The Characterization of Polymers That Mimic the Aortic Wall’s Mechanical Properties and Their Suitability for Use in the 3D Printing of Aortic Phantoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymers
2.2. Control Tissues
2.3. Generation of Test Pieces
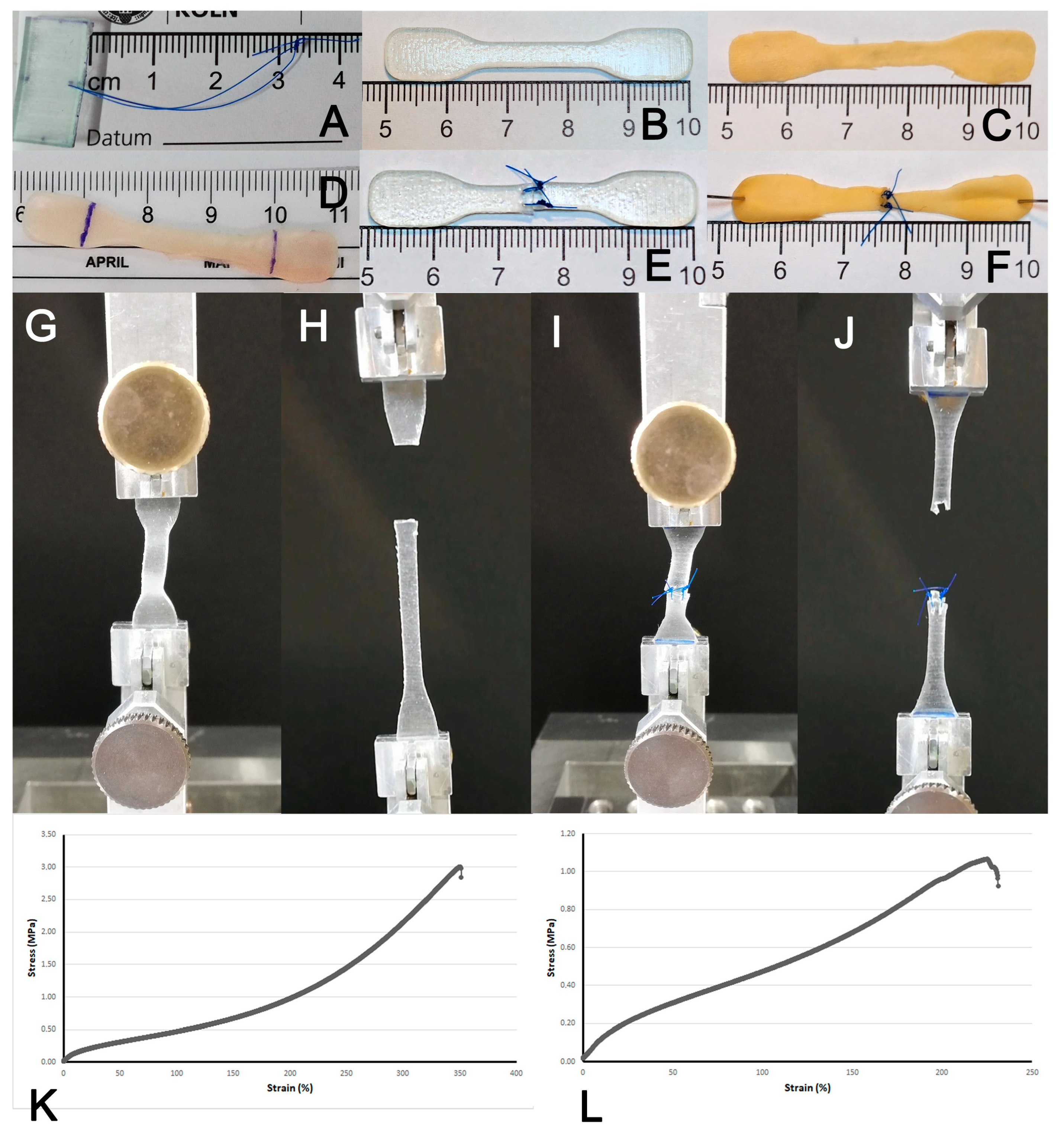
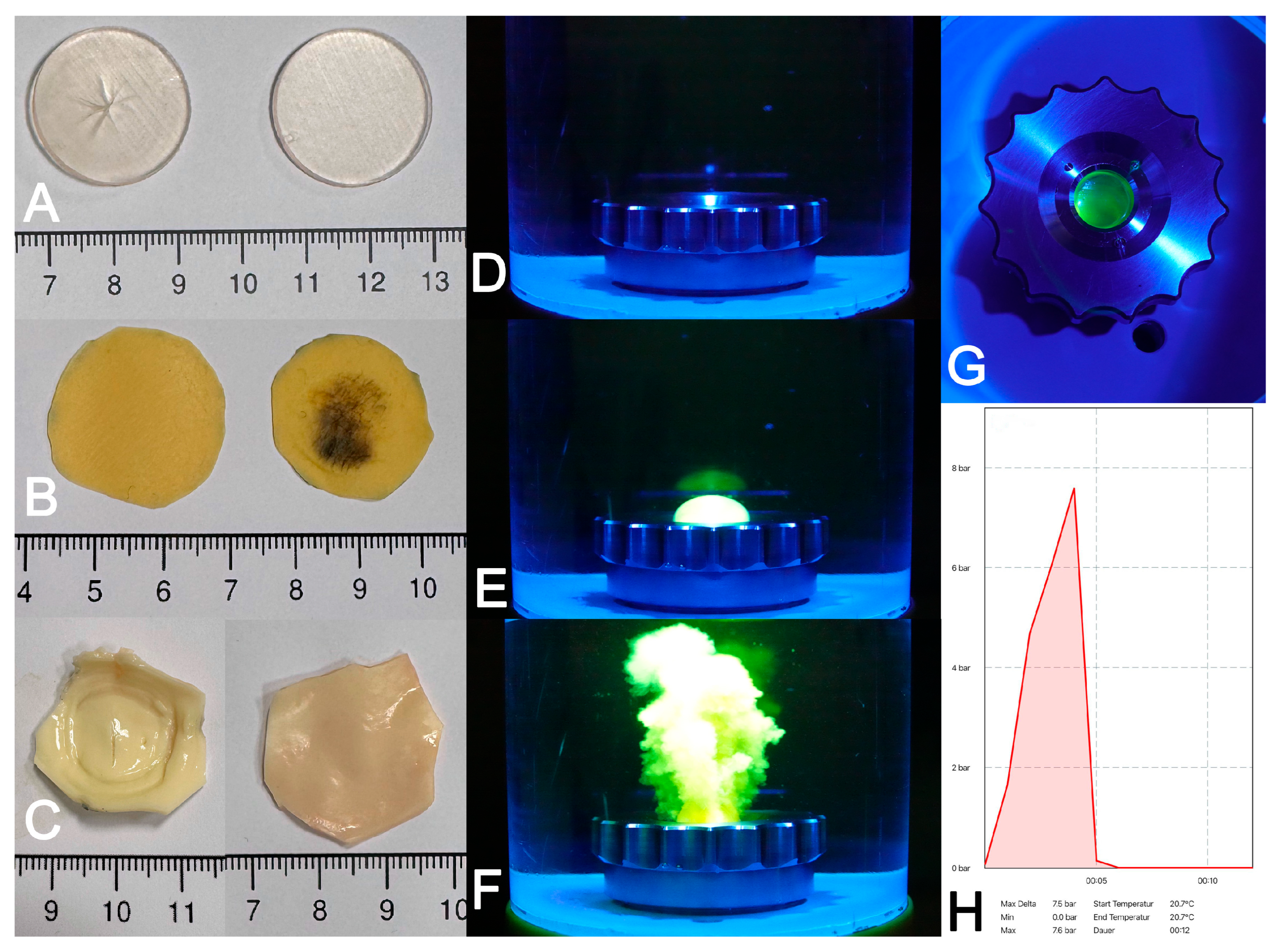
2.4. Suture Retention Strength, Uni-Axial Stretch and Burst Pressure Testing
2.5. Statistical Analysis
3. Results
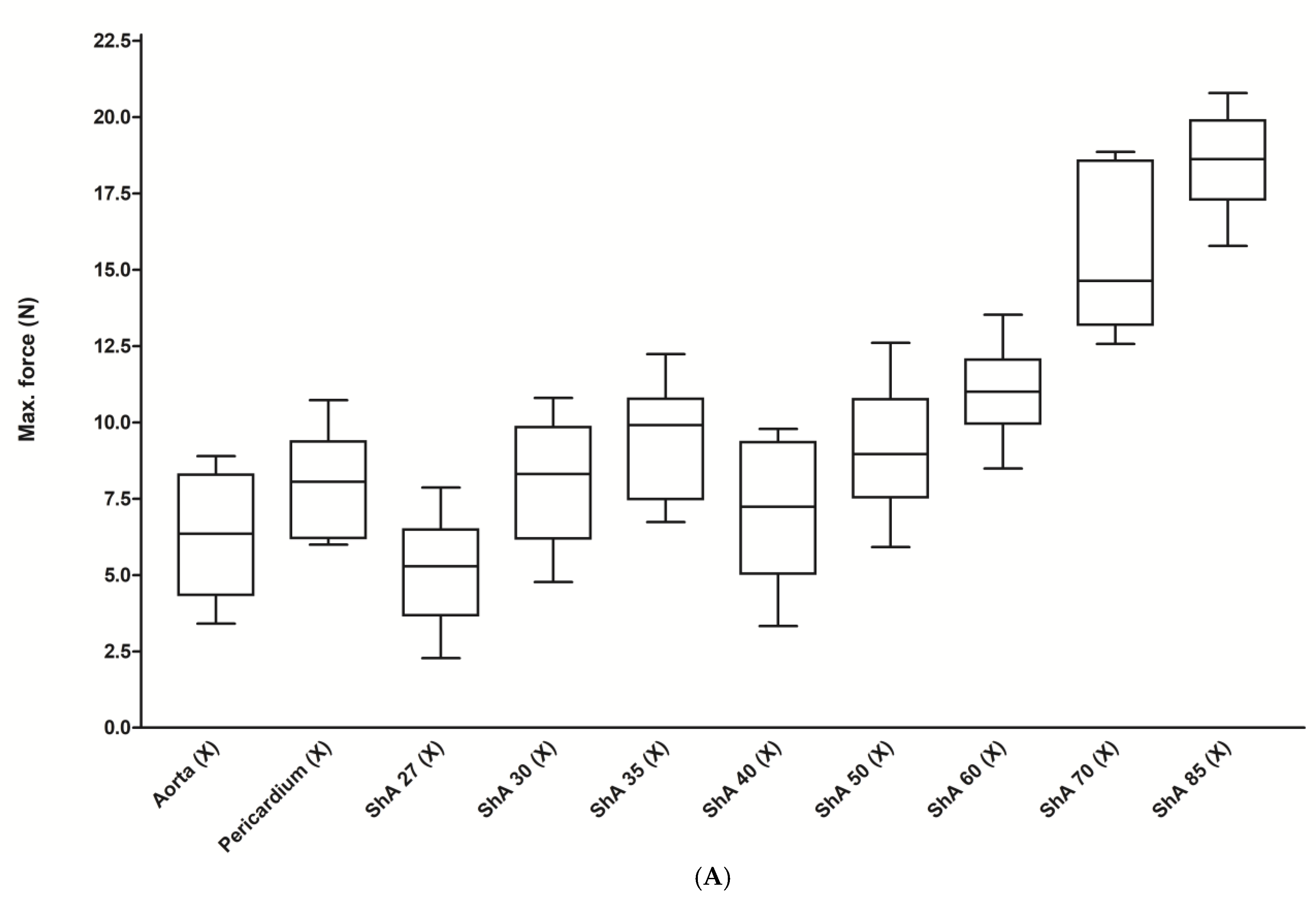
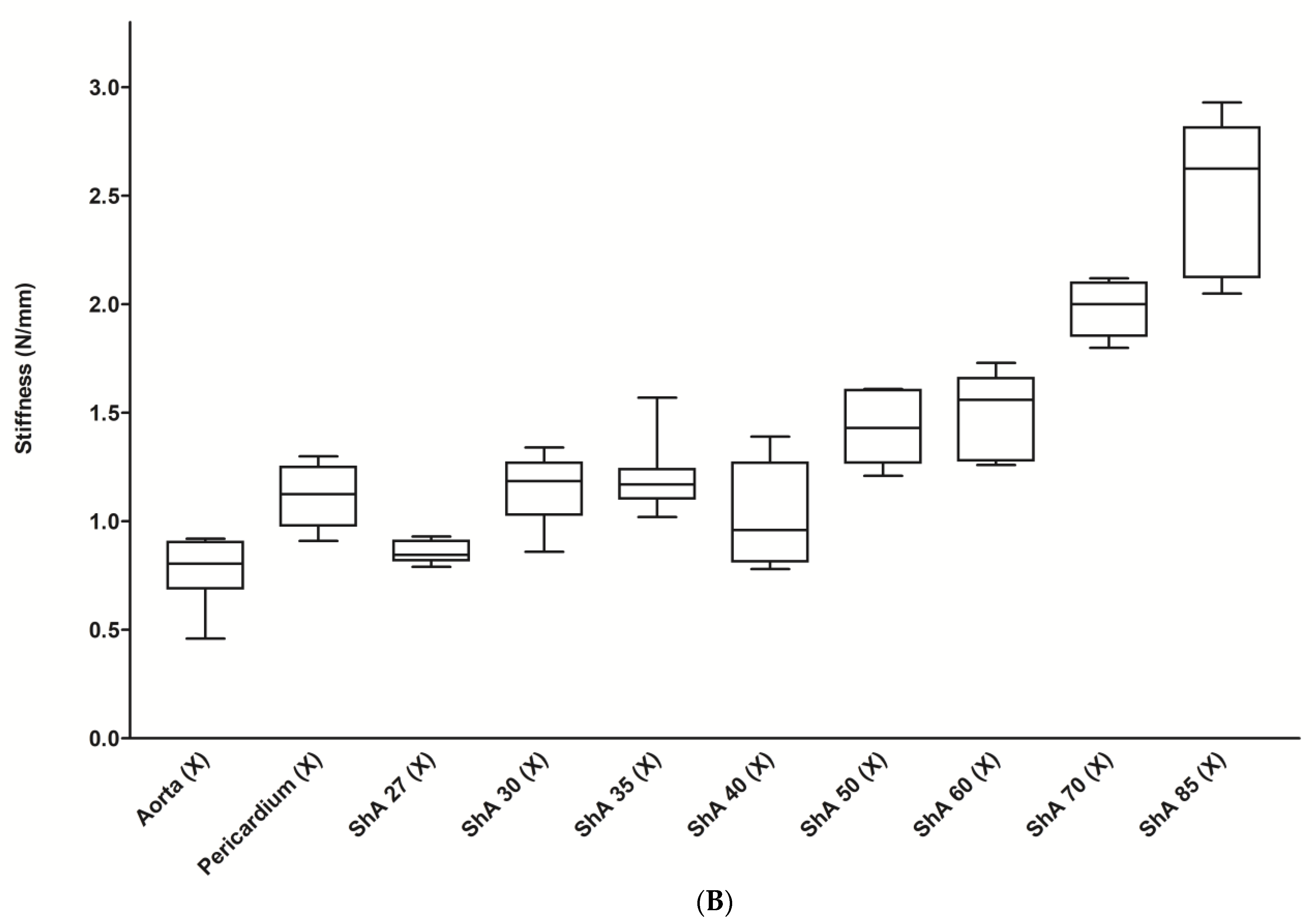
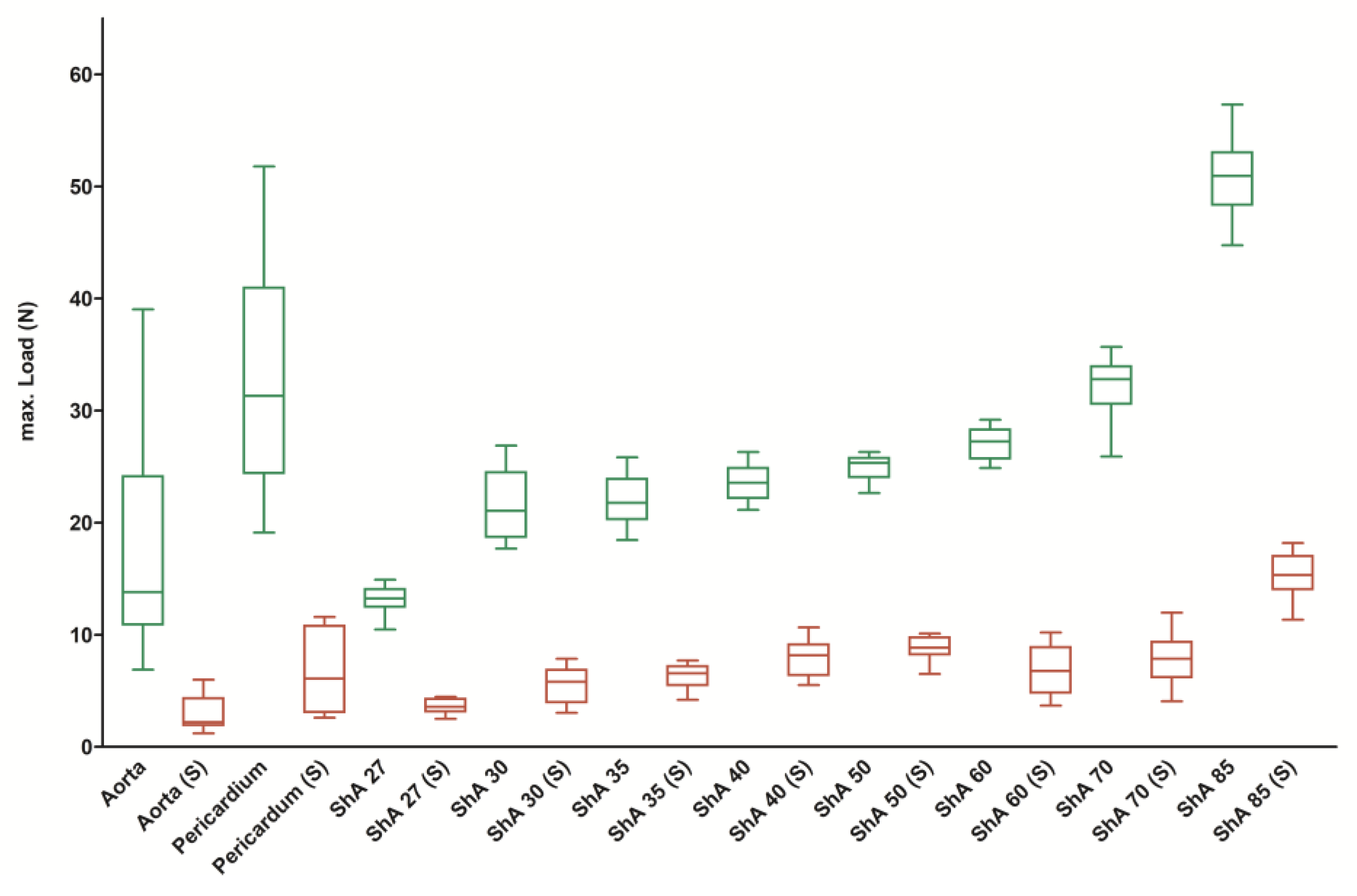
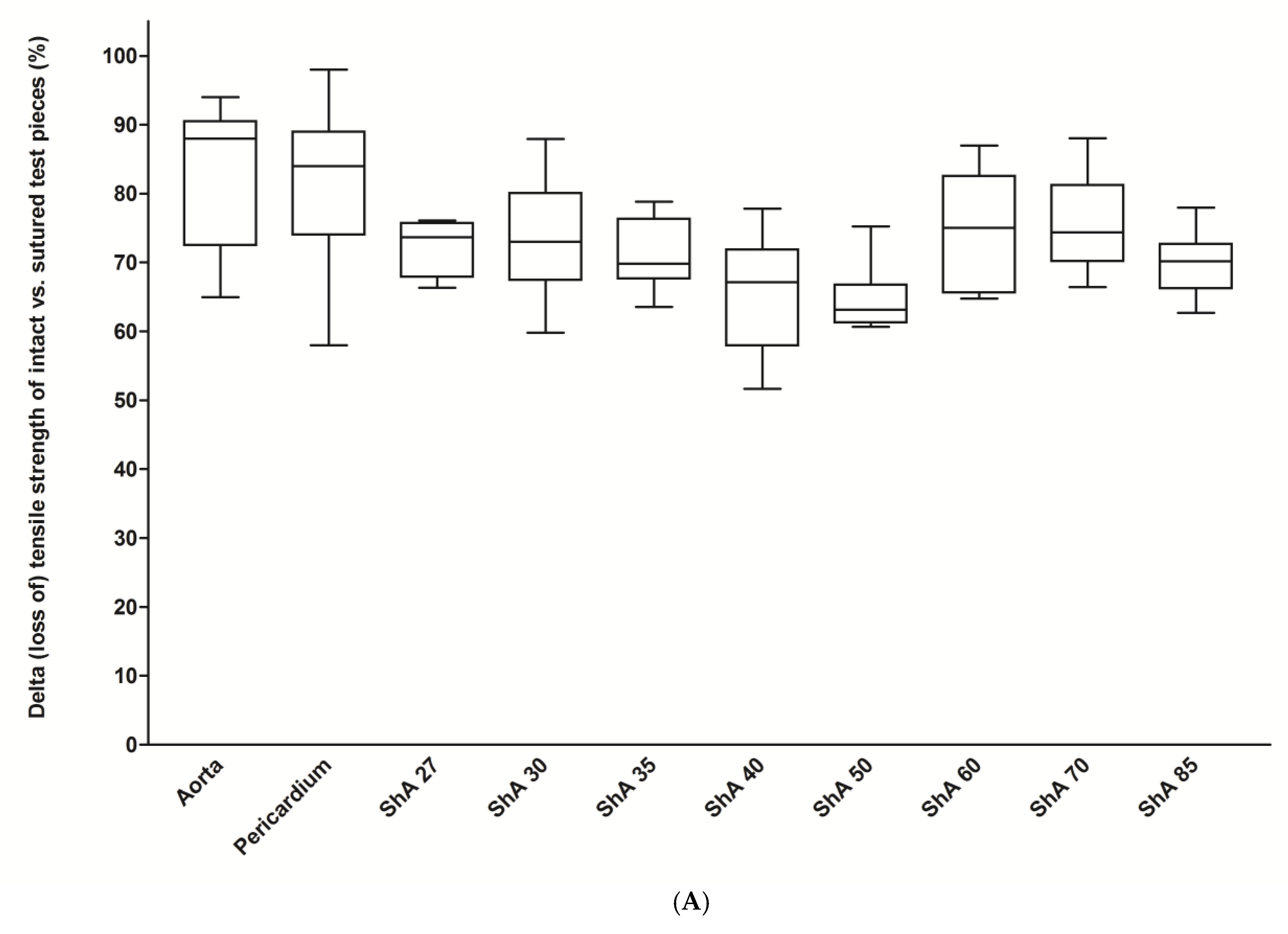
3.1. Assessment of Suture Retention Strength
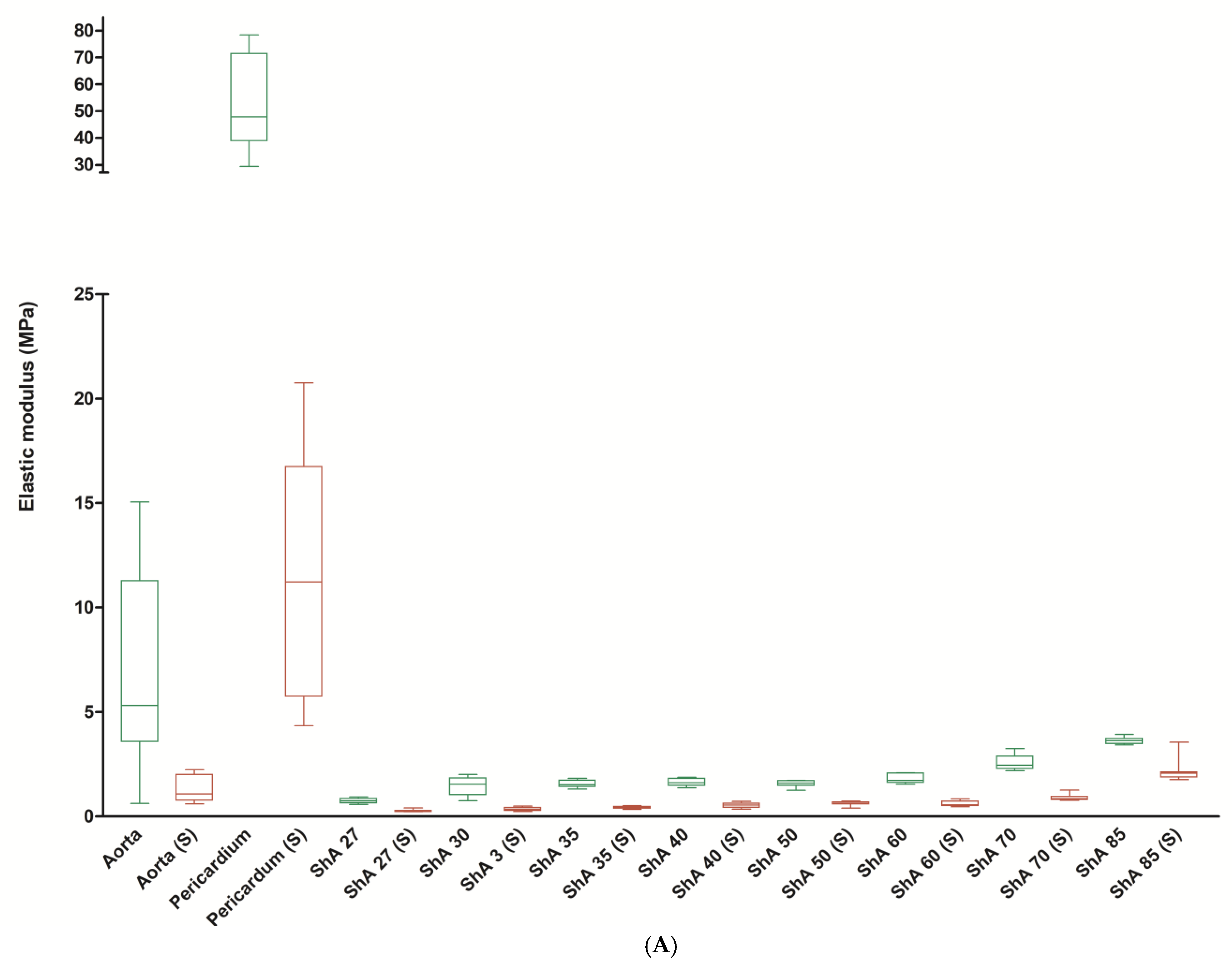
3.2. Stress–Strain Analysis
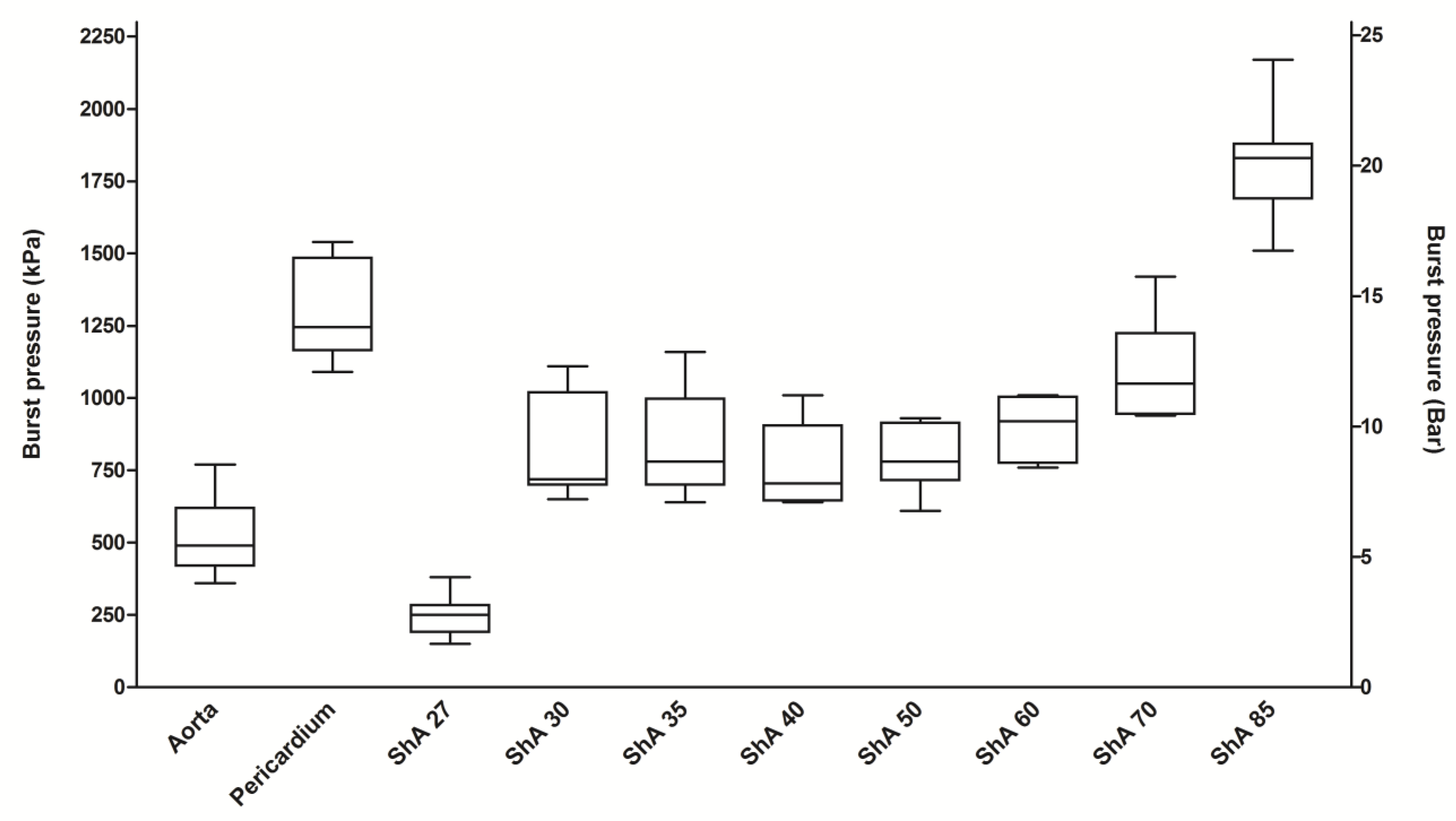
3.3. Burst Pressure Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Chernikova, O.; Heitzmann, N.; Stadler, M.; Holzberger, D.; Seidel, T.; Fischer, F. Simulation-Based Learning in Higher Education: A Meta-Analysis. Rev. Educ. Res. 2020, 90, 499–541. [Google Scholar] [CrossRef]
- Wegner, M.; Dusse, F.; Beeser, F.; Leister, N.; Lefarth, M.; Finke, S.R.; Bottiger, B.W.; Dorweiler, B.; Stoll, S.E. Comparing Simulation Training of Bronchoscopy-Guided Percutaneous Dilatational Tracheostomy Using Conventional Versus 3D Printed Simulators (TRAC-Sim Study). J. Intensive Care Med. 2024, 39, 820–828. [Google Scholar] [CrossRef]
- Wippel, D.; Ouaret, M.; Gizewski, E.R.; Kluckner, M.; Enzmann, F.K.; Wipper, S. Simulation Based Training for Iliac Endovascular Interventions: 3D Printed vs. Digital Models. Eur. J. Vasc. Endovasc. Surg. 2025, in press. [CrossRef]
- Traynor, G.; Shearn, A.I.; Milano, E.G.; Ordonez, M.V.; Velasco Forte, M.N.; Caputo, M.; Schievano, S.; Mustard, H.; Wray, J.; Biglino, G. The use of 3D-printed models in patient communication: A scoping review. J. 3D Print. Med. 2022, 6, 13–23. [Google Scholar] [CrossRef]
