Engineering to Improve Mechanical Properties of Nanocellulose Hydrogels from Aloe Vera Bagasse and Banana Pseudostem for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Their Pretreatment
2.2. Lignin and Hemicellulose Removal
2.3. Nanocellulose Isolation
2.4. Synthesis of Nanocellulose/Chitosan Hydrogels
2.5. Characterization Techniques
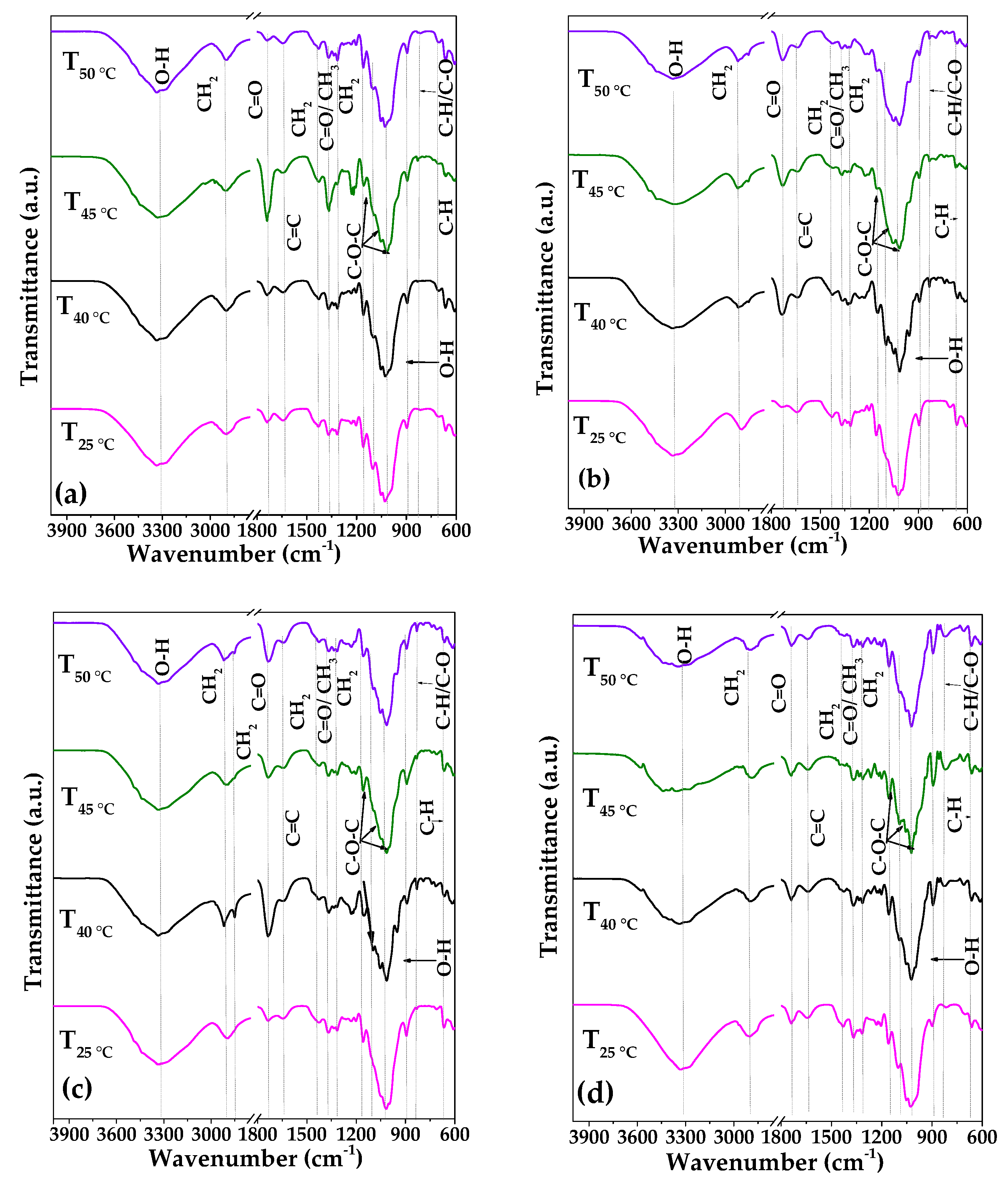
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.2. Nanocellulose Yield
2.5.3. X-Ray Diffraction (XRD)
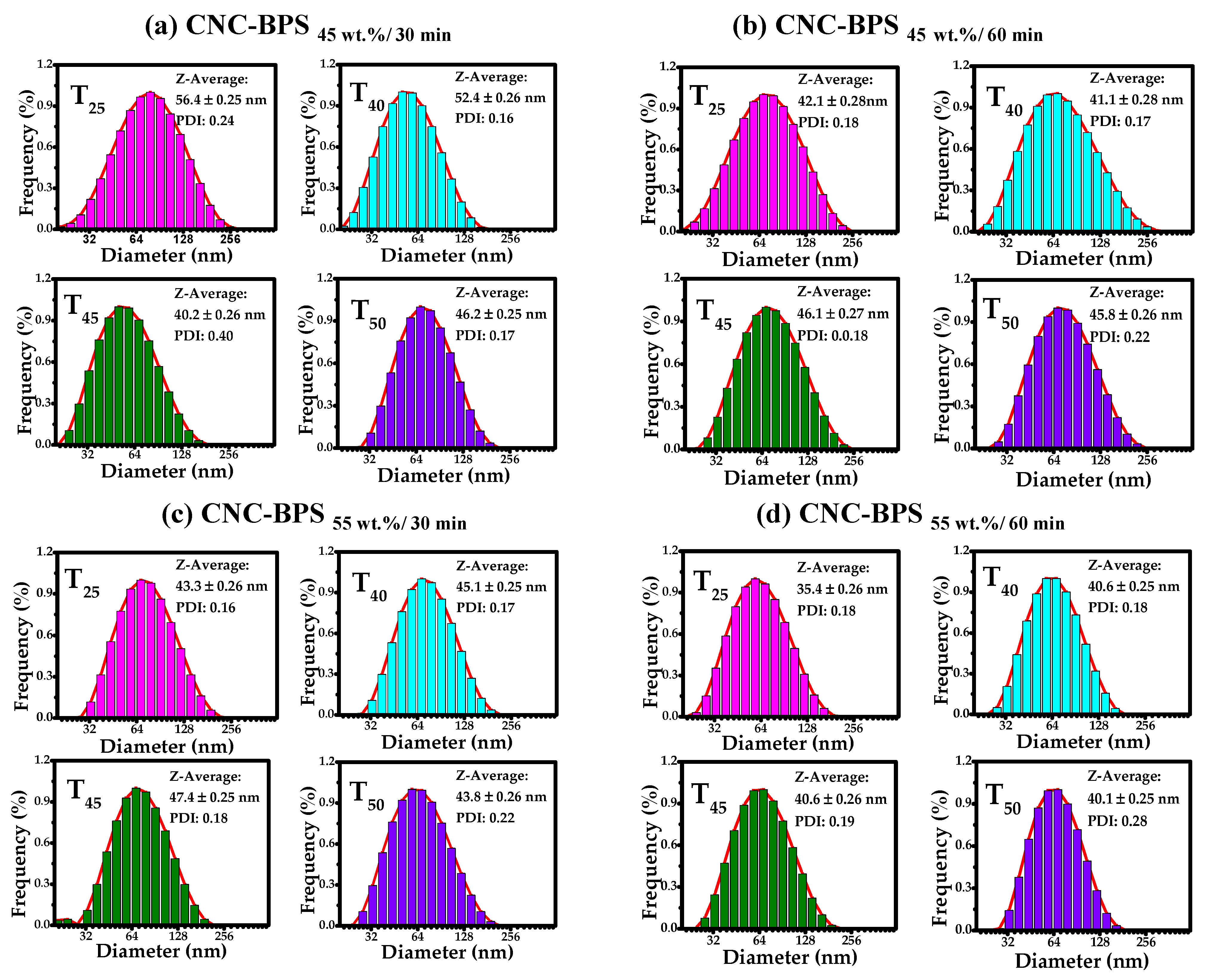
2.5.4. Dynamic Light Scattering (DLS)
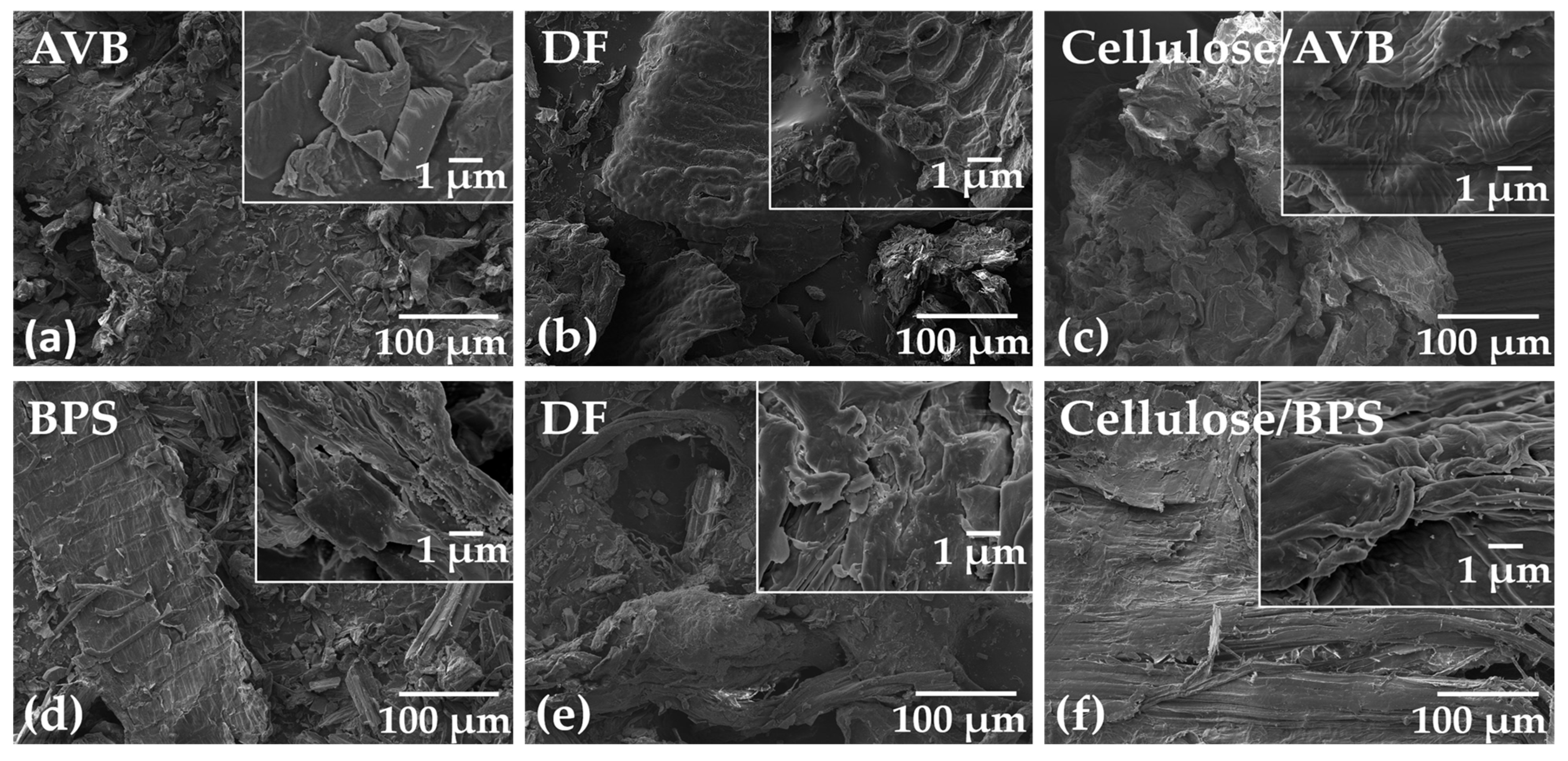
2.5.5. Scanning Electron Microscopy (SEM)
2.5.6. Determination of Gel Percentage
2.5.7. Swelling Test
2.5.8. Thermogravimetric Analysis
2.5.9. Mechanical Test
2.5.10. Porosity
2.5.11. Thermogravimetric Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy
3.2. Yield of Nanocellulose
3.2.1. Effect of Reaction Time
3.2.2. Effect of Acid Concentration
3.2.3. Effect of Temperature
3.3. Dynamic Light Scattering (DLS)
3.4. X-Ray Diffraction
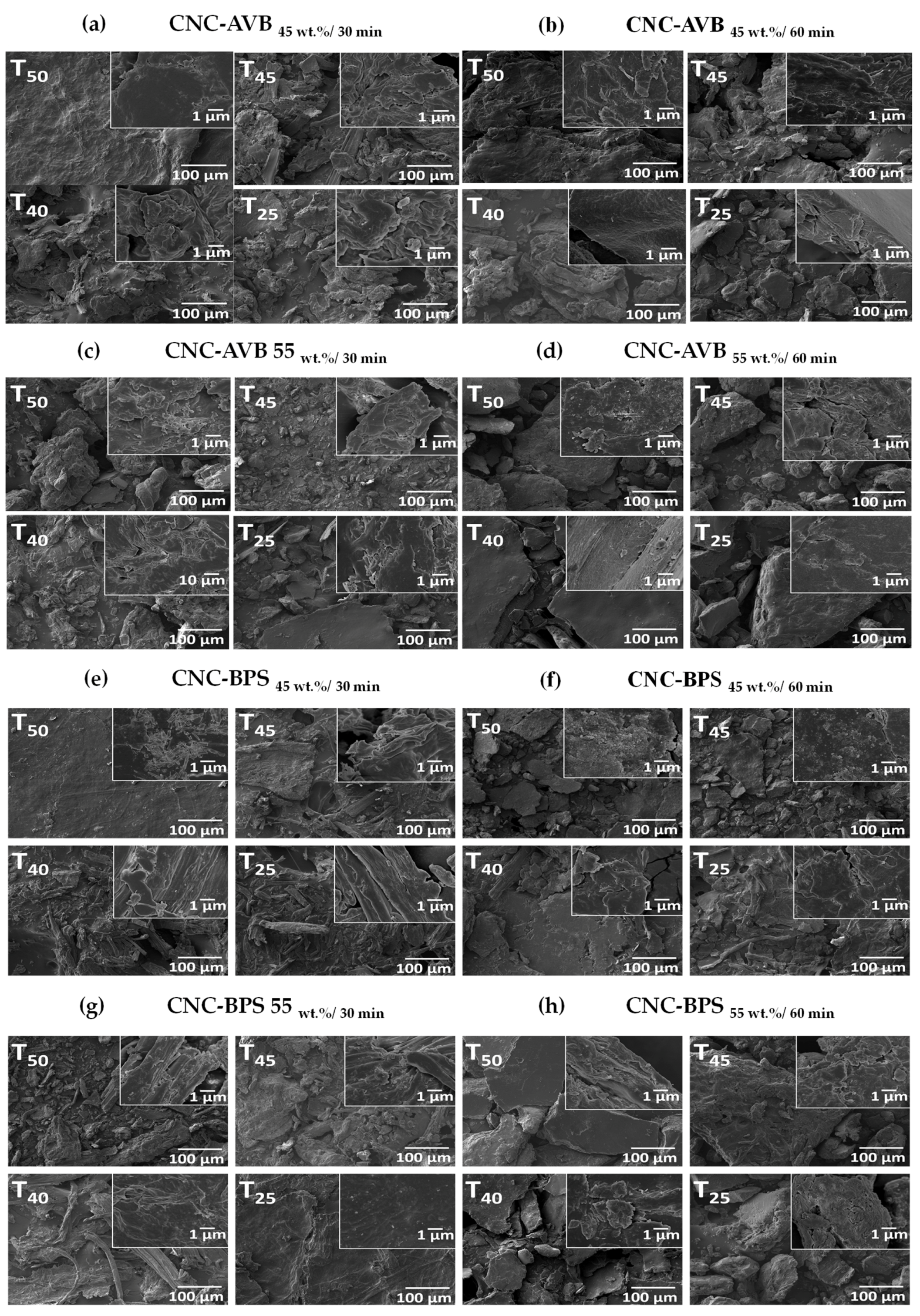
3.5. Scanning Electron Microscope (SEM)
3.6. FT-IR Analysis of Hydrogels
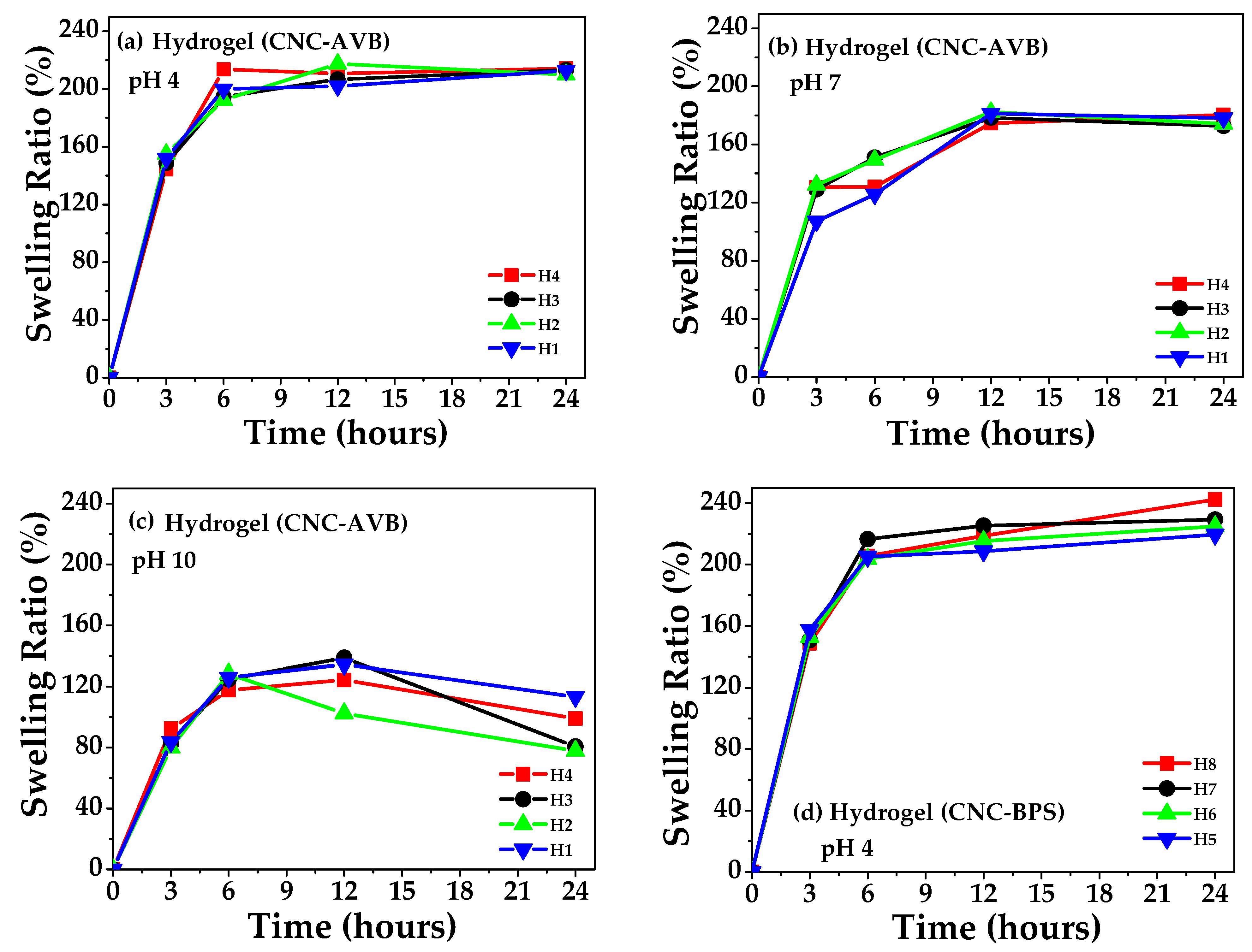
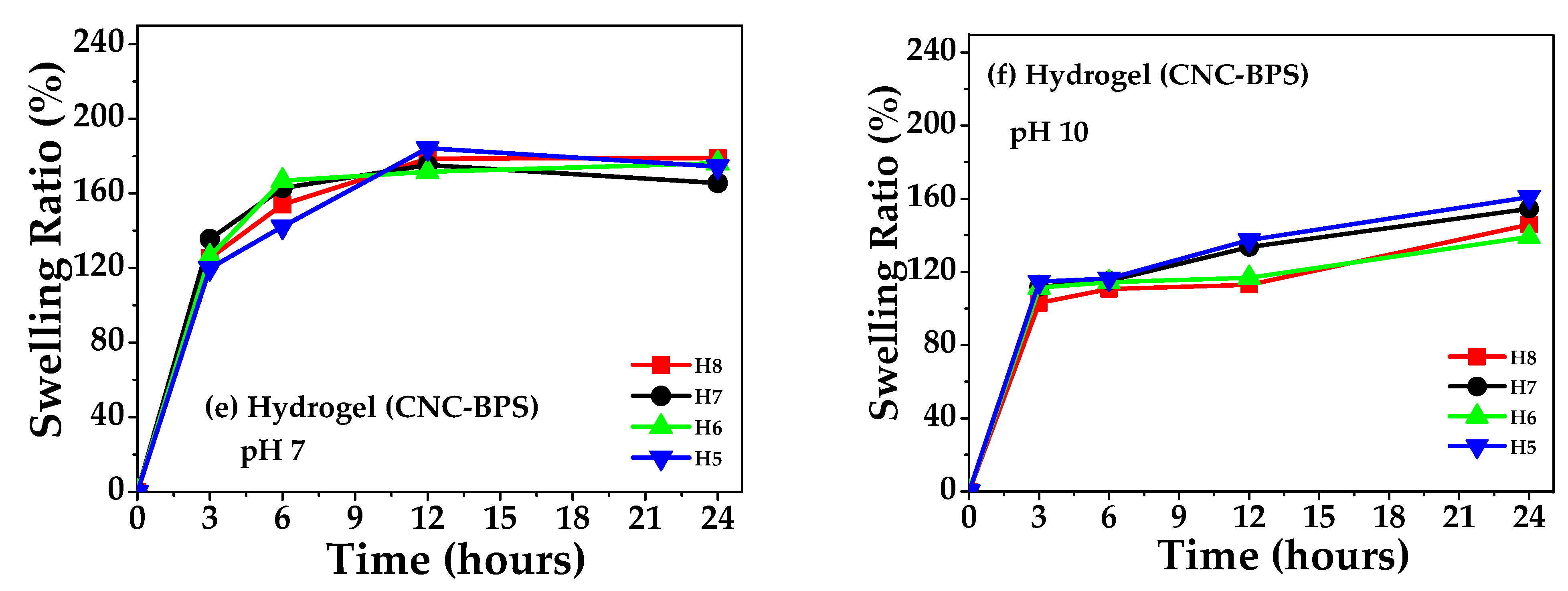
3.7. Swelling, Gel Percentage, and Porosity of Hydrogels
3.8. Morphology of Hydrogels
3.9. Thermal Analysis of Hydrogels
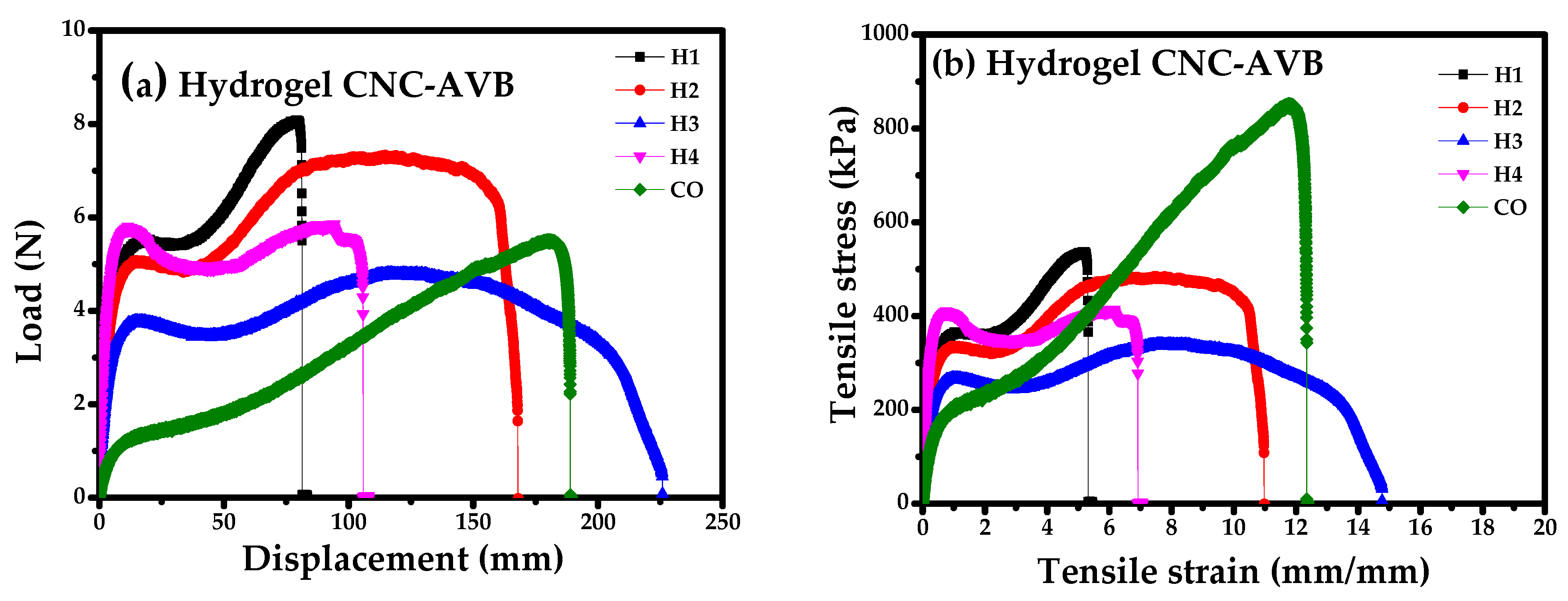
