Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Dispersion Polymerization: General Procedure
2.2. Sample Characterization
2.3. Dissociation of PTPMA-PFNA Complexes
2.4. Cloud Point Measurements
3. Results
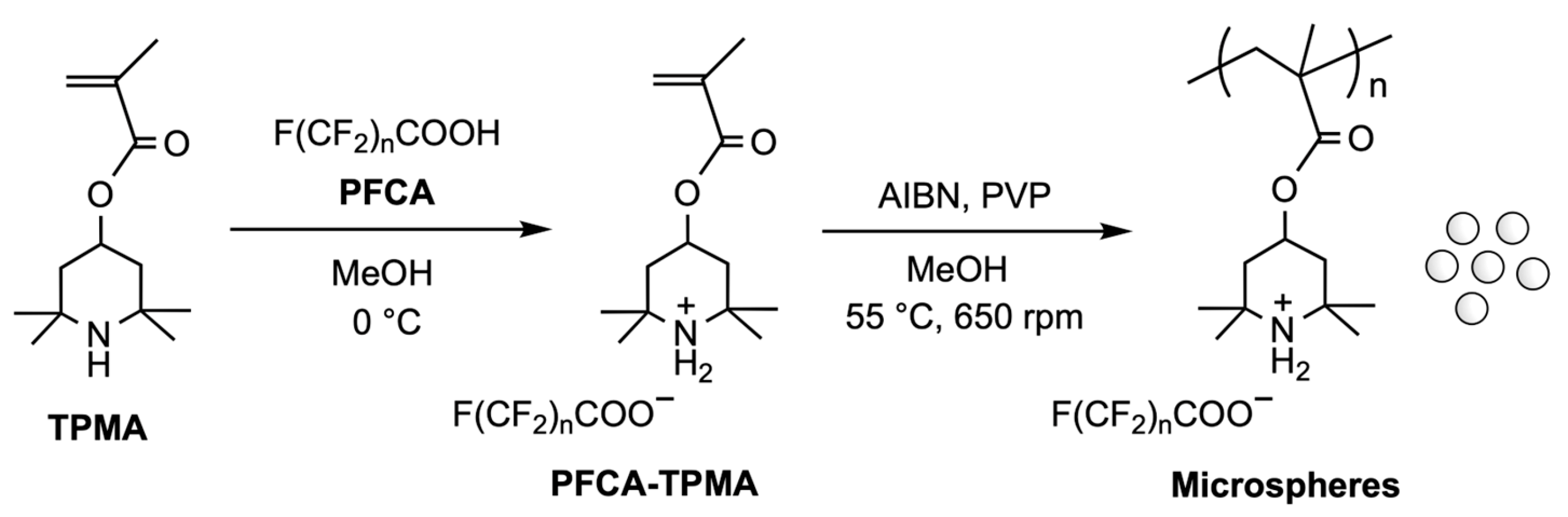
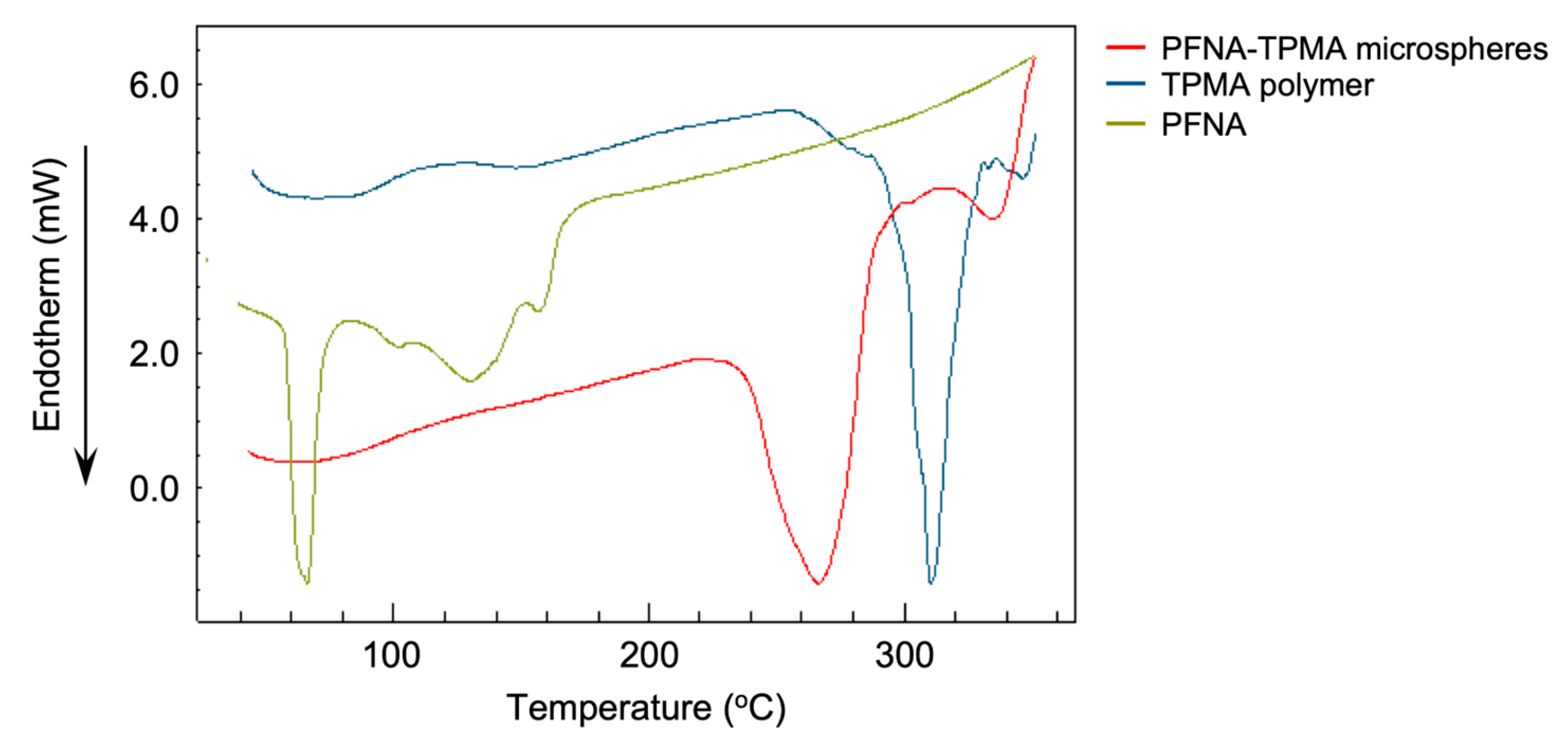
3.1. Formation of PFCA-Loaded Monomers
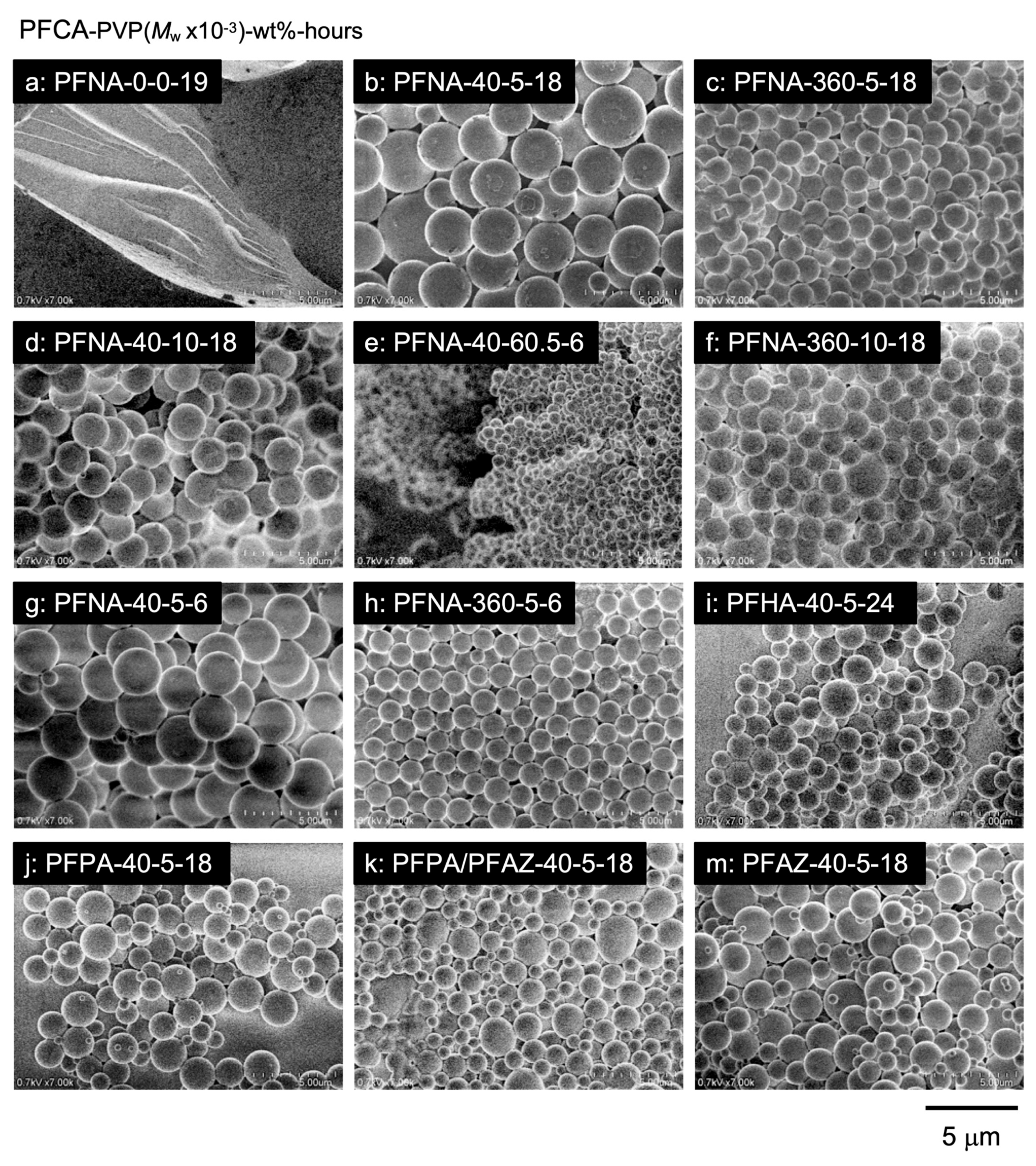
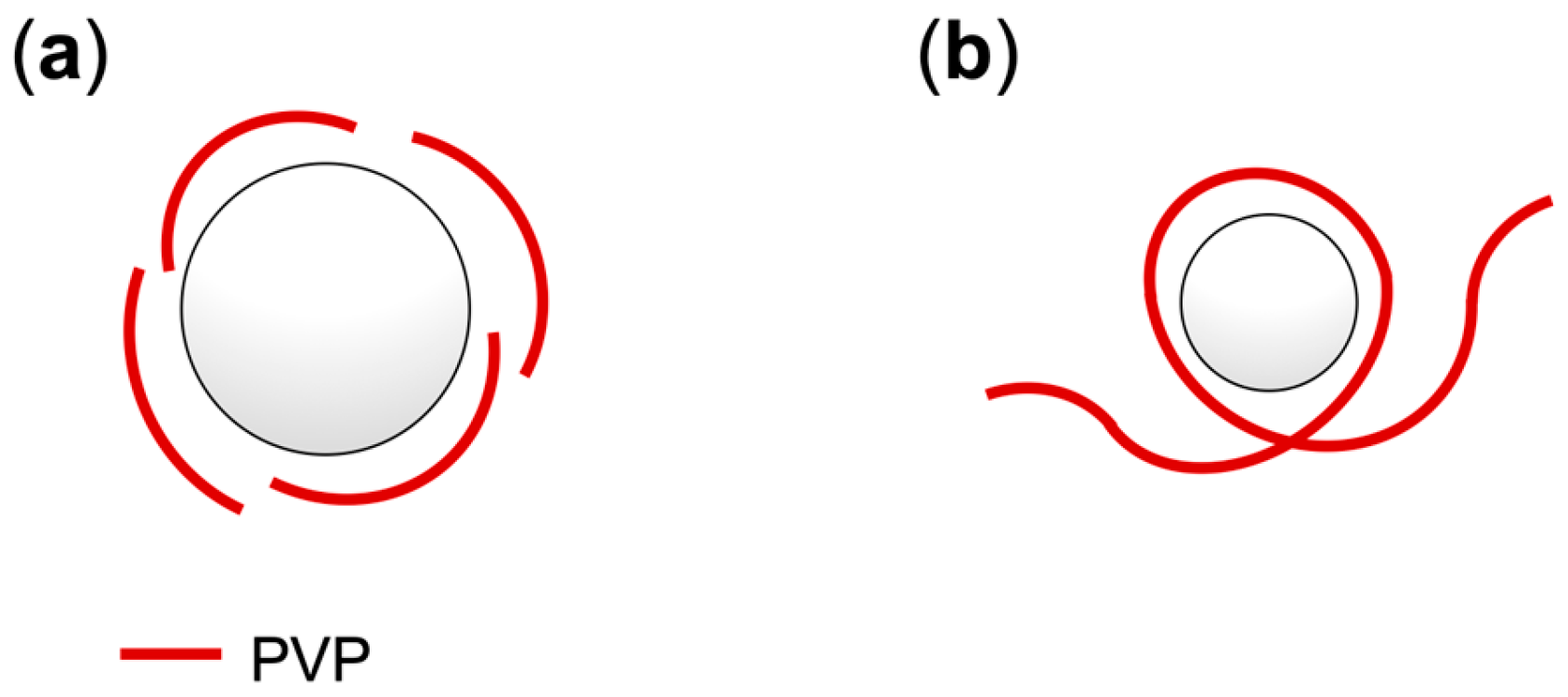
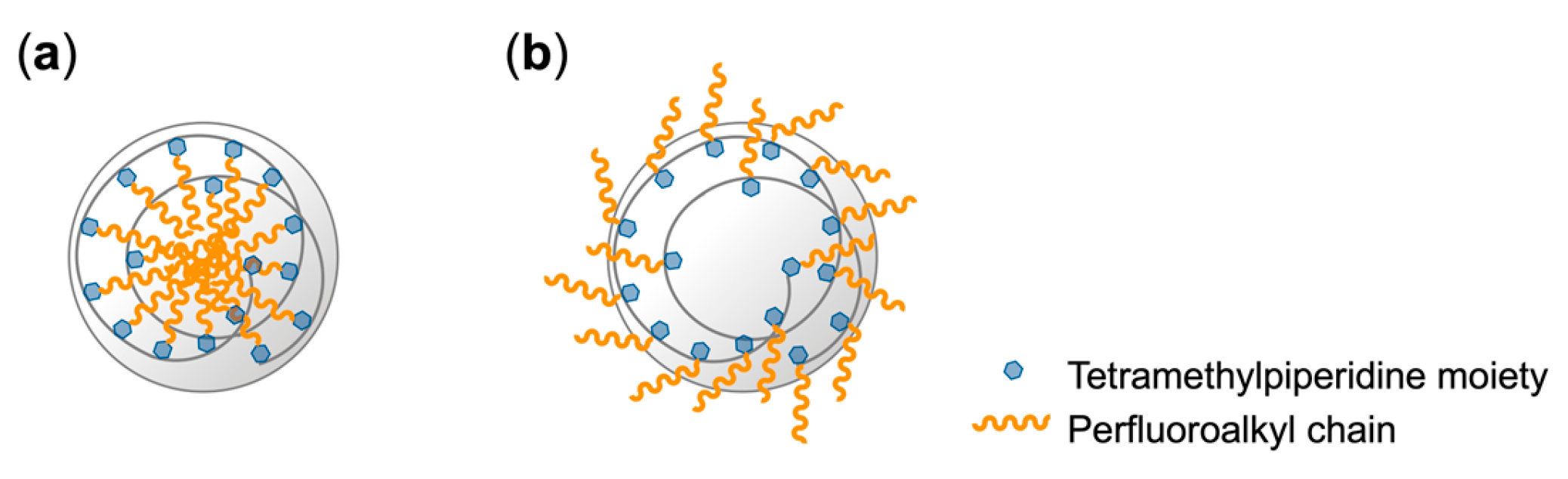
3.2. Encapsulation of PFCAs Within Microspheres via Dispersion Polymerization
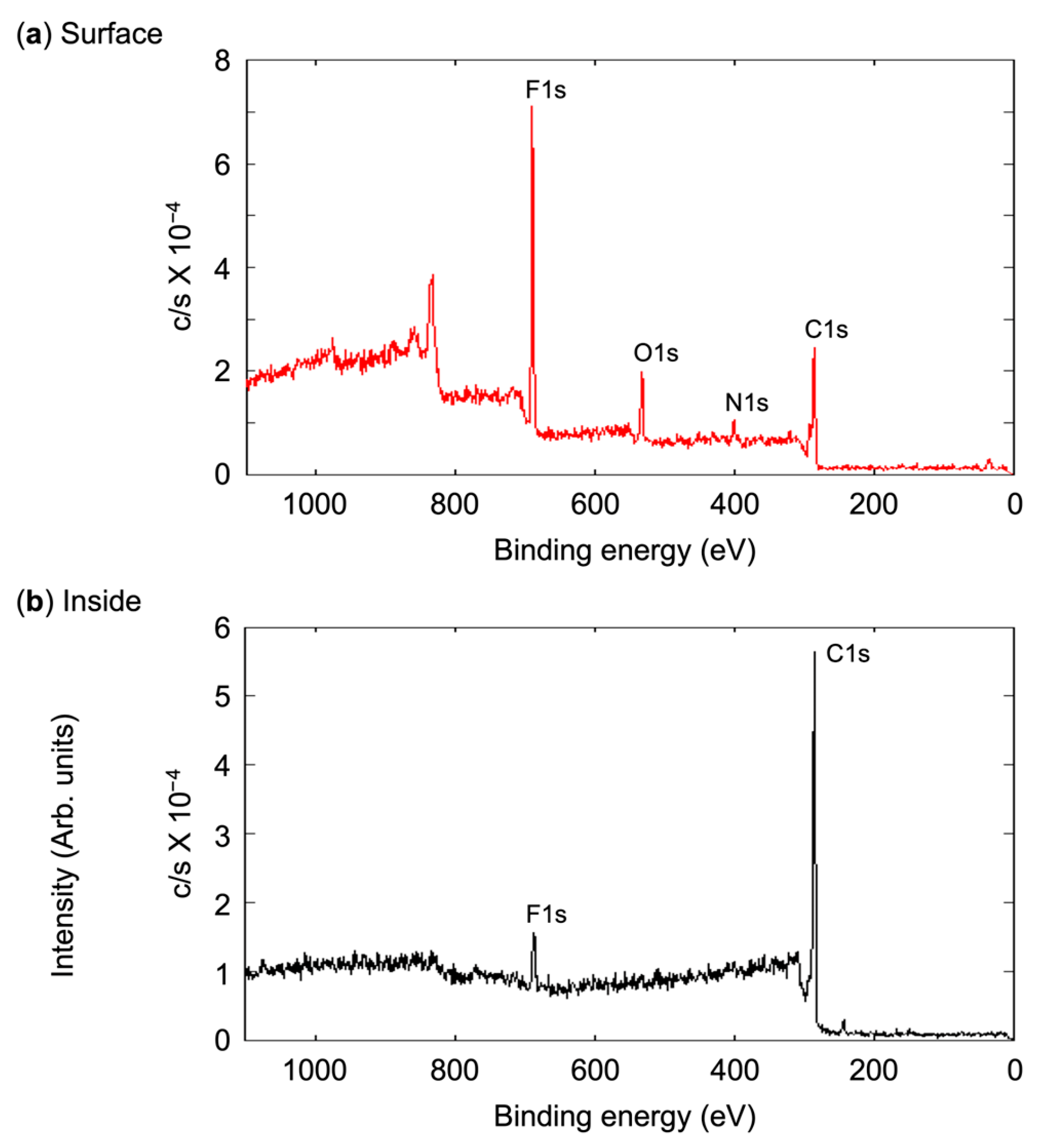
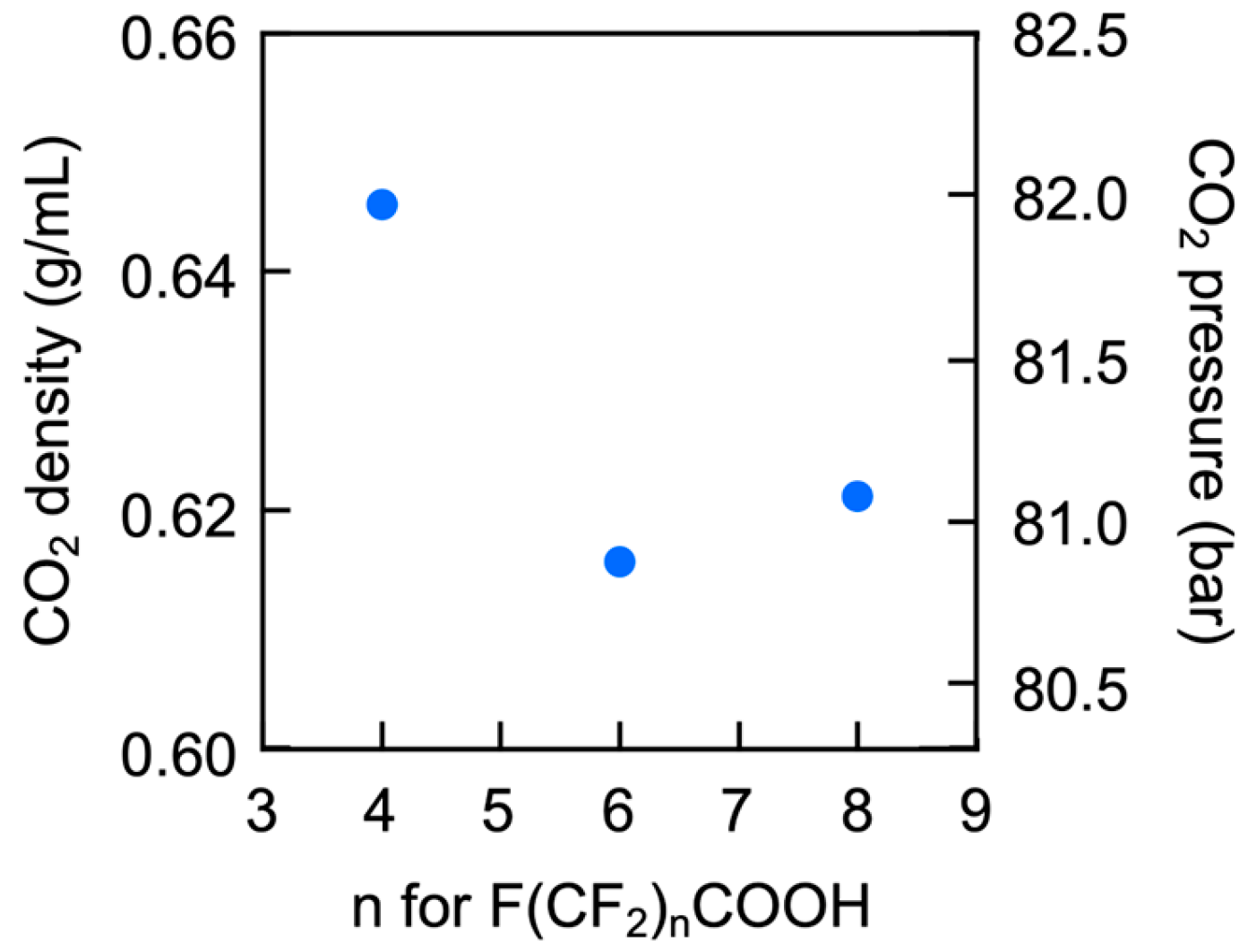
3.3. Evaluation of Microsphere Solubility in scCO2
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical properties and interactions of perfluoroalkyl substances (PFAS)—Challenges and opportunities in sensing and remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef] [PubMed]
- Kancharla, S.; Jahan, R.; Bedrov, D.; Tsianou, M.; Alexandridis, P. Role of chain length and electrolyte on the micellization of anionic fluorinated surfactants in water. Colloids Surf. A 2021, 628, 127313. [Google Scholar] [CrossRef]
- Itumoh, E.J.; Data, S.; Chen, J.L.-Y.; Kah, M.; Padhye, L.P.; Leitao, E.M. Addressing the persistence of per and poly- fluoroalkyl substances (PFAS): Current challenges and potential solutions. RSC Sustain. 2024, 2, 3183–3201. [Google Scholar] [CrossRef]
- Grunfeld, D.A.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Tohren, C.G.; Kibbey, T.C.G.; O’Carroll, D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.-E.; Karvounis, M.; Marinos, G.; Theodorakopoulou, Z.; Aloizou, E.; Petsangourakis, G.; Papakonstantinou, M.; Stoitsis, G. Comprehensive analysis of PFAS presence from environment to plate. npj Sci. Food. 2024, 8, 80. [Google Scholar] [CrossRef]
