Frontiers in Innovative Materials and Technologies for Oil–Water Separation
Abstract
1. Introduction
2. Traditional Oil–Water Separation Methods
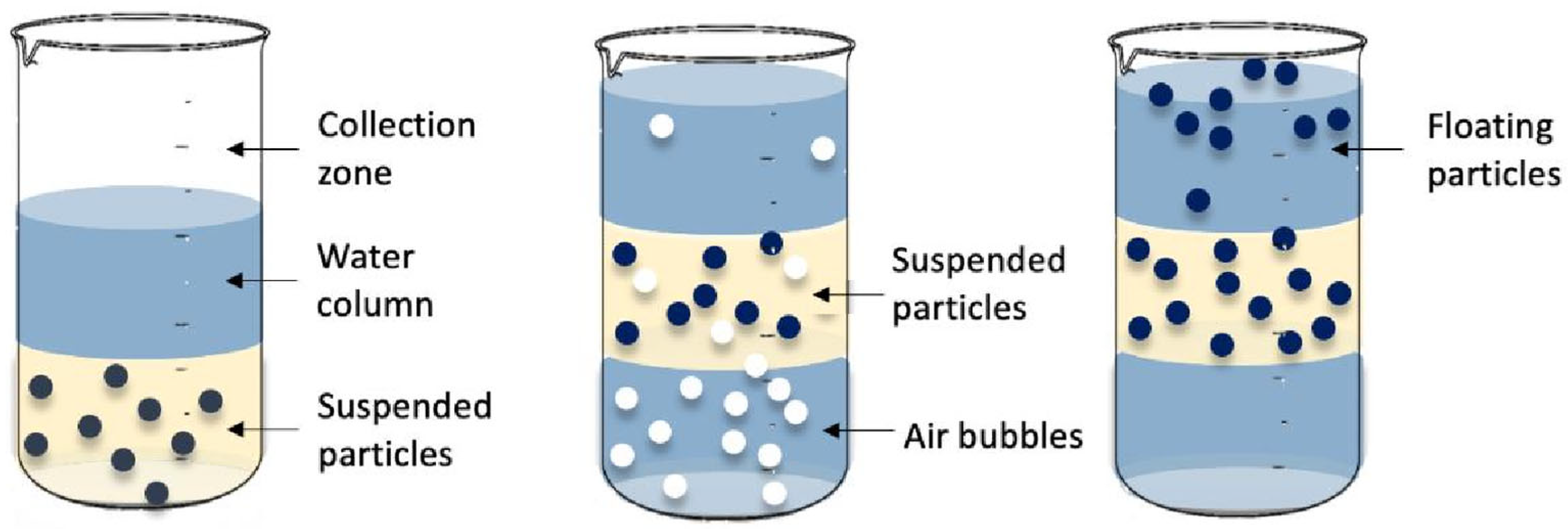
2.1. Gravity Separation Method
2.2. Centrifugal Separation Method
2.3. Flotation Method
3. Novel Oil–Water Separation Methods
3.1. Electrocoagulation
3.2. Adsorption Method
3.2.1. Activated Carbon
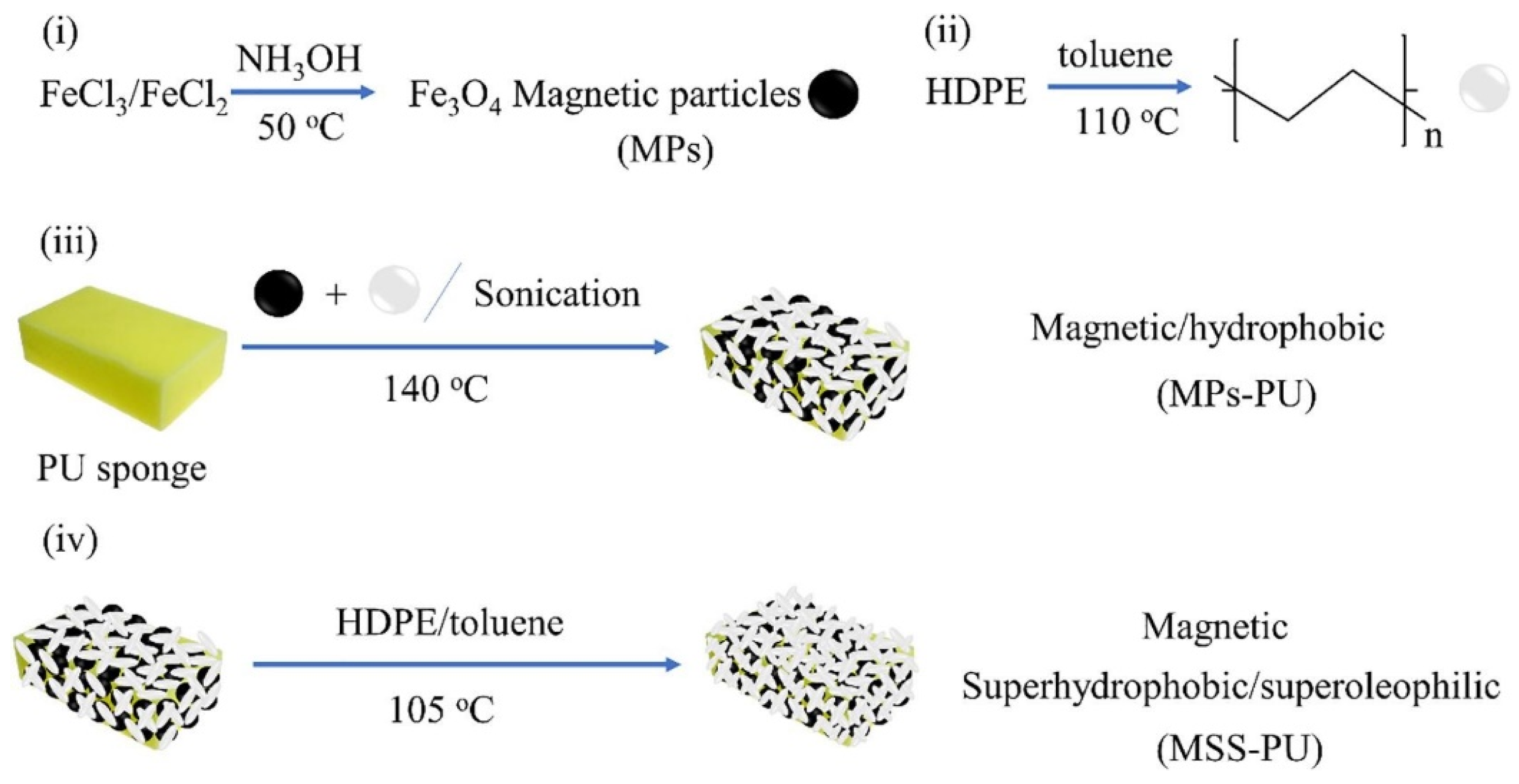
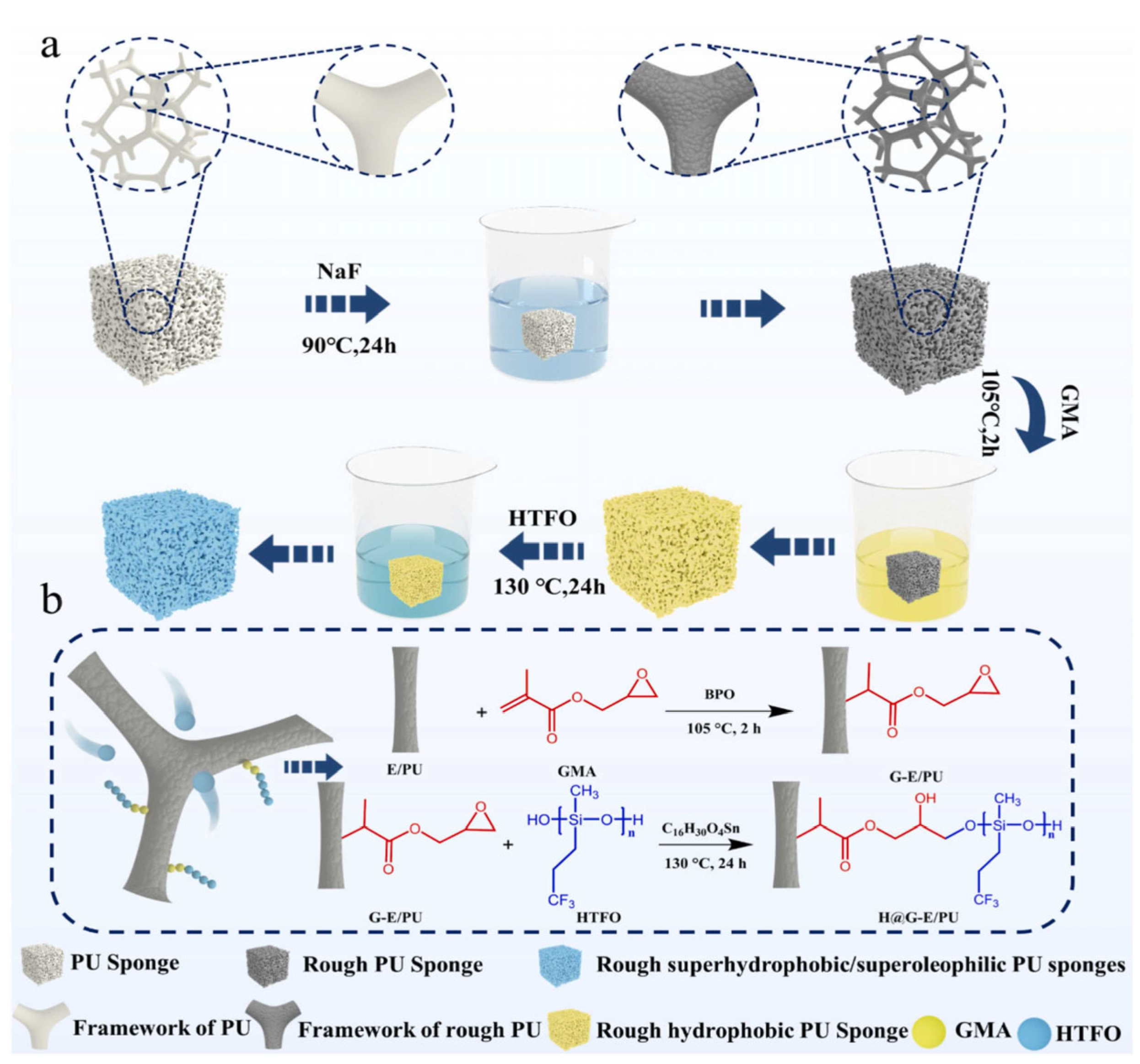
3.2.2. Sponges
- (1)
- Polyurethane Sponge
- (2)
- Melamine Sponge (MS)
| Nano-Sized Materials | Modification Method | Absorption Capacity (g/g) | WCA | Refs |
|---|---|---|---|---|
| Titanium dioxide | Spontaneous | 77–211 times its weight | 149° ± 1.5° | [63] |
| Graphene | Dip coating and chemical reduction | GO@MS: 105.3 times its weight rGO@MS: 72.3–136.5 times its weight | 151° | [68] |
| Nanodiamonds | Interfacial assembling | 26.65–55.64 | 155° ± 2° | [65] |
| Silicon dioxide | Dip coating and thermocuring | 40–90 times its weight | 162.6° | [69] |
| Carbon dots | Dip coating and thermocuring | — | — | [67] |
- (3)
- Wood-Based Sponges
3.2.3. Foam
| Modified Materials | Modification Method | Absorption Capacity (g/g) | WCA (°) | Refs |
|---|---|---|---|---|
| MCF | Dip Coating | 9–32 times its weight | 143 | [89] |
| Nano CuO and HDA | Oxidative self-polymerization | 45 times its weight | 153 | [88] |
| PDA and MWCNTs | Dip Coating | 23 times its weight | 153 | [90] |
| HS(CH2)11CH3 and HS(CH2)10COOH | Electrodeposition and Thiol Modification | - | 154.6 ± 2 | [91] |
| CB(h-BN)@Fe3O4 and acrylic resin | Dip Coating | 64–176 | 159 | [87] |
3.2.4. Natural Biomass Adsorbents
- (1)
- Cellulose-Based Adsorbent Materials
- (a)
- Cellulose-Based Aerogels: Due to their ultralow density, highly branched three-dimensional nanostructures, and exceptional absorption capacity for various oils and organic solvents, cellulose-based aerogels exhibit significant potential in oil–water separation applications. A carbon aerogel derived from winter melon, prepared via hydrothermal treatment, freeze-drying, and subsequent pyrolysis, boasts