3.10. Mechanical Studies of Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food Waste Utilization for Reducing Carbon Footprints towards Sustainable and Cleaner Environment: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Han, C.; Antoniou, M.G. Sustainable and green decomposition of cyanotoxins and cyanobacteria through the development of new photocatalytic materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100444. [Google Scholar] [CrossRef]
- Lv, H.; Chen, Y.; Xie, Q.; Wu, P.; Chen, Y.; Gu, J.; Wu, H. Performance optimization and characterization of loess-slag-based geopolymer composite: A new sustainable green material for backfill. Constr. Build. Mater. 2022, 354, 129103. [Google Scholar] [CrossRef]
- Sharma, V.S.; Mishra, S.; Sharma, A.S.; Sharma, N.; Varma, R.S.; Shrivastav, P.S.; Ammathnadu Sudhakar, A. Microcrystalline Cellulose and Cellulose Nanocrystals: Ecofriendly and Sustainable Support Materials in Heterogeneous Nanocatalysis for Green Organic Transformations. Asian J. Org. Chem. 2025, 14, e202400586. [Google Scholar] [CrossRef]
- Naseem, S.; Rizwan, M. Sustainable construction and combo nanocellulose: A synergistic approach to greener building materials. Energy Build. 2025, 328, 115218. [Google Scholar] [CrossRef]
- Martínez de León, C.; Molina, P.; Ríos, C.; Brey, J.J. Green hydrogen production’s impact on sustainable development goals. Int. J. Hydrog. Energy, 2025; in press. [Google Scholar] [CrossRef]
