Tuning Polymer–Metal Interfaces via Solvent-Engineered Electroless Nickel Coatings on Functional Fibres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Nylon-6,6 Yarns
2.3. Immobilisation of Catalysts
2.4. Metallisation by DMSO-Modified ELD
2.5. Characterisation
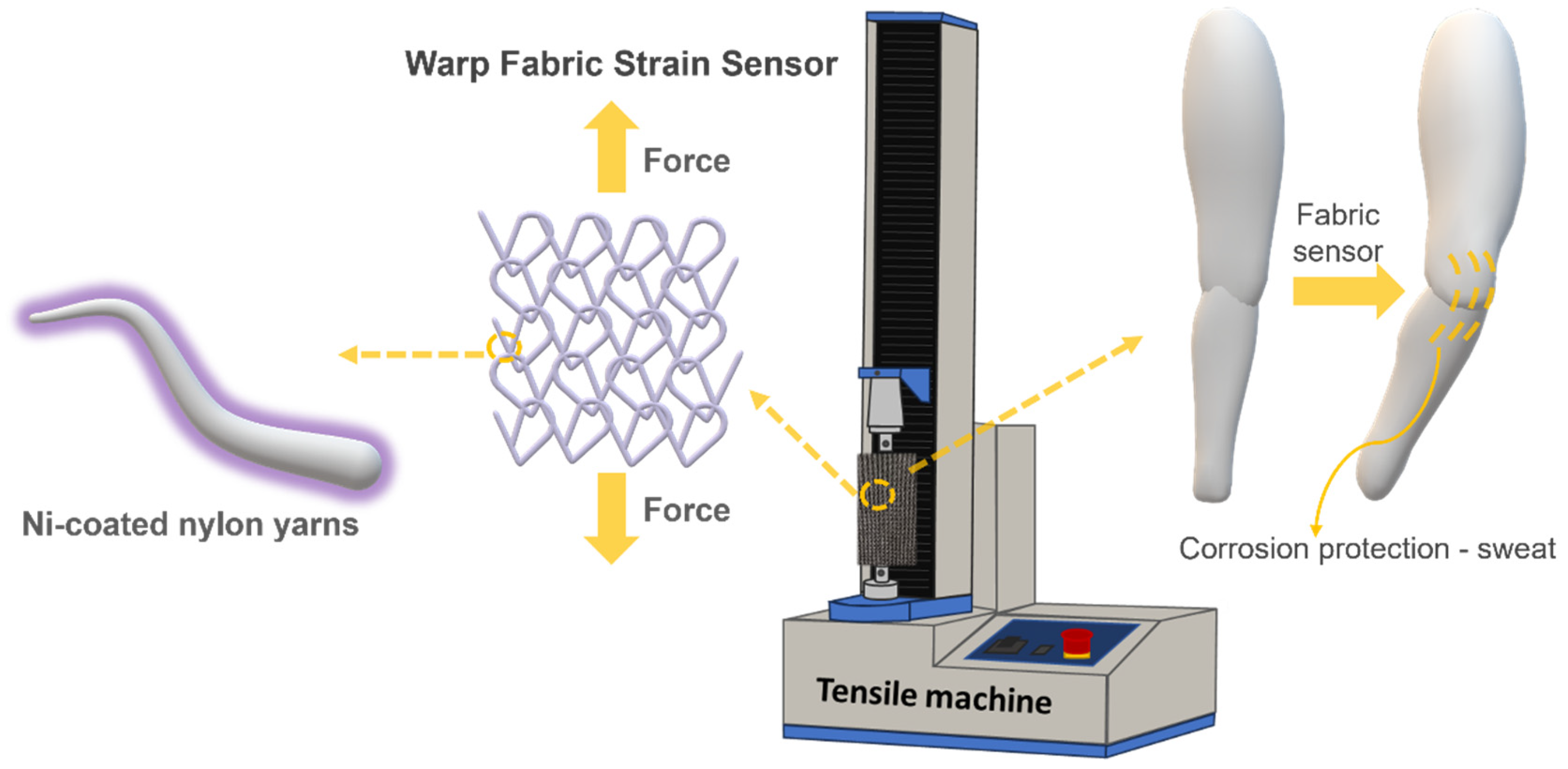
2.6. Electromechanical and Thermal Tests
2.7. Contact Angle Test
2.8. Corrosion Test
2.9. DMA Creep Behaviour Test
3. Results
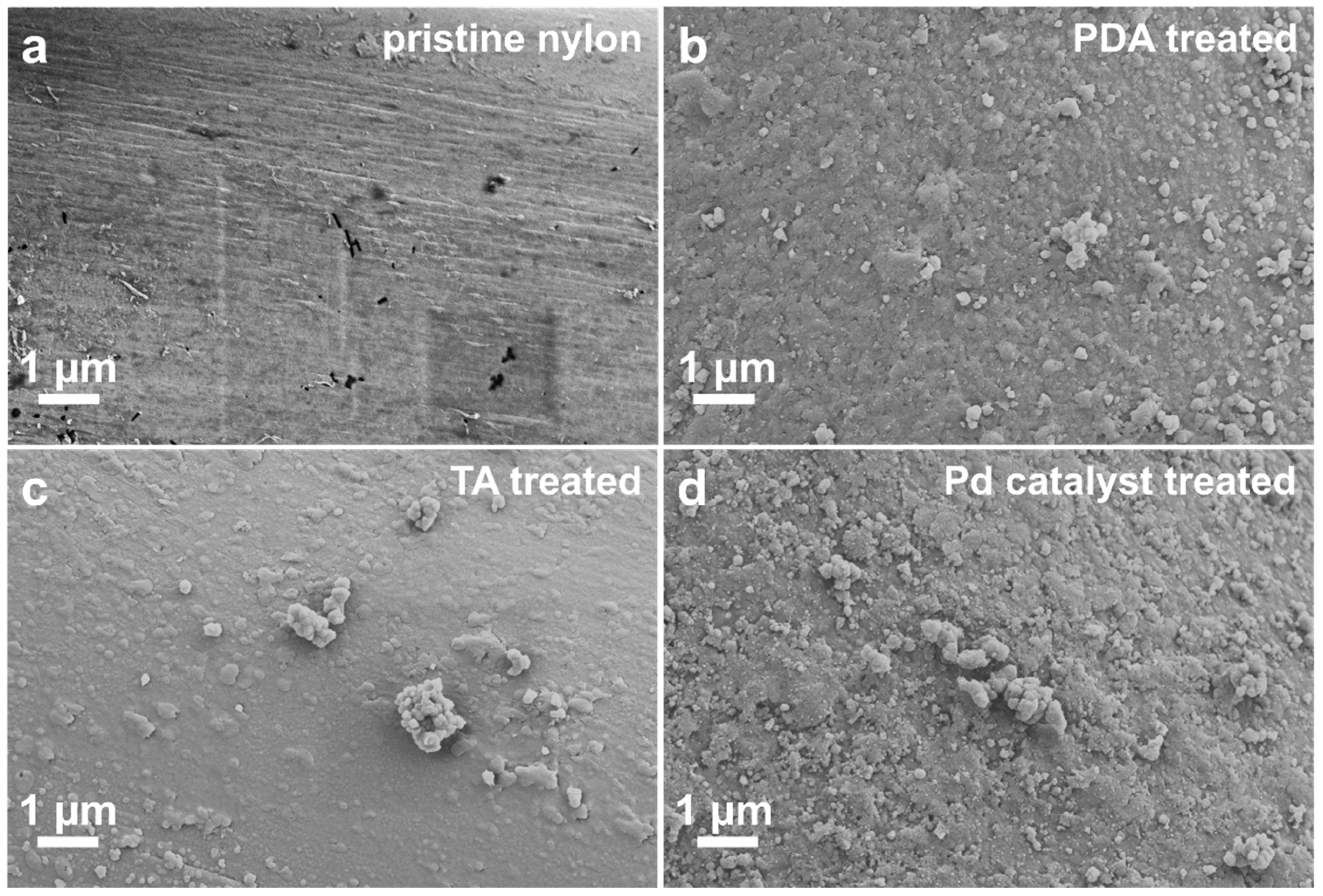
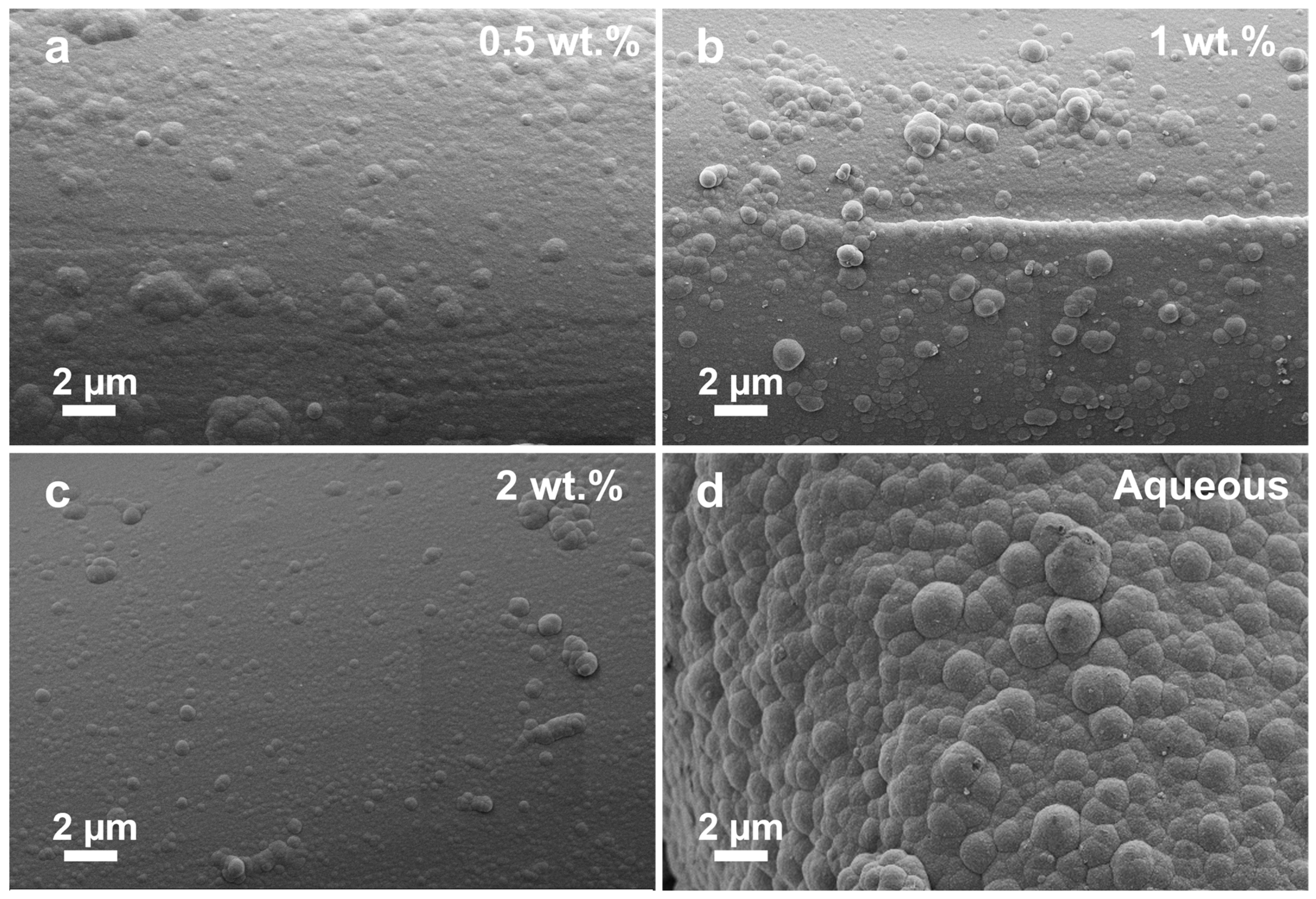
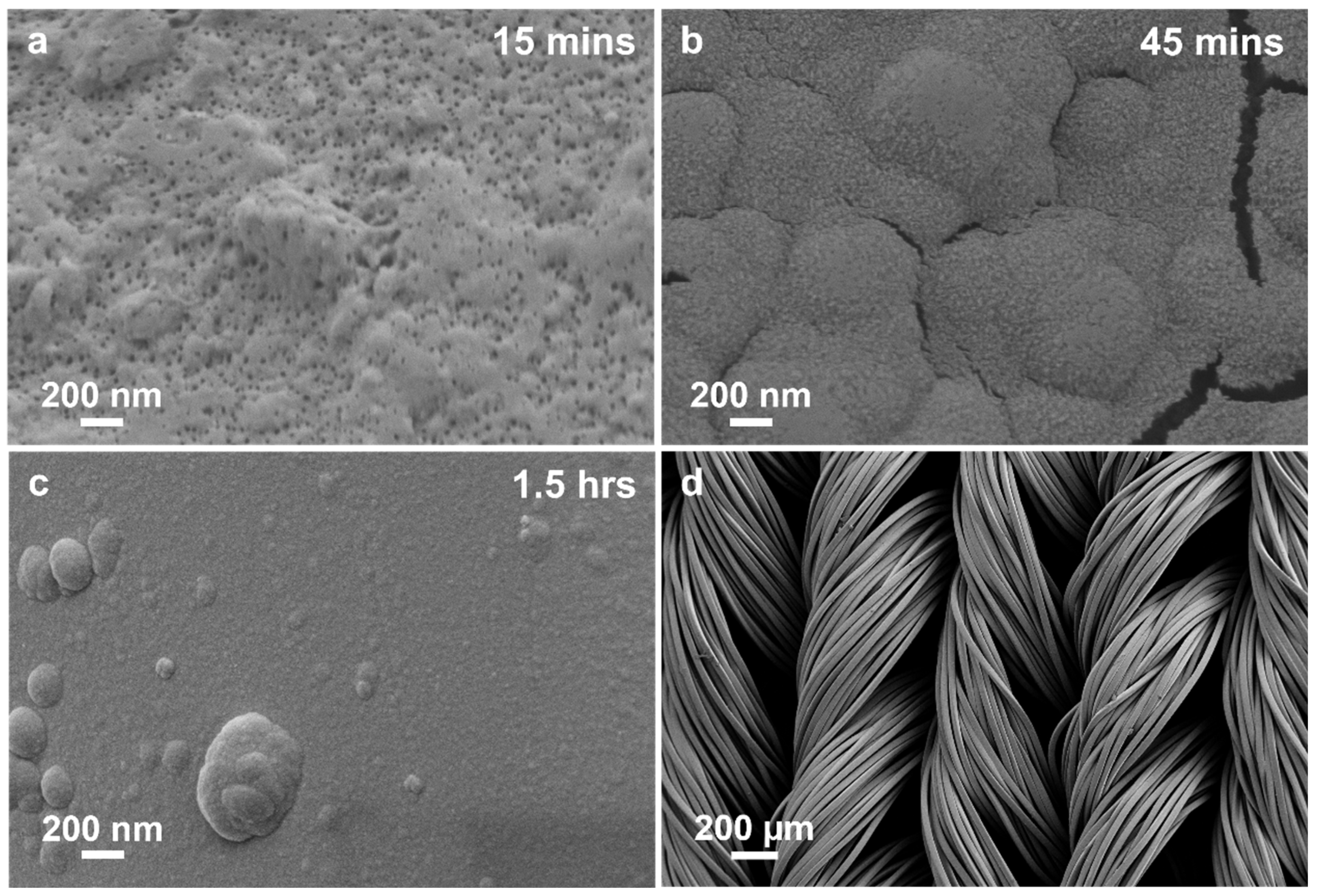
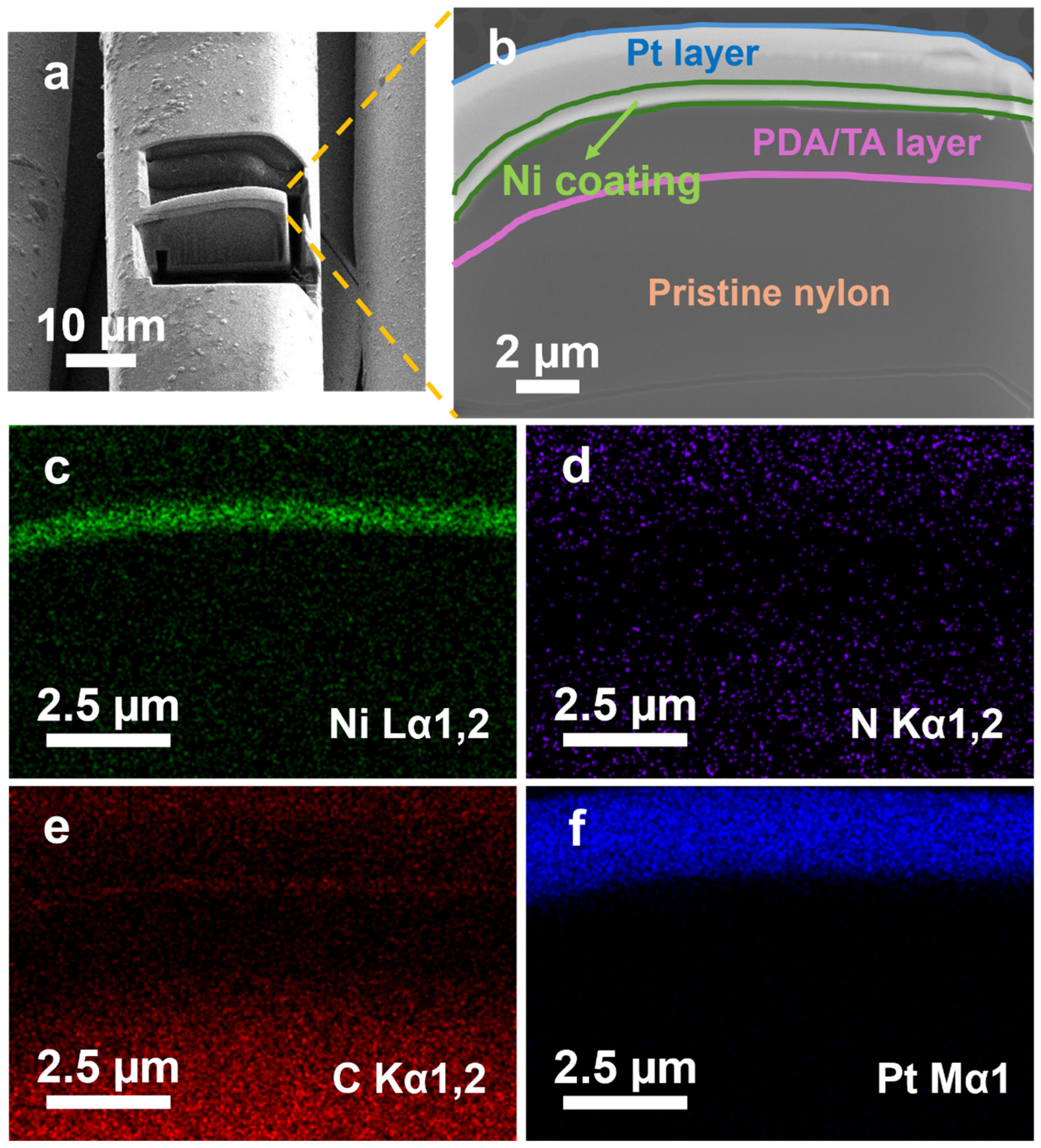
3.1. Morphological and Elemental Analysis
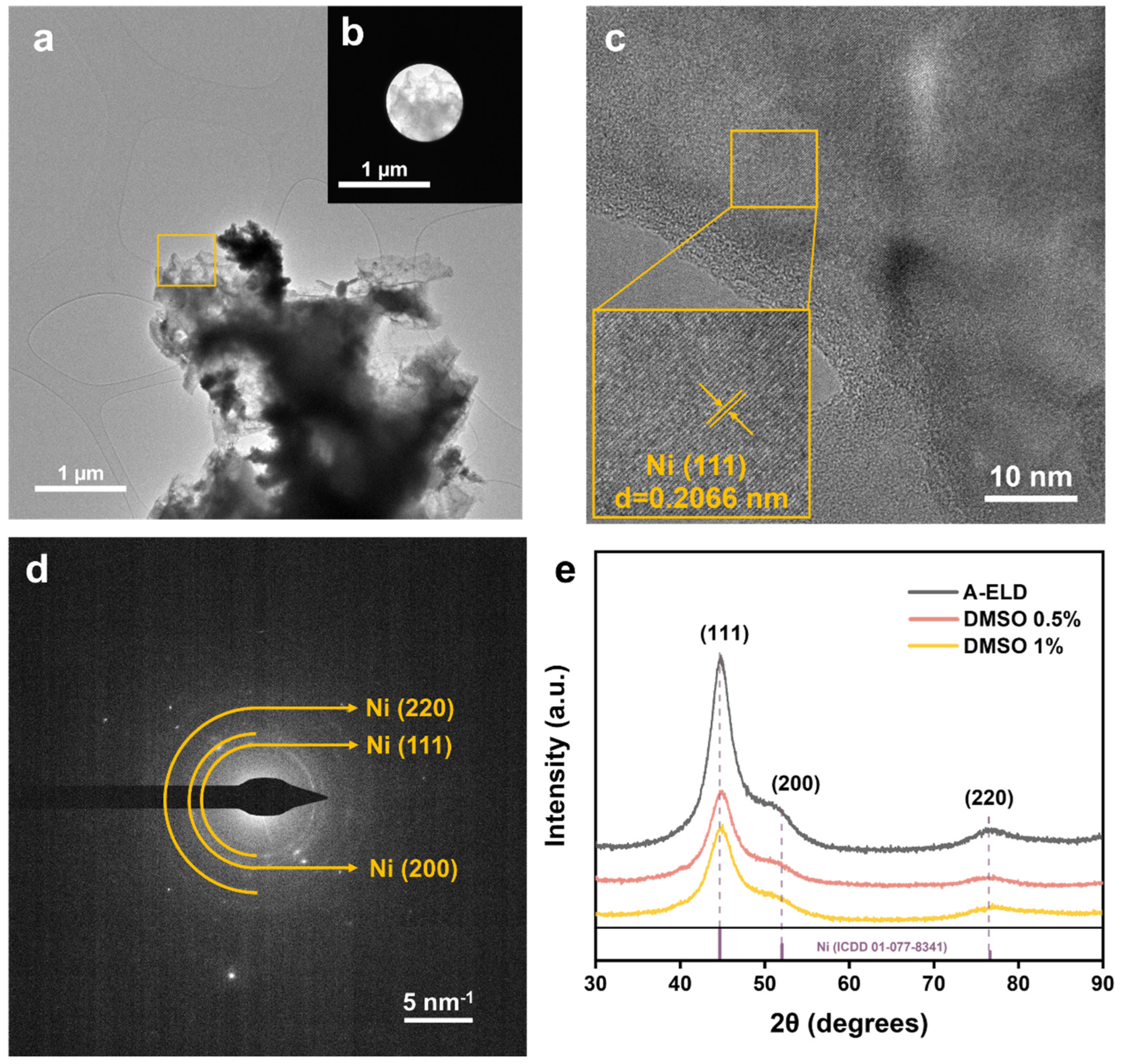
3.2. Crystallographic Structure Analysis
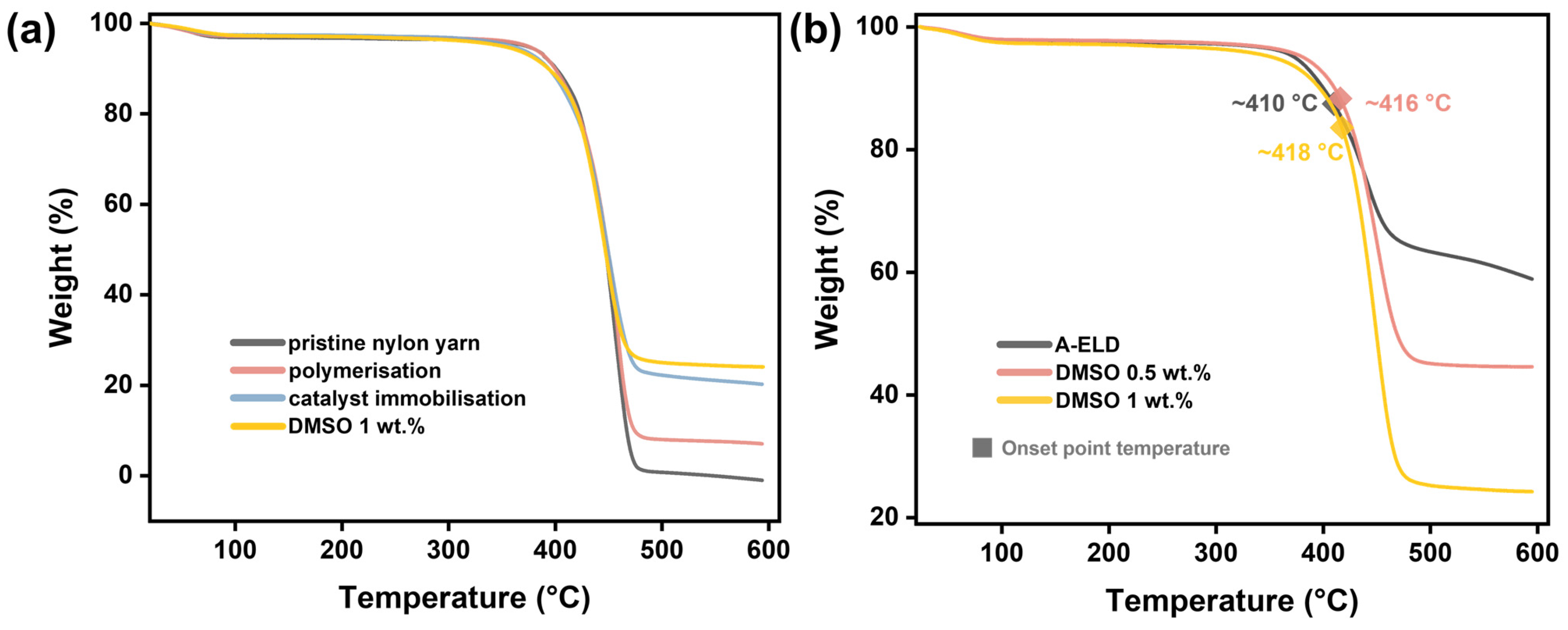