a porosity exceeding 97.5% and a density of only 0.048 g/cm³, demonstrating excellent hydrophobicity and oleophilicity [94]. This aerogel can absorb 16 to 50 times its weight in oil and retains nearly 100% of its initial oil absorption capacity even after several recycling cycles. Similarly, carbon aerogels derived from durian shells and bamboo pulp also display exceptional oil absorption performance and reusability [95,96]. Additionally, nanocellulose-based aerogels, modified with surface treatment techniques such as sulfonation, can achieve underwater superoleophobicity, along with good cyclic stability and stable wettability. These properties make cellulose-based aerogels highly promising for the development of materials for oil–water separation and purification.
- (b)
- Cellulose-Based Smart Responsive and Adsorption-Assisted Materials: The growing demand for advanced materials in various applications has led to increased interest in smart, responsive cellulose aerogels with tunable wettability, particularly for oil–water separation. Due to its highly porous network structure, cellulose not only enables control over the carbonization process for producing cellulose-derived materials but also facilitates the synthesis of cellulose-based aerogels, which can function as effective adsorbents. Li et al. [97] developed CO2-responsive cellulose nanofiber aerogels using surface-initiated atom transfer radical polymerization (ATRP) technology. The wettability of this aerogel can be modulated in response to environmental conditions. In the absence of CO2, the aerogel exhibits a water contact angle (WCA) of approximately 130°, making it hydrophobic. Upon CO2 exposure, the charged and extended PDMAEMA brushes form hydrogen bonds with water molecules, which reduces the effective pore size, enabling the aerogel to effectively separate water from oil–water mixtures. The aerogel’s porous structure and responsiveness contribute to its superior performance in oil–water separation. Moreover, its recyclability minimizes the impact of pollutants, significantly enhancing its reusability.
