Abstract
In this study, a poly [ε-caprolactam-co-bis(2-hydroxyethyl) terephthalate] copolymer (P (CL-co-BHET)) was synthesized from para-terephthalic acid (PTA), ethylene glycol (EG), and caprolactam (CL). The crystallization behavior and thermal stability of the copolymer were thoroughly investigated. With the aid of molecular simulation, this study investigated the variation in interchain hydrogen bonding in the copolymer, focusing on the direction and the number of hydrogen bonds. The results revealed a close relationship between the copolymer chain structure, the variation in interchain hydrogen bonding, and the crystallization behavior and thermal stability of the copolymer. The introduction of BHET segments disrupted the regularity of the PA6 backbone and hydrogen bonding, leading to a decrease in the melting point, crystallization temperature, and crystallinity of the copolymer. The thermal stability of the copolymers also decreased, and the crystallization form gradually shifted from the α-crystalline to the γ-crystalline phase. The findings of this study are significant for evaluating the crystallization behavior of polyester amides and for predicting and regulating the properties of polyesteramide polymers.
1. Introduction
Polyamide, commonly referred to as nylon, is a polymer characterized by amide bonds. Among various types of polyamide, polyamide 6 (PA6) is the most extensively produced. It is typically synthesized via the ring-opening polymerization of caprolactam. The intermolecular hydrogen bonding in PA6 results in strong intermolecular forces and a regular molecular structure. As a semi-crystalline polymer, PA6 exhibits favorable thermal stability and mechanical properties, making it suitable for applications in engineering plastics, films, and fibers [1,2,3,4,5]. Polyesters represent a class of polymers connected by ester bonds, which are known for their excellent hydrolytic stability and biocompatibility [6]. Polyester amide, an advanced material derived from the copolymerization of alternating ester and amide chain segments, integrates the advantages of both polyamides and polyesters. The copolymerization with polyester serves as a significant approach for modifying polyamides. This method not only retains the superior mechanical properties of polyamides but also endows them with enhanced thermal properties, biocompatibility, and biodegradability [7]. Consequently, polyester amides have broader application prospects compared to polyamides and can be utilized in hot-melt adhesives [8], foams [9], biodegradable materials [10,11], etc.
Recent investigations into polyester amides have revealed that random copolymerization typically leads to a reduction in the melting temperature and concurrently impacts the crystallization behavior [12,13]. The crystallization mechanisms of polyamides, particularly PA6, have been extensively elucidated. PA6 exhibits two primary stable crystalline forms: α-crystalline and γ-crystalline. The α-crystalline form is thermodynamically favored, whereas the γ-crystalline form is kinetically favored. The formation of these two crystalline types in PA6 is attributed to distinct hydrogen bond arrangements, and they can interconvert under specific conditions. For instance, annealing, high-temperature crystallization, and solution crystallization promote the formation of the α-crystalline form, while rapid cooling from the melt and high-speed spinning favor the γ-crystalline form [14,15,16,17]. However, the crystallization behavior of polyesteramide copolymers warrants further attention, as it is crucial for understanding and predicting material properties. Evaluating the crystallization behavior to optimize processing conditions is highly advantageous for controlling polymer stability and properties, as well as for the development of final products [18]. Current research often focuses on polyesteramide synthesis, but a comprehensive study that explores the underlying mechanisms of how BHET segments influence hydrogen bonding and induce crystalline phase transformations is lacking. This limits our ability to predict and tailor the properties of polyester amide copolymers for specific applications. To address this, our study specifically investigates the interplay between BHET-induced hydrogen bonding rearrangement and crystalline phase transformation, highlighting the mechanisms through which BHET segments influence the crystallization behavior of the copolymers.
In this study, the poly[ε-caprolactam-co-bis(2-hydroxyethyl) terephthalate] copolymer (P(CL-co-BHET)) was synthesized using para-terephthalic acid (PTA), ethylene glycol (EG), and caprolactam (CL). The crystallization properties and thermal stability of the copolymer were thoroughly investigated. Molecular simulations were employed to examine the changes in the interchain hydrogen bonding of the copolymer molecules, specifically focusing on the influence of the main chain angle and the number of hydrogen bonds. This study elucidated the relationship between the copolymer molecular chain structure, the changes in interchain hydrogen bonding, the crystallization behavior, and the thermal stability of the copolymer. These findings not only provide a deeper understanding of the structure–property relationships in polyester amides but also offer valuable insights for the development of new materials with tailored properties for specific applications.
