Segmented Polyurethanes Based on Adipate and Sebacate Biodegradable Polyesters for Use as Nerve Guide Conduits in Peripheral Nerve Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Segmented Polyurethane Synthesis
2.3. SPU Film Preparation and Characterization
2.3.1. Spectroscopic and Structural Characterization
2.3.2. Thermal Characterization
2.3.3. Accelerated Degradation
2.3.4. Uniaxial Tension Test on Films
2.4. Conduit Fabrication by Roto-Evaporation
Longitudinal and Radial Tension Tests on SPU Conduits (NGCs)
3. Results and Discission
3.1. Spectroscopy Studies
3.2. Thermal Studies
3.3. Microstructure Analysis
3.4. Degradation Studies
3.5. Mechanical Behavior
3.5.1. Uniaxial Tension Test on SPUU/SPUA Films
| Structure | (N) | (mm) | E (MPa) | (MPa) | (mm/mm) |
|---|---|---|---|---|---|
| SPU Films | |||||
| SPUU-PSC | 4.62 ± 1.21 | 2.25 ± 0.12 | 107 ± 5 | 10.3 ± 0.2 | 0.18 ± 0.01 |
| SPUA-PSC | 5.29 ± 0.53 | 2.31 ± 0.22 | 117 ± 7 | 10.8 ± 0.3 | 0.22 ± 0.04 |
| SPUU-PAD | 0.88 ± 0.08 | 10.1 ± 0.7 | 1.87 ± 0.06 | 0.95 ± 0.06 | 0.84 ± 0.06 |
| SPUA-PAD-1 | 0.18 ± 0.01 | 43.4 ± 0.1 | 0.10 ± 0.01 | 0.20 ± 0.01 | 3.91 ± 0.12 |
| SPUA-PAD-2 | 0.90 ± 0.24 | 5.07 ± 1.54 | 16.3 ± 2.2 | 2.14 ± 0.14 | 0.42 ± 0.06 |
| NGC | |||||
| NGC-SPUA-PAD-2 | 21.8 ± 2.3 | 14.3 ± 3.9 | 8.84 ± 3.8 | 1.83 ± 0.27 | 0.45 ± 0.13 |
| NGC-SPUA-PAD-2-IN ** | 11.1 ± 1.6 | 9.31 ± 1.47 | 4.46 ± 0.5 | 0.76 ± 0.11 | 0.31 ± 0.05 |
| Intact Nerve [49] | 19.85 ± 7.21 | - | 15.87 ± 2.21 | 6.78 ± 0.57 | 0.61 ± 0.02 |
| Extracted Nerve [49] | 33.56 ± 6.07 | - | 8.19 ± 7.27 | 8.54 ± 3.30 | 1.64 ± 0.34 |
| NeuraGen® Conduit ** [50] | 6.89 ± 2.6 | - | - | - | - |
| NeuroTube® Conduit [51] | - | - | 4 ± 2 | 13 ± 3 | 2.76 ± 46 |
| SilkBridgeTM Conduit * [52] | 26.7 ± 2.3 | - | 3.3 ± 0.6 | - | 0.75 ± 0.06 |
| PU NGC * [51] | - | - | 6 ± 1 | 2 | 2.55 ± 0.11 |
| PU NGC ** [53] | 4.98 ± 0.35 | - | - | 6.37 ± 0.5 | - |
3.5.2. Longitudinal and Radial Tension Tests on SPUA-PAD-2 Conduits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philips, C.; Cornelissen, M.; Carriel, V. Evaluation Methods as Quality Control in the Generation of Decellularized Peripheral Nerve Allografts. J. Neural Eng. 2018, 15, 021003. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; De Jesus, O. Neurotmesis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A Biomaterials Approach to Peripheral Nerve Regeneration: Bridging the Peripheral Nerve Gap and Enhancing Functional Recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Scionti, G.; Campos, F.; Roda, O.; Castro, B.; Cornelissen, M.; Garzón, I.; Alaminos, M. In Vitro Characterization of a Nanostructured Fibrin Agarose Bio-Artificial Nerve Substitute. J. Tissue Eng. Regen. Med. 2017, 11, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Antonini, S. Emerging Nanotechnology Approaches in Tissue Engineering for Peripheral Nerve Regeneration. Nanomedicine 2011, 7, 50–59. [Google Scholar] [CrossRef]
- Crook, B.S.; Cullen, M.M.; Pidgeon, T.S. The Role of Tissue Engineering and Three-Dimensional–Filled Conduits in Bridging Nerve Gaps: A Review of Recent Advancements. J. Hand Surg. Glob. Online 2024, 6, 700–704. [Google Scholar] [CrossRef]
- Carriel, V.; Alaminos, M.; Garzón, I.; Campos, A.; Cornelissen, M. Tissue Engineering of the Peripheral Nervous System. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef]