- Acarer Arat, S. An overview of microplastic in marine waters: Sources, abundance, characteristics and negative effects on various marine organisms. Desalination Water Treat. 2024, 317, 100138. [Google Scholar] [CrossRef]
- Sun, Y.; Teng, Y.; Zhao, L.; Li, R.; Ren, W. Non-negligibly negative role of e-waste-derived pyrogenic carbon in the soil washing of copper and polybrominated diphenyl ethers. J. Hazard. Mater. 2023, 458, 131841. [Google Scholar] [CrossRef]
- Phiri, R.; Mavinkere Rangappa, S.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Kumar, R.; Kumar, A. Environmental waste management strategies and vermi transformation for sustainable development. Environ. Chall. 2023, 13, 100747. [Google Scholar] [CrossRef]
- Viteri, G.; Aranda, A.; Díaz de Mera, Y.; Rodríguez, A.; Rodríguez, D.; Rodríguez-Fariñas, N.; Valiente, N.; Seseña, S. Effects of massive desiccation of olive waste residues on air quality. Environ. Pollut. 2024, 359, 124542. [Google Scholar] [CrossRef]
- Sathish Kumar, R.K.; Sasikumar, R.; Dhilipkumar, T. Exploiting agro-waste for cleaner production: A review focusing on biofuel generation, bio-composite production, and environmental considerations. J. Clean. Prod. 2024, 435, 140536. [Google Scholar] [CrossRef]
- UNEP: United Nations Environment Programme. Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste; United Nations Environment Programme: Nairobi, Kenya, 2024; Available online: https://wedocs.unep.org/20.500.11822/45230 (accessed on 10 March 2025).