4. Performance Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul-Bari, M.M.; McQueen, R.H.; Nguyen, H.; Wismer, W.V.; de la Mata, A.P.; Harynuk, J.J. Synthetic Clothing and the Problem With Odor: Comparison of Nylon and Polyester Fabrics. Cloth. Text. Res. J. 2018, 36, 251–266. [Google Scholar] [CrossRef]
- Liou, W.J. Effects of Moisture Content on the Creep Behavior of Nylon-6,6-6 Thermoplastic Composites. J. Reinf. Plast. Compos. 1998, 17, 39–50. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.; Mehrabanian, M. HA/Nylon 6, 6 Porous Scaffolds Fabricated by Salt-Leaching/Solvent Casting Technique: Effect of Nano-Sized Filler Content on Scaffold Properties. Indian J. Nephrol. 2011, 6, 1651–1659. [Google Scholar] [CrossRef]
- Toor, Z.S.; Kwon, J.; Kim, R.E.; Choi, Y.T.; Gu, G.H.; Seo, M.-H.; Chung, K.-H.; Wu, R.; Kim, H.S. Failure Behavior of Steel-Polymer-Steel Multi-Material Clad: Mechanical Performance and Microstructure Evolution. Met. Mater. Int. 2025, 31, 994–1008. [Google Scholar] [CrossRef]