- Isaacs, J.; Browne, T. Overcoming Short Gaps in Peripheral Nerve Repair: Conduits and Human Acellular Nerve Allograft. HAND 2014, 9, 131–137. [Google Scholar] [CrossRef]
- Sanchez Rezza, A.; Kulahci, Y.; Gorantla, V.S.; Zor, F.; Drzeniek, N.M. Implantable Biomaterials for Peripheral Nerve Regeneration–Technology Trends and Translational Tribulations. Front. Bioeng. Biotechnol. 2022, 10, 863969. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Yan, Y.; Yao, R.; Zhao, J.; Chen, K.; Duan, L.; Wang, T.; Zhang, S.; Guan, J.; Zheng, Z.; Wang, X.; et al. Implantable Nerve Guidance Conduits: Material Combinations, Multi-Functional Strategies and Advanced Engineering Innovations. Bioact. Mater. 2022, 11, 57–76. [Google Scholar] [CrossRef]
- van Uden, S.; Vanerio, N.; Catto, V.; Bonandrini, B.; Tironi, M.; Figliuzzi, M.; Remuzzi, A.; Kock, L.; Redaelli, A.C.L.; Greco, F.G.; et al. A Novel Hybrid Silk-Fibroin/Polyurethane Three-Layered Vascular Graft: Towards in Situ Tissue-Engineered Vascular Accesses for Haemodialysis. Biomed. Mater. 2019, 14, 025007. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Raza, A.; Bilal, M.; Iqbal, H.M.N.; Wang, J.-Y. Biodegradable Polymeric Conduits: Platform Materials for Guided Nerve Regeneration and Vascular Tissue Engineering. J. Drug Deliv. Sci. Technol. 2022, 67, 103014. [Google Scholar] [CrossRef]
- Szczepańczyk, P.; Szlachta, M.; Złocista-Szewczyk, N.; Chłopek, J.; Pielichowska, K. Recent Developments in Polyurethane-Based Materials for Bone Tissue Engineering. Polymers 2021, 13, 946. [Google Scholar] [CrossRef]
- Jia, W.; Li, M.; Weng, H.; Gu, G.; Chen, Z. Design and Comprehensive Assessment of a Biomimetic Tri-Layer Tubular Scaffold via Biodegradable Polymers for Vascular Tissue Engineering Applications. Mater. Sci. Eng. C 2020, 110, 110717. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.D.; Kim, S.; Wagner, W.R. Biodegradable Polyurethane Scaffolds in Regenerative Medicine: Clinical Translation Review. J. Biomed. Mater. Res. A 2022, 110, 1460–1487. [Google Scholar] [CrossRef]
- Santerre, J.P.; Woodhouse, K.; Laroche, G.; Labow, R.S. Understanding the Biodegradation of Polyurethanes: From Classical Implants to Tissue Engineering Materials. Biomaterials 2005, 26, 7457–7470. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Niu, Y.; He, T.; Chen, K.C.; Xu, K. Alternating Block Polyurethanes Based on PCL and PEG as Potential Nerve Regeneration Materials. J. Biomed. Mater. Res. A 2014, 102, 685–697. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.H.; Zhang, R.; Yan, Y. Preliminary Studies on Peripheral Nerve Regeneration Using a New Polyurethane Conduit. J. Bioact. Compat. Polym. 2007, 22, 143–159. [Google Scholar] [CrossRef]
- Joo, Y.-S.; Cha, J.-R.; Gong, M.-S. Biodegradable Shape-Memory Polymers Using Polycaprolactone and Isosorbide Based Polyurethane Blends. Mater. Sci. Eng. C 2018, 91, 426–435. [Google Scholar] [CrossRef]
- Marzec, M.; Kucińska-Lipka, J.; Kalaszczyńska, I.; Janik, H. Development of Polyurethanes for Bone Repair. Mater. Sci. Eng. C 2017, 80, 736–747. [Google Scholar] [CrossRef]
- ASTM ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. West Conshohocken: Montgomery, PA, USA, 2018.