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2022, 278, 118956. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, J.; Zhang, M.; Xu, T.; Zheng, C.; Yuan, Z.; Si, C. Cellulose-based aerogels, films, and fibers for advanced biomedical applications. Chem. Eng. J. 2024, 497, 154434. [Google Scholar] [CrossRef]
- El Bourakadi, K.; Semlali, F.-Z.; Hammi, M.; El Achaby, M. A review on natural cellulose fiber applications: Empowering industry with sustainable solutions. Int. J. Biol. Macromol. 2024, 281, 135773. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Ahn, J.-R.; Kim, B.-J. Tailoring pore structure of porous carbon fiber using cellulose crystallite structure modification and its effect on methylene chloride adsorption-desorption behaviors. Ind. Crops Prod. 2025, 225, 120451. [Google Scholar] [CrossRef]
- Nyerere, G.; Kyokusiima, S.; Nabaterega, R.; Tumusiime, G.; Kavuma, C. The synergy of maize straw cellulose and sugarcane bagasse fibre on the characteristics of bioplastic packaging film. Bioresour. Technol. Rep. 2024, 28, 102007. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.-T. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Esposito Corcione, C. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- González Martínez, M.; Anca Couce, A.; Dupont, C.; da Silva Perez, D.; Thiéry, S.; Meyer, X.-m.; Gourdon, C. Torrefaction of cellulose, hemicelluloses and lignin extracted from woody and agricultural biomass in TGA-GC/MS: Linking production profiles of volatile species to biomass type and macromolecular composition. Ind. Crops Prod. 2022, 176, 114350. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Chaisirijaroenpun, N.; Sukyai, P.; Sukatta, U.; Watthanasakphuban, N.; Nimchua, T.; Pakawanit, P.; Kamonsutthipaijit, N.; Torgbo, S. Green extraction and isolation of cellulose nanofibrils from orchid (Dendrobium sonia earsakul) stem for wound dressing application. OpenNano 2025, 22, 100229. [Google Scholar] [CrossRef]
- Cai, Y.; Pang, Z.; Hu, J.; Qu, J.; Tian, D. Cellulose-metal organic frameworks: Recent advances in design, synthesis, and application. Chem. Eng. J. 2025, 504, 158711. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Ji, Q.; Li, Z.; Su, L.; Boateng, I.D.; Zhou, C.; Liu, X. Preparation, functional modification and industrial application of nanocellulose aerogels—A comprehensive review. Biomass Bioenergy 2025, 193, 107591. [Google Scholar] [CrossRef]
- Lozano, K.P.C.; Mendes, J.F.; Ferreira, L.F.; Martins, M.A.; Mendoza, J.S.; Mendes, R.F. Hydrophobic bio-composites of stearic acid starch esters and micro fibrillated cellulose processed by extrusion. Ind. Crops Prod. 2024, 221, 119313. [Google Scholar] [CrossRef]
- Joseph, A.; Umamaheswari, S.; Vassou, M.C. Bacterial cellulose: A versatile biomaterial for biomedical application. Carbohydr. Res. 2024, 552, 109350. [Google Scholar] [CrossRef]
- Kalpana, V.P.; Perarasu, V.T. Analysis on cellulose extraction from hybrid biomass for improved crystallinity. J. Mol. Struct. 2020, 1217, 128350. [Google Scholar] [CrossRef]
- Hu, H.; Shen, X.; Zou, Y.; Dong, H.; Dai, Y.; Li, R.; Huang, X.; Li, J.; Zhou, W. Carboxylated cellulose nanofibrils from sugarcane bagasse as stabilizers for Pickering emulsions. LWT 2025, 215, 117225. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Zhang, J.; Wei, Q.; Lv, C.; Jiang, Y.; Shi, X.; Zhou, Z.; Jia, X.; Hu, Z.; et al. From agricultural waste residue to wealth support: A magnetically N-heterocyclic carbene functionalized corn cob cellulose as a new stabilizer for Pd catalyst in Suzuki reaction. Int. J. Biol. Macromol. 2024, 279, 135386. [Google Scholar] [CrossRef]
- Ajiz, H.A.; Ardiansyah, R.P.; Dwiatmaka, M.S.K.R.; Setyawan, H.; Nurtono, T.; Widiyastuti, W. Silica surface modification using cellulose as a renewable organosilane derived from coconut coir fiber for carbon capture. Results Eng. 2024, 24, 103060. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, B.; Wang, K.; Li, H.; Peng, L. Carboxymethyl cellulose-based multifunctional film integrated with polyphenol-rich extract and carbon dots from coffee husk waste for active food packaging applications. Food Chem. 2024, 448, 139143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.-L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.-T.-C.; Phan, H.-V.-T.; Dao, M.-T.; Dang, T.-T.; Suvokhiaw, S.; Do, N.-T.; Nguyen, T.-A.-M.; Nguyen, V.-K.; Hoang, L.-T.-T.-T. Facile synthesis of a 3D magnetic graphene oxide/Fe3O4/banana peel-derived cellulose composite aerogel for the efficient removal of tetracycline. RSC Adv. 2024, 14, 34457–34470. [Google Scholar] [CrossRef]
- Martinez, J.C.; Davila, L.; Zuluaga, R.; Ricardo, A.; Figueroa, J.; Oliveira, M.G.d.A.; Meriño-Cabrera, Y. Production of bacterial cellulose from banana waste (Musa paradisiaca): A sustainable material for making cardboard paper. Biocatal. Agric. Biotechnol. 2025, 63, 103484. [Google Scholar] [CrossRef]
- Campano, C.; Miranda, R.; Merayo, N.; Negro, C.; Blanco, A. Direct production of cellulose nanocrystals from old newspapers and recycled newsprint. Carbohydr. Polym. 2017, 173, 489–496. [Google Scholar] [CrossRef]
- Lam, D.-N.; Thien, D.V.H.; Nguyen, C.-N.; Nguyen, N.T.T.; Van Viet, N.; Van-Pham, D.-T. Thermally stable cellulose nanospheres prepared from office waste paper by complete removal of hydrolyzed sulfate groups. Carbohydr. Polym. 2022, 297, 120009. [Google Scholar] [CrossRef]
- Patra, B.; Gautam, R.; Das, M.T.; Pradhan, S.N.; Dash, S.R.; Mohanta, A.K. Microplastics associated contaminants from disposable paper cups and their consequence on human health. LabMed Discov. 2024, 1, 100029. [Google Scholar] [CrossRef]
- Balasubramani, V.; Nagarajan, K.J.; Karthic, M.; Pandiyarajan, R. Extraction of lignocellulosic fiber and cellulose microfibrils from agro waste-palmyra fruit peduncle: Water retting, chlorine-free chemical treatments, physio-chemical, morphological, and thermal characterization. Int. J. Biol. Macromol. 2024, 259, 129273. [Google Scholar] [CrossRef]
- Alamdari, N.E.; Aksoy, B.; Babu, R.J.; Jiang, Z. Microcrystalline cellulose from soybean hull as an excipient in solid dosage forms: Preparation, powder characterization, and tableting properties. Int. J. Biol. Macromol. 2024, 270, 132298. [Google Scholar] [CrossRef]
- Yang, W.; Li, W.; Lei, Y.; He, P.; Wei, G.; Guo, L. Functionalization of cellulose-based sponges: Design, modification, environmental applications, and sustainability analysis. Carbohydr. Polym. 2025, 348, 122772. [Google Scholar] [CrossRef]
- Liu, P.; He, C.; Chen, X.; Wang, T.; Song, W.; Liu, W.; Liu, K. Rational modulation of cellulose for zinc ion-based energy storage devices. Green Chem. 2024, 27, 325–351. [Google Scholar] [CrossRef]
- Selvaraj, S.; Chauhan, A.; Dutta, V.; Verma, R.; Rao, S.K.; Radhakrishnan, A.; Ghotekar, S. A state-of-the-art review on plant-derived cellulose-based green hydrogels and their multifunctional role in advanced biomedical applications. Int. J. Biol. Macromol. 2024, 265, 130991. [Google Scholar] [CrossRef] [PubMed]
- Chamathka, W.W.D.R.; Chai, T.-T.; Phuah, E.T.; Wong, J.X.; Chen, S.N.; Yassoralipour, A. Extraction and characterization of cellulose nanoparticles from palm kernel meal for potential application in active food packaging. Int. J. Biol. Macromol. 2024, 260, 129637. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Karchoubi, F.; Afshar Ghotli, R.; Pahlevani, H.; Baghban Salehi, M. New insights into nanocomposite hydrogels; a review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Choi, S.; Fan, Z.; Im, J.; Nguyen, T.L.; Park, N.; Choi, Y.; Lee, J.Y.; Kim, J. Tendon-mimicking anisotropic alginate-based double-network composite hydrogels with enhanced mechanical properties and high impact absorption. Carbohydr. Polym. 2025, 352, 123193. [Google Scholar] [CrossRef]
- Vasconcelos do Nascimento, C.; Simmons, R.; Feitosa, J.; Dias, C.; Costa, M. Potential of superabsorbent hydrogels to improve agriculture under abiotic stresses. J. Arid Environ. 2021, 189, 104496. [Google Scholar] [CrossRef]
- Awere, C.O.; Anadebe, V.C.; Sabapathi, D.; Mounish, B.S.C.; Rakkammal, K.; Muthuramalingam, P.; Varadharajan, V.; Arinzechukwu, M.C.; Kumar, P.; Ebenso, E.E.; et al. From waste to wealth: Plant-Derived cellulose nanocrystal hydrogel for anticancer, antibacterial, and anticorrosion studies. Carbohydr. Polym. Technol. Appl. 2025, 9, 100656. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Yan, Z.; Yang, X.; Xiao, W.; Zhang, D.; Liu, Z.; Liao, X. A review of self-healing hydrogels for bone repair and regeneration: Materials, mechanisms, and applications. Int. J. Biol. Macromol. 2025, 287, 138323. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Hart, P.; Thakur, V.K. Cellulose-alginate hydrogels and their nanocomposites for water remediation and biomedical applications. Environ. Res. 2024, 243, 117889. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.; Ng, K.R.; Chen, W.N. Food Waste Durian Rind-Derived Cellulose Organohydrogels: Toward Anti-Freezing and Antimicrobial Wound Dressing. ACS Sustain. Chem. Eng. 2021, 9, 1304–1312. [Google Scholar] [CrossRef]
- Liu, D.; Labas, A.; Long, B.; McKnight, S.; Xu, C.; Tian, J.; Xu, Y. Bacterial nanocellulose production using cost-effective, environmentally friendly, acid whey based approach. Bioresour. Technol. Rep. 2023, 24, 101629. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Balaji, A.N.; Ramanujam, N.R. Isolation and characterization of cellulose nanocrystals from Saharan aloe vera cactus fibers. Int. J. Polym. Anal. Charact. 2020, 25, 51–64. [Google Scholar] [CrossRef]
- Pereira, N.R.L.; Lopes, B.; Fagundes, I.V.; de Moraes, F.M.; Morisso, F.D.P.; Parma, G.O.C.; Zepon, K.M.; Magnago, R.F. Bio-packaging based on cellulose acetate from banana pseudostem and containing Butia catarinensis extracts. Int. J. Biol. Macromol. 2022, 194, 32–41. [Google Scholar] [CrossRef]
- Shrestha, P.; Sadiq, M.B.; Anal, A.K. Development of antibacterial biocomposites reinforced with cellulose nanocrystals derived from banana pseudostem. Carbohydr. Polym. Technol. Appl. 2021, 2, 100112. [Google Scholar] [CrossRef]
- Cheng, S.; Panthapulakkal, S.; Sain, M.; Asiri, A. Aloe vera rind cellulose nanofibers-reinforced films. J. Appl. Polym. Sci. 2014, 131, 40592. [Google Scholar] [CrossRef]
- Abera, B.; Duraisamy, R.; Birhanu, T. Study on the preparation and use of edible coating of fish scale chitosan and glycerol blended banana pseudostem starch for the preservation of apples, mangoes, and strawberries. J. Agric. Food Res. 2024, 15, 100916. [Google Scholar] [CrossRef]