- Oliny, A.; Fabre, D.; Fontaine, V.; Decante, B.; Haulon, S. Pre-clinical Evaluation of New Generation Bridging Stent for Fenestrated Endovascular Aneurysm Repair using Realistic 3D Printed Aneurysm Models. Eur. J. Vasc. Endovasc. Surg. 2025, in press. [CrossRef]
- Sparks, A.J.; Smith, C.M.; Allman, A.B.; Senko, J.L.; Meess, K.M.; Ducharme, R.W.; Springer, M.E.; Waqas, M.; Siddiqui, A.H. Compliant vascular models 3D printed with the Stratasys J750: A direct characterization of model distensibility using intravascular ultrasound. 3D Print. Med. 2021, 7, 28. [Google Scholar] [CrossRef]
- Heller, M.; Bauer, H.-K.; Goetze, E.; Gielisch, M.; Roth, K.E.; Drees, P.; Maier, G.S.; Dorweiler, B.; Ghazy, A.; Neufurth, M.; et al. Applications of patient-specific 3D printing in medicine. Int. J. Comput. Dent. 2016, 19, 323–339. [Google Scholar]
- Dorweiler, B.; El Beyrouti, H.; Vahl, C.F.; Baqué, P.E.; Ghazy, A. The Future of Vascular Medicine—Role of 3D Printing. Zentralbl Chir. 2020, 145, 448–455. [Google Scholar] [CrossRef]
- Wu, C.; Do, T.T.; Tran, P. Mechanical Properties of PolyJet 3D-Printed Composites Inspired by Space-Filling Peano Curves. Polymers 2021, 13, 3516. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Yang, Z.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printing materials and their use in medical education: A review of current technology and trends for the future. BMJ Simul. Technol. Enhanc. Learn. 2018, 4, 27–40. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Taormina, G.; Sciancalepore, C.; Messori, M.; Bondioli, F. 3D printing processes for photocurable polymeric materials: Technologies, materials, and future trends. J. Appl. Biomater. Funct. Mater. 2018, 16, 151–160. [Google Scholar] [CrossRef]