- Integra LifeSciences Corporation. Integra LifeSciences NeuraGen® 3D: Pre-Clinical Evidence Summary; Integra LifeSciences Corporation: Plainsboro, NJ, USA, 2021. [Google Scholar]
- Griffith, J.F.; Guggenberger, R. Peripheral Nerve Imaging. In Musculoskeletal Diseases 2021–2024: Diagnostic Imaging; Springer: Berlin/Heidelberg, Germany, 2021; pp. 259–268. [Google Scholar]
- ISO7198; Cardiovascular Implants—Tubular Vascular Prostheses. International Organization for Standardization: Geneva, Switzerland, 1988.
- Bronzeri, L.B.; Gauche, C.; Gudimard, L.; Courtial, E.-J.; Marquette, C.; Felisberti, M.I. Amphiphilic and Segmented Polyurethanes Based on Poly(ε-Caprolactone)Diol and Poly(2-Ethyl-2-Oxazoline)Diol: Synthesis, Properties, and a Preliminary Performance Study of the 3D Printing. Eur. Polym. J. 2021, 151, 110449. [Google Scholar] [CrossRef]
- Nguyen Dang, L.; Le Hoang, S.; Malin, M.; Weisser, J.; Walter, T.; Schnabelrauch, M.; Seppälä, J. Synthesis and Characterization of Castor Oil-Segmented Thermoplastic Polyurethane with Controlled Mechanical Properties. Eur. Polym. J. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Tereshatov, V.V.; Makarova, M.A.; Senichev, V.Y.U.; Slobodinyuk, A.I. Interrelationship between Ultimate Mechanical Properties of Variously Structured Polyurethanes and Poly(Urethane Urea)s and Stretching Rate Thereof. Colloid. Polym. Sci. 2012, 290, 641–651. [Google Scholar] [CrossRef]
- Yen, F.-S.; Lin, L.-L.; Hong, J.-L. Hydrogen-Bond Interactions between Urethane−Urethane and Urethane−Ester Linkages in a Liquid Crystalline Poly(Ester−urethane). Macromolecules 1999, 32, 3068–3079. [Google Scholar] [CrossRef]
