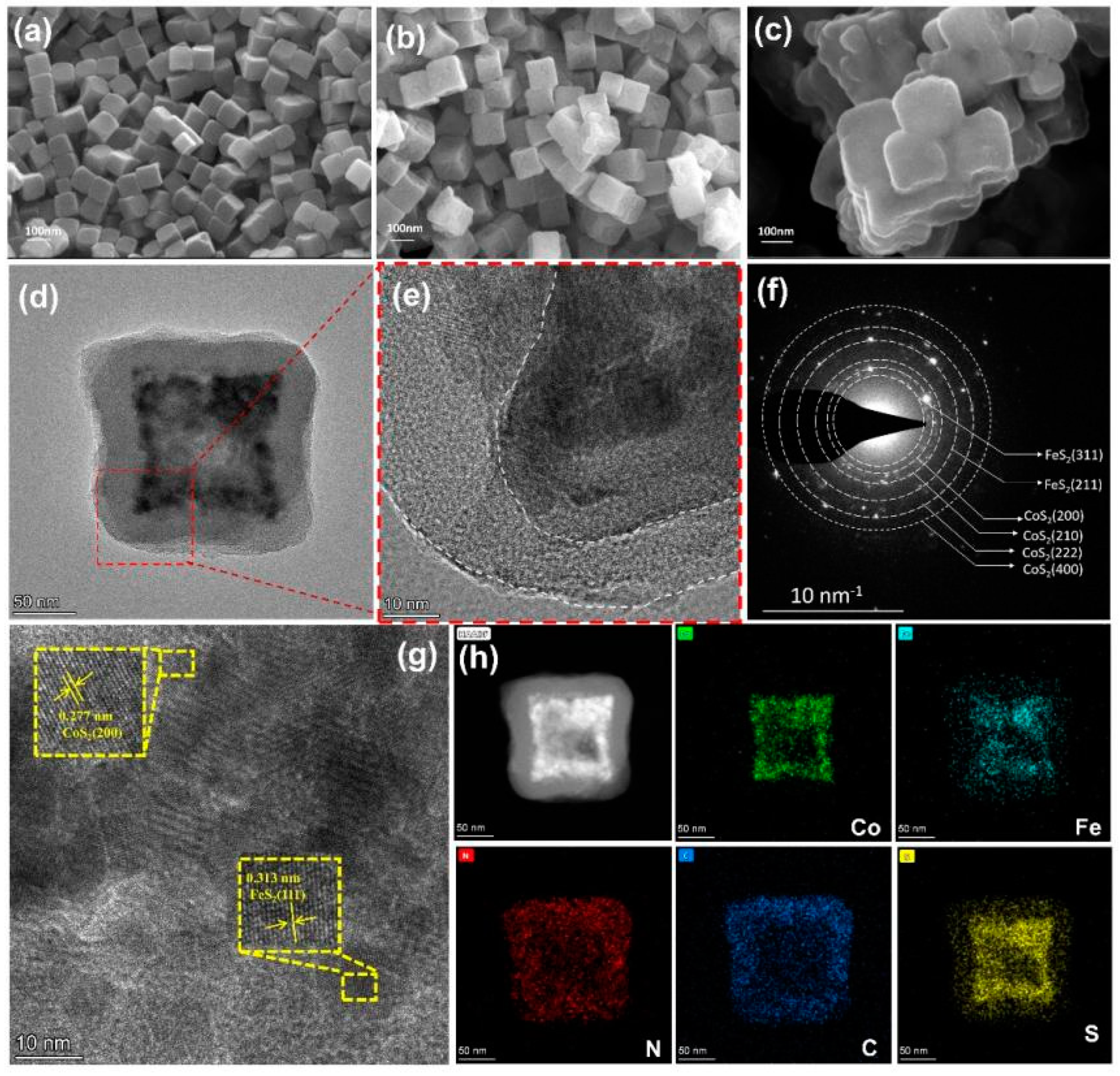
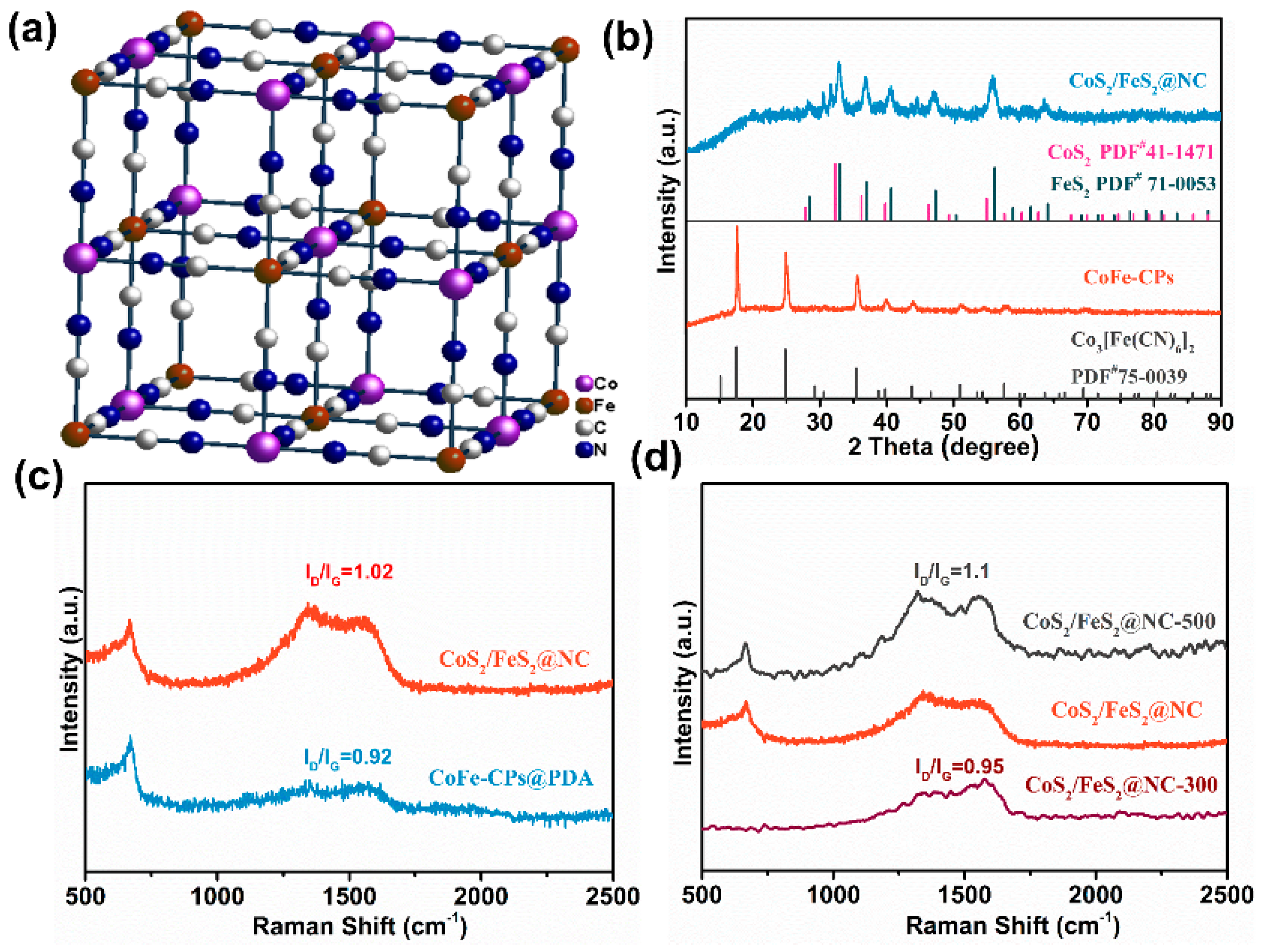
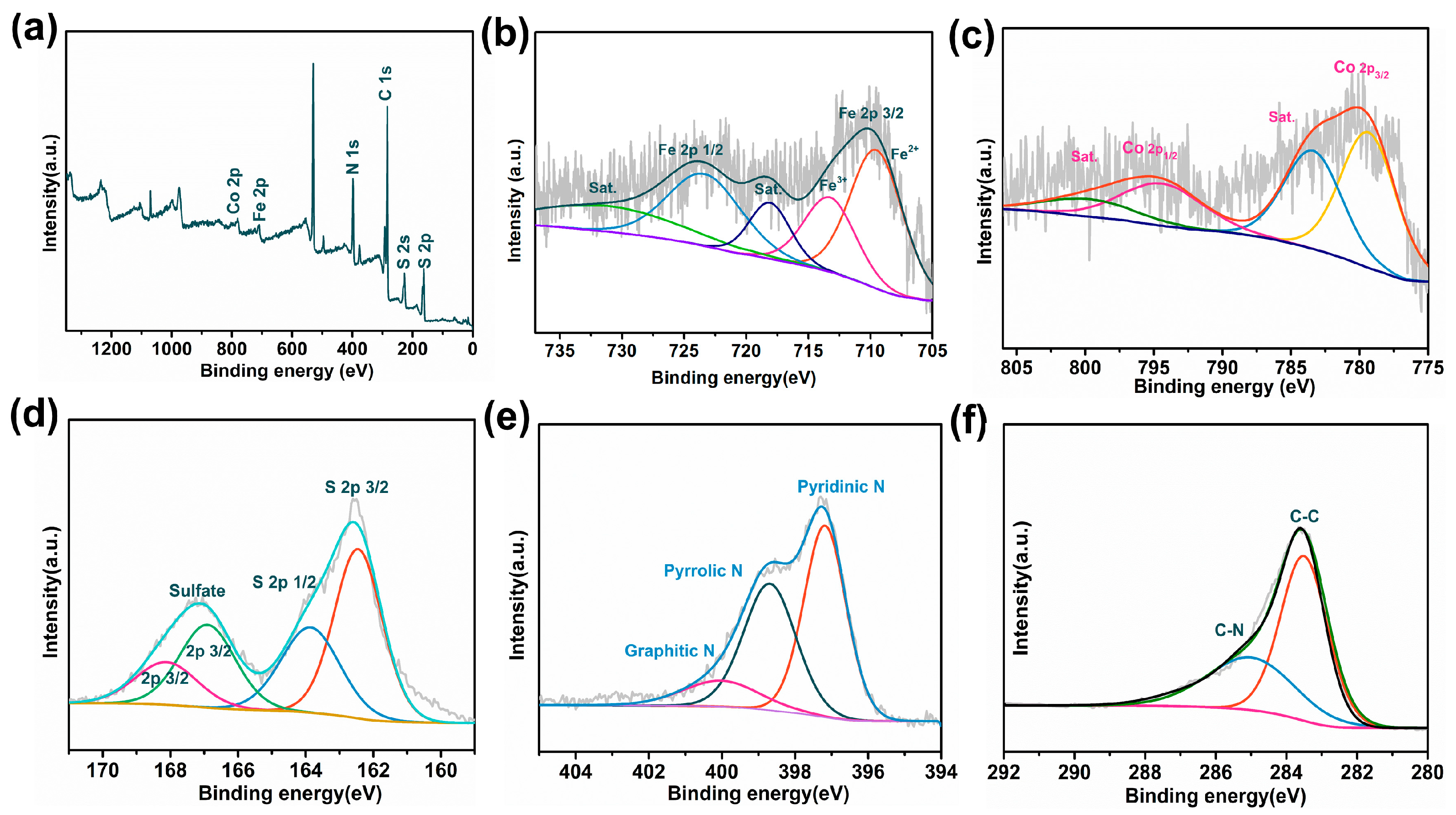
3.1. Textural and Chemical Characterization
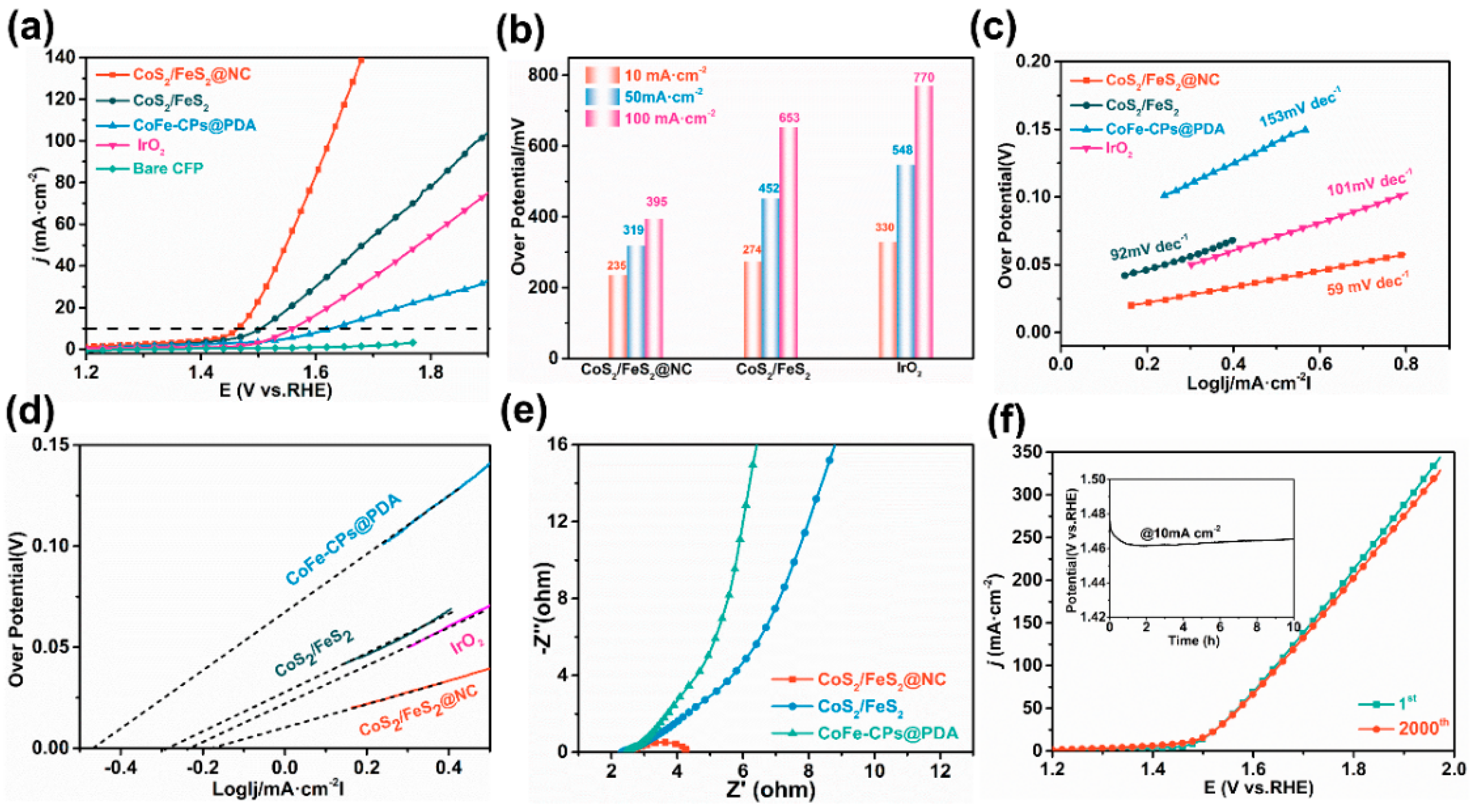
3.2. OER Catalytic Activity of CoS2/FeS2@NC in Alkaline Water
3.3. HER Catalytic Activity of CoS2/FeS2@NC in Alkaline Water
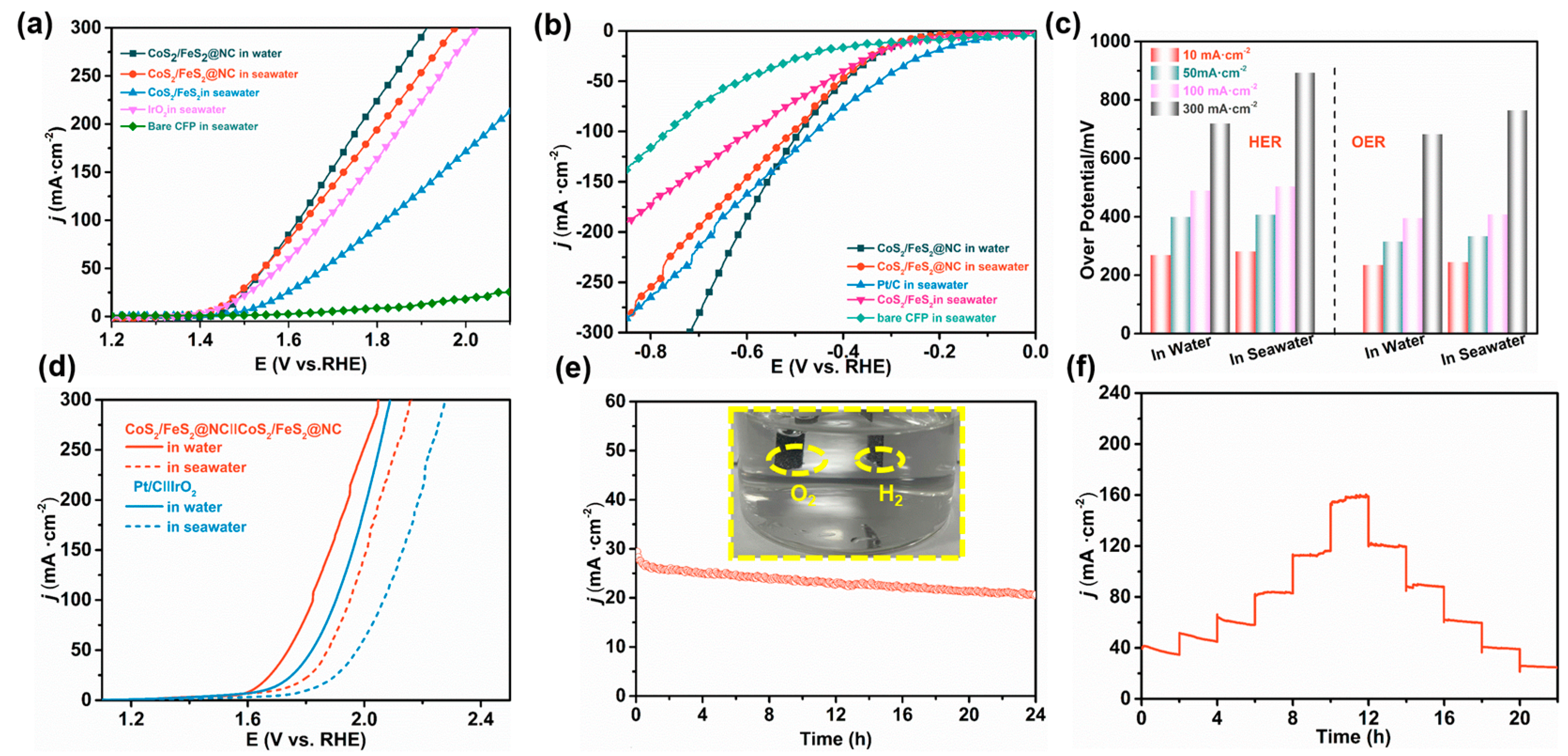
3.4. Electrocatalytic Activity of CoS2/FeS2@NC for Water/Seawater Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, F.; Li, M.; Zeng, L.; Luo, M.; Guo, S. Intermetallic Nanocrystals for Fuel-Cells-Based Electrocatalysis. Chem. Rev. 2023, 22, 12507–12593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, C.; Yang, O.; Yuan, W.; Liu, Y.; He, L.; Hu, Y.; Zhao, Z.; Zhou, L.; Wang, J.; et al. Self-Powered Seawater Electrolysis Based on a Triboelectric Nanogenerator for Hydrogen Production. ACS Nano 2022, 16, 15286–15296. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, L.; Zhuang, L. General definition of hydrogen energy and related electrochemical technologies. Chin. J. Catal. 2024, 56, 1–8. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Cagalitan, D.D.T.F.-D.; Abundo, M.L.S. A review of biohydrogen production technology for application towards hydrogen fuel cells. Renew. Sustain. Energy Rev. 2021, 151, 111413. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Liu, Y.; Sun, M.; Waterhouse, G.I.N.; Huang, B.; Zhang, K.; Zhang, T.; Lu, S. Exploiting Ru-Induced Lattice Strain in CoRu Nanoalloys for Robust Bifunctional Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2021, 60, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yu, J.; Tang, Z.; Yang, B.; Lu, S. Halogen-doped carbon dots on amorphous cobalt phosphide as robust electrocatalysts for overall water splitting. Adv. Energy Mater. 2022, 12, 2102573. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Qin, R.; Pu, Z.; Zhang, C.; Zhu, J.; Chen, D.; Feng, D.; Kou, Z.; Mu, S.; et al. Swapping catalytic active sites from cationic Ni to anionic S in nickel sulfide enables more efficient alkaline hydrogen generation. Adv. Energy Mater. 2022, 12, 2103359. [Google Scholar] [CrossRef]
- Feng, Y.; Han, J.; Xu, M.; Liang, X.; Jiang, T.; Li, H.; Wang, Z.L. Blue Energy for Green Hydrogen Fuel: A Self–Powered Electrochemical Conversion System Driven by Triboelectric Nanogenerators. Adv. Energy Mater. 2022, 12, 2103143. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Recent Development of Ni/Fe–Based Micro/Nanostructures toward Photo/Electrochemical Water Oxidation. Adv. Energy Mater. 2020, 10, 1900954. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Erami, R.S.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Urban, J.J. Emerging Scientific and Engineering Opportunities within the Water-Energy Nexus. Joule 2017, 1, 665–688. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design Criteria, Operating Conditions, and Nickel-Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef]