- Zimmermann, J.; Loecher, M.; Kolawole, F.O.; Bäumler, K.; Gifford, K.; Dual, S.A.; Levenston, M.; Marsden, A.L.; Ennis, D.B. On the impact of vessel wall stiffness on quantitative flow dynamics in a synthetic model of the thoracic aorta. Sci. Rep. 2021, 11, 6703. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Illi, J.; Ilic, M.; Stark, A.W.; Amstutz, C.; Burger, J.; Zysset, P.; Haeberlin, A.; Gräni, C. Mechanical testing and comparison of porcine tissue, silicones and 3D-printed materials for cardiovascular phantoms. Front. Bioeng. Biotechnol. 2023, 11, 1274673. [Google Scholar] [CrossRef] [PubMed]
- Reeps, C.; Maier, A.; Pelisek, J.; Härtl, F.; Grabher-Meier, V.; Wall, W.A.; Essler, M.; Eckstein, H.H.; Gee, M.W. Measuring and modeling patient-specific distributions of material properties in abdominal aortic aneurysm wall. Biomech. Model. Mechanobiol. 2013, 12, 717–733. [Google Scholar] [CrossRef] [PubMed]
- De Beaufort, H.W.L.; Ferrara, A.; Conti, M.; Moll, F.L.; Van Herwaarden, J.A.; Figueroa, C.A.; Bismuth, J.; Auricchio, F.; Trimarchi, S. Comparative Analysis of Porcine and Human Thoracic Aortic Stiffness. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 560–566. [Google Scholar] [CrossRef]
- Lelovas, P.P.; Kostomitsopoulos, N.G.; Xanthos, T.T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 432–438. [Google Scholar]
- Riedle, H.; Chaban, R.; Ghazy, A.; Piplat, C.; Dorweiler, B.; Franke, J. Experimental determination of the suture behavior of aortic tissue in comparison to 3D printed silicone modelling material. J. Mech. Behav. Biomed. Mater. 2020, 112, 104033. [Google Scholar] [CrossRef] [PubMed]
- Type 3/ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- Chen, K.; Scales, M.; Kyriakides, S.; Corona, E. Effects of anisotropy on material hardening and burst in the bulge test. Int. J. Solids Struct. 2016, 82, 70–84. [Google Scholar] [CrossRef]