- Cetina-Diaz, S.M.; Chan-Chan, L.H.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Quintana-Owen, P.; Paakinaho, K.; Kellomaki, M.; Di Silvio, L.; Deb, S.; Cauich-Rodríguez, J.V. Physicochemical Characterization of Segmented Polyurethanes Prepared with Glutamine or Ascorbic Acid as Chain Extenders and Their Hydroxyapatite Composites. J. Mater. Chem. B 2014, 2, 1966–1976. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Vlad, S. Thermogravimetric Study for Polyurethane Materials for Biomedical Applications. Compos. B Eng. 2011, 42, 1474–1479. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Janowski, B.; Apekis, L.; Pielichowski, K.; Pissis, P. Molecular Mobility and Crystallinity in Polytetramethylene Ether Glycol in the Bulk and as Soft Component in Polyurethanes. Eur. Polym. J. 2011, 47, 2120–2133. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. DSC Annealing Study of Microphase Separation and Multiple Endothermic Behavior in Polyether-Based Polyurethane Block Copolymers. Macromolecules 1986, 19, 706–713. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Klonos, P.A.; Tworzydło, P.; Ozimek, J.; Hebda, E.; Kyritsis, A.; Pielichowski, K. Effects of Segment Length and Crosslinking via POSS on the Calorimetric and Dynamic Glass Transition of Polyurethanes with Aliphatic Hard Segments. Int. J. Mol. Sci. 2023, 24, 16540. [Google Scholar] [CrossRef]
- Camberlin, Y.; Pascault, J.P. Quantitative DSC Evaluation of Phase Segregation Rate in Linear Segmented Polyurethanes and Polyurethaneureas. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 415–423. [Google Scholar] [CrossRef]