2. Experimental Section
2.1. Materials
ε-Caprolactam (CL) was supplied by Sinopec Hunan Petrochemical (Hunan, Yueyang City, China). Para-terephthalic acid (PTA), ethylene glycol (EG), 2,2,2-trifluoroethanol, and deuterotrifluoroacetic acid were purchased from Acros (Beijing, China). All the above reagents are of analytical grade and used without further purification.
2.2. Synthesis of BHET
PTA (46.2 g) and EG (25.9 g) were mixed to form a slurry. The slurry, along with antimony ethylate (0.017 g) and sodium acetate (0.005 g), was then added to a 250 mL reactor at room temperature. The air inside the reactor was removed under vacuum, and the reactor was subsequently purged with dry, high-purity nitrogen (N2). This vacuum-purging operation was repeated three times to ensure the removal of residual air. The temperature was then raised to 240 °C, and the reaction was maintained at this temperature for 2 h with the pressure controlled at 0.15 MPa and the stirring speed set at 100 rpm. During this period, water was slowly removed from the system, with approximately 98% of the theoretical water amount being expelled. At the end of the reaction, a white powder of bis(2-hydroxyethyl) terephthalate oligomer (BHET) was obtained. This oligomer was repeatedly washed with deionized water and dried under vacuum at 60 °C.
2.3. Copolymerization of CL and BHET
The dried BHET and CL were added to the 250 mL reactor in a predetermined ratio. Water (3 wt%) was introduced as a catalyst. The reactor was then evacuated at room temperature to remove air, and dry, high-purity nitrogen (N2) was introduced. This vacuum-purging operation with N2 was repeated three times to ensure the removal of residual air. Subsequently, dry high-purity N2 was continuously introduced until the pressure inside the reactor reached approximately 0.1 MPa. The reaction was conducted at 200 °C for 1 h and then at 260 °C for 5 h. Finally, the system was evacuated to −0.05 MPa, and continued to react for 1.5 h. At the conclusion of the reaction, the copolymer was extruded from the reactor in a molten state and subsequently cooled to yield the polyesteramide copolymer P(CL-co-BHET).
2.4. Characterization
The chemical structures of the polymers were investigated by 1H NMR (Bruker AVANCE NEO 500 MHz, Rheinstetten, Germany), using trifluoroacetic acid-d as the solvent.
The thermal properties of PA6 samples were tested using a Differential Scanning Calorimeter (DSC, DSC3 METLER TOLEDO, Greifensee, Switzerland) at temperatures ranging from 25 to 260 °C. The testing process consisted of two ramp-ups and one cool-down, with the first ramp-up eliminating the thermal history, and the rate of the primary cool-down versus the secondary ramp-ups was 10 °C/min. The tests were carried out in aluminum pans under a nitrogen atmosphere. The degree of crystallinity (Xc) was calculated by the following equation:
where ∆Hm is the specific enthalpy of melting, and ∆H100 is the enthalpy of melting with 100% crystalline nylon 6 (188 J/g) [19].
The crystalline structures of the PA6 samples were tested using an X-ray diffractometer (XRD Empyrean, Almelo, The Netherlands) with a radiation source of Cu-K α-rays (λ = 0.15406 nm), a scanning rate of 2°/min, a scanning area ranging from 5° to 35°, a voltage of 40 kV, and a current of 30 mA.
The thermal stability properties of the PA6 samples were tested using a thermogravimetric analyzer (TGA, TGA2 METLER TOLEDO, Greifensee, Switzerland). The tests were carried out in a ceramic crucible under an N2 atmosphere at temperatures ranging from 25 °C to 600 °C with a temperature increase rate of 10 °C/min.
2.5. Molecular Simulation
The molecular simulations were conducted using an IBM FLEX X240 clustered server, employing the Material Studio 2017R2 software. Molecular dynamics optimization was performed using the NVE system with the Andersen method for temperature control at 600 K. The Compass force field was utilized with a time step of 1 fs and a total simulation time of 500 ps. Following this initial relaxation phase, the molecular dynamics simulation was continued at 300 K for an additional 1000 ps using the NPT system to refine the structure and analyze the relevant properties. The simulation outputted one structure for every 500 steps of the calculation, with an intercept radius of 1.35 nm.
3. Results and Discussions
The PTA and EG were first esterified to produce low-molecular-weight BHET, which exhibited a degree of polymerization within 5 to 15, with an average polymerization degree of 10. Thereafter, the BHET was subjected to copolymerization with caprolactam and water in diverse molar ratios. This copolymerization procedure resulted in the formation of a series of polyester amide copolymers, designated as P(CL-co-BHET). The detailed reaction process is illustrated in Figure 1.