- Savvidou, E.K.; Sha, B.; Salter, M.E.; Cousins, I.T.; Johansson, J.H. Horizontal and vertical distribution of perfluoroalkyl acids (PFAAs) in the water column of the Atlantic Ocean. Environ. Sci. Technol. Lett. 2023, 10, 418–424. [Google Scholar] [CrossRef]
- Sobolewski, T.N.; Trousdale, R.C.; Gauvin, C.L.; Lawrence, C.M.; Walker, R.A. Nanomolar PFOA concentrations affect lipid membrane structure: Consequences for bioconcentration mechanisms. Environ. Sci. Technol. 2025, 59, 709–718. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, Y.; Zhang, Z.; Wang, F.; He, J.; Wang, R.; Xu, Y.; Keerman, M.; Zhang, S.; Zhang, Y.; et al. Plasma PFOA and PFOS levels, DNA methylation, and blood lipid levels: A pilot study. Environ. Sci. Technol. 2022, 56, 17039–17051. [Google Scholar] [CrossRef]
- Savoca, M.S.; Robuck, A.R.; Cashman, M.A.; Cantwell, M.G.; Agvent, L.C.; Wiley, D.N.; Rice, R.; Todd, S.; Hunter, N.E.; Robbins, J.; et al. Whale baleen to monitor per- and polyfluoroalkyl substances (PFAS) in marine environments. Environ. Sci. Technol. Lett. 2024, 11, 862–870. [Google Scholar] [CrossRef]
- Hedgespeth, M.L.; Taylor, D.L.; Balint, S.; Schwartz, M.; Cantwell, M.G. Ecological characteristics impact PFAS concentrations in a U.S. North Atlantic food web. Sci. Total Environ. 2023, 880, 163302. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; Jamie, C. DeWitt, J.C.; Christopher Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Tshangana, C.S.; Nhlengethwa, S.T.; Glass, S.; Denison, S.; Kuvarega, A.T.; Nkambule, T.T.I.; Mamba, B.B.; Alvarez, P.J.J.; Muleja, A.A. Technology status to treat PFAS-contaminated water and limiting factors for their effective full-scale application. npj Clean Water 2025, 8, 41. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Shetty, S.; Stritzinger, D.; Bellona, C.; Novosselov, I.V. Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor. Chemosphere 2021, 279, 130834. [Google Scholar] [CrossRef]