- (c)
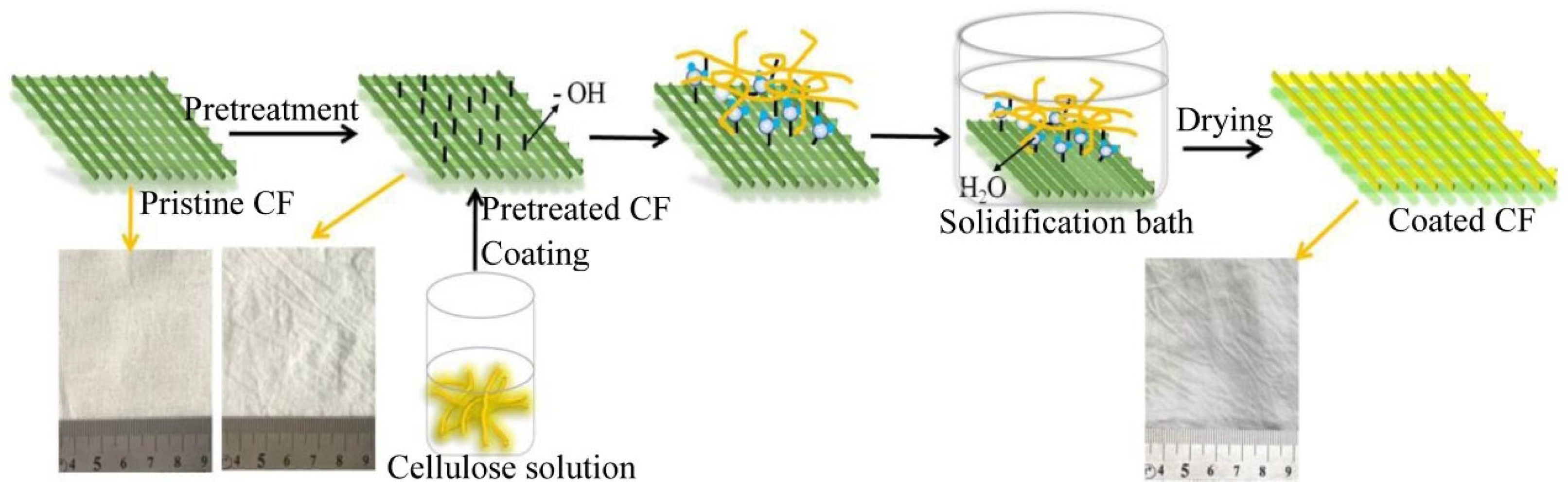
- Cellulose Superhydrophobic Sponges: Due to their excellent mechanical properties, biodegradability, environmental friendliness, and low cost, cellulose superhydrophobic sponges have gained significant attention for oil–water separation applications in recent years. Meng et al. [99] developed a hydrophobic cellulose sponge through a simple preparation process, using cellulose (C6H10O5) that was etched with HCl and subsequently modified with octadecyltrichlorosilane (OTS) to enhance the sponge’s surface roughness and hydrophobicity. The sponge exhibited a water contact angle (WCA) of 153.5° and an oil contact angle (OCA) of 0°. It achieved a separation efficiency of up to 92% for various oil–water mixture systems. In another study, Qin et al. [100] coated commercial cellulose sponges with reduced graphene oxide (rGO) and polybenzoxazine (PBZ) to create nanocomposite cellulose sponges. These sponges displayed superhydrophobicity with a WCA of 155° and excellent mechanical stability over 100 compression cycles. They maintained a high oil–water separation flux (107,428 L m−2 h−1) at 0.10 bar and achieved a separation efficiency of 99.1% even after 10 cycles. Additionally, Li et al. [101] prepared smart responsive cellulose acetate sponges with switchable wettability under varying pH conditions, utilizing dynamic covalent amide bonds. These sponges exhibited a selective oil adsorption capacity of 40–80 times their weight and an 80% desorption ability after 10 separation cycles.
- (2)
- Chitosan-Based Adsorbent Materials
- (3)
- Lignin-Based Adsorbent Materials
3.3. Filtration Methods
3.3.1. Metal Meshes
3.3.2. Paper
3.4. Membrane Separation
3.4.1. Metal-Based Filter Membranes
3.4.2. Inorganic Non-Metallic Substrate Filter Membranes
3.4.3. Polymer Filter Membranes
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.P.; Zhao, J.; Su, B.; Liu, X.; Peng, J.; Liu, Y.; Liu, H.; Yang, G.; Jiang, L.; Wen, Y.; et al. An Ion-Induced Low-Oil-Adhesion Organic/Inorganic Hybrid Film for Stable Superoleophobicity in Seawater. Adv. Mater. 2012, 25, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef]
- Bong, J.; Lim, T.; Seo, K.; Kwon, C.-A.; Park, J.H.; Kwak, S.K.; Ju, S. Dynamic graphene filters for selective gas-water-oil separation. Sci. Rep. 2015, 5, 14321. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z. Understanding the separations of oil/water mixtures from immiscible to emulsions on super-wettable surfaces. J. Bionic Eng. 2016, 13, 1–29. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water-Sui 2021, 13, 980. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, J.; Li, B.; Mi, Y.; Yang, Y.; Feng, X. The demulsification of oily wastewater by a hyperbranched polymer grafted SiO2. J. Dispers. Sci. 2023, 44, 651–659. [Google Scholar] [CrossRef]
- Wolok, E.; Barafi, J.; Joshi, N.; Girimonte, R.; Chakraborty, S. Study of bio-materials for removal of the oil spill. Arab. J. Geosci. 2020, 13, 1244. [Google Scholar] [CrossRef]
- Lewis, A.; Hopkins, J.; Carslaw, D.; Hamilton, J.; Nelson, B.S.; Stewart, G.; Dernie, J.; Passant, N.; Murrells, T. An increasing role for solvent emissions and implications for future measurements of volatile organic compounds. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2020, 378, 20190328. [Google Scholar] [CrossRef]
- Chu, J.Q.; Lu, Y.; Gan, S.X.; Qi, Q.Y.; Jia, C.; Yao, J.; Zhao, X. A Superhydrophobic Trifluoromethyl-Containing Covalent Organic Framework Membrane for Efficient Oil/Water Separation. Macromol. Rapid Commun. 2022, 44, e2200641. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Cai, M.; Xu, Q.; Zhang, J.; Cao, X.; Zhang, J. Advanced superhydrophobic and multifunctional nanocellulose aerogels for oil/water separation: A review. Carbohydr. Polym. 2023, 300, 120242. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Si, Y.; Tang, X.; Zhu, Z.; Ding, B.; Liu, L.; Zheng, G.; Luo, W.; Yu, J. Gravity driven separation of emulsified oil–water mixtures utilizing in situ polymerized superhydrophobic and superoleophilic nanofibrous membranes. J. Mater. Chem. A 2013, 1, 14071–14074. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Bio/Technol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Belachew, G.B.; Hu, C.-C.; Chang, Y.-Y.; Wang, C.-F.; Hung, W.-S.; Chen, J.-K.; Lai, J.-Y. An Eco-Friendly Manner to Prepare Superwetting Melamine Sponges with Switchable Wettability for the Separation of Oil/Water Mixtures and Emulsions. Polymers 2024, 16, 693. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, Y.A.; Crick, C. Heat-treated micronized polyethylene powder for efficient oil/water separating filters. Materials 2020, 13, 3160. [Google Scholar] [CrossRef]
- Sadler, E.; Crick, C.R. Suction or gravity-fed oil-water separation using PDMS-coated glass filters. Sustain. Mater. Technol. 2021, 29, e00321. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, P.; Wang, F.; Hu, C. Preparation of Hydrophobic Fly Ash by Surface Modification and Oil-water Separation Devices. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2023, 38, 695–700. [Google Scholar] [CrossRef]
- Gavazzoni, C.; Lazzari, D.; da Silva Ramos, I.P.; Brito, C. Optimizing oil–water separation using fractal surfaces. J. Chem. Phys. 2025, 162, 044702. [Google Scholar] [CrossRef]
- Zeevalkink, J.; Brunsmann, J. Oil removal from water in parallel plate gravity-type separators. Water Res. 1983, 17, 365–373. [Google Scholar] [CrossRef]
- Assar, M.; Asaadian, H.; Stanko, M.; Grimes, B.A. A theoretical and experimental investigation of continuous oil–water gravity separation. Chem. Eng. Sci. 2024, 298, 120375. [Google Scholar] [CrossRef]
- Baccioni, L.; Peri, C. Centrifugal separation. In Extra-Virgin Olive Oil Handbook; Wiley: Hoboken, NJ, USA, 2014; pp. 139–154. [Google Scholar]