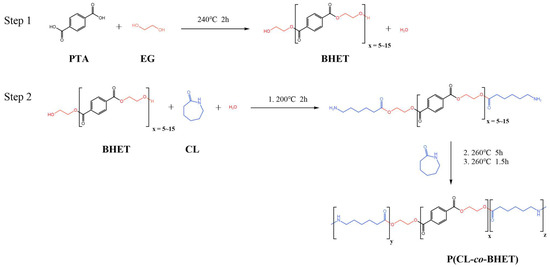
Figure 1.
Preparation process of P(CL-co-BHET).
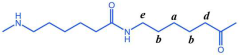
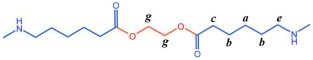
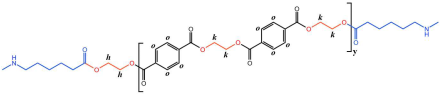
The structure of the polymer was elucidated using 1H NMR spectroscopy. Figure 2A presents the 1H NMR spectrum of the BHET, where the chemical shift peaks are as follows: δ4.34 (a) and δ4.78 (b) correspond to the hydrogen atoms on the methylene groups at the terminal ends, while δ4.98 (c) represents the hydrogen atoms on the methylene groups within the main chain of the BHET. The peak at δ8.30 (d) is attributed to the hydrogen atoms on the benzene ring. Based on the area ratio of the c peak to the a peak, the average degree of polymerization of the BHET is determined to be approximately 10. Figure 2B displays the 1H NMR spectrum of the copolymer P(CL-co-BHET). The copolymerization product is relatively complex due to the ester–amide bond exchange that occurs during the copolymerization process [20]. This bond exchange results in a more homogeneous distribution of the BHET chain segments within the P(CL) molecular chain. The chemical shift signals observed in the 1H NMR spectra provide detailed insights into the structural composition of the copolymer. The signals at δ1.50 (a), δ1.74, δ1.83 (b), δ2.72 (d), and δ3.55 (e) correspond to hydrogen atoms on the P(CL) main chain, while the signal at δ2.53 (c) corresponds to hydrogen atoms on the methylene group attached to the -C=O- of the ester bond. The signal at δ3.64 (f) corresponds to hydrogen atoms on the methylene group attached to the -NH- of the amide bond on the side of the benzene ring. The signals at δ3.95 (g), δ4.47 (h), δ4.64 (i), δ4.73 (j), and δ4.89 (k) correspond to hydrogen atoms on the two methylene groups of EG, with different signals indicating different bonding configurations. The signals at δ7.90 (l), δ7.93 (m), δ8.21 (n), and δ8.27 (o) correspond to hydrogen atoms on the benzene ring of PTA, with different positions indicating different bonding environments. These detailed chemical shifts help to elucidate the various possible structures of the copolymer, as listed in Table 1.
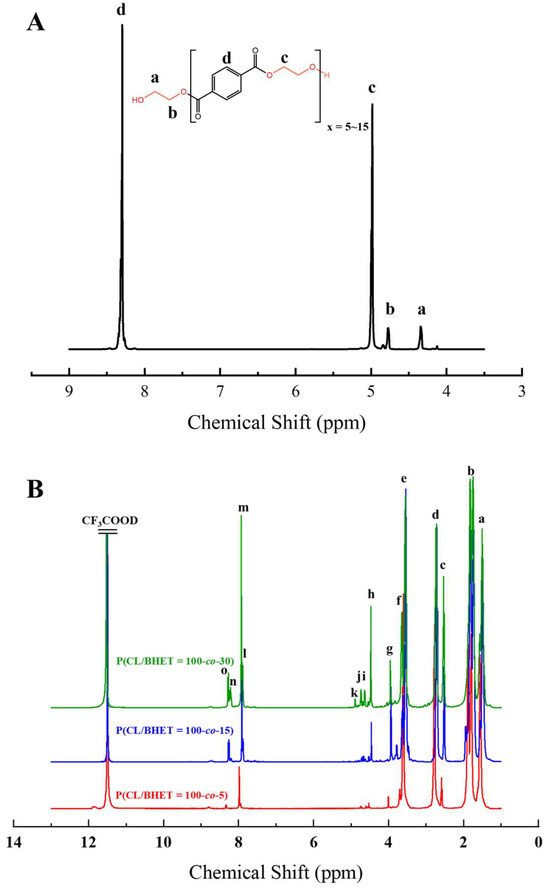
Figure 2.
The 1H NMR s of BHET (A) and P(CL-co-BHET) (B).