- Raghavendra, C.; Basavarajappa, S.; Sogalad, I.; Kumar, S. A Review on Ni Based Nano Composite Coatings. Mater. Today Proc. 2021, 39, 6–16. [Google Scholar] [CrossRef]
- Bharim, A.H.; Sarian, M.Z.; Baba, N.B. Tribology of Electroless Nickel and Interlayer Coatings: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1068, 012011. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Xiao, Y.; Dong, Y.; Wang, X.; Lin, L. A Naturally Integrated Smart Textile for Wearable Electronics Applications. Adv. Mater. Technol. 2020, 5, 1900781. [Google Scholar] [CrossRef]
- Thornton, J.A.; Hoffman, D.W. Stress-Related Effects in Thin Films. Thin Solid Film. 1989, 171, 5–31. [Google Scholar] [CrossRef]
- Etter, M.C. A New Role for Hydrogen-Bond Acceptors in Influencing Packing Patterns of Carboxylic Acids and Amides. J. Am. Chem. Soc. 1982, 104, 1095–1096. [Google Scholar] [CrossRef]
- Romero-Gilbert, S.; Castro-García, M.; Díaz-Chamorro, H.; Marambio, O.G.; Sánchez, J.; Martin-Trasancos, R.; Inostroza, M.; García-Herrera, C.; Pizarro, G.d.C. Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-Co-Acrylamide) Hydrogels. Polymers 2024, 16, 187. [Google Scholar] [CrossRef]
- Prozorovska, L.; Baker, B.A.; Laibinis, P.E.; Jennings, G.K. Surface-Initiated, Catechol-Containing Polymer Films for Effective Chelation of Aluminum Ions. Langmuir 2021, 37, 13617–13626. [Google Scholar] [CrossRef] [PubMed]
- Harandizadeh, Z.; Xie, J.; Moore, M.M.; Hohn, K.L.; Ito, T. Sensitization with Stannous Acetate in Dimethyl Sulfoxide for Silver Electroless Deposition. J. Electrochem. Soc. 2018, 165, D488. [Google Scholar] [CrossRef]
- Vaškelis, A.; Jagminien≐, A.; Prokoptchik, A. Surface Layer pH in Electroless Nickel Plating in Hypophosphite Solutions. Surf. Coat. Technol. 1986, 27, 301–310. [Google Scholar] [CrossRef]