- Patil, H.; Naik, R.; Paramasivam, S.K. Utilization of banana crop ligno-cellulosic waste for sustainable development of biomaterials and nanocomposites. Int. J. Biol. Macromol. 2024, 282, 137065. [Google Scholar] [CrossRef]
- Hernández Leal, R.; Brachetti Sibaja, S.B.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A. Aislamiento y caracterización de nanocelulosa a partir de distintos residuos agroindustriales. J. Energy Eng. Optim. Sustain. 2022, 7, 111–124. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Nazemoroaia, M.; Bagheri, F.; Mirahmadi-Zare, S.Z.; Eslami-kaliji, F.; Derakhshan, A. Asymmetric natural wound dressing based on porous chitosan-alginate hydrogel/electrospun PCL-silk sericin loaded by 10-HDA for skin wound healing: In vitro and in vivo studies. Int. J. Pharm. 2025, 668, 124976. [Google Scholar] [CrossRef] [PubMed]
- Macías-Almazán, A.; Lois-Correa, J.A.; Domínguez-Crespo, M.A.; López-Oyama, A.B.; Torres-Huerta, A.M.; Brachetti-Sibaja, S.B.; Rodríguez-Salazar, A.E. Influence of operating conditions on proton conductivity of nanocellulose films using two agroindustrial wastes: Sugarcane bagasse and pinewood sawdust. Carbohydr. Polym. 2020, 238, 116171. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.-M.; Popescu, M.-C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral Characterization of Eucalyptus Wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.; Santana, R. Structural differences between wood species: Evidence from chemical composition. FTIR Spectrosc. Thermogravim. Anal. 2012, 126, E337–E344. [Google Scholar]
- Zhao, D.; Deng, Y.; Han, D.; Tan, L.; Ding, Y.; Zhou, Z.; Xu, H.; Guo, Y. Exploring structural variations of hydrogen-bonding patterns in cellulose during mechanical pulp refining of tobacco stems. Carbohydr. Polym. 2019, 204, 247–254. [Google Scholar] [CrossRef]
- Pimentel, G.C.; Sederholm, C.H. Correlation of Infrared Stretching Frequencies and Hydrogen Bond Distances in Crystals. J. Chem. Phys. 1956, 24, 639–641. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durrani, A.I.; Naseem, S. Cellulose extraction of Alstonia scholaris: A comparative study on efficiency of different bleaching reagents for its isolation and characterization. Int. J. Biol. Macromol. 2021, 191, 964–972. [Google Scholar] [CrossRef]
- Galocha-León, C.; Antich, C.; Voltes-Martínez, A.; Marchal, J.A.; Mallandrich, M.; Halbaut, L.; Rodríguez-Lagunas, M.J.; Souto, E.B.; Clares-Naveros, B.; Gálvez-Martín, P. Development and characterization of a poloxamer hydrogel composed of human mesenchymal stromal cells (hMSCs) for reepithelization of skin injuries. Int. J. Pharm. 2023, 647, 123535. [Google Scholar] [CrossRef]
- Mardawati, E.; Rahmah, D.M.; Rachmadona, N.; Saharina, E.; Pertiwi, T.Y.R.; Zahrad, S.A.; Ramdhani, W.; Srikandace, Y.; Ratnaningrum, D.; Endah, E.S.; et al. Pineapple core from the canning industrial waste for bacterial cellulose production by Komagataeibacter xylinus. Heliyon 2023, 9, e22010. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of cellulose nanofibers from olive tree harvest—A residue with wide applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Abbasi, A.; Makhtoumi, Y.; Wu, Y.; Chen, G. Characterization of cellulose nanocrystal extracted from household waste and its application for seed germination. Carbohydr. Polym. Technol. Appl. 2024, 7, 100409. [Google Scholar] [CrossRef]
- Akinjokun, A.I.; Petrik, L.F.; Ogunfowokan, A.O.; Ajao, J.; Ojumu, T.V. Isolation and characterization of nanocrystalline cellulose from cocoa pod husk (CPH) biomass wastes. Heliyon 2021, 7, e06680. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yi, Z.; Xie, L.; Dai, L.; Su, F.; Wang, Y.; Ji, W.; Wei, X.; Hui, G.; Chang, Y.; et al. Elimination of hydrogen bonds in cellulose enables high-performance disordered carbon anode in sodium-ion batteries. Energy Storage Mater. 2024, 73, 103845. [Google Scholar] [CrossRef]
- Turki, A.; El Oudiani, A.; Msahli, S.; Sakli, F. Investigation of OH bond energy for chemically treated alfa fibers. Carbohydr. Polym. 2018, 186, 226–235. [Google Scholar] [CrossRef]
- Lee, C.M.; Kubicki, J.D.; Fan, B.; Zhong, L.; Jarvis, M.C.; Kim, S.H. Hydrogen-Bonding Network and OH Stretch Vibration of Cellulose: Comparison of Computational Modeling with Polarized IR and SFG Spectra. J. Phys. Chem. B 2015, 119, 15138–15149. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent Progress in High-Strength and Robust Regenerated Cellulose Materials. Adv. Mater. 2021, 33, 2000682. [Google Scholar] [CrossRef]
- Zainul Armir, N.A.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Che Othman, M.H.; Zakaria, S. Regenerated Cellulose Products for Agricultural and Their Potential: A Review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Cellulose Derivatives: Synthesis, Structure, and Properties; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Rajendra Bahadur, G.C.; Awasthi, G.P.; Shin, M.; Sharma, K.P.; Neupane, B.B.; Kalauni, S.K.; Bhattarai, N.; Yu, C.; Joshi, M.K. Nanocellulose from Mankamana-3 corncob biomass: Synthesis, characterization, surface modification and potential applications. Bioresour. Technol. Rep. 2024, 28, 101971. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xiang, L.; Lou, M.; Qi, J.; Jiang, Y.; Xie, J. Production of lignin-containing nanocellulose from six types of unpretreated lignocellulosic biomass by a one-step process. Eur. Polym. J. 2024, 218, 113346. [Google Scholar] [CrossRef]
- Widiarto, S.; Yuwono, S.D.; Rochliadi, A.; Arcana, I.M. Preparation and Characterization of Cellulose and Nanocellulose from Agro-industrial Waste—Cassava Peel. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012052. [Google Scholar] [CrossRef]
- Messa, L.L.; Faez, R. Spray-dried chitosan/nanocellulose microparticles: Synergistic effects for the sustained release of NPK fertilizer. Cellulose 2020, 27, 10077–10093. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Nizamuddin, S.; Xu, J.; Vancov, T.; Chen, C. Cellulose nanocrystals from agriculture and forestry biomass: Synthesis methods, characterization and industrial applications. Environ. Sci. Pollut. Res. 2024, 31, 58745–58778. [Google Scholar] [CrossRef] [PubMed]
- López-Velázquez, L.; Salgado-García, S.; Córdova-Sánchez, S.; Valerio-Cardenas, C.; Ivette, B.-L.; Castañeda-Ceja, R.; Lagunes-Espinoza, L.; Falconi, R. Characterization of cellulose and sugarcane (Saccharum spp.) straw from five cultivars grown in the humid tropic of Mexico: Characterization of cellulose and sugarcane (Saccharum spp.). Agro Product. 2021, 14, 69–78. [Google Scholar] [CrossRef]
- Sartika, D.; Firmansyah, A.P.; Junais, I.; Arnata, I.W.; Fahma, F.; Firmanda, A. High yield production of nanocrystalline cellulose from corn cob through a chemical-mechanical treatment under mild conditions. Int. J. Biol. Macromol. 2023, 240, 124327. [Google Scholar] [CrossRef]
- Coelho, C.C.d.S.; Silva, R.B.S.; Carvalho, C.W.P.; Rossi, A.L.; Teixeira, J.A.; Freitas-Silva, O.; Cabral, L.M.C. Cellulose nanocrystals from grape pomace and their use for the development of starch-based nanocomposite films. Int. J. Biol. Macromol. 2020, 159, 1048–1061. [Google Scholar] [CrossRef]
- Dahlem, M.A.; Borsoi, C.; Hansen, B.; Catto, A.L. Evaluation of different methods for extraction of nanocellulose from yerba mate residues. Carbohydr. Polym. 2019, 218, 78–86. [Google Scholar] [CrossRef]
- Csiszar, E.; Kalic, P.; Kobol, A.; de Paulo Ferreira, E. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason Sonochem. 2016, 31, 473–480. [Google Scholar] [CrossRef]
- Nugroho, R.W.N.; Tardy, B.L.; Eldin, S.M.; Ilyas, R.A.; Mahardika, M.; Masruchin, N. Controlling the critical parameters of ultrasonication to affect the dispersion state, isolation, and chiral nematic assembly of cellulose nanocrystals. Ultrason. Sonochem. 2023, 99, 106581. [Google Scholar] [CrossRef]
- Dewan, S.; Rahman, M.M.; Hossain, M.I.; Ghos, B.C.; Rabby, M.M.R.; Gafur, M.A.; Al-Amin, M.; Alam, M.A. Isolation and characterization of CNC from waste maize cob available in Bangladesh as a potential candidate for the fabrication of multifunctional bio-nanocomposites: A new approach. S. Afr. J. Chem. Eng. 2025, 51, 287–301. [Google Scholar] [CrossRef]
- Rahman, M.M.; Pk, M.E.H.; Waliullah, M.; Hossain, M.I.; Maniruzzaman, M.; Ghos, B.C. Production of cellulose nanocrystals from the waste banana (M. Oranta) tree rachis fiber as a reinforcement to fabricate useful bionanocomposite. Carbohydr. Polym. Technol. Appl. 2024, 8, 100607. [Google Scholar] [CrossRef]
- Antunes, B.d.F.; Santana, L.R.; Oliveira, R.M.; Valério Filho, A.; Carreno, N.L.V.; Wolke, S.I.; da Silva, R.; Fajardo, A.R.; Dias, A.R.G.; Zavareze, E.d.R. Cellulose, cellulose nanofibers, and cellulose acetate from Butia fruits (Butia odorata): Chemical, morphological, structural, and thermal properties. Int. J. Biol. Macromol. 2024, 281, 136151. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Prabhakar, B.; Kharkar, P.S.; Pethe, A.M. Banana peel waste: An emerging cellulosic material to extract nanocrystalline cellulose. ACS Omega 2022, 8, 1140–1145. [Google Scholar] [CrossRef]
- Merais, M.S.; Khairuddin, N.; Salehudin, M.H.; Mobin Siddique, M.B.; Lepun, P.; Chuong, W.S. Preparation and characterization of cellulose nanofibers from banana pseudostem by acid hydrolysis: Physico-chemical and thermal properties. Membranes 2022, 12, 451. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Samsalee, N.; Meerasri, J.; Sothornvit, R. Rice husk nanocellulose: Extraction by high-pressure homogenization, chemical treatments and characterization. Carbohydr. Polym. Technol. Appl. 2023, 6, 100353. [Google Scholar] [CrossRef]
- Cidreira, A.C.M.; Hatami, T.; Linan, L.Z.; Pinheiro, I.F.; Gomes, R.C.; Rocha, J.J.C.; Mei, L.H.I. Nanocellulose extraction from acai bagasse through mixed acid hydrolysis and oxidative techniques. Int. J. Biol. Macromol. 2024, 273, 133034. [Google Scholar] [CrossRef]
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Evans, S.K.; Wesley, O.N.; Nathan, O.; Moloto, M.J. Chemically purified cellulose and its nanocrystals from sugarcane baggase: Isolation and characterization. Heliyon 2019, 5, e02635. [Google Scholar] [CrossRef] [PubMed]
- Azcón-Bieto, J.; Talón, M. Fundamentos de Fisiología Vegetal; McGraw-Hill Interamericana: New York, NY, USA, 2008. [Google Scholar]
- Megías, M.M.P.; Pombal, M.A. Atlas de Histología Vegetal y Animal. Available online: http://mmegias.webs.uvigo.es/inicio.html (accessed on 27 February 2025).
