The Effect of Drying Methods on the Pore Structure of Balsa Wood Aerogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
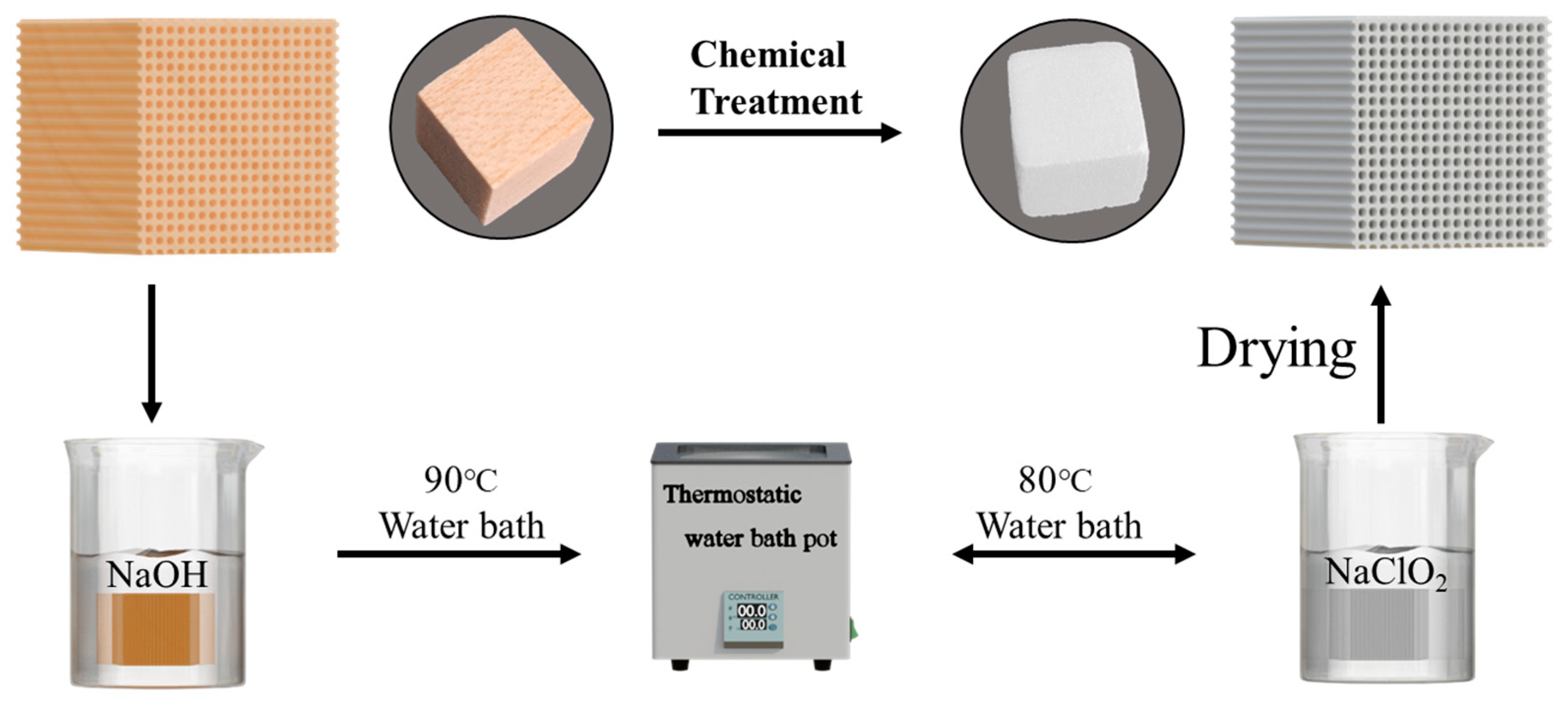
2.2. Preparation of Wood Aerogels
2.3. Freeze Drying to Prepare Wood Aerogel
2.4. Solvent Exchange
2.5. Supercritical Carbon Dioxide Drying to Prepare Wood Aerogel
2.6. Oven Drying to Prepare Wood Aerogel
2.7. Vacuum Drying to Prepare Wood Aerogel
2.8. Nature Drying to Prepare Wood Aerogel
2.9. Characterization of Scanning Electron Microscopy (SEM)
2.10. Crystallinity Testing
2.11. Characterization of Density
2.12. Characterization of Nitrogen Adsorption–Desorption
2.13. Characterization of Thermal Conductivity
2.14. Characterization of Water Absorption Performance
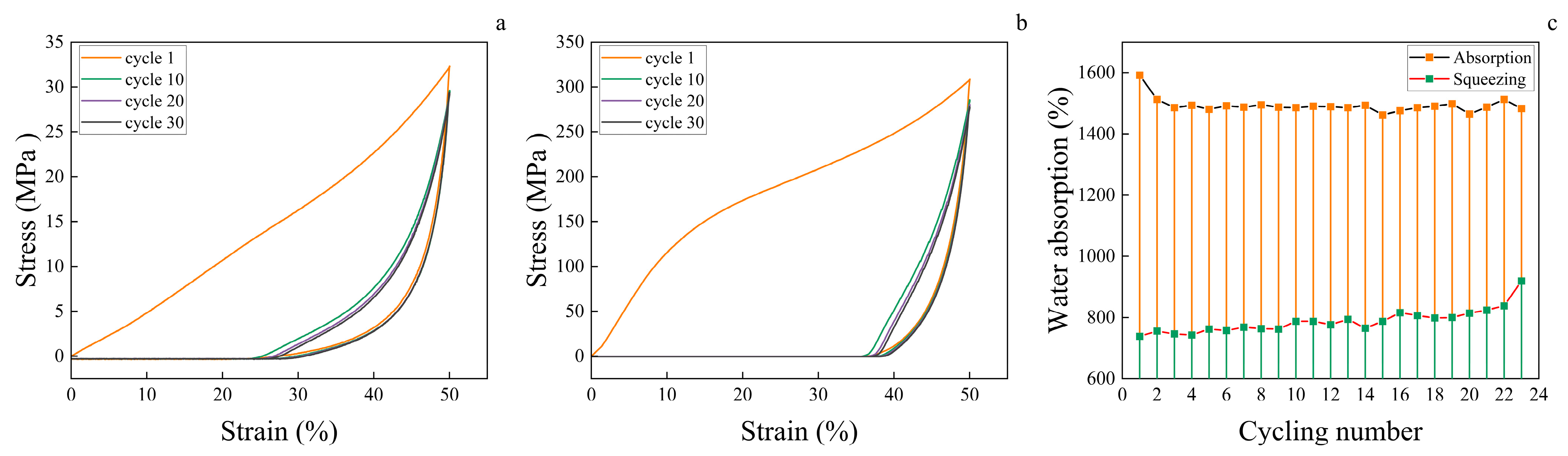
2.15. Cyclic Compression Performance
2.16. Characterization of Cyclic Water Absorption Performance
3. Results and Discussion
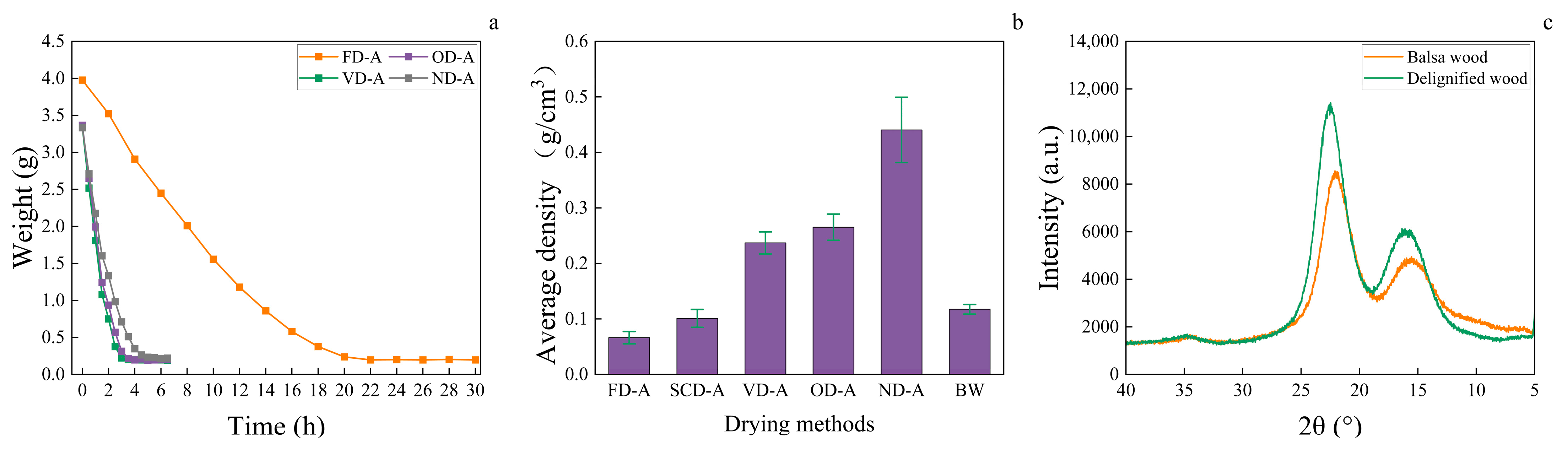
3.1. Wood Aerogel Drying Rate
3.2. Wood Aerogel Density
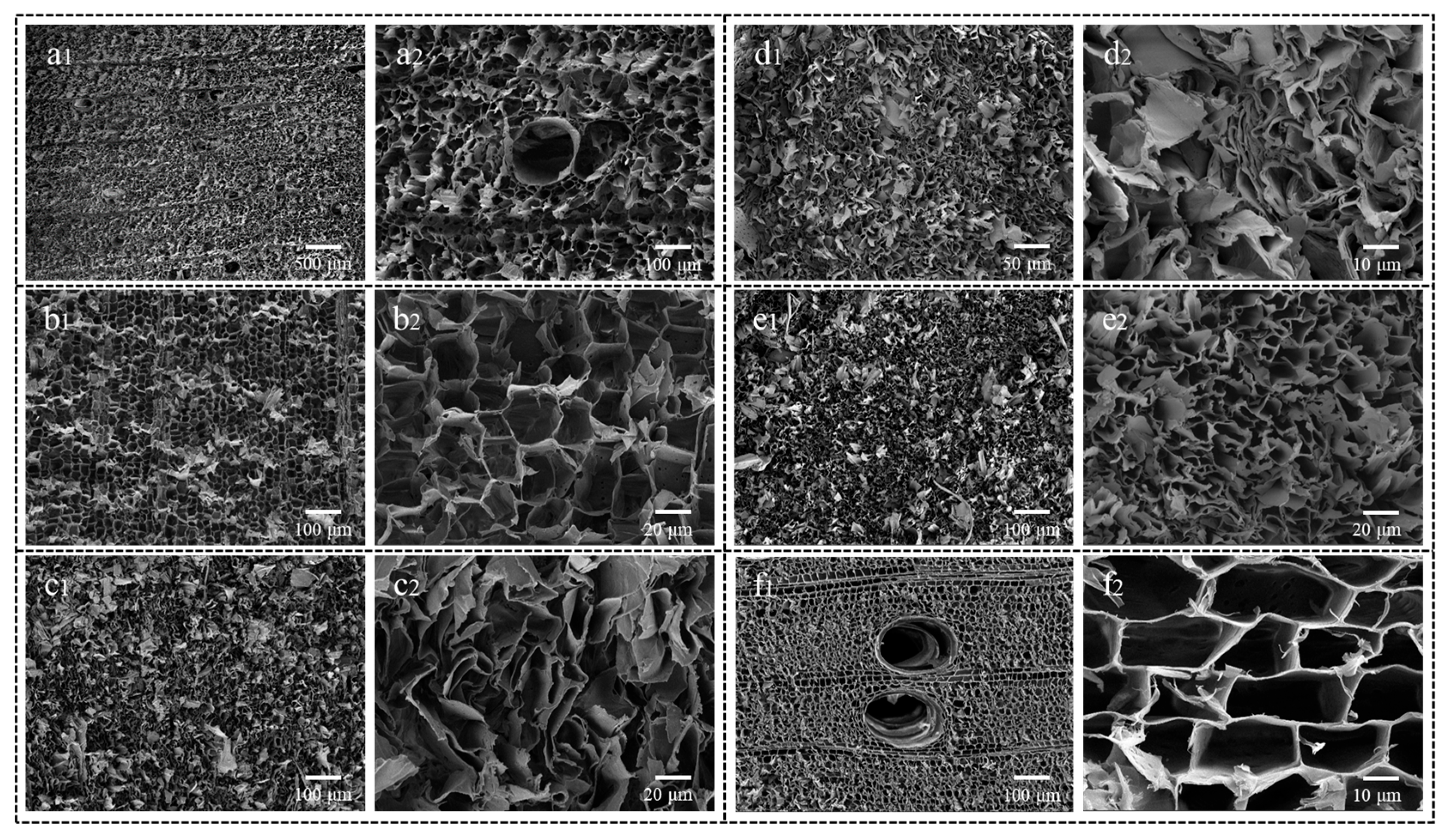
3.3. Wood Aerogel Microstructure
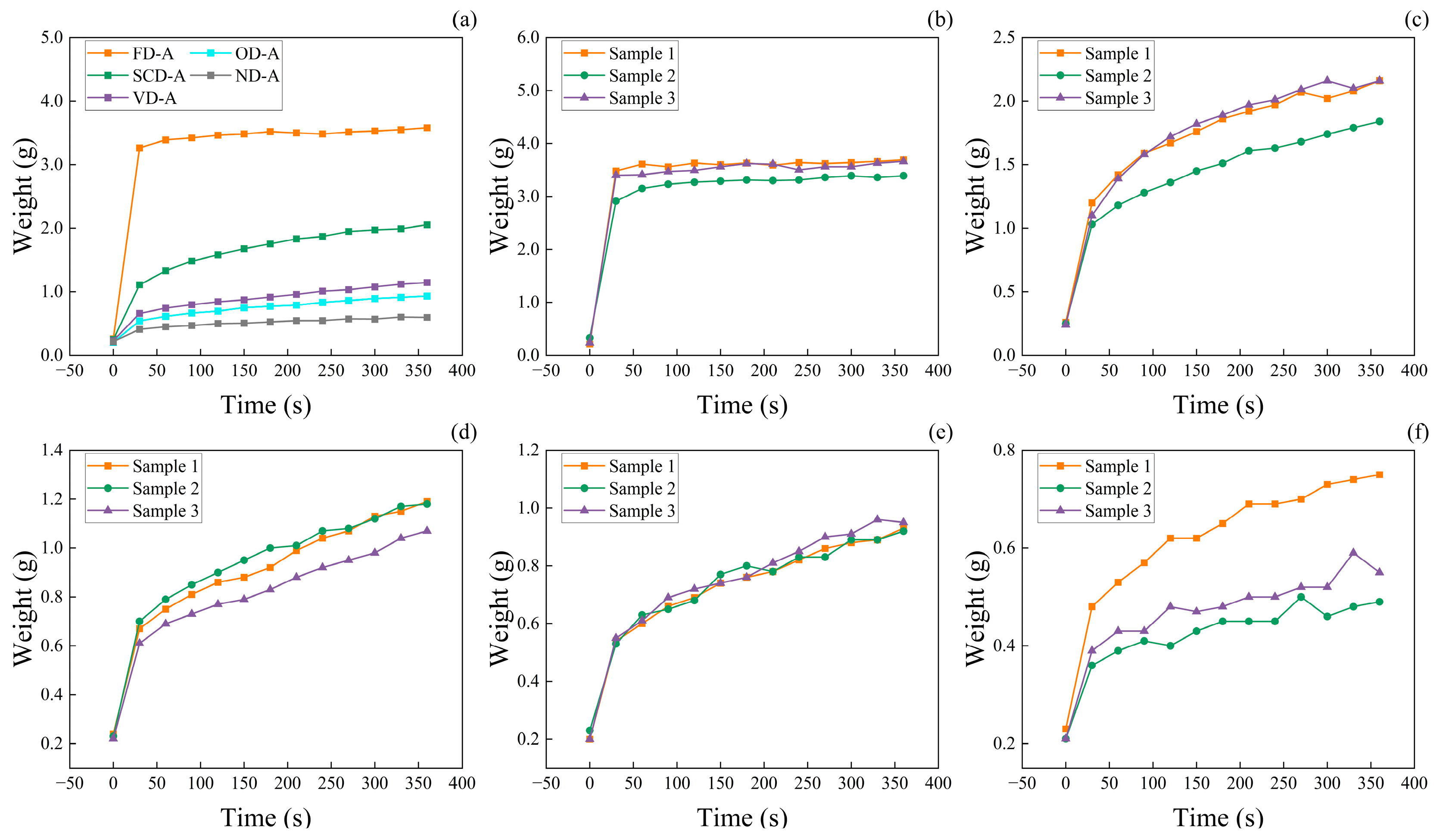
3.4. The Water Absorption Performance of Wood Aerogels
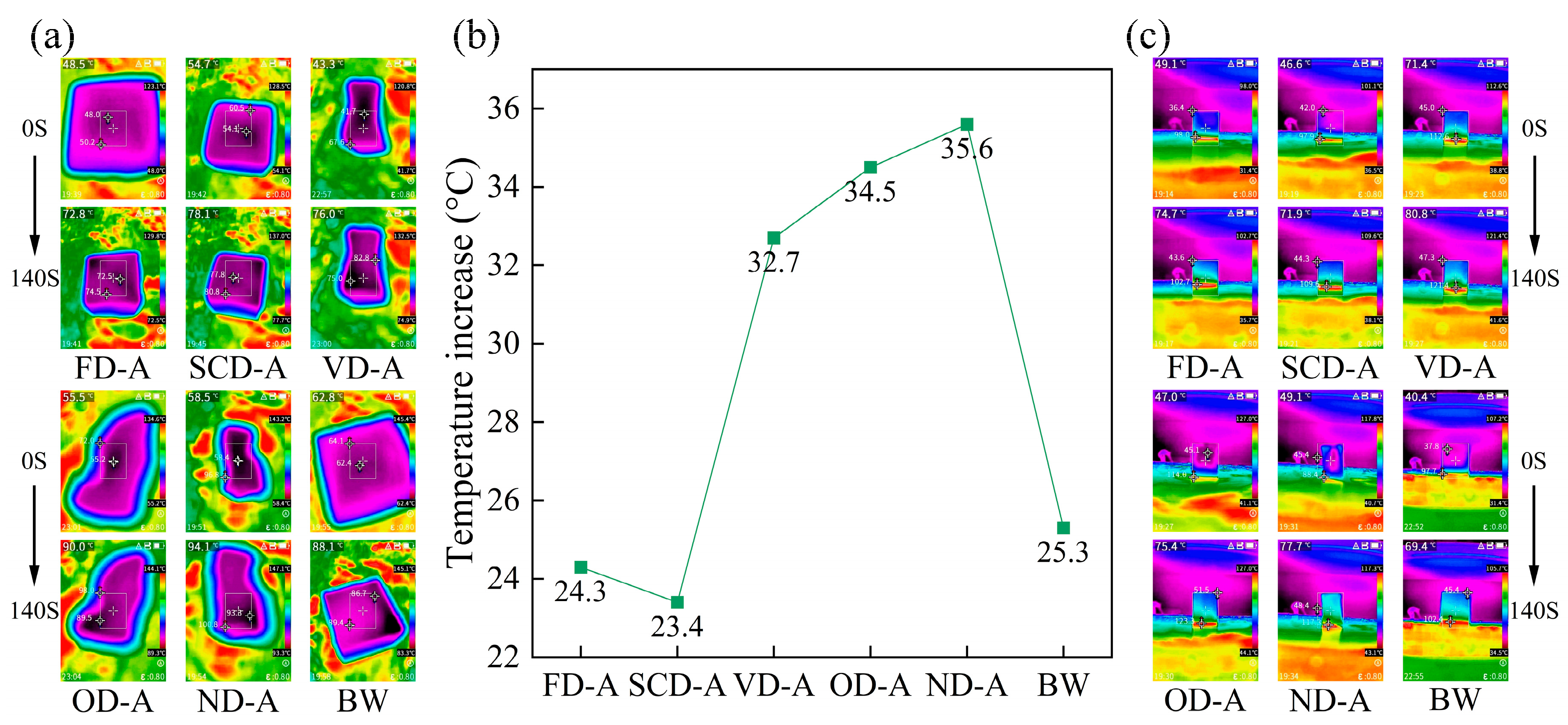
3.5. Thermal Insulation Properties of Wood Aerogels
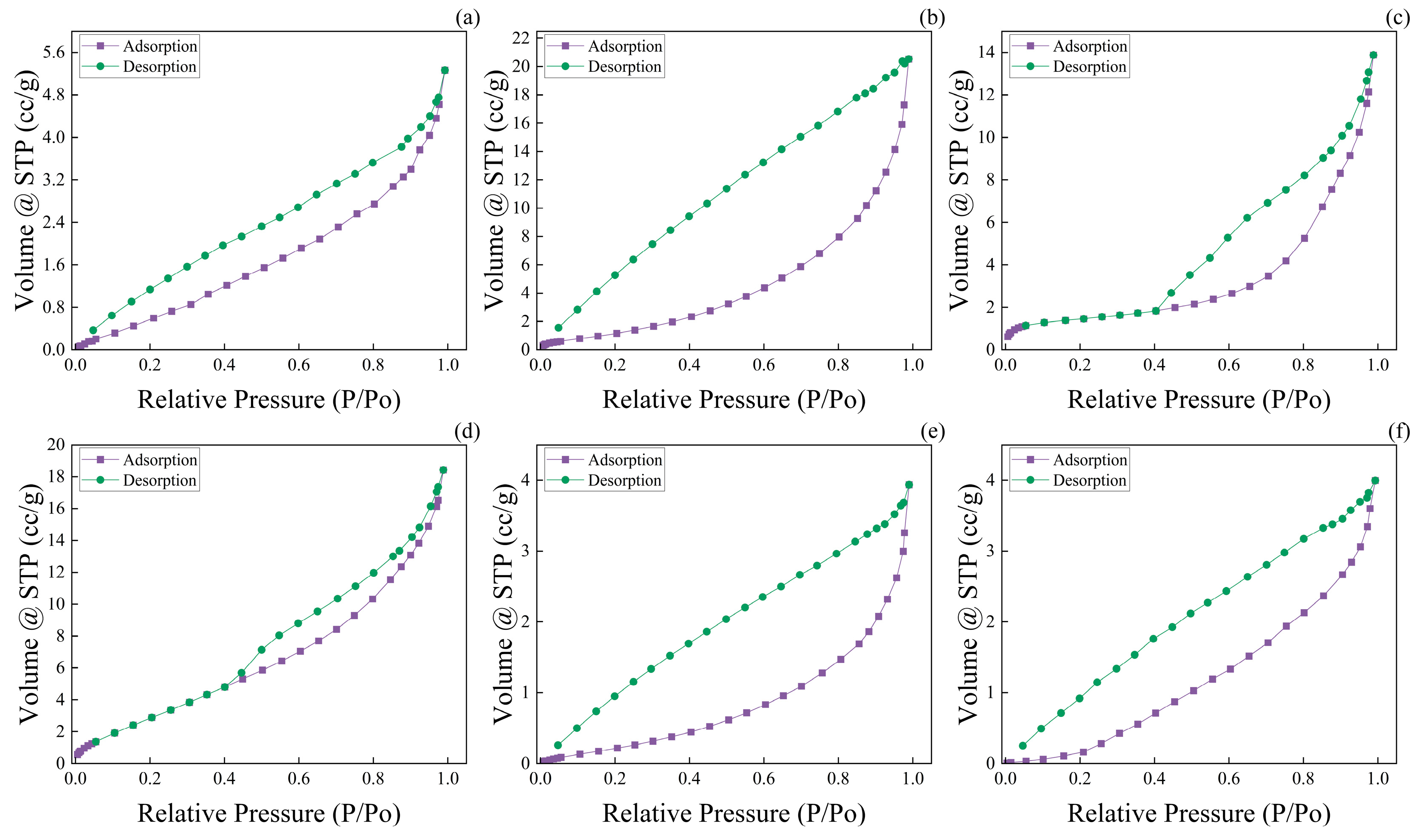
3.6. Wood Aerogel Nitrogen Adsorption
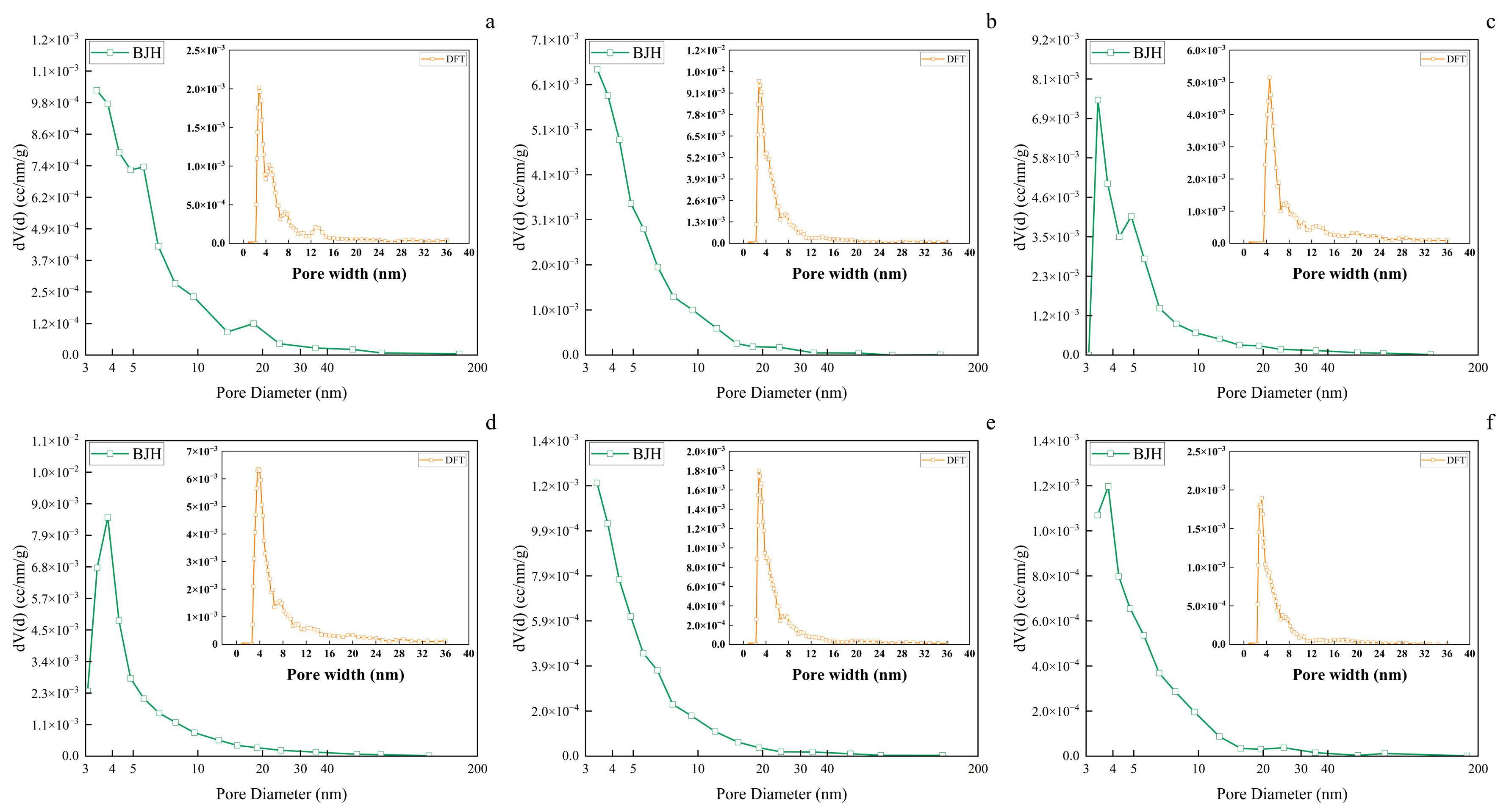
3.7. Wood Aerogel Pore Size Analysis
3.8. Wood Aerogel Cyclic Water Absorption Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Garemark, J.; Yang, X.; Sheng, X.; Cheung, O.; Sun, L.; Berglund, L.A.; Li, Y. Top-Down Approach Making Anisotropic Cellulose Aerogels as Universal Substrates for Multifunctionalization. ACS Nano 2020, 14, 7111–7120. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wei, B.; Liang, S.; Wang, R.; Ji, Q.; Hu, G.; Li, W.; He, L.; Yu, J.; Zhu, H.; et al. Highly Nanostructured and Carboxylated Wood Aerogel-Based Adsorption Membrane Reconstructed by Grafting of Polyacrylic Acid for Efficient Removal of Heavy-Metal Ions. Chem. Eng. J. 2024, 493, 152411. [Google Scholar] [CrossRef]
- He, W.; Cao, J.; Guo, F.; Guo, Z.; Zhou, P.; Wang, R.; Liang, S.; Pang, Q.; Wei, B.; Jiao, Y.; et al. Nanostructured Carboxylated-Wood Aerogel Membrane for High-Efficiency Removal of Cu (II) Ions from Wastewater. Chem. Eng. J. 2023, 468, 143747. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Che, X.; Wang, T.; Willför, S.; Li, M.; Li, C. Encapsulating Amidoximated Nanofibrous Aerogels within Wood Cell Tracheids for Efficient Cascading Adsorption of Uranium Ions. ACS Nano 2022, 16, 13144–13151. [Google Scholar] [CrossRef] [PubMed]
- Mola Ali Abasiyan, S.; Nasiri Sour, A.; Mokhtari, A.; Dashbolaghi, F.; Sabzi, M. Preparation of Chitosan/Sodium Alginate/Nano Cellulose Composite for the Efficient Removal of Cadmium (II) Cations from Wastewater and Soil Systems. Environ. Geochem. Health 2022, 44, 1259–1275. [Google Scholar] [CrossRef]
- Kong, F.; Ge, J.; Zhu, Z.; Chen, C.; Peng, J.; Li, X.; Li, B.; Ma, H. A Conjugated Microporous Polymer/Wood Aerogel with Physical Adsorption, Chemical Degradation and Antibacterial Self-Cleaning Triple Sewage Treatment Functions. Polymers 2023, 15, 3929. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Lu, K.; Hong, X.; Gao, J.; Xia, M.; Wang, F. Synergistic Adsorption of Real Phosphorus-Containing Domestic Wastewater by in-Situ Growth of MgFe-Layered Double Hydroxides Co-Doped with Dual-Functional Lignosulfonate and La(OH)3 on Wood-Derived Cellulose Aerogel. Chem. Eng. J. 2024, 493, 152725. [Google Scholar] [CrossRef]