- Hao, S.; Choi, Y.J.; Deeb, R.A.; Strathmann, T.J.; Higgins, C.P. Application of hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in contaminated groundwater and soil. Environ. Sci. Technol. 2022, 56, 6647–6657. [Google Scholar] [CrossRef]
- Zeidabadi, F.A.; Esfahani, E.B.; McBeath, S.T.; Dubrawski, K.L.; Madjid Mohseni, M. Electrochemical degradation of PFOA and its common alternatives: Assessment of key parameters, roles of active species, and transformation pathway. Chemosphere 2023, 315, 137743. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in groundwater: Kinetic effects of matrix inorganics. Environ. Sci. Technol. 2010, 44, 445–450. [Google Scholar] [CrossRef]
- Esfahani, E.B.; Zeidabadi, F.A.; Jafarikojour, M.; Mohseni, M. Photo-oxidative/reductive decomposition of perfluorooctanoic acid (PFOA) and its common alternatives: Mechanism and kinetic modeling. J. Water Process Eng. 2024, 61, 105332. [Google Scholar] [CrossRef]
- Topolovec, B.; Jovanovic, O.; Puac, N.; Skoro, N.; Lumbaque, E.C.; Petrovic, M. Plasma water treatment for PFAS: Study of degradation of perfluorinated substances and their byproducts by using cold atmospheric pressure plasma jet. J. Environ. Chem. Eng. 2024, 12, 112979. [Google Scholar] [CrossRef]
- Londhe, K.; Lee, C.-S.; Grdanovska, S.; Smolinski, R.; Hamdan, N.; McDonough, C.; Cooper, C.; Venkatesan, A.K. Application of electron beam technology to decompose per-and polyfluoroalkyl substances in water. Environ. Pollut. 2024, 348, 123770. [Google Scholar] [CrossRef]