Table 1.
Possible structures of P(CL-co-BHET) copolymers and corresponding atom numbering.
The melting and crystallization behaviors of the PA6 and P(CL-co-BHET) copolymers are illustrated in Figure 3. As the BHET content increases, the melting and crystallization temperatures of the copolymers gradually decrease, and the melting peaks become progressively broader. Specifically, when the BHET content rises from 0% to 30%, the melting point of the polymer drops from 218 °C to 175 °C, the crystallization temperature decreases from 166 °C to 120 °C, and the crystallinity reduces from 25.4% to 15.5% (Table 2). These changes are attributed to the disruption of the regularity of the PA6 molecular chain and hydrogen bonding by the incorporation of BHET segments. The presence of BHET segments hinders the close stacking of PA6 molecular chains during crystallization, thereby reducing the stability of the crystal structure and leading to a lower melting point. The rigid structure of BHET segments restricts the motion of PA6 during crystallization, resulting in a lower crystallization temperature. The addition of BHET also reduces the number of hydrogen bonds, weakens the chain regularity during crystallization, and thus lowers the degree of crystallinity. Notably, when the BHET content reaches 30%, the melting peak exhibits a tendency to split into two peaks. This may be due to the formation of a certain length of block structure in the molecular chain, leading to slight phase separation, where PBHET and P(CL-co-BHET) segments crystallize separately and show two distinct melting points. This phenomenon is also supported by the ¹H NMR characterization results of P(CL-co-BHET = 100/30) and has been similarly observed in studies of poly[(ε-caprolactone)-(ω-aminoundecanoic acid)] copolymers [21].
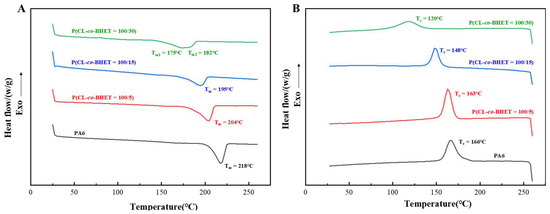
Figure 3.
(A): Melting curves of PA6 and P(CL-co-BHET) copolymers with different BHET polymerization content; (B): crystallization curves of P(CL-co-BHET) copolymers with different BHET polymerization content.

Table 2.
The thermal properties of the PA6 and P(CL-co-BHET) copolymers.
The TGA/DTA curves under a nitrogen atmosphere (Figure 4) show the thermal degradation behavior of the PA6 and P(CL-co-BHET) copolymers. The thermal decomposition temperature (Td5%) at a mass loss of 5 wt% decreases with increasing BHET content. This trend is consistent with observations in other copolymer systems such as poly[(ε-caprolactam)-(ε-caprolactone)] copolymers [22]. In contrast to the single-stage degradation observed in pure PA6, the P(CL-co-BHET) copolymers exhibit a two-stage degradation pattern characterized by a maximum thermal decomposition rate at two different temperatures (Tf). As the BHET content increases, Tf1 gradually decreases, and the proportion of the first-stage thermal weight-loss component increases, as evidenced by a gradual decrease in the residual mass percentage (W1) at the maximum rate of the first thermal decomposition. Meanwhile, Tf2 remains relatively constant, but the residual mass percentage (W2) at Tf2 also decreases. Detailed thermal stability data are shown in Table 3, where Td5% decreases from 386 °C to 315 °C, W1 decreases to 48.5%, and W2 decreases to 10.0% as the BHET content reaches 30%. Despite these changes, the Td5% of all the P(CL-co-BHET) copolymers remains above 310 °C, indicating that these copolymers are thermally stable enough for normal processing and application. The first stage of degradation is primarily attributed to the thermal degradation of the P(BHET) chain segments, with a higher BHET content leading to faster mass loss. The second stage corresponds to the thermal degradation of the P(CL) chain segments. The degradation mechanism of P(CL-co-BHET) copolymers is complex. The mass loss at each stage is not equivalent to the corresponding mass content of the P(BHET) and P(CL) chain segments. The degradation products of the P(BHET) chain segments may influence the thermal degradation of the P(CL) chain segments, and the interactions and synergistic effects between different chain segments also affect the overall thermal degradation process of the copolymers.
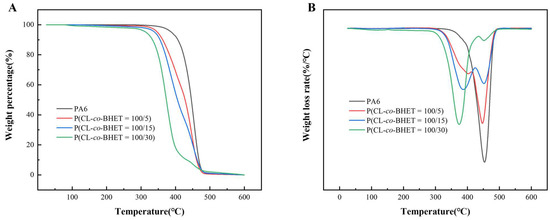
Figure 4.