- Bohl, M.A.; McBryan, S.; Spear, C.; Pais, D.; Preul, M.C.; Wilhelmi, B.; Yeskel, A.; Turner, J.D.; Kakarla, U.K.; Nakaji, P. Evaluation of a Novel Surgical Skills Training Course: Are Cadavers Still the Gold Standard for Surgical Skills Training? World Neurosurg. 2019, 127, 63–71. [Google Scholar] [CrossRef]
- Alakhtar, A.; Emmott, A.; Hart, C.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printed ascending aortic simulators with physiological fidelity for surgical simulation. BMJ Simul. Technol. Enhanc. Learn. 2021, 7, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Barabas, I.J.; Vegh, D.; Bottlik, O.; Kreuter, P.; Hartyanszky, I.; Merkely, B.; Palkovics, D. The role of 3D technology in the practical education of congenital coarctation and its treatment-a feasibility pilot study. BMC Med. Educ. 2024, 24, 357. [Google Scholar] [CrossRef]
- Vukicevic, M.; Puperi, D.S.; Jane Grande-Allen, K.; Little, S.H. 3D Printed Modeling of the Mitral Valve for Catheter-Based Structural Interventions. Ann. Biomed. Eng. 2017, 45, 508–519. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y. Application of 3D Printing in Implantable Medical Devices. Biomed. Res. Int. 2021, 2021, 6653967. [Google Scholar] [CrossRef]
- Perini, P.; Foresti, R.; Martini, C.; Massoni, C.B.; Catasta, A.; Mersanne, A.; Freyrie, A. Use of 3D Printed Models for Endovascular Planning and Training in PAD Patients Undergoing Endovascular Treatment. Eur. J. Vasc. Endovasc. Surg. 2024, 67, e41–e42. [Google Scholar] [CrossRef]
- Catasta, A.; Martini, C.; Mersanne, A.; Foresti, R.; Bianchini Massoni, C.; Freyrie, A.; Perini, P. Systematic Review on the Use of 3D-Printed Models for Planning, Training and Simulation in Vascular Surgery. Diagnostics 2024, 14, 1658. [Google Scholar] [CrossRef]
- Grimaldo Ruiz, O.; Rodriguez Reinoso, M.; Ingrassia, E.; Vecchio, F.; Maniero, F.; Burgio, V.; Civera, M.; Bitan, I.; Lacidogna, G.; Surace, C. Design and Mechanical Characterization Using Digital Image Correlation of Soft Tissue-Mimicking Polymers. Polymers 2022, 14, 2639. [Google Scholar] [CrossRef]