- Bao, Y.; Deng, S.; Jiang, X.; Qu, Y.; He, Y.; Liu, L.; Chai, Q.; Mumtaz, M.; Huang, J.; Cagnetta, G.; et al. Degradation of PFOA substitute: GenX (HFPO−DA ammonium salt): Oxidation with UV/persulfate or reduction with UV/sulfite? Environ. Sci. Technol. 2018, 52, 11728–11734. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.S.; Albalawi, S.S.; Abuhagr, A.; Althobaiti, S.A.; Hassanain, H.M.; Reeves, D.M.; Abdullah, M.R.; Sinn, E. Two-electron transfer photoreduction of methyl viologen and perfluorooctanoic acid mediated by flavin mononucleotide at colloidal titanium dioxide interfaces. New J. Chem. 2023, 47, 21661–21669. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, C.; Xing, L.; Zhou, Q.; Dong, W.; Hoffmann, M.R. UV/nitrilotriacetic acid process as a novel strategy for efficient photoreductive degradation of perfluorooctanesulfonate. Environ. Sci. Technol. 2018, 52, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Coon, C.M.; Doherty, M.E.; McHugh, E.A.; Warner, M.C.; Walters, C.L.; Orahood, O.M.; Loesch, A.E.; Hatfield, D.C.; Sitko, J.C.; et al. Engineering and characterization of dehalogenase enzymes from Delftia acidovorans in bioremediation of perfluorinated compounds. Synth. Syst. Biotechnol. 2022, 7, 671–676. [Google Scholar] [CrossRef]
- Broman, J.; Ceja, A.; Godoy, T.; Rivera, D.R.; Dionne, P.; Schipper, J.; Henkemeyer, S.; Cegielski, S.; Wong, G.; Kaur, A. Destruction of per- and polyfluoroalkyl substances (PFAS) via lacasse enzymatic degradation and electrochemical advanced oxidation. In Proceedings of the 2021 Waste-management Education Research Conference (WERC), Las Cruces, NM, USA, 11–14 April 2021; Volume 2, pp. 1–10. [Google Scholar]
- Ashley, A.; Thrope, B.; Choudhury, M.R.; Pinto, A.H. Emerging investigator series: Photocatalytic membrane reactors: Fundamentals and advances in preparation and application in wastewater treatment. Environ. Sci. Water Res. Technol. 2022, 8, 22–46. [Google Scholar] [CrossRef]