(A): The TGA curves of PA6 and P(CL-co-BHET) copolymers with different BHET polymerization content; (B): the DTA curves of PA6 and P(CL-co-BHET) copolymers with different BHET polymerization content.

Table 3.
The thermal stability properties of the PA6 and P(CL-co-BHET) copolymers.
The XRD diffraction patterns of PA6 and P(CL-co-BHET) copolymers are presented in Figure 5. Figure 5A shows the XRD pattern of pure PA6, which exhibits two characteristic diffraction peaks at 2θ = 20.1° (200) and 23.8° (002, 202), corresponding to the α-crystalline form [23]. Upon the addition of BHET, the α-crystalline form gradually transforms into the γ-crystalline form. When the polymerized content of BHET is 5%, the copolymer displays both the α- and γ-crystalline forms, with an additional characteristic peak at 2θ = 21.5° (100) (Figure 5B), indicative of the γ-crystalline form. As the BHET content increases to 15%, the copolymer continues to exhibit the coexistence of the α- and γ-crystalline phases, but the proportion of the α-crystalline form decreases, with the γ-crystalline form becoming dominant (Figure 5C). When the BHET content reaches 30%, the copolymer crystals are entirely γ-crystalline (Figure 5D). This transformation is attributed to the changes in the hydrogen bonding arrangement caused by BHET, including the number of hydrogen bonds and the deflection angle between hydrogen bonds and the main chain, which promotes the formation of the γ-crystalline form. And the stable arrangement of the methylene chain can make the γ-crystalline type more stable [24,25]. In addition, the Scherrer equation (Equation (2)) was used to determine the crystal size (LHKL) to study the relationship between the crystallization behavior of the P(CL-co-BHET) copolymers and the crystal size.
where K is the shape factor, taken as 0.89 for all peaks, and β is the full width at half peak (FWHM) of the diffraction peaks with radian (i.e., FWHM × π/180). The calculated microcrystalline parameters of P(CL-co-BHET) copolymers are shown in Table 4. With the increase in BHET content, the grain size of the α-crystalline type gradually increases, while its proportion decreases. Conversely, the grain size of the γ-crystalline type decreases, and its proportion increases. The crystallization behavior of PA6 can be attributed to the inherent regularity of its molecular chains, which promotes an orderly crystallization process. This regularity leads to more and smaller α-crystalline grains. However, as the BHET content increases, the introduction of the BHET chain segments disrupts the regularity of the PA6 molecular chain. This disruption hinders the crystallization process of the α-crystalline form and slows down the growth rate of the grains. Although the growth rate is slow, the relatively long crystallization time allows more time for the grains to grow, leading to an increase in grain size. At the same time, the proportion of α-crystalline forms in the copolymer decreases, resulting in the formation of more γ-crystalline grains. These grains have limited space to grow, resulting in a gradual decrease in the γ-crystalline grain size.
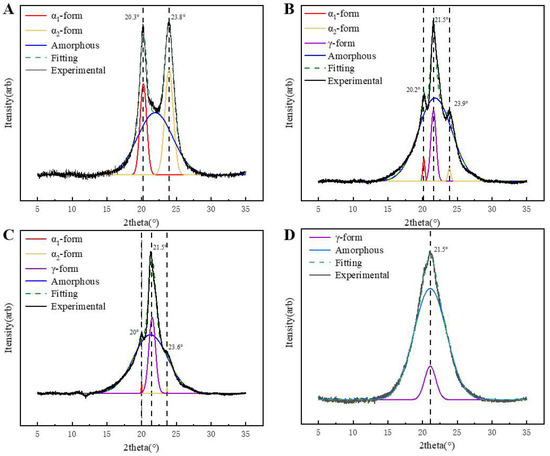
Figure 5.
The wide-angle X-ray diffraction (WAXD) profiles of the (A): pure polyamide 6; (B): P(CL-co-BHET = 100/5); (C): P(CL-co-BHET = 100/15); (D): P(CL-co-BHET = 100/30).

Table 4.
The crystallographic parameters of the PA6 and P(CL-co-BHET) copolymers.