- Cambiella, A.; Benito, J.; Pazos, C.; Coca, J. Centrifugal separation efficiency in the treatment of waste emulsified oils. Chem. Eng. Res. Des. 2006, 84, 69–76. [Google Scholar] [CrossRef]
- Kassymbekov, Z.; Akmalaiuly, K.; Kassymbekov, G. Application of hydrocyclones to improve membrane technologies for urban wastewater treatment. J. Ecol. Eng. 2021, 22, 148–155. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Cen, C.; Wu, X.; Mao, R.; Zheng, Y. Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Luo, X.; Gao, Q.; Liu, M.; Yang, L.; He, L. Separation characteristics of axial-flow gas-liquid cyclone separator. Acta Pet. Sin. 2020, 36, 592–599. [Google Scholar]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Chi, Y.; Wang, F.; Yan, J.; Ni, M.; Cen, K. The effect of microbubbles on gas-liquid mass transfer coefficient and degradation rate of COD in wastewater treatment. Water Sci. Technol. 2016, 73, 1969–1977. [Google Scholar] [CrossRef]
- Fan, W.; Li, Y.; Lyu, T.; Chen, Z.; Jarvis, P.; Huo, Y.; Xiao, D.; Huo, M.J.W.R. A modelling approach to explore the optimum bubble size for micro-nanobubble aeration. Water Res. 2023, 228, 119360. [Google Scholar] [CrossRef]
- Wang, C.; Lü, Y.; Song, C.; Zhang, D.; Rong, F.; He, L. Separation of emulsified crude oil from produced water by gas flotation: A review. Sci. Total Environ. 2022, 845, 157304. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. A critical review in electrocoagulation technology applied for oil removal in industrial wastewater. Chemosphere 2022, 288, 132355. [Google Scholar] [CrossRef]
- Fotovat, F.; Hosseini, M. Treatment of oily wastewater by electrocoagulation: Simultaneous optimization of oil removal efficiency and specific energy consumption. J. Water Process Eng. 2023, 55, 104221. [Google Scholar] [CrossRef]
- Sasson, M.B.; Calmano, W.; Adin, A. Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J. Hazard. Mater. 2009, 171, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Theydan, S.K.; Mohammed, W.T. Refinery Wastewater Treatment by a Novel Three-Dimensional Electrocoagulation System Design. Eng. Technol. Appl. Sci. Res. 2022, 12, 9590–9600. [Google Scholar] [CrossRef]
- Treviño-Reséndez, J.; Razo-Negrete, M.; Godínez, L.A.; Meas, Y.; García-Espinoza, J.D. Integrated system of electrocoagulation, activated sludge, and electrooxidation for the treatment of oil refinery wastewater. Chem. Eng. Process.-Process Intensif. 2025, 208, 110135. [Google Scholar] [CrossRef]
- Ji, M.; Jiang, X.; Wang, F. A mechanistic approach and response surface optimization of the removal of oil and grease from restaurant wastewater by electrocoagulation and electroflotation. Desalination Water Treat. 2015, 55, 2044–2052. [Google Scholar] [CrossRef]
- Adedotun, T.A.; Ewemoje, T.A. A preliminary study on the treatment of restaurant wastewater using electrocoagulation technique. J. Degrad. Min. Lands Manag. 2020, 7, 2029–2033. [Google Scholar]
- Akansha, J.; Nidheesh, P.V.; Gopinath, A.; Anupama, K.V.; Suresh Kumar, M. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 2020, 253, 126652. [Google Scholar] [CrossRef]
- García-Morales, M.A.; Barrera-Díaz, C.; Martínez Miranda, V.; Roa-Morales, G.; Martín del Campo Lopez, E.; Torres Blancas, T. Wastewater Treatment of Chocolate Manufacture Industry through an Integrated Process of Electrocoagulation and Filtration. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2014; p. 941. [Google Scholar]
- Ngobeni, P.V.; Basitere, M.; Thole, A. Treatment of poultry slaughterhouse wastewater using electrocoagulation: A review. Water Pract. Technol. 2021, 17, 38–59. [Google Scholar] [CrossRef]
- Aiyd Jasim, M.; AlJaberi, F.Y. Investigation of oil content removal performance in real oily wastewater treatment by electrocoagulation technology: RSM design approach. Results Eng. 2023, 18, 101082. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sardari, E.; Javadian, H.R.; Katal, R.; Sefti, M.V. Removal of oil from biodiesel wastewater by electrocoagulation method. Korean J. Chem. Eng. 2013, 30, 634–641. [Google Scholar] [CrossRef]
- Rahmani, Z.; Rashidi, A.M.; Samadi, M.T.; Rahmani, A.R. N-doped reduced graphene oxide aerogel for the selective adsorption of oil pollutants from water: Isotherm and kinetic study. J. Ind. Eng. Chem. 2018, 61, 416–426. [Google Scholar] [CrossRef]
- Behera, U.S.; Poddar, S.; Byun, H.-S. Electrocoagulation treatment of wastewater collected from Haldia industrial region: Performance evaluation and comparison of process optimization. Water Res. 2025, 268, 122716. [Google Scholar] [CrossRef]
- Nassar, S.O.A.; Yusoff, M.S.; Halim, H.; Mokhtar Kamal, N.H.; Bashir, M.J.K.; Manan, T.S.B.A.; Aziz, H.A.; Mojiri, A. Ultrasonic (US)-Assisted Electrocoagulation (EC) Process for Oil and Grease (O&G) Removal from Restaurant Wastewater. Separations 2023, 10, 61. [Google Scholar]
- Oliveira, L.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.d.S.; McKay, G.; Meili, L. Sorption as a rapidly response for oil spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2021, 407, 124842. [Google Scholar] [CrossRef]
- Ravi, Y.; Prasanthi, I.; Behera, S.; Datta, K. MIL-101 (Fe) networks supported on fluorinated graphene nanosheets as coatings for oil sorption. ACS Appl. Nano Mater. 2022, 5, 5857–5867. [Google Scholar] [CrossRef]
- Torres, C.E.I.; Quezada, T.E.S.; Kharissova, O.V.; Kharisov, B.I.; de la Fuente, M.I.G. Carbon-based aerogels and xerogels: Synthesis, properties, oil sorption capacities, and DFT simulations. J. Environ. Chem. Eng. 2021, 9, 104886. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Some theoretical aspects of tertiary treatment of water/oil emulsions by adsorption and coalescence mechanisms: A review. Water-Sui 2021, 13, 652. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Liu, Q.; Lu, Q.; Cheng, R.; Zhan, K.; Wan, H.; Yang, L.; Du, G.; Gao, W. Superhydrophobic Poplar Scrimber Via In Situ Synthesis of Cu7Cl4 (OH) 10· H2O Heterostructure Inspired by Pine Cone with Superultraviolet Resistance. ACS Sustain. Chem. Eng. 2022, 10, 16169–16181. [Google Scholar] [CrossRef]