- Yeh, F.; Hsiao, B.S.; Sauer, B.B.; Michel, S.; Siesler, H.W. In-Situ Studies of Structure Development during Deformation of a Segmented Poly(Urethane−urea) Elastomer. Macromolecules 2003, 36, 1940–1954. [Google Scholar] [CrossRef]
- Yilgör, I.; Yilgör, E.; Wilkes, G.L. Critical Parameters in Designing Segmented Polyurethanes and Their Effect on Morphology and Properties: A Comprehensive Review. Polymer 2015, 58, A1–A36. [Google Scholar] [CrossRef]
- Garrett, J.T.; Siedlecki, C.A.; Runt, J. Microdomain Morphology of Poly(Urethane Urea) Multiblock Copolymers. Macromolecules 2001, 34, 7066–7070. [Google Scholar] [CrossRef]
- Garrett, J.T.; Runt, J.; Lin, J.S. Microphase Separation of Segmented Poly(Urethane Urea) Block Copolymers. Macromolecules 2000, 33, 6353–6359. [Google Scholar] [CrossRef]
- Bajsic, E.G.; Rek, V.; Sendijarevic, A.; Sendijarevic, V.; Frisch, K.C. DSC Study of Morphological Changes in Segmented Polyurethane Elastomers. J. Elastomers Plast. 2000, 32, 162–182. [Google Scholar] [CrossRef]
- Hwang, K.K.S.; Lin, S.B.; Tsay, S.Y.; Cooper, S.L. Properties of Polyurethane Oligomeric Blends versus High Molecular Weight Block Copolymers. Polymer 1984, 25, 947–955. [Google Scholar] [CrossRef]
- Meijs, G.F.; McCarthy, S.J.; Rizzardo, E.; Chen, Y.; Chatelier, R.C.; Brandwood, A.; Schindhelm, K. Degradation of Medical—Grade Polyurethane Elastomers: The Effect of Hydrogen Peroxide in Vitro. J. Biomed. Mater. Res. 1993, 27, 345–356. [Google Scholar] [CrossRef]