- Tian, S.; Yi, Z.; Chen, J.; Fu, S. In Situ Growth of UiO-66-NH2 in Wood-Derived Cellulose for Iodine Adsorption. J. Hazard. Mater. 2023, 443, 130236. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Wang, X.; Liu, Y.; Wu, W.; Xiao, H.; Huang, L.; Dai, H.; Zhou, X.; Bian, H. Aerogels Fabricated from Wood-Derived Functional Cellulose Nanofibrils for Highly Efficient Separation of Microplastics. ACS Sustain. Chem. Eng. 2023, 11, 13928–13938. [Google Scholar] [CrossRef]
- Zheng, D.; Yao, W.; Sun, C.; Chen, X.; Wang, Z.; Wang, B.; Tan, H.; Zhang, Y. Solar-Assisted Self-Heating Ti3C2Tx-Decorated Wood Aerogel for Adsorption and Recovery of Highly Viscous Crude Oil. J. Hazard. Mater. 2022, 435, 129068. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, T.; Ge-Zhang, S.; Mu, P.; Liu, Y.; Cui, J. Wood Sponge for Oil–Water Separation. Polymers 2024, 16, 2362. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X.; et al. In-Situ Immobilization of ZIF-67 on Wood Aerogel for Effective Removal of Tetracycline from Water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, Z.; Zhang, X.-F.; Yao, J. Metal-Organic Frameworks Decorated Wood Aerogels for Efficient Particulate Matter Removal. J. Colloid Interface Sci. 2023, 629, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, D.; Hu, Z.; Zhang, H.; Zhang, Z.; Wang, F.; Xie, Y.; Liu, S.; Wang, Q.; Pittman, C.U. In Situ Growth of Zn-Based Metal–Organic Frameworks in Ultra-High Surface Area Nano-Wood Aerogel for Efficient CO2 Capture and Separation. J. Mater. Chem. A 2023, 11, 16878–16888. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Dong, H.; Yang, B.; Li, X.; Li, X.; Wu, Y.; Xu, K. Diatom-Inspired Nanoscale Heterogeneous Assembly Strategy for Constructing Thermal Insulating Wood-Based Aerogels with Exceptional Strength, Resilience, Degradability, and Flame Retardancy. ACS Nano 2025, 19, 6826–6839. [Google Scholar] [CrossRef]
- Shahid, A.M.; Sangeetha, U.K.; Sahoo, S.K. Facile Fabrication of Green and Sustainable Functionalized Bombax ceiba L. Wood Based Aerogel for Multifunctional Applications. Ind. Crops Prod. 2023, 202, 117076. [Google Scholar] [CrossRef]
- Chang, A.-J.; Xue, C.-H.; Sun, J.-J.; Cheng, J.; Huang, M.-C.; Liu, B.-Y.; Wang, H.-D.; Guo, X.-J.; Ma, C.-Q.; Wan, L.; et al. A Superhydrophobic Wood Aerogel for Radiative Cooling and Sound Absorption. J. Mater. Chem. A 2025, 13, 6440–6450. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.; Ouyang, S.; Liu, Y.; Hu, Z.; Wu, Y.; Qian, J.; Li, Z.; Wang, L.; Ma, S. A Comprehensive Review on Preparation and Functional Application of the Wood Aerogel with Natural Cellulose Framework. Int. J. Biol. Macromol. 2024, 275, 133340. [Google Scholar] [CrossRef]
- Rege, A.; Aney, S.; Milow, B. Influence of Pore-Size Distributions and Pore-Wall Mechanics on the Mechanical Behavior of Cellular Solids like Aerogels. Phys. Rev. E 2021, 103, 043001. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray Freeze-Dried Nanofibrillated Cellulose Aerogels with Thermal Superinsulating Properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Chen, L.; Duan, G.; Mei, C.; Huang, C.; Han, J.; Jiang, S. Anisotropic Nanocellulose Aerogels with Ordered Structures Fabricated by Directional Freeze-Drying for Fast Liquid Transport. Cellulose 2019, 26, 6653–6667. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Huang, Q.; Chen, Z.; Wang, W.; Li, W. Tailorable Lignocellulose-Based Aerogel to Achieve the Balance between Evaporation Enthalpy and Water Transport Rate for Efficient Solar Evaporation. ACS Appl. Mater. Interfaces 2023, 15, 11827–11836. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-Q.; Xie, K.-Y.; Wan, J.-N.; Chen, Q.-Y.; Zuo, X.; Li, X.; Wu, X.; Fei, C.; Yao, S. Effects of Freeze-Drying Processes on the Acoustic Absorption Performance of Sustainable Cellulose Nanocrystal Aerogels. Gels 2024, 10, 141. [Google Scholar] [CrossRef]
- Wu, K.; Yue, X.; Wu, S.; Peng, X.; Zhang, T.; Qiu, F. All Cellulose-Based Superwetting Aerogel/Wood Composite with Disordered/Ordered Pores Structure for Rapid Emulsion Separation. J. Environ. Chem. Eng. 2024, 12, 113905. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, L.; Pang, Z.; Wu, J.; Dong, Y.; Pan, X.; Hu, J.; Qu, J.; Li, J.; Tian, D.; et al. Multifunctional Wood Composite Aerogel with Integrated Radiant Cooling and Fog–Water Harvesting for All-Day Building Energy Conservation. Adv. Funct. Mater. 2025, 35, 2414590. [Google Scholar] [CrossRef]
- Baraka, F.; Ganesan, K.; Milow, B.; Labidi, J. Cellulose Nanofiber Aerogels: Effect of the Composition and the Drying Method. Cellulose 2024, 31, 9699–9713. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Z.; Hu, J.; Wang, W.; Li, W. Facile Preparation of Lignocellulosic Xerogels by Alkali Freezing and Ambient Drying. Green Chem. 2024, 26, 6501–6510. [Google Scholar] [CrossRef]
