Flame Retardance and Antistatic Polybutylene Succinate/Polybutylene Adipate-Co-Terephthalate/Magnesium Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biodegradable Polymer Composite
2.3. Preparation of Biodegradable Polymer Composite Blown Film
2.4. Preparation of Plasma Technology (Sputtering) on Biodegradable Polymer Composite Films for Antistatic Properties
2.5. Preparation of Nano-Metal-Particles (NMP) Sparking Process on Biodegradable Polymer Composite Films for Antistatic Properties
2.6. Flame-Retardant Characterization
2.7. Mechanical Properties
2.8. Thermogravimetric Analysis (TGA)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Morphological Properties
2.11. Water Contact Angle
2.12. Chemical Structure Characterization by Fourier-Transform Infrared (FTIR) Spectroscopy
2.13. Determine the Antistatic Properties of the Plasma Technology and Nano-Metal-Particles (NMP) Sparking Method on Biodegradable Polymer Composite Films
2.14. Statistical Analysis
3. Results and Discussion
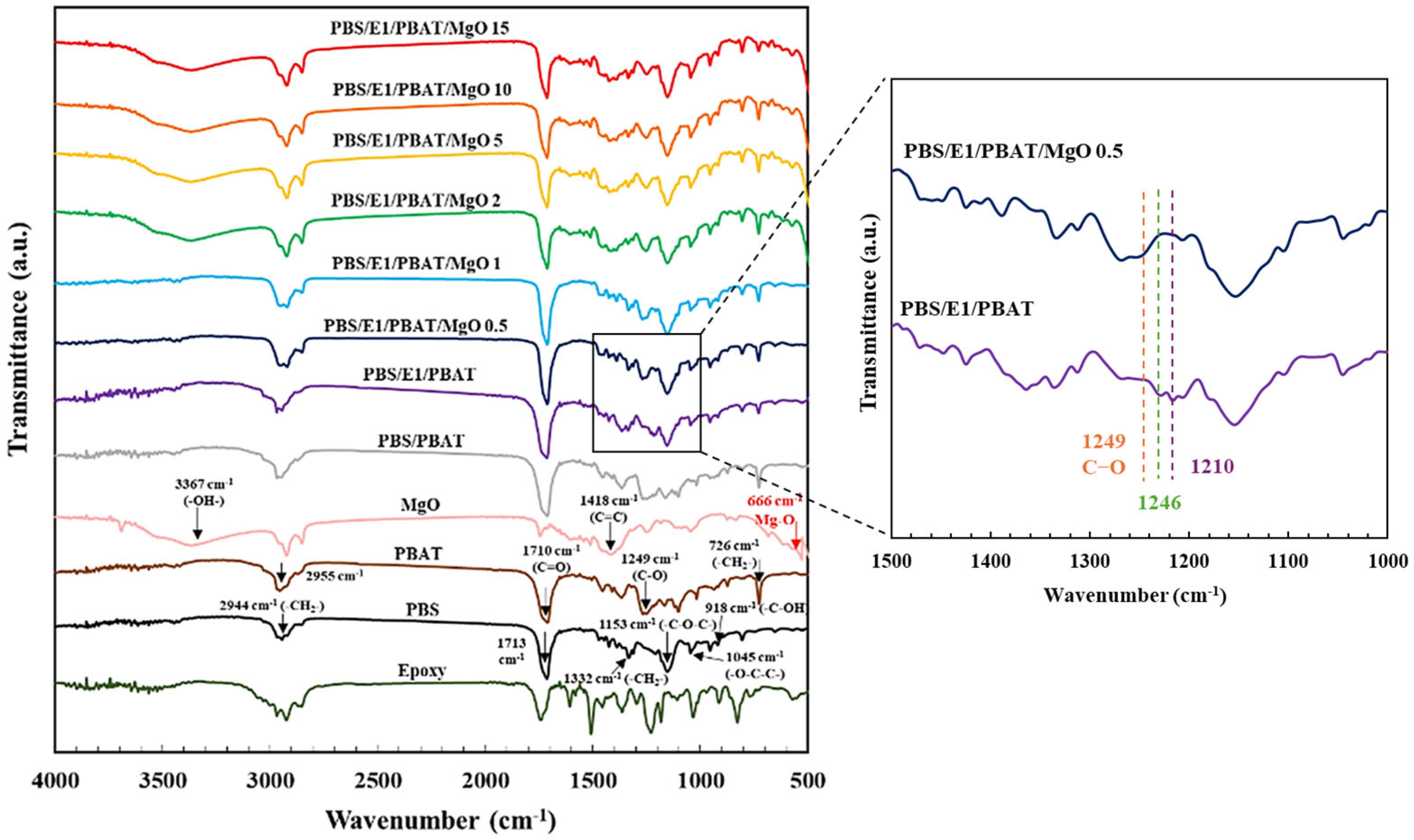
3.1. Chemical Structure
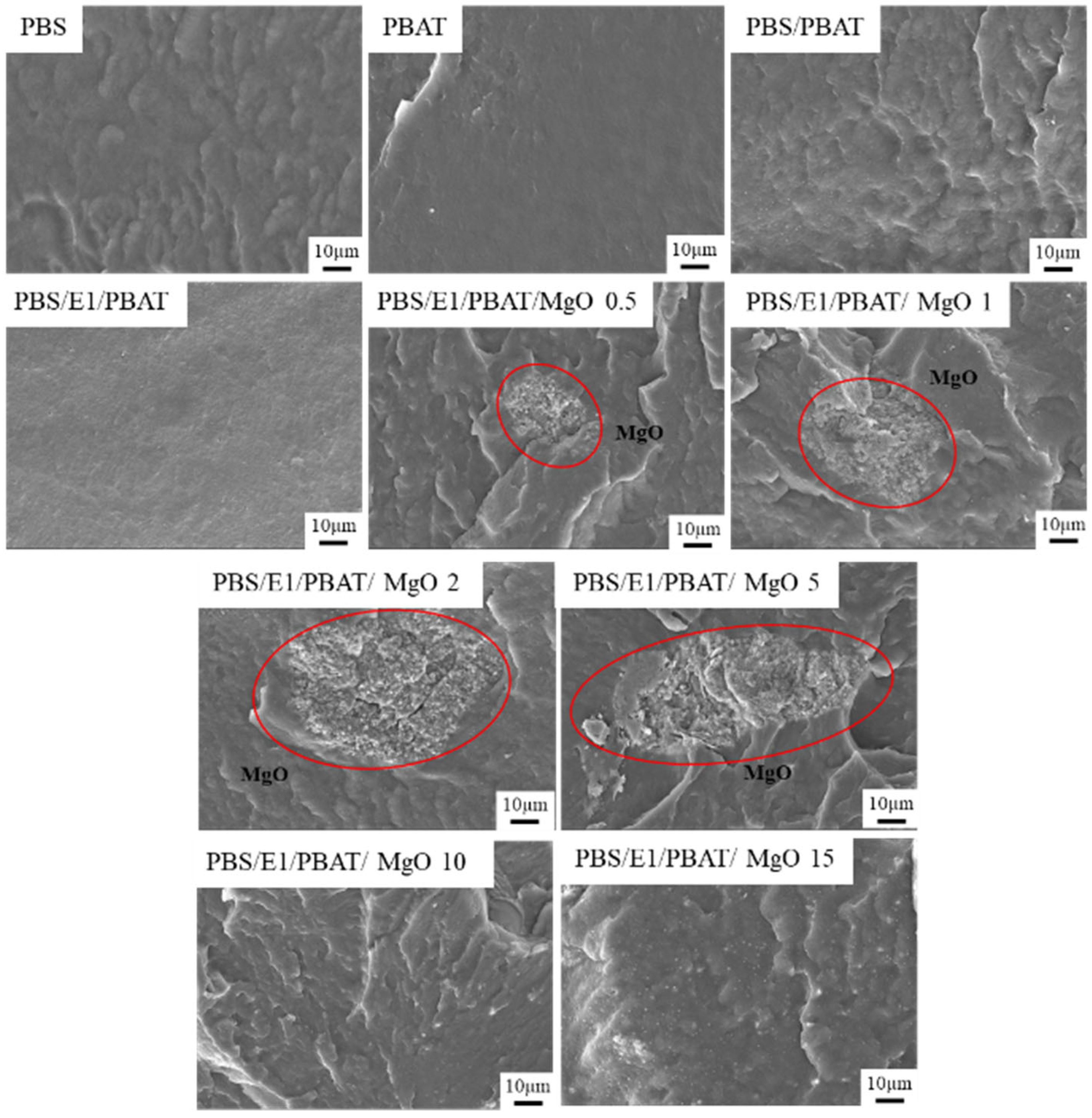
3.2. Morphology
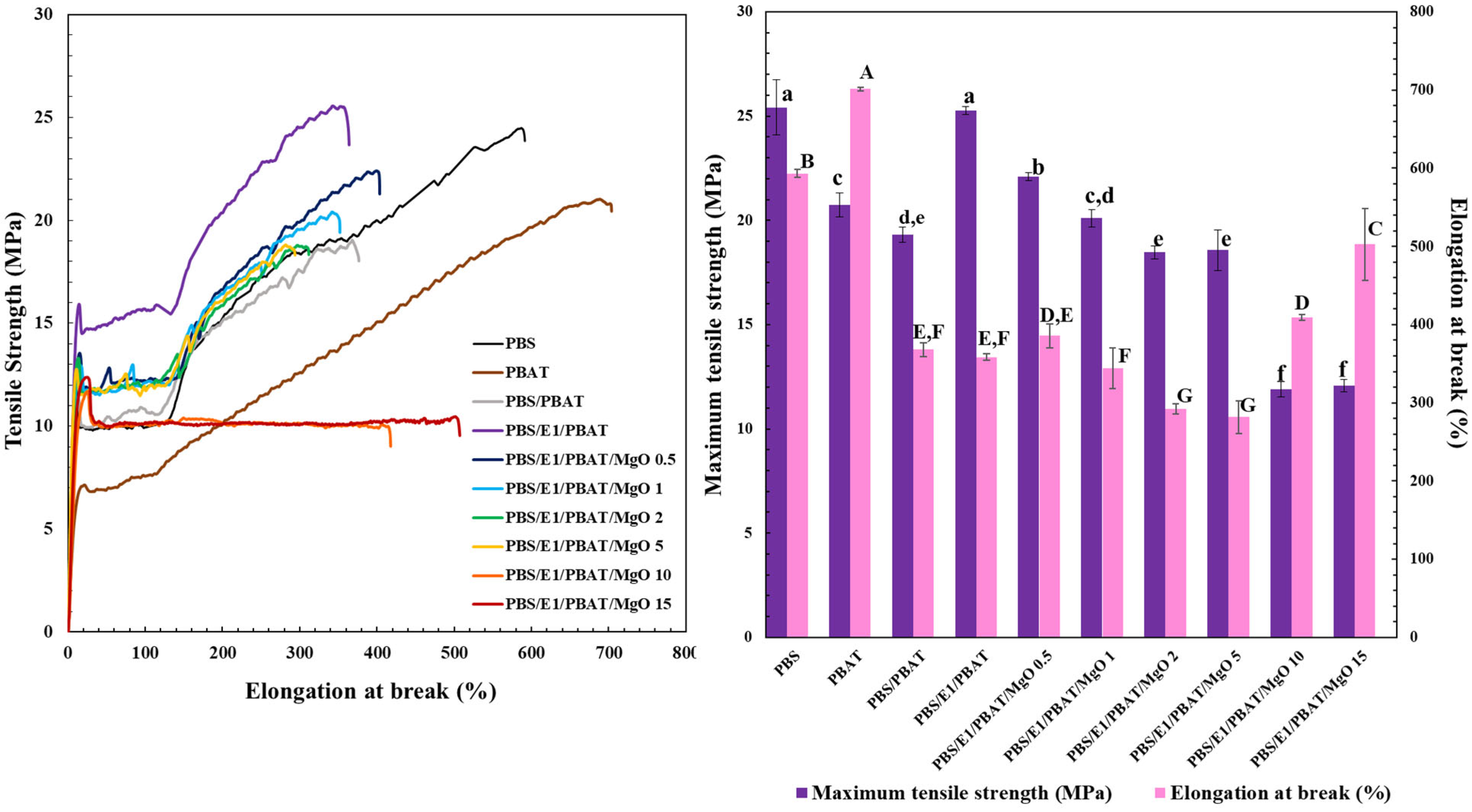
3.3. Mechanical Properties
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetric Analysis (TGA)
3.6. Water Contact Angles
3.7. Flame-Retardant Characterization
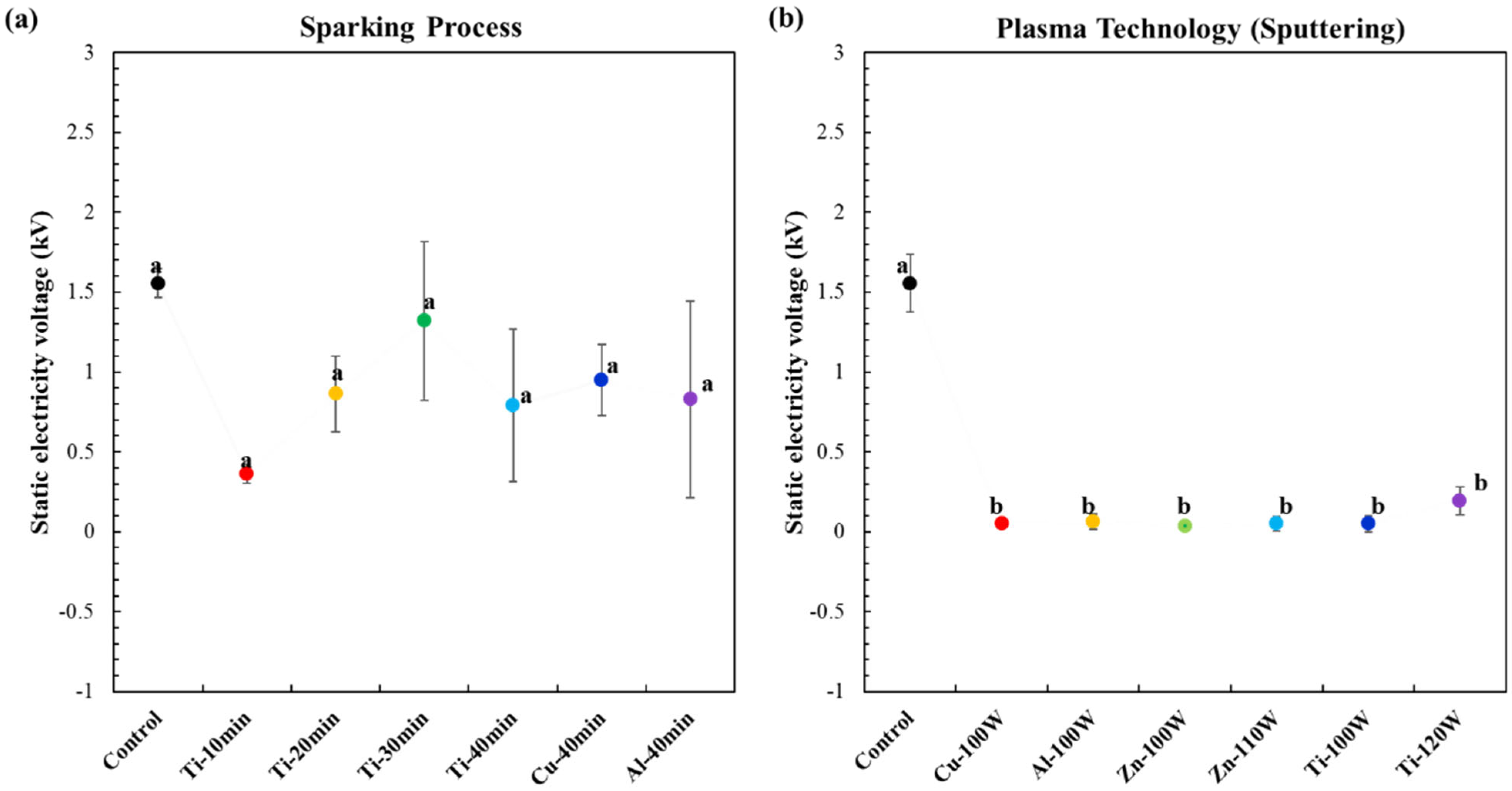
3.8. Antistatic Properties of the Plasma Technology (Sputtering) and Nano-Metal-Particles (NMP) Sparking Process Method for Coating on Biodegradable Polymer Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlMaadeed, M.A.A.; Ponnamma, D.; El-Samak, A.A. Chapter 1—Polymers to Improve the World and Lifestyle: Physical, Mechanical, and Chemical Needs. In Polymer Science and Innovative Applications; AlMaadeed, M.A.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Montemor, M.F. Corrosion Issues in Joining Lightweight Materials: A Review of the Latest Achievements. Phys. Sci. Rev. 2016, 1, 20150011. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive Extrusion of Bio-Based Polymer Blends and Composites-Current Trends and Future Developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(butylene succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- de Matos Costa, A.R.; Crocitti, A.; Hecker de Carvalho, L.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef]
- Liminana, P.; Sanoguera, D.G.; Carrillo, L.Q.; Balart, R.; Montanes, N. Development and Characterization of Environmentally Friendly Composites from Poly(butylene succinate) (PBS) and Almond Shell Flour with Different Compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, C.; Wongwiwattana, P.; Thomas, N.L. Optimising Ductility of Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends Through Co-Continuous Phase Morphology. J. Polym. Environ. 2018, 26, 3802–3816. [Google Scholar] [CrossRef]
- Rodrigues, B.V.M.; Silva, A.S.; Melo, G.F.S.; Vasconscellos, L.M.R.; Marciano, F.R.; Lobo, A.O. Influence of Low Contents of Superhydrophilic MWCNT on The Properties and Cell Viability of Electrospun Poly(butylene adipate-co-terephthalate) Fibers. Mater. Sci. Eng. C 2016, 59, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and Permeability of Bio-Based Poly(butylene adipate-co-terephthalate)(PBAT), Poly(butylene succinate)(PBS) and Linear Low-Density Polyethylene (LLDPE) Blend Films Control Shelf-Life of Packaged Bread. Food Control 2022, 132, 108541. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Brennan, C.S.; Regenstain, J.M.; Jantanasakulwong, K.; Boonyawan, D.; Phimolsiripol, Y. Gliding Arc Discharge Non-Thermal Plasma for Retardation of Mango Anthracnose. LWT 2019, 105, 142–148. [Google Scholar] [CrossRef]
- Könczöl, L.; Döll, W.; Buchholz, U.; Mülhaupt, R. Ultimate Properties of Epoxy Resins Modified with a Polysiloxane–Polycaprolactone Block Copolymer. J. Appl. Polym. Sci. 1994, 54, 815–826. [Google Scholar] [CrossRef]