- Jin, H.; Xu, J.; Liu, H.; Shen, H.; Yu, H.; Jaroniec, M.; Zheng, Y.; Qiao, S.Z. Emerging materials and technologies for electrocatalytic seawater splitting. Sci. Adv. 2023, 9, eadi7755. [Google Scholar] [CrossRef]
- Hwang, S.M.; Park, J.S.; Kim, Y.; Go, W.; Han, J.; Kim, Y.; Kim, Y. Rechargeable Seawater Batteries—From Concept to Applications. Adv. Mater. 2019, 31, 1804936. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cheng, S.; Yu, L.; Zhang, W.; Zhou, J.; Zhang, Y.; Yu, Y. Electrolyzer with hierarchical transition metal sulfide and phosphide towards overall water splitting. Mater. Today Phys. 2019, 11, 100162. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Raaijman, S.; Kortlever, R.; Kooyman, P.J.; Wezendonk, T.; Gascon, J.; Fu, W.T.; Koper, M.T. Iridium-based double perovskites for efficient water oxidation in acid media. Nat. Commun. 2016, 7, 12363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. Metal–organic-framework-derived Co–Febimetallic oxygen reduction electrocatalysts for alkaline fuel cells. J. Am. Chem. Soc. 2019, 141, 10744–10750. [Google Scholar] [CrossRef]