- Ruskic, D.; Hopfgartner, G. Modifier Selectivity Effect on Differential Ion Mobility Resolution of Isomeric Drugs and Multidimensional Liquid Chromatography Ion Mobility Analysis. Anal. Chem. 2019, 91, 11670–11677. [Google Scholar] [CrossRef]
- Osti, N.C.; Van Aken, K.L.; Thompson, M.W.; Tiet, F.; Jiang, D.; Cummings, P.T.; Gogotsi, Y.; Mamontov, E. Solvent Polarity Governs Ion Interactions and Transport in a Solvated Room-Temperature Ionic Liquid. J. Phys. Chem. Lett. 2017, 8, 167–171. [Google Scholar] [CrossRef]
- Huffman, B.A.; Poltash, M.L.; Hughey, C.A. Effect of Polar Protic and Polar Aprotic Solvents on Negative-Ion Electrospray Ionization and Chromatographic Separation of Small Acidic Molecules. Anal. Chem. 2012, 84, 9942–9950. [Google Scholar] [CrossRef]
- MacGregor, W.S. The Chemical and Physical Properties of DMSO. Ann. N. Y. Acad. Sci. 1967, 141, 3–12. [Google Scholar] [CrossRef]
- Guo-Zhu, J.; Jie, Q. Dielectric Constant of Dimethyl Sulfoxide-Monohydric Alcohol Mixture Solution at the Microwave Frequency. Fluid Phase Equilibria 2014, 365, 5–10. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ma, L.; Huang, W. Solvation Structures in Aqueous Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2202068. [Google Scholar] [CrossRef]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; de Witte, P.A.M.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 Organic Solvents and Carriers for Screening Applications in Zebrafish Embryos and Larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef]
- Kim, J.-M.; Jia, H.; Koirala, K.P.; Liu, D.; Simmons, A.; Engelhard, M.H.; Ahmed, R.A.; Zhang, Y.; Wang, C.; Zhang, J.