- Mohl, L.; Karl, R.; Hagedorn, M.N.; Runz, A.; Skornitzke, S.; Toelle, M.; Bergt, C.S.; Hatzl, J.; Uhl, C.; Böckler, D.; et al. Simulation of thoracic endovascular aortic repair in a perfused patient-specific model of type B aortic dissection. Int. J. Comput. Assist. Radiol. Surg. 2025, 20, 391–404. [Google Scholar] [CrossRef]
- Schneider, K.H.; Oberoi, G.; Unger, E.; Janjic, K.; Rohringer, S.; Heber, S.; Agis, H.; Schedle, A.; Kiss, H.; Podesser, B.K.; et al. Medical 3D printing with polyjet technology: Effect of material type and printing orientation on printability, surface structure and cytotoxicity. 3D Print. Med. 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.; Tierney, P.; Hynes, N.; Sultan, S. An in vitro Assessment of the Haemodynamic Features Occurring Within the True and False Lumens Separated by a Dissection Flap for a Patient-Specific Type B Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 797829. [Google Scholar] [CrossRef]
- Illi, J.; Bernhard, B.; Nguyen, C.; Pilgrim, T.; Praz, F.; Gloeckler, M.; Windecker, S.; Haeberlin, A.; Gräni, C. Translating Imaging Into 3D Printed Cardiovascular Phantoms: A Systematic Review of Applications, Technologies, and Validation. JACC Basic. Transl. Sci. 2022, 7, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Ock, J.; Kim, N. Mimicking the Mechanical Properties of Aortic Tissue with Pattern-Embedded 3D Printing for a Realistic Phantom. Materials 2020, 13, 5042. [Google Scholar] [CrossRef]
- Lee, V.; Severseike, L.; Bakken, C.; Bermel, E.; Bhatia, V. PolyJet 3D Printing of Tissue Mimicking Materials: An Investigation of Characteristic Properties of 3D Printed Synthetic Tissue. bioRxiv, 2020; ahead of print. [Google Scholar] [CrossRef]