- Khurana, N.; Arora, P.; Pente, A.S.; Pancholi, K.C.; Kumar, V.; Kaushik, C.P.; Rattan, S. Surface modification of zinc oxide nanoparticles by vinyltriethoxy silane (VTES). Inorg. Chem. Commun. 2021, 124, 108347. [Google Scholar] [CrossRef]
- Li, Z.; Jin, Y.; Chen, T.; Tang, F.; Cai, J.; Ma, J. Trimethylchlorosilane modified activated carbon for the adsorption of VOCs at high humidity. Sep. Purif. Technol. 2021, 272, 118659. [Google Scholar] [CrossRef]
- Kumar, P.G.; Kanmani, S.; Kumar, P.S. Treatability studies on the optimization of ozone and carbon dosages for the effective removal of contaminants from secondary treated effluent. Adsorpt. Sci. Technol. 2022, 2022, 1998549. [Google Scholar] [CrossRef]
- Mariana, M.; Hps, A.K.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Sun, R.; Yu, N.; Zhao, J.; Mo, J.; Pan, Y.; Luo, D. Chemically stable superhydrophobic polyurethane sponge coated with ZnO/epoxy resin coating for effective oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125850. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N. Preparation of magnetic, superhydrophobic/superoleophilic polyurethane sponge: Separation of oil/water mixture and demulsification. Chem. Eng. J. 2020, 384, 123339. [Google Scholar] [CrossRef]
- Parsaie, A.; Mohammadi-Khanaposhtani, M.; Riazi, M.; Tamsilian, Y. Magnesium stearate-coated superhydrophobic sponge for oil/water separation: Synthesis, properties, application. Sep. Purif. Technol. 2020, 251, 117105. [Google Scholar] [CrossRef]
- Xu, Y.; Liao, J.; He, R.; Yang, S.; Luo, Z.; Xu, M.; Tao, Y.; Wang, X. Superhydrophobic and oleophilic polyurethane sponge for oil/water separation. Mater. Today Commun. 2024, 38, 107658. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Li, H.; Lai, X.; Zeng, X. Superhydrophobic, flame-retardant and magnetic polyurethane sponge for oil-water separation. J. Environ. Chem. Eng. 2022, 10, 107580. [Google Scholar] [CrossRef]
- Peng, L.; Li, H.; Zhang, Y.; Su, J.; Yu, P.; Luo, Y. A superhydrophobic 3D porous material for oil spill cleanup. Rsc Adv. 2014, 4, 46470–46475. [Google Scholar] [CrossRef]
- Ge, B.; Men, X.; Zhu, X.; Zhang, Z. A superhydrophobic monolithic material with tunable wettability for oil and water separation. J. Mater. Sci. 2015, 50, 2365–2369. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, J.; Yan, H.; Xiao, J.; Wang, J. Reversible wettability switching of melamine sponges for oil/water separation. Mater. Chem. Phys. 2021, 257, 123772. [Google Scholar] [CrossRef]
- Luo, X.; He, Z.; Gong, H.; He, L. Recent advances in oil-water separation materials with special wettability modified by graphene and its derivatives: A review. Chem. Eng. Process.-Process Intensif. 2022, 170, 108678. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Q.; Zhang, K.; Wang, F.; Zhi, J.; Shan, C.-X. Superhydrophobic nanodiamond-functionalized melamine sponge for oil/water separation. Langmuir 2022, 38, 11304–11313. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, J.; Niu, X.; Wang, J.; Dong, G.; Shan, M.; Zhang, B.; Matsuyama, H.; Zhang, Y. Interfacial assembly of micro/nanoscale nanotube/silica achieves superhydrophobic melamine sponge for water/oil separation. Sep. Purif. Technol. 2022, 280, 119920. [Google Scholar] [CrossRef]
- Li, J.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Carbon dots efficiently enhance photothermal conversion and storage of organic phase change materials through interfacial interaction. Carbon 2023, 203, 21–28. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zhen, C.; Li, F.; Sha, S.; Hou, C.; Lu, H.; Wu, J.; Sheng, Z.; Ma, J. Graphene-based melamine sponges with reverse wettability for oil/water separation through absorption and filtration. J. Environ. Chem. Eng. 2022, 10, 107543. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y.; Ma, S.; Shi, H.; Wang, Y.; Ren, H.; Xu, K. Enhanced oil/water separation using superhydrophobic nano SiO2-modified porous melamine sponges. Chemosphere 2024, 369, 143879. [Google Scholar] [CrossRef]
- Dong, X.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Polydopamine-assisted surface coating of MIL-53 and dodecanethiol on a melamine sponge for oil–water separation. Langmuir 2020, 36, 1212–1220. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Q.; Zeng, Q.; Jiang, Q.; Ren, Q. Solar interfacial evaporation based oil/water separation from emulsion using a wood-melamine/calcium alginate composite structure. Sol. Energy 2023, 250, 59–69. [Google Scholar] [CrossRef]
- Lei, Z.; Deng, Y.; Wang, C. Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J. Mater. Chem. A 2018, 6, 3258–3263. [Google Scholar] [CrossRef]
- Bauza, M.; Palomino, G.T.; Cabello, C.P. MIL-100 (Fe)-derived carbon sponge as high-performance material for oil/water separation. Sep. Purif. Technol. 2021, 257, 117951. [Google Scholar] [CrossRef]
- Zhou, X.; Li, D.; Wang, L.; Wang, Q.; Wang, Z.; Jing, Q.; Marisol, R.; Li, L. Recent advances in the modification of melamine sponge for oil-water separation. J. Mater. Sci. Technol. 2025, 207, 209–224. [Google Scholar] [CrossRef]
- Hadji, E.M.; Fu, B.; Abebe, A.; Bilal, H.M.; Wang, J. Sponge-based materials for oil spill cleanups: A review. Front. Chem. Sci. Eng. 2020, 14, 749–762. [Google Scholar] [CrossRef]
- Lam, S.S.; Xia, C.; Sonne, C. Plastic crisis underscores need for alternative sustainable-renewable materials. J. Bioresour. Bioprod. 2022, 7, 145–147. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, T.; Ge-Zhang, S.; Mu, P.; Liu, Y.; Cui, J. Wood sponge for oil–water separation. Polymers 2024, 16, 2362. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem. Eng. J. 2019, 358, 1539–1551. [Google Scholar] [CrossRef]