- Chiou, K.C.; Chang, F.C. Reactive Compatibilization of Polyamide-6 (PA 6)/Polybutylene terephthalate (PBT) Blends by a Multifunctional Epoxy Resin. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 23–33. [Google Scholar] [CrossRef]
- Kodsangma, A.; Homsaard, N.; Nadon, S.; Rachtanapun, P.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Effect of Sodium Benzoate and Chlorhexidine Gluconate on a Bio-Thermoplastic Elastomer Made from Thermoplastic Starch-Chitosan Blended with Epoxidized Natural Rubber. Carbohydr. Polym. 2020, 242, 116421. [Google Scholar] [CrossRef]
- Thomas, R.; Yumei, D.; Yuelong, H.; Le, Y.; Moldenaers, P.; Weimin, Y.; Czigany, T.; Thomas, S. Miscibility, Morphology, Thermal, and Mechanical Properties of A DGEBA Based Epoxy Resin Toughened with A Liquid Rubber. Polymer 2008, 49, 278–294. [Google Scholar] [CrossRef]
- Li, H.; Ning, N.; Zhang, L.; Wang, Y.; Liang, W.; Tian, M. Different Flame Retardancy Effects and Mechanisms of Aluminium Phosphinate in PPO, TPU and PP. Polym. Degrad. Stab. 2014, 105, 86–95. [Google Scholar] [CrossRef]
- Battegazzore, D.; Frache, A.; Carosio, F. Layer-by-Layer Nanostructured Interphase Produces Mechanically Strong and Flame Retardant Bio-Composites. Compos. Part B Eng. 2020, 200, 108310. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Song, P.; Lei, W.; Wu, Q.; Liu, L.; Yu, Y.; Wang, H. Fire-Resistant, Strong, and Green Polymer Nanocomposites Based on Poly(lactic acid) and Core–Shell Nanofibrous Flame Retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Jiang, D.; Pan, M.; Cai, X.; Zhao, Y. Flame Retardancy of Rice Straw-Polyethylene Composites Affected by In Situ Polymerization of Ammonium Polyphosphate/Silica. Compos. Part A Appl. Sci. Manuf. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Pradhan, S.P.; Shubhadarshinee, L.; Mohapatra, P.; Mohanty, P.; Jali, B.R.; Mohapatra, P.; Barick, A.K. Conducting Polymer Composites for Antistatic Application in Aerospace. In Aerospace Polymeric Materials; Inamuddin, Altalhi, T., Adnan, S.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 155–187. [Google Scholar]
- Bharati, B.; Vijaykumar, B.T.; Ramabai, N.; Basavaraj, S. Synthesis, Characterizations, and Physical Properties of Magnesium Oxide with Poly Aniline Nanocomposites. Mater. Today Proc. 2023, 92, 1640–1645. [Google Scholar] [CrossRef]
- Stöckel, S.; Ebert, S.; Böttcher, M.; Seifert, A.; Wamser, T.; Krenkel, W.; Schulze, S.; Hietschold, M.; Gnaegi, H.; Goedel, W.A. Coating of Alumina Fibres with Aluminium Phosphate by a Continuous Chemical Vapour Deposition Process. Chem. Vap. Depos. 2014, 20, 388–398. [Google Scholar] [CrossRef]
- Turkoglu, S.H.; Alazzawi, M.; Kadim, A.A.N. Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation. Molecules 2021, 26, 1665. [Google Scholar] [CrossRef] [PubMed]
- Can-Herrera, L.A.; Ávila-Ortega, A.; de la Rosa-García, S.; Oliva, A.I.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Surface Modification of Electrospun Polycaprolactone Microfibers by Air Plasma Treatment: Effect of Plasma Power and Treatment Time. Eur. Polym. J. 2016, 84, 502–513. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Trimukhe, A.M.; Deshmukh, R.; Joshi, G.M. Disparity in Hydrophobic to Hydrophilic Nature of Polymer Blend Modified by K2Ti6O13 as a Function of Air Plasma Treatment. Prog. Org. Coat. 2017, 111, 371–380. [Google Scholar] [CrossRef]
- Al Ghufais, I.A.; Rahaman, M.; Aldalbahi, A. Evaluation of Physical Properties of Recycled Polyethylene Waste Films and Application of Its Carbon Filled Composites as Anti-Static Material in Electronic Packaging. Bachelor’s Thesis, King Saud University, Riyadh, Saudi Arabia, 2018. [Google Scholar]