- Jin, W.; Lu, X.; Wang, Y.; Xiao, W.; Zhang, X. Bamboo-like N-doped carbon tubes encapsulated Co2P-Fe2P derived from zeolitic imidazolate framework as an efficient trifunctional electrocatalyst for water splitting and Zn-air batteries. Int. J. Hydrogen Energy 2023, 48, 25250–25262. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhuo, S.; Liu, S.; Han, A.; Li, L.; Tian, Y. Flower-like CoO@Cu2S nanocomposite for enhanced oxygen evolution reaction. Appl. Surf. Sci. 2021, 555, 149441. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Gao, Y.; Chen, Z.; Li, Z.; Ma, T.; Wu, Z.; Wang, L.; Feng, S. Heterostructure of RuO2-RuP2/Ru Derived from HMT-based Coordination Polymers as Superior pH-Universal Electrocatalyst for Hydrogen Evolution Reaction. Small 2022, 18, 2105168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Z.; Kong, X.; Xu, H.; Jin, W. Rational design of MOFs-derived Co-Ru species embedded N-doped carbon/carbon matrix for highly-efficient and multifunctional electrocatalysis. Appl. Surf. Sci. 2022, 606, 154818. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, Z.; Li, D.; Ullah, S.; Hai, Y.; Xin, H.; Liao, W.; Yang, B.; Fan, H.; Xu, J.; et al. NiS2@CoS2 nanocrystals encapsulated in N-doped carbon nanocubes for high performance lithium/sodium ion batteries. Energy Storage Mater. 2018, 11, 67–74. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; An, L.; Lu, M.; Sun, K.; Zhao, Q.Y.; Gao, D.; Cheng, F.; Xi, P. FeS2/CoS2 interface nanosheets as efficient bifunctional electrocatalyst for overall water splitting. Small 2018, 14, 1801070. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Yu, Y.; Sun, H.; Liu, X.; Li, Y.; Zhang, L.; Yang, X.; Zhong, S.; Wang, R. Flexible Potassium-Ion Batteries Enabled by Encapsulating Hollow NiSe/SnSe Nanocubes within Freestanding N-doped Carbon Nanofibers. Energy Storage Mater. 2024, 74, 103908. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Li, Z.; Yang, Y.; Tamirat, A.G.; Qi, H.; Han, J.; Li, W.; Wang, L.; Feng, S. Lithiophilic Co/Co4N nanoparticles embedded in hollow N-doped carbon nanocubes stabilizing lithium metal anodes for Li–air batteries. J. Mater. Chem. A 2018, 6, 22096–22105. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Zhou, Y.; Lu, J.; Zhou, K.; Yang, L.; Tang, Z.; Li, L.; Chen, S. N-doped carbon-wrapped cobalt nanoparticles on N-doped graphene nanosheets for high-efficiency hydrogen production. Chem. Mater. 2015, 27, 2026–2032. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, J.; Tang, Z.; Bi, D.; Zhao, J.; Liang, Y. Optimal configuration of N-doped carbon defects in 2D turbostratic carbon nanomesh for advanced oxygen reduction electrocatalysis. Angew. Chem. 2020, 132, 12097–12104. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Shen, H.; Song, J.; Gao, H.; Yang, X.; Yu, J.; Wu, Z.; Lei, W.; Hao, Q. Hollow Porous CoSnOx Nanocubes Encapsulated in One-Dimensional N-Doped Carbon Nanofibers as Anode Material for High-Performance Lithium Storage. ACS Appl. Mater. Interfaces 2020, 13, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.R.; Amal, H. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910. [Google Scholar] [CrossRef]
- Hong, W.; Jian, C.; Wang, G.; He, X.; Li, J.; Cai, Q.; Wen, Z.; Liu, W. Self-supported nanoporous cobalt phosphosulfate electrodes for efficient hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 251, 213–219. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Lian, Z.; Araujo, A.; Li, Y.; Wei, B.; Yu, Z.; Bondarchuk, O.; Amorim, I.; Tileli, V.; et al. Atomic-step enriched ruthenium–iridium nanocrystals anchored homogeneously on MOF-derived support for efficient and stable oxygenevolution in acidic and neutral media. ACS Catal. 2021, 11, 3402–3413. [Google Scholar] [CrossRef]
- Su, J.; Ge, R.; Jiang, K.; Dong, Y.; Hao, F.; Tian, Z.; Chen, G.; Chen, L. Assemblingultra small copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: Highly robust electrocatalysts for oxygen evolution in acidic media. Advanced. Mater. 2018, 30, 1801351. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-rich RuCu nanosheets for pH-universal overall water splitting electrocatalysis. Angew. Chem. Int. Ed. Engl. 2019, 58, 13983–13988. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Zeng, Z.; Zou, Y.; Wang, W.; Zhang, J.; Wang, J.; Kong, X.; Meng, X. Core–Shell CoS2/FeS2 Heterojunction Encapsulated in N-Doped Carbon Nanocubes Derived from Coordination Polymers for Electrocatalytic Alkaline Water/Seawater Splitting. Polymers 2025, 17, 1701. https://doi.org/10.3390/polym17121701
Zhang X, Liu Y, Zeng Z, Zou Y, Wang W, Zhang J, Wang J, Kong X, Meng X. Core–Shell CoS2/FeS2 Heterojunction Encapsulated in N-Doped Carbon Nanocubes Derived from Coordination Polymers for Electrocatalytic Alkaline Water/Seawater Splitting. Polymers. 2025; 17(12):1701. https://doi.org/10.3390/polym17121701
Chicago/Turabian StyleZhang, Xiaoyin, Yan Liu, Zihan Zeng, Yan Zou, Wanzhen Wang, Jing Zhang, Jing Wang, Xiangfeng Kong, and Xiangmin Meng. 2025. "Core–Shell CoS2/FeS2 Heterojunction Encapsulated in N-Doped Carbon Nanocubes Derived from Coordination Polymers for Electrocatalytic Alkaline Water/Seawater Splitting" Polymers 17, no. 12: 1701. https://doi.org/10.3390/polym17121701
APA StyleZhang, X., Liu, Y., Zeng, Z., Zou, Y., Wang, W., Zhang, J., Wang, J., Kong, X., & Meng, X. (2025). Core–Shell CoS2/FeS2 Heterojunction Encapsulated in N-Doped Carbon Nanocubes Derived from Coordination Polymers for Electrocatalytic Alkaline Water/Seawater Splitting. Polymers, 17(12), 1701. https://doi.org/10.3390/polym17121701