- Ray, U.; Zhu, S.; Pang, Z.; Li, T. Mechanics design in cellulose-enabled high-performance functional materials. Adv. Mater. 2021, 33, 2002504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Huang, Q.; Li, F.; Liu, X. Liquid-phase deposition functionalized wood sponges for oil/water separation. J. Mater. Sci. 2021, 56, 19075–19092. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S.; Li, X.; Zou, H.; Zhuo, B.; Ti, P.; Yuan, Q.J.J.o.M.S. Optimization and absorption performance of wood sponge. J. Mater. Sci. 2021, 56, 8479–8496. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, Y.; Yang, F.; Gan, J.; Wang, Y.; Zhang, J.J.A.o. Wood sponge reinforced with polyvinyl alcohol for sustainable oil–water separation. ACS Omega 2021, 6, 12866–12876. [Google Scholar] [CrossRef]
- Wu, M.-B.; Huang, S.; Liu, C.; Wu, J.; Agarwal, S.; Greiner, A.; Xu, Z.-K. Carboxylated wood-based sponges with underoil superhydrophilicity for deep dehydration of crude oil. J. Mater. Chem. A 2020, 8, 11354–11361. [Google Scholar] [CrossRef]
- Król, P.; Król, B. Structures, properties and applications of the polyurethane ionomers. J. Mater. Sci. 2020, 55, 73–87. [Google Scholar] [CrossRef]
- Terban, M.W.; Dabbous, R.; Debellis, A.D.; Pselt, E.; Billinge, S.J.L. Structures of Hard Phases in Thermoplastic Polyurethanes. Macromolecules 2016, 49, 7350–7358. [Google Scholar] [CrossRef]
- Terban, M.W.; Seidel, K.; Pöselt, E.; Malfois, M.; Baumann, R.-P.; Sander, R.; Paulus, D.; Hinrichsen, B.; Dinnebier, R.E. Cross-examining polyurethane nanodomain formation and internal structure. Macromolecules 2020, 53, 9065–9073. [Google Scholar] [CrossRef]
- Habibi, N.; Pourjavadi, A. Magnetic, thermally stable, and superhydrophobic polyurethane sponge: A high efficient adsorbent for separation of the marine oil spill pollution. Chemosphere 2022, 287, 132254. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Feng, X.; Pan, Q. Preparation of superhydrophobic and superoleophilic polyurethane foam for oil spill cleanup. J. Macromol. Sci. Part A 2021, 58, 758–768. [Google Scholar] [CrossRef]
- Alazab, A.A.; Saleh, T.A. Magnetic hydrophobic cellulose-modified polyurethane filter for efficient oil-water separation in a complex water environment. J. Water Process Eng. 2022, 50, 103125. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, H.; Gong, Q.; Xie, Y.; Fei, F.; Fan, L. Preparation and modification of antibacterial polyurethane foam for oil-water separation. J. Mater. Res. 2023, 38, 2701–2712. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, Y.; Zhan, B.; Han, Z.; Ren, L. pH-responsive smart superwetting Fe foam for efficient in situ on-demand oil/water separation. J. Mater. Sci. 2021, 56, 13372–13385. [Google Scholar] [CrossRef]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a process for produced water treatment: A review. Processes 2020, 8, 1657. [Google Scholar] [CrossRef]
- Ke, W.; Ge, F.; Shi, X.; Zhang, Y.; Wu, T.; Zhu, X.; Cheng, Y.; Shi, Y.; Wang, Z.; Yuan, L. Superelastic and superflexible cellulose aerogels for thermal insulation and oil/water separation. Int. J. Biol. Macromol. 2024, 260, 129245. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhu, F.; You, L.; Shen, X.; Li, S. Removal of organic solvents/oils using carbon aerogels derived from waste durian shell. J. Taiwan Inst. Chem. Eng. 2017, 78, 351–358. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Zhao, J.; Li, Q.; Ao, C.; Xia, T.; Zhang, W.; Lu, C. Ultra-lightweight and highly porous carbon aerogels from bamboo pulp fibers as an effective sorbent for water treatment. Results Phys. 2017, 7, 2919–2924. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Grishkewich, N.; Tam, K.C.; Yuan, J.; Mao, Z.; Sui, X. CO2-responsive cellulose nanofibers aerogels for switchable oil–water separation. ACS Appl. Mater. Interfaces 2019, 11, 9367–9373. [Google Scholar] [CrossRef]
- Li, Z.; Lei, S.; Xi, J.; Ye, D.; Hu, W.; Song, L.; Hu, Y.; Cai, W.; Gui, Z. Bio-based multifunctional carbon aerogels from sugarcane residue for organic solvents adsorption and solar-thermal-driven oil removal. Chem. Eng. J. 2021, 426, 129580. [Google Scholar] [CrossRef]
- Meng, X.; Dong, Y.; Zhao, Y.; Liang, L. Preparation and modification of cellulose sponge and application of oil/water separation. RSC Adv. 2020, 10, 41713–41719. [Google Scholar] [CrossRef]
- Qin, Y.; Li, S.; Li, Y.; Pan, F.; Han, L.; Chen, Z.; Yin, X.; Wang, L.; Wang, H. Mechanically robust polybenzoxazine/reduced graphene oxide wrapped-cellulose sponge towards highly efficient oil/water separation, and solar-driven for cleaning up crude oil. Compos. Sci. Technol. 2020, 197, 108254. [Google Scholar] [CrossRef]
- Li, L.; Rong, L.; Xu, Z.; Wang, B.; Feng, X.; Mao, Z.; Xu, H.; Yuan, J.; Liu, S.; Sui, X. Cellulosic sponges with pH responsive wettability for efficient oil-water separation. Carbohydr. Polym. 2020, 237, 116133. [Google Scholar] [CrossRef]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from Water. J. Hazard. Mater. 2020, 385, 121507. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, X.; Bao, M.; Li, Y. Construction of a hydrophobic magnetic aerogel based on chitosan for oil/water separation applications. Int. J. Biol. Macromol. 2020, 165, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, G.; Cao, J.; Fan, L.; Cai, W.; Yin, Y. Underwater superoleophobic and salt-tolerant sodium alginate/N-succinyl chitosan composite aerogel for highly efficient oil–water separation. ACS Appl. Polym. Mater. 2020, 2, 1124–1133. [Google Scholar] [CrossRef]
- Liang, F.; Hou, T.; Li, S.; Liao, L.; Li, P.; Li, C. Elastic, super-hydrophobic and biodegradable chitosan sponges fabricated for oil/water separation. J. Environ. Chem. Eng. 2021, 9, 106027. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.; Tsang, D.C.; Hunt, A. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef]
- Partow, A.J.; Meng, S.; Wong, A.J.; Savin, D.A.; Tong, Z. Recyclable & highly porous organo-aerogel adsorbents from biowaste for organic contaminants’ removal. Sci. Total Environ. 2022, 827, 154051. [Google Scholar]