- Silva, T.F.d.; Menezes, F.; Montagna, L.S.; Lemes, A.P.; Passador, F.R. Preparation and Characterization of Antistatic Packaging for Electronic Components Based on Poly(lactic acid)/Carbon Black Composites. J. Appl. Polym. Sci. 2019, 136, 47273. [Google Scholar] [CrossRef]
- Zoubek, M.; Kudláček, J.; Kreibich, V.; Jirout, T.; Abramov, A. The Influence of Mixing Method and Mixing Parameters in Process of Preparation of Anti-static Coating Materials Containing Nanoparticles. In Advances in Manufacturing II. Lecture Notes in Mechanical Engineering; Gapiński, B., Szostak, M., Ivanov, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 154–196. [Google Scholar]
- Kumpika, T.; Kantarak, E.; Sroila, W.; Panthawan, A.; Jhuntama, N.; Sanmuangmoon, P.; Thongsuwan, W.; Singjai, P. Superhydrophilic/Superhydrophobic Surfaces Fabricated by Spark-coating. Surf. Interface Anal. 2018, 50, 827–834. [Google Scholar] [CrossRef]
- Kumpika, T.; Thongsuwan, W.; Singjai, P. Optical and Electrical Properties of ZnO Nanoparticle Thin Films Deposited on Quartz by Sparking Process. Thin Solid Film. 2008, 516, 5640–5644. [Google Scholar] [CrossRef]
- Jantanasakulwong, K.; Thanakkasaranee, S.; Seesuriyachan, P.; Singjai, P.; Saenjaiban, A.; Photphroet, S.; Pratinthong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Chaiyaso, T.; et al. Sparking Nano-Metals on a Surface of Polyethylene Terephthalate and Its Application: Anti-Coronavirus and Anti-Fogging Properties. Int. J. Mol. Sci. 2022, 23, 10541. [Google Scholar] [CrossRef]
- Underwriters Laboratories. UL 94 Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances, 7th ed.; Underwriters Laboratories: Northbrook, IL, USA, 2023; Available online: https://omnexus.specialchem.com/polymer-property/flammability-ul94 (accessed on 12 May 2025).
- JIS K 6251-7; Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties. Japanese Industrial Standards Committee: Tokyo, Japan, 2017.