- Riehle, N.; Athanasopulu, K.; Kutuzova, L.; Götz, T.; Kandelbauer, A.; Tovar, G.E.M.; Lorenz, G. Influence of Hard Segment Content and Diisocyanate Structure on the Transparency and Mechanical Properties of Poly(Dimethylsiloxane)-Based Urea Elastomers for Biomedical Applications. Polymers 2021, 13, 212. [Google Scholar] [CrossRef]
- Asplund, J.O.B.; Bowden, T.; Mathisen, T.; Hilborn, J. Synthesis of Highly Elastic Biodegradable Poly(Urethane Urea). Biomacromolecules 2007, 8, 905–911. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in Waterborne Polyurethane and Polyurethane-Urea Dispersions and Their Eco-Friendly Derivatives: A Review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Ahmed, W.; Yao, Y.; Bai, J.; Chen, Y.; Gao, C. Design and Applications of Cell-Selective Surfaces and Interfaces. Biomacromolecules 2018, 19, 1746–1763. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Perera, T.H.; Aria, A.B.; Smith Callahan, L.A. Polyethylene Glycol in Spinal Cord Injury Repair: A Critical Review. J Exp Pharmacol. 2018, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Mosley, M.C.; Lim, H.J.; Chen, J.; Yang, Y.; Li, S.; Liu, Y.; Smith Callahan, L.A. Neurite Extension and Neuronal Differentiation of Human Induced Pluripotent Stem Cell Derived Neural Stem Cells on Polyethylene Glycol Hydrogels Containing a Continuous Young’s Modulus Gradient. J. Biomed. Mater. Res. A 2017, 105, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.E.; Born, W. Stimulation of Neurite Outgrowth in a Human Nerve Scaffold Designed for Peripheral Nerve Reconstruction. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73B, 194–202. [Google Scholar] [CrossRef]