- Olimattel, K.; Zhai, L.; Sadmani, A.H.M.A. Enhanced removal of perfluorooctane sulfonic acid and perfluorooctanoic acid via polyelectrolyte functionalized ultrafiltration membrane: Effects of membrane modification and water matrix. J. Hazard. Mater. Lett. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Shohel, M.; Bays, N.R.; LaFond, J.A.; Kruse, S.M.; Bryant, Z.K.; Krawchuck, J.A.; Salinas, P.A.; Roman-Kustas, J.K.; Rigali, M.J.; Knight, A.W.; et al. Mesoporous carbons and their modification with aliphatic quaternary amines for adsorption, thermal treatment, and preconcentration of perfluorooctanoic acid (PFOA). Ind. Eng. Chem. Res. 2025, 64, 5123–5133. [Google Scholar] [CrossRef]
- Xin, W.; Song, Y. Mesoporous carbons: Recent advances in synthesis and typical applications. RSC Adv. 2015, 5, 83239–83285. [Google Scholar] [CrossRef]
- Chen, L.; He, K.; Li, W.; Ma, D.; Xin, X.; Wang, G.; Liu, Q.; Yang, L.; Cheng, F.; Lv, S.; et al. Fast perfluorooctanoic acid (PFOA) removal with honeycomb-like nitrogen-doped carbon nanosheets: Mechanisms for the selective adsorption of PFOA over competing contaminants/water matrix. ACS ES&T Eng. 2024, 4, 3092–3104. [Google Scholar]
- Karbassiyazdi, E.; Kasula, M.; Modak, S.; Pala, J.; Kalantari, M.; Altaee, A.; Esfahani, M.R.; Razmjou, A. A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents. Chemosphere 2023, 311, 136933. [Google Scholar] [CrossRef]
- Li, R.; Alomari, S.; Stanton, R.; Wasson, M.C.; Islamoglu, T.; Farha, O.K.; Holsen, T.M.; Thagard, S.M.; Trivedi, D.J.; Wriedt, M. Efficient removal of per- and polyfluoroalkyl substances from water with zirconium-based metal−organic frameworks. Chem. Mater. 2021, 33, 3276–3285. [Google Scholar] [CrossRef]