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins-Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. Infrared Spectrosc.-Mater. Sci. Eng. Technol. 2012, 2, 261–284. [Google Scholar]
- Cecen, V.; Seki, Y.; Sarikanat, M.; Tavman, I.H. FTIR and SEM Analysis of Polyester- and Epoxy-Based Composites Manufactured by VARTM Process. J. Appl. Polym. Sci. 2008, 108, 2163–2170. [Google Scholar] [CrossRef]
- Yin, Q.; Chen, F.; Zhang, H.; Liu, C. Fabrication and Characterisation of Thermoplastic Starch/Poly(butylene succinate) Blends with Maleated Poly(butylene succinate) as Compatibiliser. Plast. Rubber Compos. 2015, 44, 362–367. [Google Scholar] [CrossRef]
- Ostrowska, J.; Sadurski, W.; Paluch, M.; Tyński, P.; Bogusz, J. The Effect of Poly(butylene succinate) Content on the Structure and Thermal and Mechanical Properties of Its Blends with Polylactide. Polym. Int. 2019, 68, 1271–1279. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2012, 21, 108–114. [Google Scholar] [CrossRef]
- Boonprasertpoh, A.; Pentrakoon, D.; Junkasem, J. Effect of PBAT on Physical, Morphological, and Mechanical Properties of PBS/PBAT Foam. Cell. Polym. 2019, 39, 31–41. [Google Scholar] [CrossRef]
- Liu, B.; Guan, T.; Wu, G.; Fu, Y.; Weng, Y. Biodegradation Behavior of Degradable Mulch with Poly (Butylene Adipate-co-Terephthalate) (PBAT) and Poly (Butylene Succinate) (PBS) in Simulation Marine Environment. Polymers 2022, 14, 1515. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Mousavi, M.; Shabanian, M.; Habibnejad, N.; Vahabi, H. Design and Preparation of New Polypropylene/Magnesium Oxide Micro Particles Composites Reinforced with Hydroxyapatite Nanoparticles: A Study of Thermal Stability, Flame Retardancy and Mechanical Properties. Mater. Chem. Phys. 2021, 258, 123917. [Google Scholar] [CrossRef]
- Dong, Q.; Gao, C.; Ding, Y.; Wang, F.; Wen, B.; Zhang, S.; Wang, T.; Yang, M. A Polycarbonate/Magnesium Oxide Nanocomposite with High Flame Retardancy. J. Appl. Polym. Sci. 2012, 123, 1085–1093. [Google Scholar] [CrossRef]
- Thajai, N.; Rachtanapun, P.; Thanakkasaranee, S.; Chaiyaso, T.; Phimolsiripol, Y.; Leksawasdi, N.; Sommano, S.R.; Sringarm, K.; Chaiwarit, T.; Ruksiriwanich, W.; et al. Antimicrobial Thermoplastic Starch Reactive Blend with Chlorhexidine Gluconate and Epoxy Resin. Carbohydr. Polym. 2023, 301 Part B, 120328. [Google Scholar] [CrossRef]
- Zhou, X.; He, T.; Jiang, Y.; Chang, S.; Yu, Y.; Fang, X.; Zhang, Y. A Novel Network-Structured Compatibilizer for Improving the Interfacial Behavior of PBS/Lignin. ACS Sustain. Chem. Eng. 2021, 9, 8592–8602. [Google Scholar] [CrossRef]
- Promhuad, K.; Phothisarattana, D.; Laorenza, Y.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Zinc Oxide Enhanced the Antibacterial Efficacy of Biodegradable PBAT/PBS Nanocomposite Films: Morphology and Food Packaging Properties. Food Biosci. 2023, 55, 103077. [Google Scholar] [CrossRef]
- Kontou, E.; Christopoulos, A.; Koralli, P.; Mouzakis, D.E. The Effect of Silica Particle Size on the Mechanical Enhancement of Polymer Nanocomposites. Nanomaterials 2023, 13, 1095. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.; Gupta, A. Influence of Metal Ions on Structure and Properties of Acrylic Fibers. J. Appl. Polym. Sci. 1998, 67, 1647–1659. [Google Scholar] [CrossRef]
- Jia, J.; Yang, J.; Zhao, Y.; Liang, H.; Chen, M. The Crystallization Behaviors and Mechanical Properties of Poly(L-lactic acid)/Magnesium Oxide Nanoparticle Composites. RSC Adv. 2016, 6, 43855–43863. [Google Scholar] [CrossRef]
- John, J.; Mani, R.; Bhattacharya, M. Evaluation of Compatibility and Properties of Biodegradable Polyester Blends. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2003–2014. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Poly (butylene succinate) and Poly (butylene adipate-co-terephthalate) Blends: Reactive Extrusion and Performance Evaluation. J. Polym. Environ. 2014, 22, 336–349. [Google Scholar] [CrossRef]
- Thajai, N.; Rachtanapun, P.; Thanakkasaranee, S.; Punyodom, W.; Worajittiphon, P.; Phimolsiripol, Y.; Leksawasdi, N.; Ross, S.; Jantrawut, P.; Jantanasakulwong, K. Reactive Blending of Modified Thermoplastic Starch Chlorhexidine Gluconate and Poly(butylene succinate) Blending with Epoxy Compatibilizer. Polymers 2023, 15, 3487. [Google Scholar] [CrossRef]
- Kiattipornpithak, K.; Thajai, N.; Kanthiya, T.; Rachtanapun, P.; Leksawasdi, N.; Phimolsiripol, Y.; Rohindra, D.; Ruksiriwanich, W.; Sommano, S.R.; Jantanasakulwong, K. Reaction Mechanism and Mechanical Property Improvement of Poly(Lactic Acid) Reactive Blending with Epoxy Resin. Polymers 2021, 13, 2429. [Google Scholar] [CrossRef]