-G.; et al. Three-Dimensional Polymeric-Scaffold-Based Current Collector for a Lithium Metal Anode toward High-Energy-Density Batteries. ACS Energy Lett. 2024, 9, 919–926. [Google Scholar] [CrossRef]
- Broudin, M.; Le Gac, P.Y.; Le Saux, V.; Champy, C.; Robert, G.; Charrier, P.; Marco, Y. Water Diffusivity in PA66: Experimental Characterization and Modeling Based on Free Volume Theory. Eur. Polym. J. 2015, 67, 326–334. [Google Scholar] [CrossRef]
- Uyanık, S. A Comparative Study on Moisture Management Properties of Cotton/Elastane Plain Knit Fabrics Having Different Elastane Ratio. Fibers Polym. 2022, 23, 2316–2329. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhai, H.; Liu, X.; Lewis, D.; Huang, Y.; Ai, L.; Guan, X.; Gong, H.; Liu, X.; Fernando, A. Tuning Polymer–Metal Interfaces via Solvent-Engineered Electroless Nickel Coatings on Functional Fibres. Polymers 2025, 17, 1693. https://doi.org/10.3390/polym17121693
Wang C, Zhai H, Liu X, Lewis D, Huang Y, Ai L, Guan X, Gong H, Liu X, Fernando A. Tuning Polymer–Metal Interfaces via Solvent-Engineered Electroless Nickel Coatings on Functional Fibres. Polymers. 2025; 17(12):1693. https://doi.org/10.3390/polym17121693
Chicago/Turabian StyleWang, Chenyao, Heng Zhai, Xuzhao Liu, David Lewis, Yuhao Huang, Ling Ai, Xinyi Guan, Hugh Gong, Xuqing Liu, and Anura Fernando. 2025. "Tuning Polymer–Metal Interfaces via Solvent-Engineered Electroless Nickel Coatings on Functional Fibres" Polymers 17, no. 12: 1693. https://doi.org/10.3390/polym17121693
APA StyleWang, C., Zhai, H., Liu, X., Lewis, D., Huang, Y., Ai, L., Guan, X., Gong, H., Liu, X., & Fernando, A. (2025). Tuning Polymer–Metal Interfaces via Solvent-Engineered Electroless Nickel Coatings on Functional Fibres. Polymers, 17(12), 1693. https://doi.org/10.3390/polym17121693