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer: Sunderland, MA, USA, 2014. [Google Scholar]
- Balaji, A.N.; Nagarajan, K.J. Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar] [CrossRef]
- Thi Thuy Van, N.; Gaspillo, P.-A.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Thi Truc Van, N.; Nguyen, T.-T.; Ky Phuong Ha, H. Cellulose from the banana stem: Optimization of extraction by response surface methodology (RSM) and charaterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Preparation and characterization of okara nanocellulose fabricated using sonication or high-pressure homogenization treatments. Carbohydr. Polym. 2021, 255, 117364. [Google Scholar] [CrossRef]
- Nang Vu, A.; Hoang Nguyen, L.; Yoshimura, K.; Duy Tran, T.; Van Le, H. Cellulose nanocrystals isolated from sugarcane bagasse using the formic/peroxyformic acid process: Structural, chemical, and thermal properties. Arab. J. Chem. 2024, 17, 105841. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Tariq, Z.; Philips, B.; Sadiqa, A.; Ahmad, M.; Al-Ahmary, K.M.; Ali, I.; Ahmed, M. Nanocellulose/wood ash-reinforced starch–chitosan hydrogel composites for soil conditioning and their impact on pea plant growth††Electronic supplementary information (ESI) available. RSC Adv. 2024, 14, 8652–8664. [Google Scholar] [CrossRef]
- Rafiee, A.; Mozafari, N.; Fekri, N.; Memarpour, M.; Azadi, A. Preparation and characterization of a nanohydroxyapatite and sodium fluoride loaded chitosan-based in situ forming gel for enamel biomineralization. Heliyon 2024, 10, e24217. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Vega-Cázarez, C.A.; Hernández-Ruiz, K.L.; Campas-Baypoli, O.N.; Soto-Cota, A.; Madera-Santana, T.J. Functional and antibacterial characterization of electrospun nanofiber membranes made of chitosan and polyvinyl alcohol. Results Chem. 2024, 7, 101314. [Google Scholar] [CrossRef]
- Majiya, H.; Clegg, F.; Sammon, C. Bentonite-Chitosan composites or beads for lead (Pb) adsorption: Design, preparation, and characterisation. Appl. Clay Sci. 2023, 246, 107180. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Zdanowicz, M. The Effect of Citric Acid on Physicochemical Properties of Hydrophilic Carboxymethyl Starch-Based Films. J. Polym. Environ. 2019, 27, 1379–1387. [Google Scholar] [CrossRef]
- Agu, A.B.; Benablo, P.J.; Mesias, V.; Penaloza, D. Synthesis and characterization of a chitosan-based citric-acid crosslinked encapsulant system. J. Chil. Chem. Soc. 2019, 64, 4610–4612. [Google Scholar] [CrossRef]
- Latif, M.; Jiang, Y.; Kumar, B.; Song, J.; Cho, H.; Kim, J. High Content Nanocellulose 3D-Printed and Esterified Structures with Strong Interfacial Adhesion, High Mechanical Properties, and Shape Fidelity. Adv. Mater. Interfaces 2022, 9, 2200280. [Google Scholar] [CrossRef]
- Waheed, Z.; Ahmad, F.; Mushtaq, B.; Ahmad, S.; Habib, S.R.; Rasheed, A.; Zafar, M.S.; Sefat, F.; Saeinasab, M.; Azam, F. Biowaste rice husk derived cellulosic hydrogel incorporating industrial cotton waste nonwoven for wound dressing. Int. J. Biol. Macromol. 2024, 281, 136412. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Peng, Z.; Peng, Z.; Shen, Y. Fabrication and properties of gelatin/chitosan composite hydrogel. Polym.-Plast. Technol. Eng. 2011, 50, 1160–1164. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Scott, A.J.; Xu, C.C.; Gagnon, G.; Kermanshahi-pour, A. Green synthesis of self-assembly, self-healing, and injectable polyelectrolyte complex hydrogels using chitosan, sulphated polysaccharides, hydrolyzed collagen and nanocellulose. Int. J. Biol. Macromol. 2025, 288, 138566. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A. An Overview on the Recent Advances in the Treatment of Infected Wounds: Antibacterial Wound Dressings. Macromol. Biosci. 2022, 22, 2200014. [Google Scholar] [CrossRef]
- Soleimani, F.; Sadeghi, M. Synthesis of pH-Sensitive Hydrogel Based on Starch-Polyacrylate Superabsorbent. J. Biomater. Nanobiotechnology 2012, 3, 310–314. [Google Scholar] [CrossRef]
- Carvalho, R.S.F.d.; Mahnke, L.C.; Palácio, S.B.; Barbosa, W.T.; Hodel, K.V.S.; Barbosa, J.D.V.; Melo, F.d.A.D.; Chorilli, M.; Meneguin, A.B.; Pinto, F.C.M.; et al. Bacterial cellulose hydrogel produced by Gluconacetobacter hansenii using sugarcane molasses as medium: Physicochemical characterization for wound healing applications. Carbohydr. Polym. Technol. Appl. 2025, 9, 100632. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Gálvez-Gómez, M.P.; González-Perez, L.; Pinedo-Rangel, V.; Pineda-Vasquez, T.; Hotza, D. Production of Bacterial Cellulose Hydrogel and its Evaluation as a Proton Exchange Membrane. J. Polym. Environ. 2023, 31, 2462–2472. [Google Scholar] [CrossRef]
- Niknia, N.; Kadkhodaee, R. Factors affecting microstructure, physicochemical and textural properties of a novel Gum tragacanth-PVA blend cryogel. Carbohydr. Polym. 2017, 155, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, Y.; Wang, J.; Zhu, Y.; Zhu, Y.; Zhang, X.; Liao, J.; Li, X.; Wu, X.; Qin, Y.-X.; et al. A Composite Hydrogel with High Mechanical Strength, Fluorescence, and Degradable Behavior for Bone Tissue Engineering. Polymers 2019, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Polez, R.T.; Kimiaei, E.; Madani, Z.; Österberg, M.; Baniasadi, H. Tragacanth gum hydrogels with cellulose nanocrystals: A study on optimizing properties and printability. Int. J. Biol. Macromol. 2024, 280, 136182. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. A Brief Evaluation of Pore Structure Determination for Bioaerogels. Gels 2022, 8, 438. [Google Scholar] [CrossRef]
- Rizwan, M.; Naseem, S.; Gilani, S.R.; Durrani, A.I. Optimization of swelling and mechanical behavior of Acer platanoides cellulose combo hydrogel. Kuwait J. Sci. 2024, 51, 100177. [Google Scholar] [CrossRef]
- Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef]
- Juan, A.S.; Montembault, A.; Gillet, D.; Say, J.P.; Rouif, S.; Bouet, T.; Royaud, I.; David, L. Degradation of chitosan-based materials after different sterilization treatments. IOP Conf. Ser. Mater. Sci. Eng. 2012, 31, 012007. [Google Scholar] [CrossRef]
- Berglund, L.; Squinca, P.; Baş, Y.; Zattarin, E.; Aili, D.; Rakar, J.; Junker, J.; Starkenberg, A.; Diamanti, M.; Sivlér, P.; et al. Self-Assembly of Nanocellulose Hydrogels Mimicking Bacterial Cellulose for Wound Dressing Applications. Biomacromolecules 2023, 24, 2264–2277. [Google Scholar] [CrossRef]