- Szlachetka, I.; Dobrev, J.W.; Baryła, A.; Dohojda, M. Low-Density Polyethylene (LDPE) Building Films—Tensile Properties and Surface Morphology. J. Build. Eng. 2021, 44, 103386. [Google Scholar] [CrossRef]
- Yongcheng, Y. The Thermal Stability of Polyethylene Blends During Heat-Processing and Short-Term Photooxidation. Polym. Degrad. Stab. 1993, 39, 193–198. [Google Scholar] [CrossRef]
- Sanchis, M.R.; Blanes, V.; Blanes, M.; Garcia, D.; Balart, R. Surface Modification of Low Density Polyethylene (LDPE) Film by Low Pressure O2 Plasma Treatment. Eur. Polym. J. 2006, 42, 1558–1568. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer Nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and Crystallization Behaviour of Biodegradable Blends of Two Aliphatic Polyesters. Poly(3-hydroxybutyrate-co-hydroxyvalerate) and Poly(butylene succinate) Blends. Polymer 2003, 44, 7519–7527. [Google Scholar] [CrossRef]
- Kennouche, S.; Moigne, N.L.; Kaci, M.; Quantin, J.C.; Caro-Bretelle, A.S.; Delaite, C.; Lopez-Cuesta, J.M. Morphological Characterization and Thermal Properties of Compatibilized Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/Poly(butylene succinate) (PBS)/Halloysite Ternary Nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.; Bian, X.; Li, G.; Chen, X. Evaluation of PLA Content in PLA/PBAT Blends Using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Jakić, M.; Vrandečić, N.S.; Klarić, I. Thermal Degradation of Poly(vinyl chloride)/Poly(ethylene oxide) Blends: Thermogravimetric Analysis. Polym. Degrad. Stab. 2013, 98, 1738–1743. [Google Scholar] [CrossRef]
- Borhade, A.V.; Kanade, K.G.; Tope, D.R.; Patil, M.D. A Comparative Study on Synthesis, Characterization and Photocatalytic Activities of MgO and Fe/MgO Nanoparticles. Res. Chem. Intermed. 2012, 38, 1931–1946. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Thorat, S.; Desale, A.; Desai, P.; Kulkarni, M. Structure and Properties of PBS/PBAT Blends and Nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1248, 012013. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Jiao, L.; Li, Y.-D.; Srinivasan, M.; Li, T.; Wang, Y.-Z. Bio-Based Blends of Starch and Poly(butylene succinate) with Improved Miscibility, Mechanical Properties, and Reduced Water Absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, C.; Chauhan, B.S.; Srikrishna, S.; Yadav, R.S.; Rai, S.B. Enhanced Mechanical Properties and Hydrophilic Behavior of Magnesium Oxide Added Hydroxyapatite Nanocomposite: A Bone Substitute Material for Load Bearing Applications. Ceram. Int. 2020, 46 Part B, 16235–16248. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Muchtadi, T.R.; Iriani, E.S. Preparation and Characterization of Bionanocomposite Films Made from Carrageenan, Beeswax and ZnO Nanoparticles. In Materials Science Forum; Trans Tech Publications Ltd.: Zürich, Switzerland, 2016; Volume 872, pp. 157–161. [Google Scholar]
- Wen, W.; Luo, B.; Qin, X.; Li, C.; Liu, M.; Ding, S.; Zhou, C. Strengthening and Toughening of Poly(L-lactide) Composites by Surface Modified MgO Whiskers. Appl. Surf. Sci. 2015, 332, 215–223. [Google Scholar] [CrossRef]
- Gravogl, G.; Knoll, C.; Welch, J.M.; Artner, W.; Freiberger, N.; Nilica, R.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Werner, A.; et al. Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology. Nanomaterials 2018, 8, 795. [Google Scholar] [CrossRef]
- Morgan, A.B.; Bundy, M. Cone Calorimeter Analysis of UL-94 V-Rated Plastics. Fire Mater. Int. J. 2007, 31, 257–283. [Google Scholar] [CrossRef]
- Najim, M.N.; Sabr, O.H.; Kadhim, B.J. The Effect of MgO Nanoparticle on PVA/PEG-Based Membranes for Potential Application in Wound Healing. J. Biomater. Sci. Polym. Ed. 2024, 13, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Bagheripour, E.; Ansari, M. Adapting the Performance and Physico-Chemical Properties of PES Nanofiltration Membrane by Using of Magnesium Oxide Nanoparticles. Korean J. Chem. Eng. 2017, 34, 1774–1780. [Google Scholar] [CrossRef]
- Hong, S.; Yang, J.; Ahn, S.; Mun, Y.; Lee, G. Flame Retardant Performance of Various UL94 Classified Materials Exposed to External Ignition Sources. Fire Mater. 2004, 28, 25–31. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Wang, G.; Wang, Y.; Han, Z. Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene–Vinyl Acetate Copolymer. Polymers 2023, 15, 4440. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Azizi, M.; Seyed Dorraji, M.S. A Promising Ternary Nanohybrid of Copper@ Zinc Oxide Intercalated with Polyaniline for Simultaneous Antistatic and Antibacterial Applications. J. Coat. Technol. Res. 2019, 16, 1411–1422. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Marracci, M.; Piccinelli, F.; Tellini, B. Novel Microwave-Synthesis of Cu Nanoparticles in the Absence of Any Stabilizing Agent and Their Antibacterial and Antistatic Applications. Appl. Surf. Sci. 2013, 280, 610–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, H.; Yan, W.; Chen, S.; Qiu, M.; Liao, B. Atomic-Oxygen-Durable and Antistatic α-Al x Ti y O/γ-NiCr Coating on Kapton for Aerospace Applications. ACS Appl. Mater. Interfaces 2021, 13, 58179–58192. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Domaradzki, J.; Borna, A. Electrical and Antistatic Properties of Magnetron Sputtered Thin Films Based on TiO2:(V, Ta). In Proceedings of the 2011 International Students and Young Scientists Workshop “Photonics and Microsystems”, Cottbus, Germany, 8–10 July 2011; pp. 89–93. [Google Scholar]