- Guan, H.; Cheng, Z.; Wang, X. Highly Compressible Wood Sponges with a Spring-like Lamellar Structure as Effective and Reusable Oil Absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef]
- Sivaraman, D.; Siqueira, G.; Maurya, A.K.; Zhao, S.; Koebel, M.M.; Nyström, G.; Lattuada, M.; Malfait, W.J. Superinsulating Nanocellulose Aerogels: Effect of Density and Nanofiber Alignment. Carbohydr. Polym. 2022, 292, 119675. [Google Scholar] [CrossRef]
- Mao, Y.; Sheng, Y.; Fan, Z.; Yang, J.; Liu, J.; Tang, C.; Fu, S. Atmospheric Pressure Dried Discontinuous Pore Gradient Structured CNF-Based Aerogel for Ultra-Low Reflection, Broadband, and Super-High EMI Shielding. Adv. Funct. Mater. 2025, 2421492. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, D.; Chen, X.; Wang, X.; Dong, B.; Dai, X. Vacuum Ammonia Stripping from Liquid Digestate: Effects of pH, Alkalinity, Temperature, Negative Pressure and Process Optimization. J. Environ. Sci. 2025, 149, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Zhu, K.; Yuan, Z.; Zhu, Y. Effect of Density and Thickness on Sound Absorption Performance of Flexible Porous Material-Aerogel. J. Phys. Conf. Ser. 2024, 2827, 012015. [Google Scholar] [CrossRef]
- Sun, H.; Bi, H.; Lin, X.; Cai, L.; Xu, M. Lightweight, Anisotropic, Compressible, and Thermally-Insulating Wood Aerogels with Aligned Cellulose Fibers. Polymers 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Qin, Q.; Hu, R.; Liang, Z.; Han, X.; Yang, H.; Huang, Y.; Zhang, C.; He, S.; Jiang, S. Density Modifications Toward High Mechanical Performance Nanocellulose Aerogels. J. Polym. Sci. 2024. [Google Scholar] [CrossRef]