The crystallization behavior of the P(CL-co-BHET) copolymers was simulated using the Materials Studio (MS) software. Initially, the molecular chains of pure PA6 and P(CL-co-BHET) copolymers were modeled. The pure PA6 molecular chain consisted of 100 caprolactam repeating units (e.g., Figure 6 P(CL) chain). Due to ester–amide exchange, the distribution of the BHET chain segments in the molecular chain could result in four possible configurations (A, B, C, and D), as shown in Table 1. Therefore, four distinct structures were modeled (A1, B1, C1, and D1), each containing 36 caprolactam repeating units and 1 BHET structural unit. This configuration ensured that the mass fraction of BHET was approximately 5%, aligning closely with the experimental value. Taking P(CL) as an example, two P(CL) molecular chains were placed into a 3D periodic cell for structural optimization. The size of the 3D periodic cell was 37.3 Å × 37.3 Å × 37.3 Å. Figure 7A shows the optimization results at 300 K. After 1000 ps, the energy has already stabilized. The optimized density is 1.109 g/cm3, which is close to the experimental density of PA6 (1.13 g/cm3). Figure 7B shows the simulation results in the 3D periodic cell, with one P(CL) molecular chain in red and the other P(CL) molecular chain depicted with gray C atoms, white H atoms, red O atoms, and blue N atoms. The N-H┄C=O angle was found to be 164.8°, and the dihedral angle formed by the hydrogen bond to the main chain was 163.9°. Figure 8 shows the results of the crystallization simulation of the P(CL) molecular chain with the A1, B1, C1, and D1 molecular chains in a 3D periodic cell. The detailed data are summarized in Table 5. The number of hydrogen bonds is determined from the average counts over the last 800 steps of the optimization process, which tends to stabilize. Compared to hydrogen bonds formed by two P(CL) chains, the addition of BHET in the four structures resulted in a slight decrease in the N-H┄C=O angle, a significant reduction in the dihedral angle of hydrogen bonds with the backbone, and fewer hydrogen bonds. This is likely due to the BHET repeating unit altering the molecular chain structure, changing interactions between neighboring atoms, and disrupting the regularity of hydrogen bonding, thus affecting the crystalline shape. In the previous study on the crystallization mechanism of nylon by Siddharth et al. [26], simulations on the α and γ-crystalline forms of PA6 showed that while the N-H┄C=O angles did not differ significantly, the dihedral angle formed by hydrogen bonding with the main chain in the γ-crystalline form was significantly reduced compared to that in the α-crystalline form. The introduction of BHET changed the geometrical configuration of hydrogen bonding between molecular chains, altering the directionality of hydrogen bonding and significantly impacting the spatial conformation of the molecular chains. This change affected the stacking of molecular chains during crystallization, causing the crystalline type of PA6 to shift from α-crystalline to γ-crystalline and impeding the formation of hydrogen bonds, leading to a significant decrease in their number. This aligns with the experimental observations of a crystalline phase transition, decreased melting point, reduced crystallinity, and increased crystallization temperature in P(CL-co-BHET) copolymers.
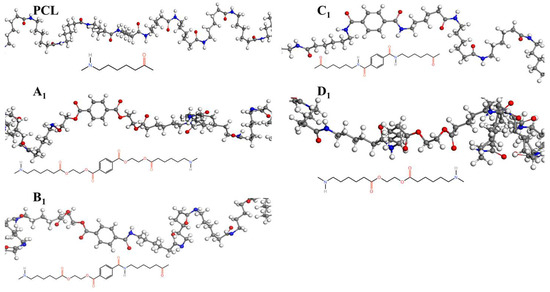
Figure 6.
Molecular chain modeling of P(CL-co-BHET) copolymers.
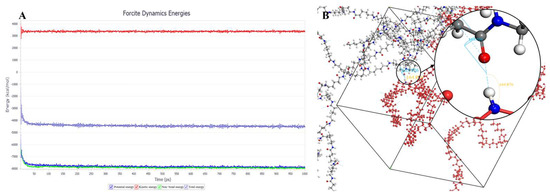
Figure 7.
(A): Results of energy optimization of P(CL) chains in 3D periodic cells in molecular dynamics calculations; (B): simulation results of hydrogen bond formation of P(CL) chains in 3D periodic cells.
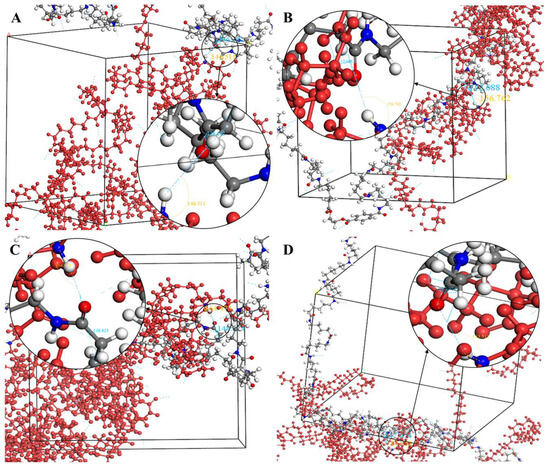
Figure 8.