- Yao, L.; Billiar, K.L.; Windebank, A.J.; Pandit, A. Multichanneled Collagen Conduits for Peripheral Nerve Regeneration: Design, Fabrication, and Characterization. Tissue Eng. Part C Methods 2010, 16, 1585–1596. [Google Scholar] [CrossRef]
- Hsu, S.; Chang, W.; Yen, C. Novel Flexible Nerve Conduits Made of Water—Based Biodegradable Polyurethane for Peripheral Nerve Regeneration. J. Biomed. Mater. Res. A 2017, 105, 1383–1392. [Google Scholar] [CrossRef]
- Fregnan, F.; Muratori, L.; Bassani, G.A.; Crosio, A.; Biagiotti, M.; Vincoli, V.; Carta, G.; Pierimarchi, P.; Geuna, S.; Alessandrino, A.; et al. Preclinical Validation of SilkBridgeTM for Peripheral Nerve Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 835. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, K.C.; He, T.; Yu, W.; Huang, S.; Xu, K. Scaffolds from Block Polyurethanes Based on Poly(ε-Caprolactone) (PCL) and Poly(Ethylene Glycol) (PEG) for Peripheral Nerve Regeneration. Biomaterials 2014, 35, 4266–4277. [Google Scholar] [CrossRef]
- Mankavi, F.; Ibrahim, R.; Wang, H. Advances in Biomimetic Nerve Guidance Conduits for Peripheral Nerve Regeneration. Nanomaterials 2023, 13, 2528. [Google Scholar] [CrossRef]




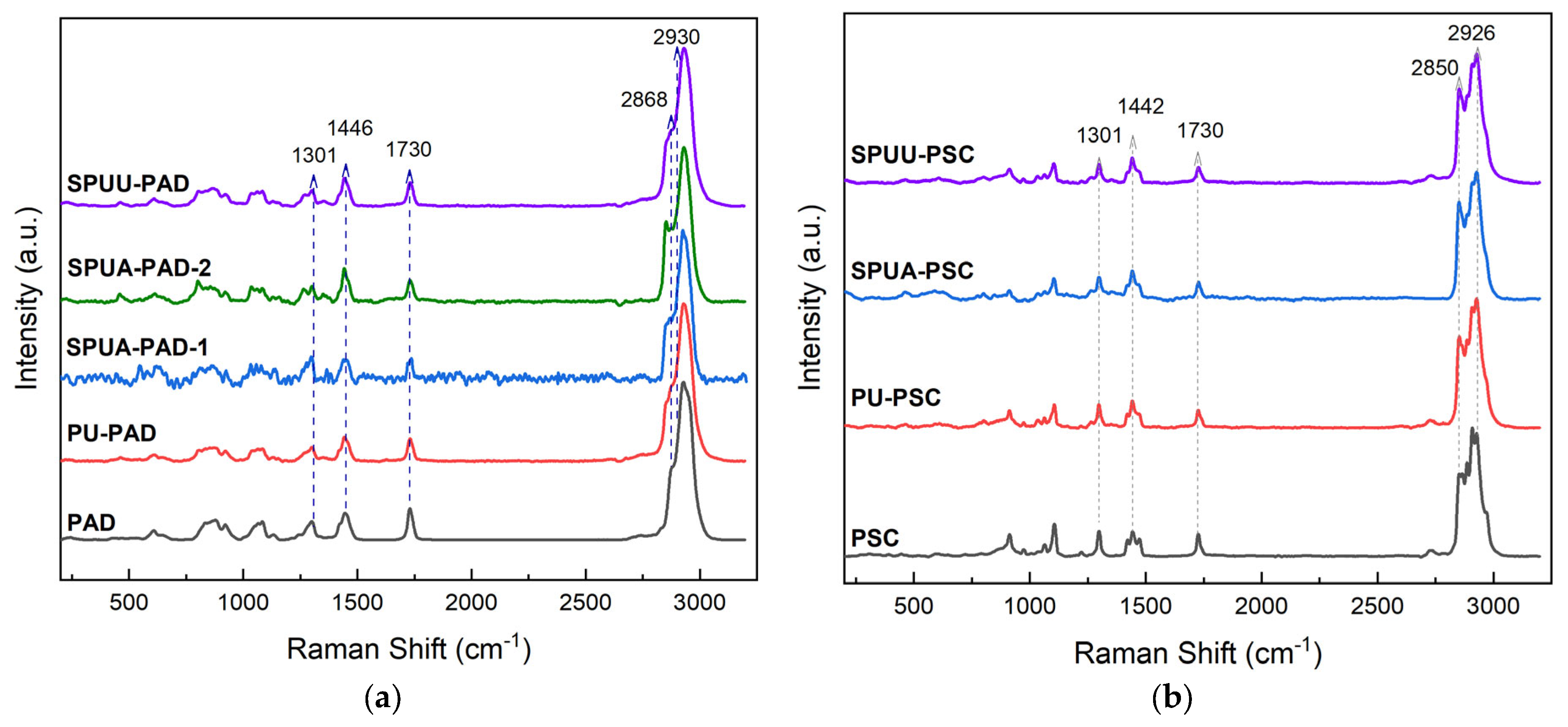

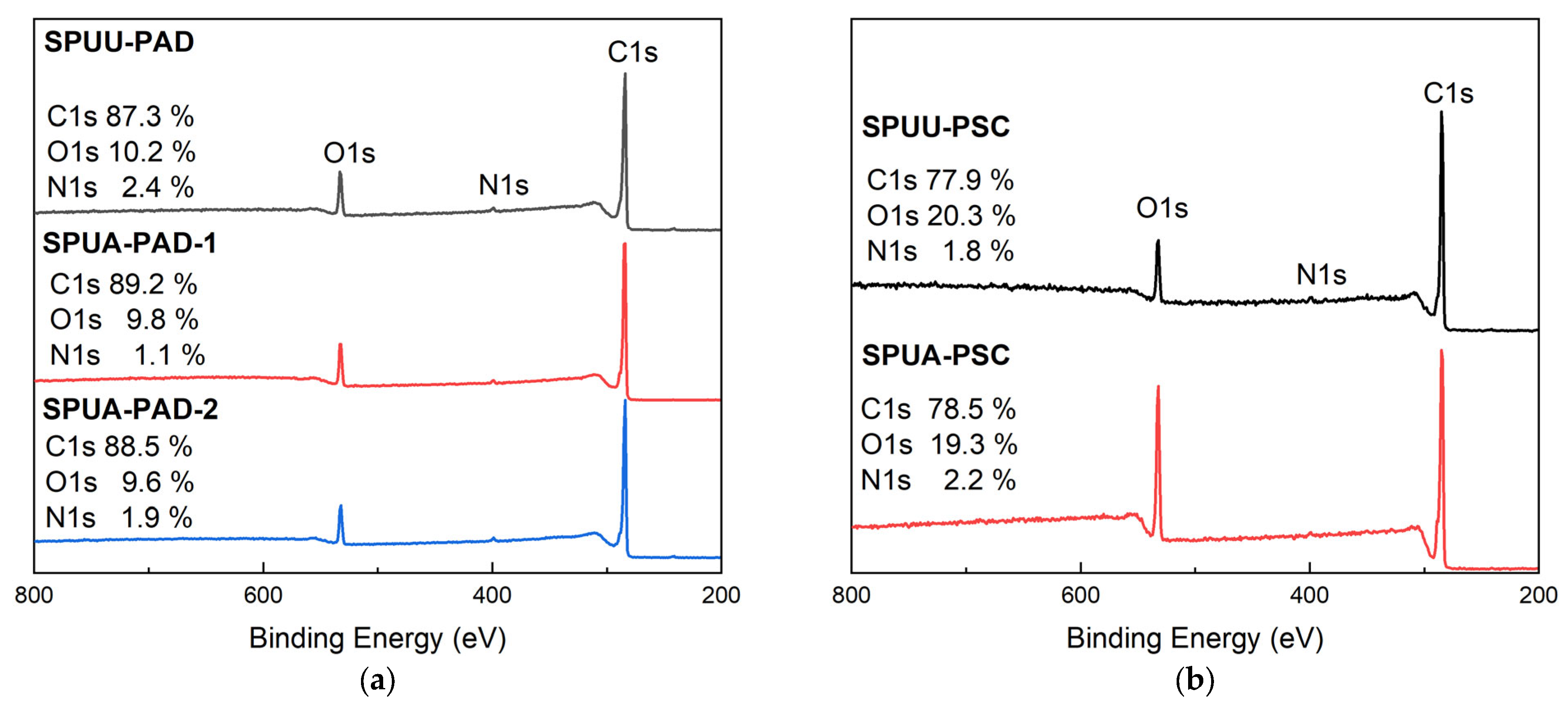
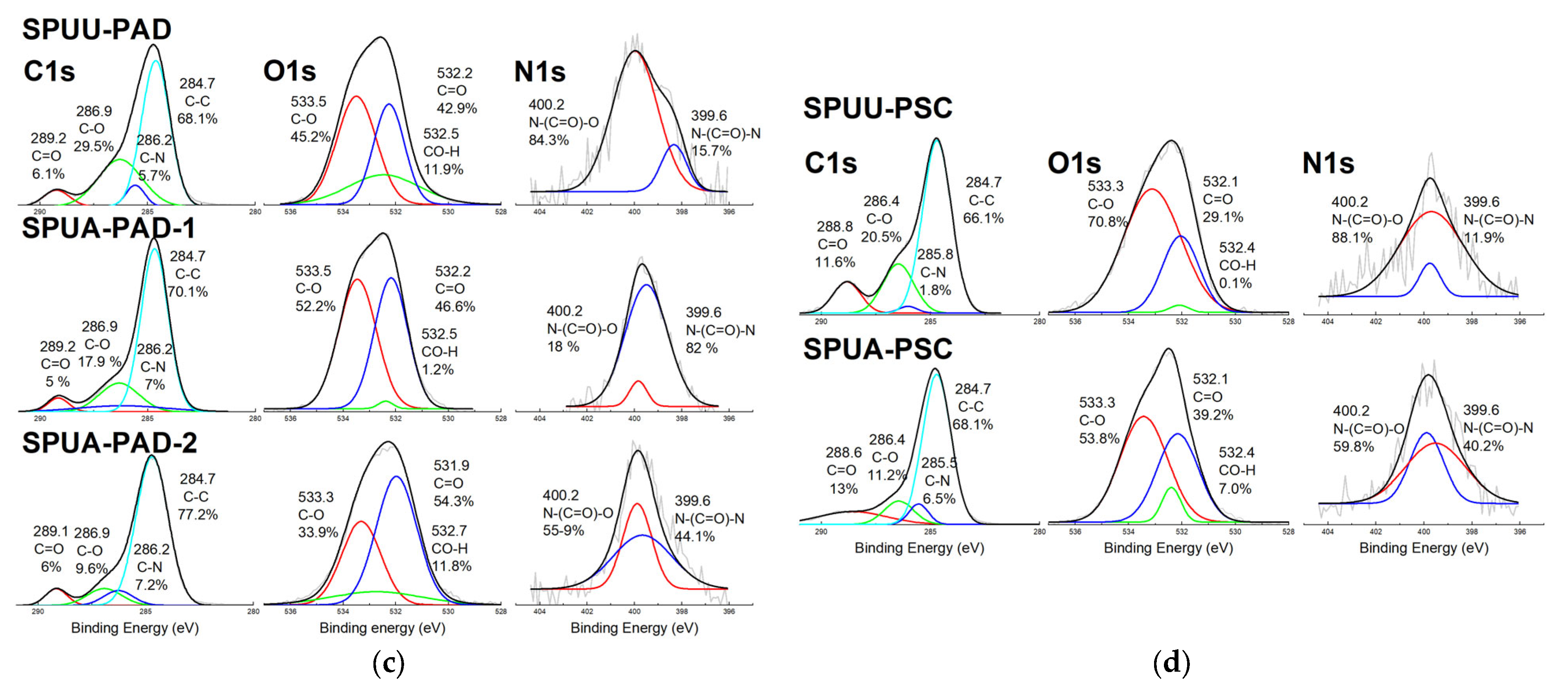




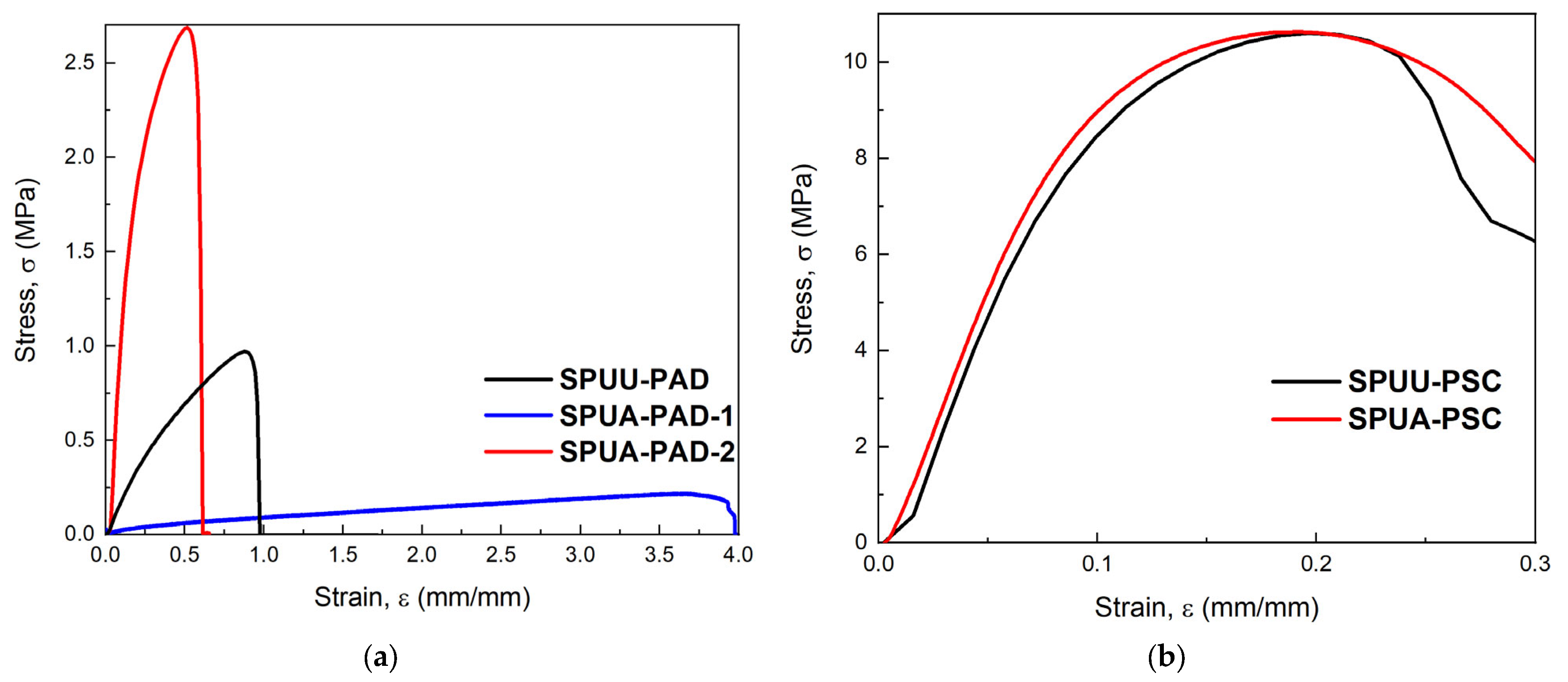


| Designation | Composition * | SS % | HS % |
|---|---|---|---|
| PU-PAD | PAD:HMDI (1:1) | 100 | 0 |
| SPUU-PAD | PAD:HMDI:BO (1:2:1) | 86.6 | 13.4 |
| SPUA-PAD-1 | PAD:HMDI:BA (1:2:1) | 86.6 | 13.4 |
| SPUA-PAD-2 | PAD:HMDI:BA (1:3:2) | 76.4 | 23.6 |
| PU-PSC | PSC:HMDI (1:1) | 100 | 0 |
| SPUU-PSC | PSC:HMDI:BO (1:2:1) | 88.1 | 11.9 |