- Sakai, A.K.F.; Cestari, I.N.; de Sales, E.; Mazzetto, M.; Cestari, I.A. Metamaterial design for aortic aneurysm simulation using 3D printing. 3D Print. Med. 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, A.J.; Shahmirzadi, D.; Li, R.X.; Doyle, B.J.; Konofagou, E.E.; McGloughlin, T.M. 3D-Printed Tissue-Mimicking Phantoms for Medical Imaging and Computational Validation Applications. 3D Print. Addit. Manuf. 2014, 1, 14–23. [Google Scholar] [CrossRef]
- Kornfellner, E.; Königshofer, M.; Krainz, L.; Krause, A.; Unger, E.; Moscato, F. Measured and simulated mechanical properties of additively manufactured matrix-inclusion multimaterials fabricated by material jetting. 3D Print. Med. 2024, 10, 4. [Google Scholar] [CrossRef]
- Zhalmuratova, D.; La, T.G.; Yu, K.T.; Szojka, A.R.A.; Andrews, S.H.J.; Adesida, A.B.; Kim, C.I.; Nobes, D.S.; Freed, D.H.; Chung, H.J. Mimicking “J-Shaped” and Anisotropic Stress-Strain Behavior of Human and Porcine Aorta by Fabric-Reinforced Elastomer Composites. ACS Appl. Mater. Interfaces 2019, 11, 33323–33335. [Google Scholar] [CrossRef]
- Severseike, L.; Lee, V.; Brandon, T.; Bakken, C.; Bhatia, V. Polyjet 3D printing of tissue-mimicking materials: How well can 3D printed synthetic myocardium replicate mechanical properties of organic myocardium? bioRxiv, 2019; ahead of print. [Google Scholar] [CrossRef]
- Dorweiler, B.; Baqué, P.E.; Chaban, R.; Ghazy, A.; Salem, O. Quality Control in 3D Printing: Accuracy Analysis of 3D-Printed Models of Patient-Specific Anatomy. Materials 2021, 14, 1021. [Google Scholar] [CrossRef]
- Wu, C.A.; Squelch, A.; Sun, Z. Investigation of Three-dimensional Printing Materials for Printing Aorta Model Replicating Type B Aortic Dissection. Curr. Med. Imaging 2021, 17, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, J.; Hatoum, H.; Flemister, D.C.; Krull, C.M.; Walter, B.A.; Zhang, W.; Mery, C.M.; Molossi, S.; Jadhav, S.; Dasi, L.P.; et al. Assessment of transfer of morphological characteristics of Anomalous Aortic Origin of a Coronary Artery from imaging to patient specific 3D Printed models: A feasibility study. Comput. Methods Programs Biomed. 2021, 201, 105947. [Google Scholar] [CrossRef] [PubMed]