- Klemes, M.; Ling, Y.; Ching, C.; Wu, V.; Helbling, D.; Dichtel, W. Reduction of a tetrafluoroterephthalonitrile-β-cyclodextrin polymer to remove anionic micropollutants and perfluorinated alkyl substances from Water. Angew. Chem. Int. Ed. 2019, 131, 12177–12181. [Google Scholar] [CrossRef]
- Yang, A.; Ching, C.; Easler, M.; Helbling, D.E.; Dichtel, W.R. Cyclodextrin polymers with nitrogen-containing tripodal crosslinkers for efficient pfas adsorption. ACS Mater. Lett. 2020, 2, 1240–1245. [Google Scholar] [CrossRef]
- Hatinoglu, M.D.; Perreault, F.; Apul, O.G. Modified linear solvation energy relationships for adsorption of perfluorocarboxylic acids by polystyrene microplastics. Sci. Total Environ. 2023, 860, 160524. [Google Scholar] [CrossRef]
- Mu, T.; Kim, K.-Y. Emerging investigator series: Impacts of aeration flow rates and bubble sizes on PFOA/PFOS removal in electrocoagulation. Environ. Sci. Water Res. Technol. 2023, 9, 1783–1791. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Östlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Y.; Tan, X.; Gunjal, S.J.J.; Dewapriya, P.; Wang, Y.; Xin, R.; Fu, C.; Liu, K.; Macintosh, K.; et al. Fluoropolymer sorbent for efficient and selective capturing of per- and polyfluorinated compounds. Nat. Commun. 2024, 15, 8269. [Google Scholar] [CrossRef]
- O’Neill, M.L.; Cao, Q.; Fang, M.; Johnston, K.P.; Wilkinson, S.P.; Smith, C.D.; Kerschner, J.L.; Jureller, S.H. Solubility of homopolymers and copolymers in carbon dioxide. Ind. Eng. Chem. Res. 1998, 37, 3067–3079. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Valle, J.M.D.; Fuente, J.C.D.L. Supercritical CO2 extraction of oilseeds: Review of kinetic and equilibrium models. Crit. Rev. Food Sci. Nutr. 2006, 46, 131–160. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Yan, J.; Zheng, L. An industrial scale multiple supercritical carbon dioxide apparatus and its eco-friendly dyeing production. J. CO2 Util. 2016, 16, 272–281. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Ryan, K.M.; Padrela, L. Production of biopharmaceutical dried-powders using supercritical CO2 technology. J. Supercrit. Fluids 2022, 187, 105645. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jiang, H.; Song, Y.; Zhou, Z.; Zhao, H. Fabrication and characterization of nano-cellulose aerogels via supercritical CO2 drying technology. Mater. Lett. 2016, 183, 179–182. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Yamamoto, I.; Matsumoto, Y. Preparation of monodispersed poly(methyl methacrylate)particle in the size of micron range. Polym. J. 1990, 22, 759–761. [Google Scholar] [CrossRef]
- Gauthier, J.R.; Mabury, S.A. Identifying unknown fluorine-containing compounds in environmental samples using 19F NMR and spectral database matching. Environ. Sci. Technol. 2023, 57, 8760–8767. [Google Scholar] [CrossRef]
- van de Grampel, R.D.; Ming, W.; Gildenpfennig, A.; van Gennip, W.J.H.; Laven, J.; Niemantsverdriet, J.W.; Brongersma, H.H.; de With, G.; van der Linde, R. The outermost atomic layer of thin films of fluorinated polymethacrylates. Langmuir 2004, 20, 6344–6351. [Google Scholar] [CrossRef]
- Yoshida, E. Preparation of micro- and nanospheres with superamphiphobic surfaces. Colloid Polym. Sci. 2012, 290, 525–530. [Google Scholar] [CrossRef]



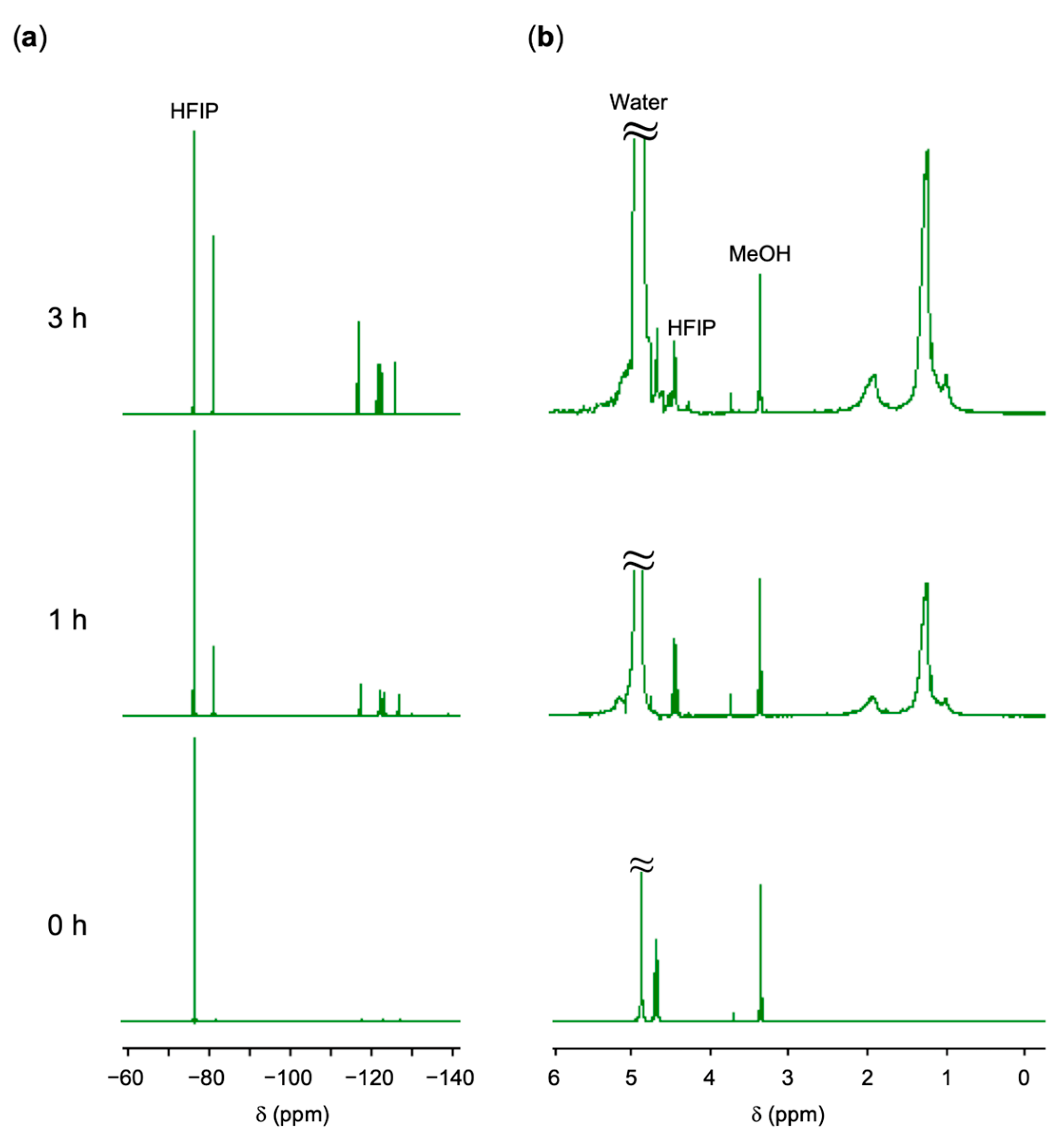
| PFCA | n a | PVP | Time (h) | Conv. b(%) | Mn c | Mw/Mn c | Dn(μm) | Dw/Dn | |
|---|---|---|---|---|---|---|---|---|---|
| Mn | wt% | ||||||||
| – | – | – | 0 | 21 | 85 | 17,800 | 3.75 | – | – |
| PFNA | 8 | – | 0 | 19 | 87 | 26,800 | 2.11 | – | – |
| PFNA | 8 | 40,000 | 5 | 18 | 81 | 25,200 | 1.77 | 2.57 | 1.13 |
| PFNA | 8 | 360,000 | 5 | 18 | 83 | 25,400 | 1.74 | 1.45 | 1.03 |