- Timusk, M.; Kangur, T.; Locs, J.; Šutka, A.; Järvekülg, M. Aerogel-like Silica Powders by Combustion of Sol-Gel Derived Alcogels. Microporous Mesoporous Mater. 2021, 315, 110895. [Google Scholar] [CrossRef]
- Liu, K.-P.; Panda, A.S.; Huang, W.-C.; Ho, R.-M. Vacuum-Driven Orientation of Nanostructured Polystyrene-Block-Poly(L-Lactide) Block Copolymer Thin Films for Nanopatterning. Giant 2024, 19, 100303. [Google Scholar] [CrossRef]
- Zamruddin, N.D.; Salleh, K.M.; Mutalib, H.A.A. Insight to Critical Role of Surface Tension for Cellulose-Based Film: A Review. Int. J. Biol. Macromol. 2025, 303, 140680. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, X.; Chai, Y.; Li, X.; Chen, L.; Feng, X. Superabsorbent Whey Protein Isolates/Chitosan-Based Antibacterial Aerogels: Preparation, Characterization and Application in Chicken Meat Preservation. Int. J. Biol. Macromol. 2024, 259, 128961. [Google Scholar] [CrossRef]
- Ciuffarin, F.; Négrier, M.; Plazzotta, S.; Libralato, M.; Calligaris, S.; Budtova, T.; Manzocco, L. Interactions of Cellulose Cryogels and Aerogels with Water and Oil: Structure-Function Relationships. Food Hydrocoll. 2023, 140, 108631. [Google Scholar] [CrossRef]
- Mubarak, S.A.; Kim, Y.; Elsayed, I.; Hassan, E.B. Cellulose Nanofibril Aerogels Derived from Pickering Emulsion Templates with Anisotropic Droplet Sizes. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136393. [Google Scholar] [CrossRef]