- Cui, L.; Wang, W.; Zheng, J.; Hu, C.; Zhu, Z.; Liu, B. Wide-humidity, anti-freezing and stretchable multifunctional conductive carboxymethyl cellulose-based hydrogels for flexible wearable strain sensors and arrays. Carbohydr. Polym. 2024, 342, 122406. [Google Scholar] [CrossRef]
- He, X.; Luo, X.; Wang, Y.; Zhu, J.; Li, Y.; Zhu, S.; Zhao, H. Nanoarchitectonics of nanocomposite hydrogels based on cellulose nanocrystals for biomedical applications: A review. Adv. Nanocompos. 2024, 1, 110–119. [Google Scholar] [CrossRef]
- Hafemann, E.; Battisti, R.; Marangoni, C.; Machado, R.A.F. Valorization of royal palm tree agroindustrial waste by isolating cellulose nanocrystals. Carbohydr. Polym. 2019, 218, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Jawaid, M.; Parveez, B.; Zuriyati, A.; Khan, A. Morphological, chemical and thermal analysis of cellulose nanocrystals extracted from bamboo fibre. Int. J. Biol. Macromol. 2020, 160, 183–191. [Google Scholar] [CrossRef]
- Wei, Z.; Ye, H.; Li, Y.; Li, X.; Liu, Y.; Chen, Y.; Yu, J.; Wang, J.; Ye, X. Mechanically tough, adhesive, self-healing hydrogel promotes annulus fibrosus repair via autologous cell recruitment and microenvironment regulation. Acta Biomater. 2024, 178, 50–67. [Google Scholar] [CrossRef]
- Dugam, S.; Jain, R.; Dandekar, P. Silver nanoparticles loaded triple-layered cellulose-acetate based multifunctional dressing for wound healing. Int. J. Biol. Macromol. 2024, 276, 133837. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Gea, S.; Ilyas, S.; Tamrin, T.; Radecka, I. Characterization of Bacterial Cellulose-Based Wound Dressing in Different Order Impregnation of Chitosan and Collagen. Biomolecules 2020, 10, 1511. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]



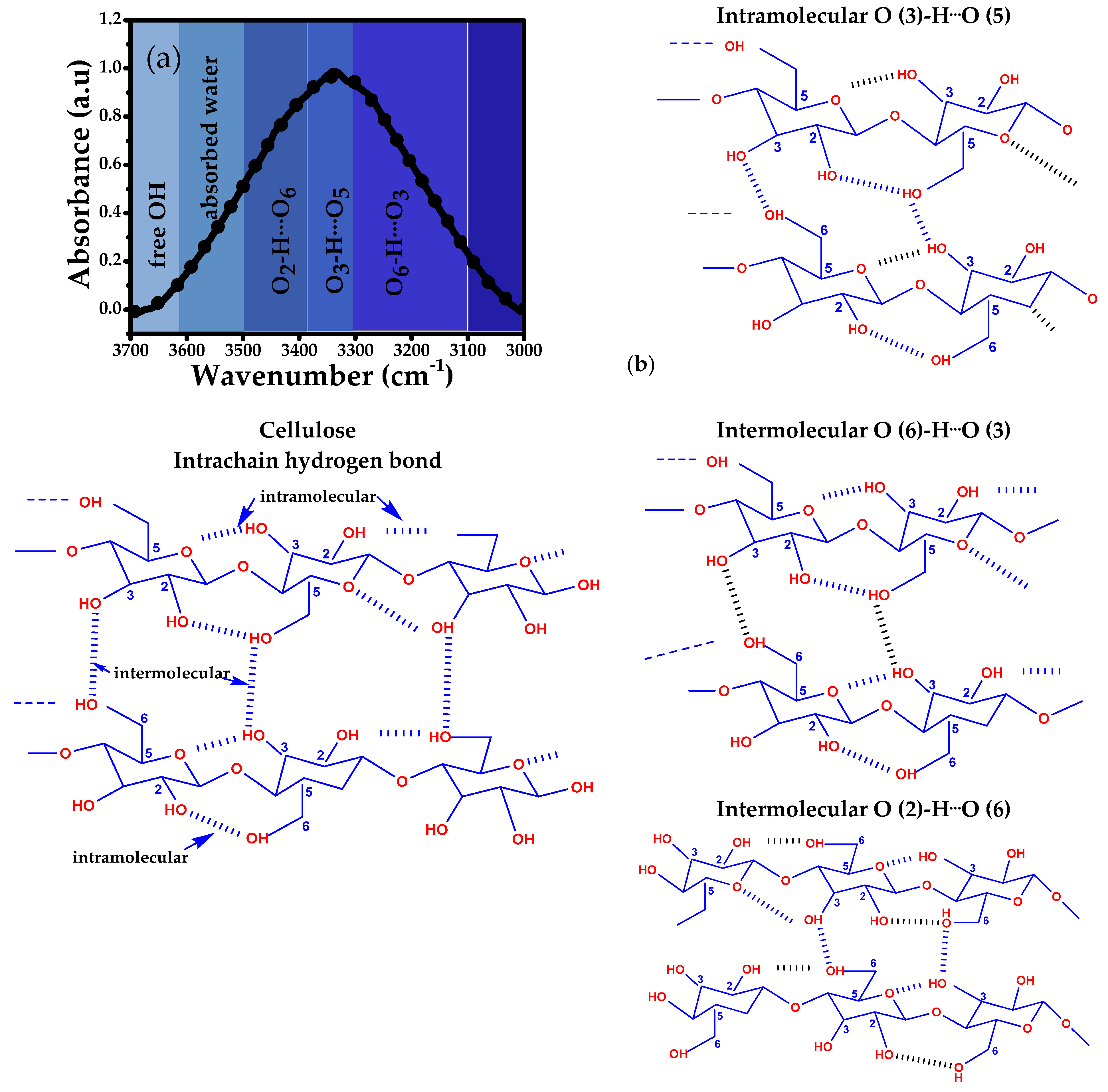






| Source AVB | Group Assignment O6H·O3 3100–3310 cm−1 | Group Assignment O3H·O5 3310–3340 cm−1 | Group Assignment O2H·O6 3340–3500 cm−1 | Group Assignment Free OH Groups OH (2) 3560–3513 cm−1 OH (6) 3577–3579 cm−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Band Position (cm−1) | EH (kJ/mol) | R (Å) | Band Position (cm−1) | EH (kJ/mol) | R (Å) | Band Position (cm−1) | EH (kJ/mol) | R (Å) | Band Position (cm−1) | EH (kJ/mol) | R (Å) | |
| C45t30T25 | 3142 | 36.53 | 2.73 | 3312 | 24.58 | 2.77 | 3470 | 13.09 | 2.81 | 3571 | 5.74 | 2.83 |
| C45t30T40 | 3149 | 36.03 | 2.73 | 3314 | 24.44 | 2.77 | 3472 | 12.94 | 2.81 | 3568 | 5.96 | 2.83 |
| C45t30T45 | 3140 | 36.67 | 2.73 | 3319 | 24.07 | 2.77 | 3477 | 12.58 | 2.81 | 3570 | 5.81 | 2.83 |
| C45t30T50 | 3151 | 35.88 | 2.73 | 3325 | 23.64 | 2.77 | 3478 | 12.51 | 2.81 | 3570 | 5.81 | 2.83 |
| C45t60T25 | 3134 | 37.11 | 2.73 | 3320 | 24.00 | 2.77 | 3477 | 12.58 | 2.81 | 3564 | 6.25 | 2.83 |
| C45t60T40 | 3127 | 37.61 | 2.73 | 3320 | 24.00 | 2.77 | 3464 | 13.53 | 2.80 | 3549 | 7.34 | 2.82 |
| C45t60T45 | 3139 | 36.75 | 2.73 | 3322 | 23.85 | 2.77 | 3485 | 12.00 | 2.81 | 3572 | 5.67 | 2.83 |
| C45t60T50 | 3135 | 37.03 | 2.73 | - | - | - | 3445 | 14.91 | 2.80 | 3545 | 7.63 | 2.82 |
| 3297 | 25.67 | 2.77 | ||||||||||
| C55t30T25 | 3159 | 35.31 | 2.74 | 3333 | 23.05 | 2.78 | 3493 | 11.42 | 2.81 | 3570 | 5.81 | 2.83 |
| C55t30T40 | - | - | - | 3327 | 23.22 | 2.77 | 3395 | 18.54 | 2.79 | 3562 | 6.40 | 2.83 |
| 3495 | 11.27 | 2.81 | ||||||||||
| C55t30T45 | 3115 | 38.47 | 2.73 | - | - | - | 3425 | 16.36 | 2.80 | 3552 | 7.12 | 2.82 |
| 3284 | 26.62 | 2.76 | - | - | - | |||||||
| C55t30T50 | 3224 | 30.63 | 2.75 | 3394 | 18.62 | 2.79 | 3511 | 10.11 | 2.82 | 3579 | 5.16 | 2.83 |
| C55t60T25 | 3145 | 36.31 | 2.73 | 3442 | 15.13 | 2.80 | 3569 | 5.89 | 2.83 | |||
| 3294 | 25.89 | 2.77 | ||||||||||
| C55t60T40 | 3155 | 35.59 | 2.74 | 3324 | 23.71 | 2.77 | 3477 | 12.58 | 2.81 | 3561 | 6.44 | 2.83 |
| C55t60T45 | 3153 | 35.74 | 2.73 | 3328 | 23.42 | 2.77 | 3479 | 12.43 | 2.81 | 3561 | 6.47 | 2.83 |
| C55t60T50 | 3145 | 36.31 | 2.73 | 3339 | 22.62 | 2.78 | 3443 | 15.05 | 2.80 | 3568 | 5.96 | 2.83 |
| 3299 | 25.53 | 2.77 | ||||||||||
| Source BPS | Group Assignment O6H·O3 3100–3310 cm−1 | Group Assignment O3H·O5 3310–3340 cm−1 | Group Assignment O2H·O6 3340–3500 cm−1 | Group Assignment Free OH Groups OH (2) 3560–3513 cm−1 OH (6) 3577–3600 cm−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Band Position (cm−1) | EH (kJ/mol) | R (Å) | Band Position (cm−1) | EH (kJ/mol) | R (Å) | Band Position (cm−1) | EH (kJ/mol) | R (Å) | Band Position (cm−1) | EH (kJ/mol) | R (Å) | |
| C45t30T25 | 3183 | 33.58 | 2.74 | 3339 | 22.62 | 2.781 | 3354 | 21.53 | 2.78 | 3549 | 7.34 | 2.82 |
| 3275 | 27.27 | 2.76 | ||||||||||
| C45t30T40 | 3153 | 35.74 | 2.73 | 3341 | 22.47 | 2.78 | 3554 | 6.98 | 2.75 | |||
| 3292 | 26.04 | 2.77 | 3426 | 16.29 | 2.80 | |||||||
| 3493 | 11.42 | 2.81 | ||||||||||
| C45t30T45 | 3140 | 36.67 | 2.73 | 3337 | 22.76 | 2.78 | 3440 | 15.27 | 2.80 | 3565 | 6.18 | 2.83 |
| 3282 | 26.76 | 2.76 | ||||||||||
| C45t30T50 | 3154 | 35.67 | 2.73 | 3340 | 22.54 | 2.78 | 3564 | 6.25 | 2.83 | |||
| 3280 | 26.91 | 2.76 | 3401 | 18.11 | 2.79 | |||||||
| C45t60T25 | 3138 | 36.82 | 2.73 | 3339 | 22.62 | 2.78 | 3427 | 16.22 | 2.80 | 3571 | 5.74 | 2.83 |
| 3283 | 26.69 | 2.76 | ||||||||||
| C45t60T40 | 3130 | 37.39 | 2.73 | 3339 | 22.62 | 2.78 | 3429 | 16.07 | 2.80 | 3545 | 7.63 | 2.82 |
| 3281 | 26.84 | 2.76 | ||||||||||
| C45t60T45 | 3144 | 36.39 | 2.73 | 3327 | 23.49 | 2.77 | 3440 | 15.27 | 2.80 | 3526 | 9.02 | 2.82 |
| 3492 | 11.49 | 2.81 | ||||||||||
| C45t60T50 | 3138 | 36.82 | 2.73 | 3341 | 22.47 | 2.78 | 3572 | 5.67 | 2.83 | |||
| 3299 | 25.53 | 2.77 | 3458 | 13.96 | 2.80 | |||||||
| C55t30T25 | 3138 | 36.82 | 2.73 | 3312 | 24.58 | 2.77 | 3461 | 13.74 | 2.80 | 3543 | 7.78 | 2.82 |
| C55t30T40 | 3173 | 34.30 | 2.74 | - | - | - | 3346 | 22.11 | 2.78 | 3519 | 9.52 | 2.75 |
| 3304 | 25.16 | 2.77 | 3383 | 19.42 | 2.79 | |||||||
| 3441 | 15.20 | 2.80 | ||||||||||
| C55t30T45 | 3153 | 35.74 | 2.73 | - | - | - | 3354 | 21.53 | 2.78 | 3522 | 9.31 | 2.82 |
| 3300 | 25.45 | 2.77 | 3445 | 14.91 | 2.80 | 3596 | 3.92 | 2.75 | ||||
| C55t30T50 | 3155 | 35.59 | 2.74 | - | - | - | 3350 | 21.82 | 2.78 | 3524 | 9.16 | 2.82 |
| 3292 | 26.04 | 2.77 | 3435 | 15.63 | 2.80 | 3591 | 4.29 | 2.75 | ||||