(A): Simulation results of hydrogen bond formation between a P(CL) chain and an A1 chain in a 3D periodic cell; (B): simulation results of hydrogen bond formation between a P(CL) chain and a B1 chain in a 3D periodic cell; (C): simulation results of hydrogen bond formation between a P(CL) chain and a C1 chain in a 3D periodic cell; (D): simulation results of hydrogen bond formation between a P(CL) chain and a D1 chain in a 3D periodic cell.

Table 5.
Geometric properties and number of hydrogen bonds obtained from simulations.
4. Conclusions
In this study, P(CL-co-BHET) copolymers were synthesized, and the interplay between the molecular structure, hydrogen bonding changes, thermal properties, and crystalline behavior of the copolymers was explored. The incorporation of BHET segments affects the regularity of hydrogen bonding, leading to a slight decrease in the N-H┄C=O angle, a marked reduction in the dihedral angle formed by hydrogen bonds with the main chain, and a significant decrease in the number of hydrogen bonds. These changes result in a lower melting point, reduce thermal stability, and cause a gradual shift in the crystalline type from α-crystalline to γ-crystalline. The grain size of the α-crystalline form gradually increases until it disappears, while the grain size of the γ-crystalline form decreases. This study provides valuable insights into the crystallization behavior of polyester amides and offers a foundation for predicting and tailoring the properties of polyester amide polymers. These findings highlight the potential of P(CL-co-BHET) copolymers for applications in fields where adjustable thermal properties and crystalline structures are desired, such as in the development of low-melting-point hot-melt adhesive materials and biodegradable plastics. Future work will focus on exploring the mechanical properties and long-term stability of these copolymers to further assess their suitability for practical applications.
Author Contributions
Conceptualization, B.Z. and W.L.; methodology, Z.L. and W.L.; validation, Z.L., Q.R., and G.Z.; formal analysis, Z.L.; writing—original draft preparation, Z.L.; writing—review and editing, Z.L. and S.M.; supervision, B.Z. and W.L.; project administration, S.M.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 52403020).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The authors will supply the relevant data in response to reasonable requests.
Conflicts of Interest
Authors Zeyang Li, Qiang Ren, Shan Mei, Wei Liu, Guangyi Zhou, and Baoning Zong were employed by the company Research Institute of Petroleum Progressing, SINOPEC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kohan, M.I. Nylon Plastics Handbook; Hanser: New York, NY, USA, 1995. [Google Scholar]
- Holmes, D.R.; Bunn, C.W.; Smith, D.J. The crystal structure of polycaproamide: Nylon 6. J. Polym. Sci. 1955, 17, 159–177. [Google Scholar] [CrossRef]
- Liu, J.; Liao, G.; Xie, Z.; Li, B.; Cui, L.; Liu, Y. Investigation on the rheological behavior of PA6 film during biaxial stretching. Mater. Today Commun. 2024, 38, 107616. [Google Scholar] [CrossRef]
- Li, B.; Liao, G.; Liu, Y.; Cao, K.; Li, Y.; Xie, Z.; Yin, H.; Cui, L. Investigation on the creep mechanism of PA6 films based on quasi point defect theory. Sci. Rep. 2024, 14, 22169. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Liu, Y.; Zhang, K.; Qin, S.; Qin, J.; He, M.; Yu, J. Simultaneously Enhanced Antioxidation and Plasticization of PA6 by Hindered Phenol Bonded with Aromatic Amide. ACS Appl. Polym. Mater. 2025, 7, 2430–2438. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Gil, M.H.; Simoes, P.N. Biodegradable poly(ester amide)s—A remarkable opportunity for the biomedical area: Review on the synthesis, characterization and applications. Prog. Polym. Sci. 2014, 39, 1291–1311. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, C.W.; Way, T.F.; Rwei, S.-P. Synthesis and characterization of low-temperature polyamide 6 (PA6) copolyamides used as hot melt adhesives and derived from the comonomer of novel aliphatic diamine bis (2-aminoethyl) adipamide and adipic acid. Int. J. Adhes. Adhes. 2020, 101, 102619. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, C.W.; Tasi, M.C.; Rwei, S.-P.; Lee, Y.-H. Biomass Thermoplastic (Co) polyamide Elastomers Synthesized from a Fatty Dimer Acid: A Sustainable Route toward a New Era of Uniform and Bimodal Foams. Ind. Eng. Chem. Res. 2021, 60, 12139–12154. [Google Scholar] [CrossRef]