- Tan, Z.; Hu, L.; Yang, D.; Zheng, D.; Qiu, X. Lignin: Excellent Hydrogel Swelling Promoter Used in Cellulose Aerogel for Efficient Oil/Water Separation. J. Colloid Interface Sci. 2023, 629, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yoo, C.G.; Yang, D.; Liu, W.; Qiu, X.; Zheng, D. “Rigid-Flexible” Anisotropic Biomass-Derived Aerogels with Superior Mechanical Properties for Oil Recovery and Thermal Insulation. ACS Appl. Mater. Interfaces 2023, 15, 42080–42093. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Heidari, N.; Fathi, M.; Hamdami, N.; Taheri, H.; Siqueira, G.; Nyström, G. Thermally Insulating Cellulose Nanofiber Aerogels from Brewery Residues. ACS Sustain. Chem. Eng. 2023, 11, 10698–10708. [Google Scholar] [CrossRef]
| Drying Method | FD-A | SCD-A | VD-A | OD-A | ND-A | BW |
|---|---|---|---|---|---|---|
| Specific Surface Area m2/g | 4.4 | 5.4 | 5.3 | 14.3 | 1.2 | 1.1 |
| Pore width (HK) nm | 1.1 | 0.4 | 0.4 | 0.4 | 1.6 | 1.9 |
| Pore width (BJH) nm | 3.4 | 3.4 | 3.4 | 3.8 | 3.4 | 3.8 |
| Pore width (DFT) nm | 2.8 | 2.8 | 4.5 | 3.8 | 2.8 | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Fu, Z.; Yu, X.; Wang, X.; Lu, Y. The Effect of Drying Methods on the Pore Structure of Balsa Wood Aerogels. Polymers 2025, 17, 1686. https://doi.org/10.3390/polym17121686
Yin M, Fu Z, Yu X, Wang X, Lu Y. The Effect of Drying Methods on the Pore Structure of Balsa Wood Aerogels. Polymers. 2025; 17(12):1686. https://doi.org/10.3390/polym17121686
Chicago/Turabian StyleYin, Min, Zongying Fu, Xia Yu, Ximing Wang, and Yun Lu. 2025. "The Effect of Drying Methods on the Pore Structure of Balsa Wood Aerogels" Polymers 17, no. 12: 1686. https://doi.org/10.3390/polym17121686
APA StyleYin, M., Fu, Z., Yu, X., Wang, X., & Lu, Y. (2025). The Effect of Drying Methods on the Pore Structure of Balsa Wood Aerogels. Polymers, 17(12), 1686. https://doi.org/10.3390/polym17121686