| SPUA-PSC | PSC:HMDI:BA (1:2:1) | 88.1 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabido-Barahona, A.B.; Vargas-Coronado, R.F.; Hernández-Sánchez, F.; Martínez-Richa, A.; Gómez Ribelles, J.L.; Cauich-Rodríguez, J.V.; Marcos-Fernández, A. Segmented Polyurethanes Based on Adipate and Sebacate Biodegradable Polyesters for Use as Nerve Guide Conduits in Peripheral Nerve Regeneration. Polymers 2025, 17, 1692. https://doi.org/10.3390/polym17121692
Sabido-Barahona AB, Vargas-Coronado RF, Hernández-Sánchez F, Martínez-Richa A, Gómez Ribelles JL, Cauich-Rodríguez JV, Marcos-Fernández A. Segmented Polyurethanes Based on Adipate and Sebacate Biodegradable Polyesters for Use as Nerve Guide Conduits in Peripheral Nerve Regeneration. Polymers. 2025; 17(12):1692. https://doi.org/10.3390/polym17121692
Chicago/Turabian StyleSabido-Barahona, Alexis B., Rossana F. Vargas-Coronado, Fernando Hernández-Sánchez, Antonio Martínez-Richa, José L. Gómez Ribelles, Juan V. Cauich-Rodríguez, and Angel Marcos-Fernández. 2025. "Segmented Polyurethanes Based on Adipate and Sebacate Biodegradable Polyesters for Use as Nerve Guide Conduits in Peripheral Nerve Regeneration" Polymers 17, no. 12: 1692. https://doi.org/10.3390/polym17121692
APA StyleSabido-Barahona, A. B., Vargas-Coronado, R. F., Hernández-Sánchez, F., Martínez-Richa, A., Gómez Ribelles, J. L., Cauich-Rodríguez, J. V., & Marcos-Fernández, A. (2025). Segmented Polyurethanes Based on Adipate and Sebacate Biodegradable Polyesters for Use as Nerve Guide Conduits in Peripheral Nerve Regeneration. Polymers, 17(12), 1692. https://doi.org/10.3390/polym17121692