- Tan, Z.; Hu, L.; Yang, D.; Zheng, D.; Qiu, X. Lignin: Excellent hydrogel swelling promoter used in cellulose aerogel for efficient oil/water separation. J. Colloid Interface Sci. 2023, 629, 422–433. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Liu, K.; Gibril, M.E.; Kong, F.; Wang, S. Lignin-based superhydrophobic melamine resin sponges and their application in oil/water separation. Ind. Crops Prod. 2021, 170, 113798. [Google Scholar] [CrossRef]
- Lehtonen, J.; Chen, X.; Beaumont, M.; Hassinen, J.; Orelma, H.; Dumée, L.F.; Tardy, B.L.; Rojas, O.J. Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration. Carbohydr. Polym. 2021, 251, 117073. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.A.; Silva, W.E.; Belian, M.F.; Lins, L.S.G.; Galembeck, A. Bacterial cellulose membranes for environmental water remediation and industrial wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3997–4008. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Faisal, I.; Alwan, M.M. Oily wastewater treatment using expanded beds of activated carbon and zeolite. Iraqi J. Chem. Pet. Eng. 2011, 12, 1–12. [Google Scholar] [CrossRef]
- Saengkaew, J.; Le, D.; Samart, C.; Sawada, H.; Nishida, M.; Chanlek, N.; Kongparakul, S.; Kiatkamjornwong, S. Superhydrophobic coating from fluoroalkylsilane modified natural rubber encapsulated SiO2 composites for self-driven oil/water separation. Appl. Surf. Sci. 2018, 462, 164–174. [Google Scholar] [CrossRef]
- Zhang, X.; Chai, W.; Liu, X.; Beibei, L. Stainless steel mesh coated withsilica for oil–water separation. Eur. Polym. J. 2015, 73, 374–379. [Google Scholar]
- Zhang, L.; Zhong, Y.; Cha, D.; Wang, P. A self-cleaning underwater superoleophobic mesh for oil-water separation. Sci. Rep. 2013, 3, 2326. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Y.; Xu, J.; Chen, B.; Tang, H.; Li, C. Durable superhydrophobic/superoleophilic epoxy/attapulgite nanocomposite coatings for oil/water separation. Surf. Coat. Technol. 2015, 272, 285–290. [Google Scholar] [CrossRef]
- Dunderdale, G.J.; England, M.W.; Sato, T.; Urata, C.; Hozumi, A. Programmable Oil/Water Separation Meshes: Water or Oil Selectivity Using Contact Angle Hysteresis. Macromol. Mater. Eng. 2016, 301, 1032–1036. [Google Scholar] [CrossRef]
- Lee, C.H.; Johnson, N.; Drelich, J.; Yap, Y.K. The performance of superhydrophobic and superoleophilic carbon nanotube meshes in water–oil filtration. Carbon 2011, 49, 669–676. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Liu, Q. ZnO nanorod array-coated mesh film for the separation of water and oil. Nanoscale Res. Lett. 2013, 8, 183. [Google Scholar] [CrossRef]
- Deng, D.; Prendergast, D.P.; Macfarlane, J.; Bagatin, R.; Stellacci, F.; Gschwend, P.M. Hydrophobic Meshes for Oil Spill Recovery Devices. Appl. Mater. Interfaces 2013, 5, 774–781. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Yao, W.; Liu, J.; Han, Z.; Ren, L. Bioinspired structured superhydrophobic and superoleophilic stainless steel mesh for efficient oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 54–63. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Xue, M.; Ou, J.; Li, C.; Li, W. Superhydrophobic and Superoleophilic Miniature Device for the Collection of Oils from Water Surfaces. J. Phys. Chem. C 2014, 118, 6344–6351. [Google Scholar] [CrossRef]
- Varshney, P.; Nanda, D.; Satapathy, M.; Mohapatra, S.S.; Kumar, A. A facile modification of steel mesh for oil–water separation. N. J. Chem. 2017, 41, 7463–7471. [Google Scholar] [CrossRef]
- Shen, L.; Wang, X.; Zhang, Z.; Jin, X.; Jiang, M.; Zhang, J. Design and Fabrication of the Evolved Zeolitic Imidazolate Framework-Modified Polylactic Acid Nonwoven Fabric for Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 14653–14661. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Girard, M.; Dumont, M.-J.; Chouinard, G.; Tavares, J.R. Surface Modification of Commercially Available PLA Polymer Mesh. Ind. Eng. Chem. Res. 2022, 61, 17297–17305. [Google Scholar] [CrossRef]
- Ren, D.; Guo, Z.; Song, W.; Guo, Z.; Liu, H.; Huang, M.; Liu, W. Modification of Poly(Lactic Acid) Non-Woven Fabric for Enhanced Oil–Water Separation. Fibers Polym. 2024, 25, 1727–1736. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple Method for Preparing Superhydrophobic Paper: Spray-Deposited Hydrophobic Silica Nanoparticle Coatings Exhibit High Water-Repellency and Transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, M.; Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shen, C.; Zhu, W.; Zhang, S.; Xu, Y.; Yang, Y.; Gao, M.; Dong, F. A facile procedure to modify filter paper for oil–water separation. RSC Adv. 2017, 7, 30495–30499. [Google Scholar] [CrossRef]
- Cai, Y.; Shi, S.Q.; Fang, Z.; Li, J. Design, development, and outlook of superwettability membranes in oil/water emulsions separation. Adv. Mater. Interfaces 2021, 8, 2100799. [Google Scholar] [CrossRef]
- Cheng, X.; Li, T.; Yan, L.; Jiao, Y.; Zhang, Y.; Wang, K.; Cheng, Z.; Ma, J.; Shao, L. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil-water separation. Sci. Adv. 2023, 9, eadh8195. [Google Scholar] [CrossRef]
- Qi, G.; Guo, K.; Yang, J.; Wang, Y.; Wang, Z.; Yuan, Z. A stable underwater superoleophobic membrane constructed by CuO oriented rods and PAA water-adsorbent resin for fast and high efficient oil–water separation. Sep. Purif. Technol. 2022, 294, 121175. [Google Scholar] [CrossRef]
- Avornyo, A.; Thanigaivelan, A.; Krishnamoorthy, R.; Hassan, S.W.; Banat, F. Ag-CuO-decorated ceramic membranes for effective treatment of oily wastewater. Membranes 2023, 13, 176. [Google Scholar] [CrossRef]
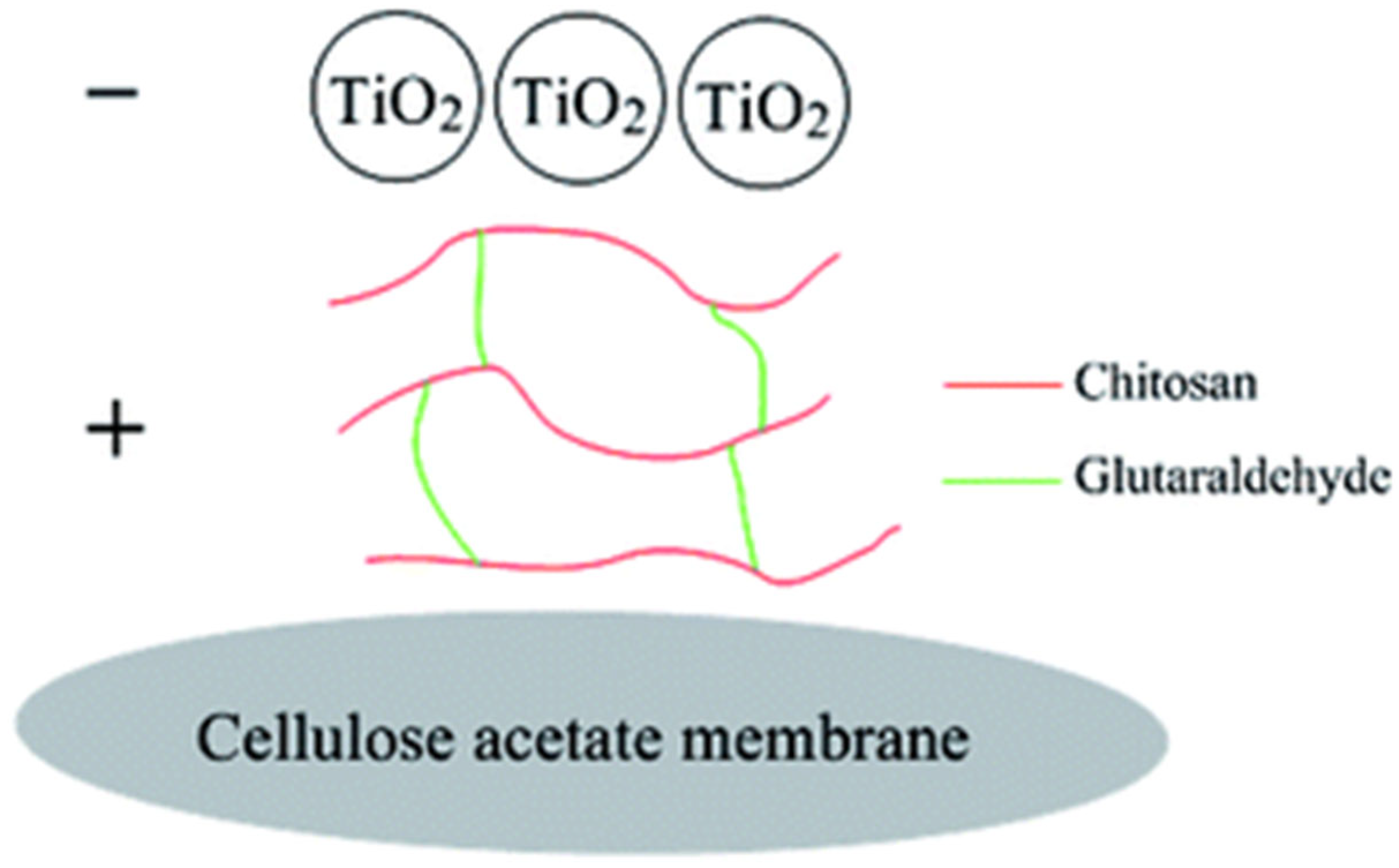