- Samanta, K.K.; Jassal, M.; Agrawal, A.K. Antistatic Effect of Atmospheric Pressure Glow Discharge Cold Plasma Treatment on Textile Substrates. Fibers Polym. 2010, 11, 431–437. [Google Scholar] [CrossRef]
- Belkind, A.; Freilich, A.; Lopez, J.; Zhao, Z.; Zhu, W.; Becker, K. Characterization of Pulsed DC Magnetron Sputtering Plasmas. New J. Phys. 2005, 7, 90. [Google Scholar] [CrossRef]
- Abdelrahman, M. Study of Plasma and Ion Beam Sputtering Processes. J. Phys. Sci. Appl. 2015, 5, 128–142. [Google Scholar]
- Han, J.G. Recent Progress in Thin Film Processing by Magnetron Sputtering with Plasma Diagnostics. J. Phys. D Appl. Phys. 2009, 42, 043001. [Google Scholar] [CrossRef]




| Samples | Composition (g) | |
|---|---|---|
| PBS/E1/PBAT | MgO | |
| 1. PBS/E1/PBAT | 100 | - |
| 2. PBS/E1/PBAT/MgO 0.5 | 99.5 | 0.5 |
| 3. PBS/E1/PBAT/MgO 1 | 99 | 1 |
| 4. PBS/E1/PBAT/MgO 2 | 98 | 2 |
| 5. PBS/E1/PBAT/MgO 5 | 95 | 5 |
| 6. PBS/E1/PBAT/MgO 10 | 90 | 10 |
| 7. PBS/E1/PBAT/MgO 15 | 85 | 15 |
| No. | Metal Wire: Repeated Times |
|---|---|
| 1 | Control (Untreated) |
| 2 | Titanium (Ti:10) |
| 3 | Titanium (Ti:20) |
| 4 | Titanium (Ti:30) |
| 5 | Titanium (Ti:40) |
| 6 | Copper (Cu:40) |
| 7 | Aluminum (Al:40) |
| UL 94 Test (Vertical Burning Test) | |||
|---|---|---|---|
| Test Criteria | V-0 | V-1 | V-2 |
| Burning time for each individual test specimen (s) (after first and second flame applications T1 or T2) | ≤10 s | ≤30 s | ≤30 s |
| Total burning time (s) (T1 + T2) | ≤50 s | ≤250 s | ≤250 s |
| Dripping of burning specimen (ignition of cotton batting) | No | No | Yes |
| Combustion up to holding clamp (specimens completely burned) | No | No | No |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | ΔHm (J/g) | ΔHc (J/g) | ΔXc (%) |
|---|---|---|---|---|---|---|
| PBS | - | - | 87.5 | 47.1 | - | 42.8 |
| PBAT | −28.2 | - | 121.5 | 12.9 | - | 11.3 |
| PBS/PBAT | - | - | 87.1 | 33.9 | - | 26.3 |
| PBS/E1/PBAT | - | - | 87.1 | 31.4 | - | 28.6 |
| PBS/E1/PBAT/MgO15 | - | - | 86.8 | 21.0 | - | 19.1 |
| Samples | Class UL 94 (V0–V2) | Ignition (Flaming Drip) (Yes/No) | Specimen Burns up to Holding Clamp. (Yes/No) |
|---|---|---|---|
| PBS | V-2 | Yes | No |
| PBAT | V-2 | Yes | No |
| PBS/PBAT | V-2 | Yes | No |
| PBS/E1/PBAT | V-2 | Yes | No |
| PBS/E1/PBAT/MgO 0.5 | - | Yes | No |
| PBS/E1/PBAT/MgO 1 | - | Yes | No |
| PBS/E1/PBAT/MgO 2 | - | Yes | No |
| PBS/E1/PBAT/MgO 5 | V-2 | Yes | No |
| PBS/E1/PBAT/MgO 10 | V-2 | Yes | No |
| PBS/E1/PBAT/MgO 15 | V-1 | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachtanapun, P.; Suhr, J.; Oh, E.; Thajai, N.; Kanthiya, T.; Kiattipornpithak, K.; Kaewapai, K.; Photphroet, S.; Worajittiphon, P.; Tanadchangsaeng, N.; et al. Flame Retardance and Antistatic Polybutylene Succinate/Polybutylene Adipate-Co-Terephthalate/Magnesium Composite. Polymers 2025, 17, 1675. https://doi.org/10.3390/polym17121675
Rachtanapun P, Suhr J, Oh E, Thajai N, Kanthiya T, Kiattipornpithak K, Kaewapai K, Photphroet S, Worajittiphon P, Tanadchangsaeng N, et al. Flame Retardance and Antistatic Polybutylene Succinate/Polybutylene Adipate-Co-Terephthalate/Magnesium Composite. Polymers. 2025; 17(12):1675. https://doi.org/10.3390/polym17121675
Chicago/Turabian StyleRachtanapun, Pornchai, Jonghwan Suhr, Eunyoung Oh, Nanthicha Thajai, Thidarat Kanthiya, Krittameth Kiattipornpithak, Kannikar Kaewapai, Siriphan Photphroet, Patnarin Worajittiphon, Nuttapol Tanadchangsaeng, and et al. 2025. "Flame Retardance and Antistatic Polybutylene Succinate/Polybutylene Adipate-Co-Terephthalate/Magnesium Composite" Polymers 17, no. 12: 1675. https://doi.org/10.3390/polym17121675
APA StyleRachtanapun, P., Suhr, J., Oh, E., Thajai, N., Kanthiya, T., Kiattipornpithak, K., Kaewapai, K., Photphroet, S., Worajittiphon, P., Tanadchangsaeng, N., Wattanachai, P., Jantanasakulwong, K., & Sawangrat, C. (2025). Flame Retardance and Antistatic Polybutylene Succinate/Polybutylene Adipate-Co-Terephthalate/Magnesium Composite. Polymers, 17(12), 1675. https://doi.org/10.3390/polym17121675