| C55t60T25 | 3136 | 36.96 | 2.73 | - | - | - | 3340 | 22.54 | 2.78 | 3568 | 5.96 | 2.83 |
| 3299 | 25.53 | 2.77 | 3450 | 14.54 | 2.80 | |||||||
| C55t60T40 | 3145 | 36.31 | 2.73 | 3339 | 22.62 | 2.78 | 3462 | 13.67 | 2.80 | 3560 | 6.54 | 2.83 |
| 3309 | 24.80 | 2.77 | ||||||||||
| C55t60T45 | 3145 | 36.31 | 2.73 | 3323 | 23.78 | 2.77 | 3460 | 13.82 | 2.80 | 3545 | 7.63 | 2.82 |
| C55t60T50 | 3140 | 36.67 | 2.73 | 3338 | 22.69 | 2.78 | 3453 | 14.33 | 2.80 | 3565 | 6.18 | 2.83 |
| 3296 | 25.75 | 2.77 | ||||||||||
| (a) | (b) | ||
|---|---|---|---|
| Source CNC-AVB | Nanocellulose Yield (%) | Source CNC-BPS | Nanocellulose Yield (%) |
| C45t30T25 | 47.63 ± 1.29 | C45t30T25 | 57.22 ± 1.45 |
| C45t30T40 | 47.57 ± 0.03 | C45t30T40 | 57.99 ± 2.81 |
| C45t30T45 | 49.90 ± 5.86 | C45t30T45 | 59.21 ± 2.50 |
| C45t30T50 | 48.65 ± 5.1 | C45t30T50 | 58.75 ± 1.04 |
| C45t60T25 | 48.72 ± 5.13 | C45t60T25 | 57.2 ± 1.29 |
| C45t60T40 | 49.65 ± 5.21 | C45t60T40 | 51.98 ± 9.77 |
| C45t60T45 | 48.47 ± 1.19 | C45t60T45 | 50.03 ± 6.40 |
| C45t60T50 | 48.77 ± 4.98 | C45t60T50 | 49.22 ± 5.70 |
| C55t30T25 | 46.61 ± 1.27 | C55t30T25 | 54.31 ± 2.41 |
| C55t30T40 | 48.85 ± 1.99 | C55t30T40 | 56.79 ± 5.60 |
| C55t30T45 | 48.50 ± 5.19 | C55t30T45 | 58.26 ± 2.09 |
| C55t30T50 | 49.60 ± 2.73 | C55t30T50 | 58.78 ± 0.94 |
| C55t60T25 | 49.61 ± 4.79 | C55t60T25 | 55.18 ± 0.03 |
| C55t60T40 | 45.06 ± 9.01 | C55t60T40 | 55.8 ± 0.20 |
| C55t60T45 | 46.19 ± 0.92 | C55t60T45 | 56.82 ± 0.49 |
| C55t60T50 | 46.65 ± 1.30 | C55t60T50 | 57.54 ± 0.54 |
| Nanocellulose from BPS | Nanocellulose from AVB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose Iα | Cellulose Iβ | Cellulose II | C.S. (nm) | CI (%) | Cellulose Iα | Cellulose Iβ | Cellulose II | C.S. (nm) | CI (%) | |
| C45t30T25 | 15.42° 20.22° 21.80° | 13.80°(011) 14.85° 16.66°(110) 22.98°(200) | 12.26° 19.92° | 3.65 | 73.13 | 14.26° 5.24° 18.65° 21.80°) | 13.8° 16.6° | 4.94° 19.77° | 30.70 | 88.97 |
| C45t30T40 | 37.20 | 72.47 | 27.20 | 72.68 | ||||||
| C45t30T45 | 31.50 | 76.12 | 34.00 | 88.88 | ||||||
| C45t30T50 | 33.10 | 87.76 | 27.50 | 89.66 | ||||||
| C45t60T25 | 15.42° 20.22° 21.80° | 14.27°(101) 14.85° 16.66°(110) 22.98°(200) | 12.26° 17.18°(002) 19.92° 21.58°(110) | 36.30 | 83.92 | 15.42° 20.63° | 13.80° 14.85° 16.66° 20.27°, 22.98° | 21.13° | 32.00 | 85.19 |
| C45t60T40 | 43.70 | 93.6 | 35.70 | 88.33 | ||||||
| C45t60T45 | 42.40 | 86.79 | 28.10 | 73.13 | ||||||
| C45t60T50 | 40.20 | 81.47 | 28.70 | 70.67 | ||||||
| C55t30T25 | 15.42° 20.22° 21.80° | 13.80°(011), 14.85° 16.66°(110) 22.98°(200) | 12.26° 19.92° | 33.10 | 77.86 | 15.42° 21.80° | 13.80°(011) 14.27°(101) 16.66°(110) | 17.18°(002) 19.77° 19.92°(110) | 31.10 | 88.52 |
| C55t30T40 | 33.50 | 76.33 | 33.40 | 88.01 | ||||||
| C55t30T45 | 23.40 | 87.64 | 34.90 | 81.74 | ||||||
| C55t30T50 | 31.20 | 97.58 | 33.60 | 78.91 | ||||||
| C55t60T25 | 15.42° 18.65° | 13.80°(011) 14.85° 16.66°(110) 22.98°(200) | 12.26° 19.92° | 38.50 | 79.66 | 20.22° 21.80° | 13.80°(011) 14.27°(101) 14.85° 18.74°(111) 22.98°(200) | 12.26°(100) 14.94°(101) 17.18°(002) 19.77° 21.58° | 36.20 | 83.93 |
| C55t60T40 | 68.30 | 88.41 | 32.20 | 82.64 | ||||||
| C55t60T45 | 43.20 | 88.47 | 37.50 | 73.08 | ||||||
| C55t60T50 | 49.10 | 87.46 | 39.10 | 86.07 | ||||||
| Hydrogel | Swelling % (pH 4 and 12 h) | Gel (%) | Porosity (%) | Pore Size (μm) |
|---|---|---|---|---|
| H1 | 202.01 | 81.91 ± 2.86 | 53.45 ± 1.24 | 2.38 ± 0.39 |
| H2 | 217.50 | 79.13 ± 1.42 | 55.09 ± 0.90 | 2.26 ± 0.39 |
| H3 | 210.67 | 78.19 ± 1.85 | 54.89 ± 0.67 | 2.53 ± 0.55 |
| H4 | 206.72 | 77.93 ± 1.88 | 53.37 ± 6.28 | 3.15 ± 0.10 |
| H5 | 208.63 | 84.26 ± 1.81 | 58.20 ± 1.46 | 2.52 ± 0.87 |
| H6 | 215.26 | 85.94 ± 1.86 | 60.77 ± 2.6 | 2.60 ± 0.34 |
| H7 | 225.39 | 83.45 ± 2.67 | 58.80 ± 0.97 | 2.81 ± 0.28 |
| H8 | 218.68 | 86.60 ± 2.62 | 57.37 ± 0.86 | 2.45 ± 0.11 |
| Hydrogel | Maximum Load (N) | Extension at Maximum Load (mm) | Extension at Break (mm) | Tensile Stress at Maximum Load (kPa) | Tensile Strain at Maximum Load (mm) | Tensile Strain at Break (mm/mm) |
|---|---|---|---|---|---|---|
| H1 | 8.08 | 79.45 | 83.45 | 537.15 | 5.19 | 5.45 |
| H2 | 7.33 | 114.75 | 168.25 | 484.74 | 7.50 | 11.00 |
| H3 | 4.82 | 117.35 | 226.10 | 341.30 | 7.67 | 14.78 |
| H4 | 5.86 | 94.20 | 108.03 | 413.87 | 6.16 | 7.06 |
| H5 | 5.55 | 39.87 | 42.63 | 615.06 | 2.61 | 2.79 |
| H6 | 7.10 | 40.13 | 43.98 | 707.67 | 2.62 | 2.88 |
| H7 | 5.36 | 35.20 | 43.33 | 609.40 | 2.30 | 2.83 |
| H8 | 6.25 | 38.30 | 41.93 | 644.17 | 2.50 | 2.74 |
| Commercial (CO) | 5.52 | 179.82 | 189.33 | 852.97 | 11.75 | 12.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Leal, R.; Licona-Aguilar, Á.I.; Domínguez-Crespo, M.A.; Ramírez-Meneses, E.; Rodríguez-Salazar, A.E.; Juárez-Balderas, C.; Brachetti-Sibaja, S.B.; Torres-Huerta, A.M. Engineering to Improve Mechanical Properties of Nanocellulose Hydrogels from Aloe Vera Bagasse and Banana Pseudostem for Biomedical Applications. Polymers 2025, 17, 1642. https://doi.org/10.3390/polym17121642
Hernández-Leal R, Licona-Aguilar ÁI, Domínguez-Crespo MA, Ramírez-Meneses E, Rodríguez-Salazar AE, Juárez-Balderas C, Brachetti-Sibaja SB, Torres-Huerta AM. Engineering to Improve Mechanical Properties of Nanocellulose Hydrogels from Aloe Vera Bagasse and Banana Pseudostem for Biomedical Applications. Polymers. 2025; 17(12):1642. https://doi.org/10.3390/polym17121642
Chicago/Turabian StyleHernández-Leal, Rocío, Ángeles Iveth Licona-Aguilar, Miguel Antonio Domínguez-Crespo, Esther Ramírez-Meneses, Adela Eugenia Rodríguez-Salazar, Carlos Juárez-Balderas, Silvia Beatriz Brachetti-Sibaja, and Aidé Minerva Torres-Huerta. 2025. "Engineering to Improve Mechanical Properties of Nanocellulose Hydrogels from Aloe Vera Bagasse and Banana Pseudostem for Biomedical Applications" Polymers 17, no. 12: 1642. https://doi.org/10.3390/polym17121642
APA StyleHernández-Leal, R., Licona-Aguilar, Á. I., Domínguez-Crespo, M. A., Ramírez-Meneses, E., Rodríguez-Salazar, A. E., Juárez-Balderas, C., Brachetti-Sibaja, S. B., & Torres-Huerta, A. M. (2025). Engineering to Improve Mechanical Properties of Nanocellulose Hydrogels from Aloe Vera Bagasse and Banana Pseudostem for Biomedical Applications. Polymers, 17(12), 1642. https://doi.org/10.3390/polym17121642