| PFNA | 8 | 40,000 | 10 | 18 | 82 | 23,800 | 1.85 | 2.01 | 1.02 |
| PFNA | 8 | 40,000 | 60.5 | 6 | 65 | 36,300 | 1.70 | 0.632 | 1.14 |
| PFNA | 8 | 360,000 | 10 | 18 | 81 | 25,300 | 1.71 | 1.55 | 1.11 |
| PFNA | 8 | 40,000 | 5 | 6 | 51 | 51,800 | 1.28 | 2.50 | 1.09 |
| PFNA | 8 | 360,000 | 5 | 6 | 53 | 34,100 | 1.57 | 1.33 | 1.05 |
| PFHA | 6 | 40,000 | 5 | 24 | 84 | 54,700 | 1.53 | 1.28 | 1.27 |
| PFPA | 4 | 40,000 | 5 | 18 d | 86 | 44,400 | 2.86 | 1.15 | 2.86 |
| PFPA/PFAZ e | 4/7b f | 40,000 | 5 | 18 | 87 | 56,600 | 2.11 | 1.10 | 1.42 |
| PFAZ | 7b f | 40,000 | 5 | 18 | 97 | 133,000 | 1.98 | 1.25 | 1.42 |
| Dn (μm) | (CF2)n a n | Surface (%) | Inside (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1s | N1s | O1s | F1s | C1s | N1s | O1s | F1s | |||
| 1.45 | 8 | 51.21 | 5.71 | 10.55 | 32.53 | 86.11 | 2.49 | 1.01 | 10.39 | |
| 2.57 | 8 | 55.68 | 3.28 | 11.16 | 29.88 | 93.12 | 0.37 | 0.63 | 5.88 | |
| 1.28 | 6 | 53.79 | 3.26 | 10.06 | 32.89 | 93.86 | 1.23 | 2.06 | 2.85 | |
| 1.15 | 4 | 57.41 | 5.86 | 10.75 | 25.98 | 93.59 | 1.83 | 2.00 | 2.58 | |
| 1.10 | 4/7b b | 58.63 | 2.72 | 12.50 | 26.15 | 93.80 | 1.77 | 1.49 | 2.94 | |
| 1.25 | 7b b | 66.84 | 6.27 | 13.52 | 13.37 | 91.80 | 4.84 | 1.69 | 1.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, E. Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers. Polymers 2025, 17, 1688. https://doi.org/10.3390/polym17121688
Yoshida E. Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers. Polymers. 2025; 17(12):1688. https://doi.org/10.3390/polym17121688
Chicago/Turabian StyleYoshida, Eri. 2025. "Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers" Polymers 17, no. 12: 1688. https://doi.org/10.3390/polym17121688
APA StyleYoshida, E. (2025). Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers. Polymers, 17(12), 1688. https://doi.org/10.3390/polym17121688