- Asciak, L.; Domingo-Roca, R.; Dow, J.R.; Brodie, R.; Paterson, N.; Riches, P.E.; Shu, W.; McCormick, C. Exploiting light-based 3D-printing for the fabrication of mechanically enhanced, patient-specific aortic grafts. J. Mech. Behav. Biomed. Mater. 2024, 154, 106531. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, E.; Bosques-Palomo, B.; Carmona-Arriaga, F.; Fabiani, M.A.; Aguirre-Soto, A. Fabrication of Soft Transparent Patient-Specific Vascular Models with Stereolithographic 3D printing and Thiol-Based Photopolymerizable Coatings. Macromol. Rapid Commun. 2024, 45, e2300611. [Google Scholar] [CrossRef]
- Martin, C.; Pham, T.; Sun, W. Significant differences in the material properties between aged human and porcine aortic tissues. Eur. J. Cardiothorac. Surg. 2011, 40, 28–34. [Google Scholar] [CrossRef]
- Weiss, S.; Hugas Mallorqui, M.; Czerny, M.; Walter, T.; Biro, G.; Puttini, I.; Almasi-Sperling, V.; Lang, W.; Schmidli, J.; Wyss, T.R. Physician Made Bovine Pericardial Tube Grafts in Aortic Infection: A European Multicentre Study. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 997–1005. [Google Scholar] [CrossRef]
- Noronen, K.; Söderström, M.; Kouhia, S.; Venermo, M. Bovine pericardial patch: A good alternative in femoral angioplasty. J. Vasc. Surg. 2023, 77, 225–230. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, Z.; Guo, X.; Gu, H.; Tang, D.; Wang, L. Comparison of Biomechanical and Microstructural Properties of Aortic Graft Materials in Aortic Repair Surgeries. J. Funct. Biomater. 2024, 15, 248. [Google Scholar] [CrossRef]