- Basterretxea, A.; Gabirondo, E.; Sanchez-Sanchez, A.; Etxeberria, A.; Coulembier, O.; Mecerreyes, D.; Sardon, H. Synthesis and characterization of poly (ε-caprolactam-co-lactide) polyesteramides using Brønsted acid or Brønsted base organocatalyst. Eur. Polym. J. 2017, 95, 650–659. [Google Scholar] [CrossRef]
- Lu, D.; Duan, P.; Guo, R.; Yang, L.; Zhang, H. Synthesis and characterization of starch graft biodegradable polyester and polyesteramide by direct polycondensation. Polym. Plast. Technol. Eng. 2013, 52, 200–205. [Google Scholar] [CrossRef]
- Wu, B.; Gong, Y.; Yang, G. Non-isothermal crystallization of polyamide 6 matrix in all-polyamide composites: Crystallization kinetic, melting behavior, and crystal morphology. J. Mater. Sci. 2011, 46, 5184–5191. [Google Scholar] [CrossRef]
- Brucato, V.; Piccarolo, S.; Titomanlio, G. Crystallization kinetics in relation to polymer processing. Makromol. Chem. Macromol. Symp. 1993, 68, 245–255. [Google Scholar] [CrossRef]
- Parodi, E.; Govaert, L.E.; Peters, G.W.M. Glass transition temperature versus structure of polyamide 6: A flash-DSC study. Thermochim. Acta 2017, 657, 110–122. [Google Scholar] [CrossRef]
- Murthy, N.S.; Aharoni, S.M.; Szollosi, A.B. Stability of the γ form and the development of the α form in nylon 6. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 2549–2565. [Google Scholar] [CrossRef]
- Miyasaka, K.; Ishikawa, K. Effects of temperature and water on the γ → α crystalline transition of nylon 6 caused by stretching in the chain direction. J. Polym. Sci. Part A-2 Polym. Phys. 1968, 6, 1317–1329. [Google Scholar] [CrossRef]
- Haberkorn, H.; Hahn, K.; Breuer, H.; Dorrer, H.-D.; Matthies, P. On the neck-like deformation in high-speed spun polyamides. J. Appl. Polym. Sci. 1993, 47, 1551–1579. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, T.; Yang, G. Isothermal crystallization and melting behaviors of nylon 11/nylon 66 alloys by in situ polymerization. Polymer 2006, 47, 2116–2126. [Google Scholar] [CrossRef]
- Kruissink, C.A.; Van der Want, G.M.; Staverman, A.J. On the mechanism of the polymerization of ϵ-caprolactam. I. The polymerization initiated by ϵ-aminocaproic acid. J. Polym. Sci. 1958, 30, 67–80. [Google Scholar] [CrossRef]
- Evstatiev, M.; Schultz, J.M.; Petrovich, S.; Georgiev, G.; Fakirov, S.; Friedrich, K. In situ polymer/polymer composites from poly (ethylene terephthalate), polyamide-6, and polyamide-66 blends. J. Appl. Polym. Sci. 1998, 67, 723–737. [Google Scholar]
- Qian, Z.; Li, S.; He, Y.; Li, C.; Liu, X. Synthesis and thermal degradation of biodegradable polyesteramide based on ε-caprolactone and 11-aminoundecanoic acid. Polym. Degrad. Stab. 2003, 81, 279–286. [Google Scholar] [CrossRef]
- Draye, A.C.; Persenaire, O.; Brožek, J.; Roda, J. Thermogravimetric analysis of poly (ε-caprolactam) and poly [(ε-caprolactam)-co-(ε-caprolactone)] polymers. Polymer 2001, 42, 8325–8332. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, S.; Huang, A. Phase stability and melting behavior of the α and γ phases of nylon 6. J. Appl. Polym. Sci. 2011, 120, 1885–1891. [Google Scholar] [CrossRef]
- Loo, L.S.; Gleason, K.K. Insights into structure and mechanical behavior of α and γ crystal forms of nylon-6 at low strain by infrared studies. Macromolecules 2003, 36, 6114–6126. [Google Scholar] [CrossRef]
- Sakurai, K.; Amador, G.; Takahashi, T. Block copolymers based on nylon 6 and poly (propylene glycol). I. Structure and thermal properties. Polymer 1998, 39, 4089–4094. [Google Scholar] [CrossRef]
- Dasgupta, S.; Hammond, W.B.; Goddard, W.A. Crystal structures and properties of nylon polymers from theory. J. Am. Chem. Soc. 1996, 118, 12291–12301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).