- Recco, D.P.; Kizilski, S.B.; Marshall, L.E.; Earley, P.D.; Kneier, N.E.; Del Nido, P.J.; Hammer, P.E.; Hoganson, D.M. Mechanical failure analysis of patch materials used in aortic arch reconstruction: Implications for clinical practice. Eur. J. Cardiothorac. Surg. 2023, 64, ezad366. [Google Scholar] [CrossRef]
- Lin, S.; Tarris, G.; Bernard, C.; Kafi, M.; Walker, P.M.; Marín-Castrillón, D.M.; Gobled, C.; Boucher, A.; Presles, B.; Morgant, M.C.; et al. Biomechanical properties of 3D printable material usable for synthetic personalized healthy human aorta. Int. J. Bioprint 2023, 9, 736. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegner, M.; Karagoez, B.S.; Wippel, D.; Enzmann, F.K.; Niehoff, A.; Salem, O.; Dorweiler, B. The Characterization of Polymers That Mimic the Aortic Wall’s Mechanical Properties and Their Suitability for Use in the 3D Printing of Aortic Phantoms. Polymers 2025, 17, 1700. https://doi.org/10.3390/polym17121700
Wegner M, Karagoez BS, Wippel D, Enzmann FK, Niehoff A, Salem O, Dorweiler B. The Characterization of Polymers That Mimic the Aortic Wall’s Mechanical Properties and Their Suitability for Use in the 3D Printing of Aortic Phantoms. Polymers. 2025; 17(12):1700. https://doi.org/10.3390/polym17121700
Chicago/Turabian StyleWegner, Moritz, Benan Sahin Karagoez, David Wippel, Florian K. Enzmann, Anja Niehoff, Oroa Salem, and Bernhard Dorweiler. 2025. "The Characterization of Polymers That Mimic the Aortic Wall’s Mechanical Properties and Their Suitability for Use in the 3D Printing of Aortic Phantoms" Polymers 17, no. 12: 1700. https://doi.org/10.3390/polym17121700
APA StyleWegner, M., Karagoez, B. S., Wippel, D., Enzmann, F. K., Niehoff, A., Salem, O., & Dorweiler, B. (2025). The Characterization of Polymers That Mimic the Aortic Wall’s Mechanical Properties and Their Suitability for Use in the 3D Printing of Aortic Phantoms. Polymers, 17(12), 1700. https://doi.org/10.3390/polym17121700