- Du, Y.; Li, Y.; Wu, T. A superhydrophilic and underwater superoleophobic chitosan–TiO2 composite membrane for fast oil-in-water emulsion separation. RSC Adv. 2017, 7, 41838–41846. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A.; Abussaud, B.; Aljundi, I.H. A simple approach to fabricate composite ceramic membranes decorated with functionalized carbide-derived carbon for oily wastewater treatment. Membranes 2022, 12, 394. [Google Scholar] [CrossRef]
- Naseeb, N.; Mohammed, A.A.; Laoui, T.; Khan, Z. A novel PAN-GO-SiO2 hybrid membrane for separating oil and water from emulsified mixture. Materials 2019, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Obaid, M.; Barakat, N.A.M.; Fadali, O.A.; Motlak, M.; Almajid, A.A.; Khalil, K.A. Effective and reusable oil/water separation membranes based on modified polysulfone electrospun nanofiber mats. Chem. Eng. J. 2015, 259, 449–456. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Effect of reduced graphene oxide/TiO2 nanocomposite with different molar ratios on the performance of PVDF ultrafiltration membranes. Sep. Purif. Technol. 2015, 140, 32–42. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun Nanofibrous Membranes for High Flux Microfiltration. J. Membr. Sci. 2012, 392–393, 167–174. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152. [Google Scholar] [CrossRef] [PubMed]
- Jinyou, L.; Feng, T.; Yanwei, S.; Fujun, W.; Bin, D. Facile control of intra-fiber porosity and inter-fiber voids in electrospun fibers for selective adsorption. Nanoscale 2012, 4, 5316–5320. [Google Scholar]
- Lin, J.; Ding, B.; Yang, J.; Yu, J.; Sun, G. Subtle regulation of the micro- and nanostructures of electrospun polystyrene fibers and their application in oil absorption. Nanoscale 2012, 4, 176–182. [Google Scholar] [CrossRef]
- Ning, L.Q.; Xu, N.K.; Wang, R.; Liu, Y. Fibrous membranes electrospun from the suspension polymerization product of styrene and butyl acrylate for oil-water separation. RSC Adv. 2015, 5, 57101–57113. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Highly Permeable Polymer Membranes Containing Directed Channels for Water Purification. ACS Macro Lett. 2012, 1, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lan, Y.; Zhu, W.; Li, W.; Xu, D.; Cui, J.; Shena, D.; Li, G. Polydopamine-coated nanofibrous mats as a versatile platform for producing porous functional membranes. J. Mater. Chem. 2012, 22, 16994–17001. [Google Scholar] [CrossRef]
- Tang, X.; Si, Y.; Ge, J.; Ding, B.; Liu, L.; Zheng, G.; Luo, W.; Yu, J. In situ polymerized superhydrophobic and superoleophilic nanofibrous membranes for gravity driven oil–water separation. Nanoscale 2013, 5, 11657–11664. [Google Scholar] [CrossRef]
- Feng, L.; Gao, Y.; Dai, Z.; Dan, H.; Xiao, F.; Yue, Q.; Gao, B.; Wang, S. Preparation of a rice straw-based green separation layer for efficient and persistent oil-in-water emulsion separation. J. Hazard. Mater. 2021, 415, 125594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Teng, C.; Zhai, R.; Jiang, L.; Wang, J.; Zhou, J.; Wang, R.; Wang, H.; Wang, X.; Ma, X. Easy preparation of wood-base membrane with fouling resistance in complex environments for efficient oil–water emulsion separation. Cellulose 2023, 30, 11041–11054. [Google Scholar] [CrossRef]
- Hu, R.; Yang, J.; Li, S.; Zhang, T.; Xiao, H.; Liu, Y.; Lu, M. Fabrication of special wettability functionalized Mg (OH) 2@ cotton fabric for oil/water mixtures and emulsions separation. Cellulose 2020, 27, 7739–7749. [Google Scholar] [CrossRef]
- Ou, X.; Ren, Y.; Guo, J.; Zhou, Y.; Yuan, Y.; Liu, Q.; Yan, F. ZIF-8@ poly (ionic liquid)-grafted cotton cloth for switchable water/oil emulsion separation. ACS Appl. Polym. Mater. 2020, 2, 3433–3439. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Chen, J.-T.; Hao, B.; Wang, R.; Ma, P.-C. Preparation of cellulose-coated cotton fabric and its application for the separation of emulsified oil in water. Carbohydr. Polym. 2020, 240, 116318. [Google Scholar] [CrossRef]
- Kuga, S.; Brown, R.M., Jr. Silver labeling of the reducing ends of bacterial cellulose. Carbohydr. Res. 1988, 180, 345–350. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Abd Rahman, N. Overview of bacterial cellulose production and application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Nascimento, V.; De Amorim, J.; Gomes, E.; Araujo, L.; Sarubbo, L. Residue from the production of sugar cane: An alternative nutrient used in biocellulose production by Gluconacetobacter hansenii. Chem. Eng. Trans. 2018, 64, 7–12. [Google Scholar]
- Albuquerque, R.M.; Meira, H.M.; Silva, I.D.; Silva, C.J.G.; Almeida, F.C.G.; Amorim, J.D.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of a bacterial cellulose/poly (3-hydroxybutyrate) blend activated with clove essential oil for food packaging. Polym. Polym. Compos. 2021, 29, 259–270. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Donini, Í.A.; De Salvi, D.T.; Fukumoto, F.K.; Lustri, W.R.; Barud, H.S.; Marchetto, R.; Messaddeq, Y.; Ribeiro, S.J. Biossíntese e recentes avanços na produção de celulose bacteriana. Eclética Química 2010, 35, 165–178. [Google Scholar] [CrossRef]
- Neera; Ramana, K.V.; Batra, H.V. Occurrence of cellulose-producing Gluconacetobacter spp. in fruit samples and kombucha tea, and production of the biopolymer. Appl. Biochem. Biotechnol. 2015, 176, 1162–1173. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]

| Technology | Advantages | Disadvantages |
|---|---|---|
| Gravity Separation |
|
|
| Centrifugal Separation |
|
|
| Flotation |
|
|
| Electrocoagulation |
|
|
| Adsorption |
|
|
| Filtration |
|
|
| Membrane Separation |
|
|
| Superhydrophobic/ Superoleophilic materials |
|
|
| Stimuli-Responsive Materials |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Wan, S.; Wen, C.; Tang, L.; Xu, N. Frontiers in Innovative Materials and Technologies for Oil–Water Separation. Polymers 2025, 17, 1635. https://doi.org/10.3390/polym17121635
Jiang J, Wan S, Wen C, Tang L, Xu N. Frontiers in Innovative Materials and Technologies for Oil–Water Separation. Polymers. 2025; 17(12):1635. https://doi.org/10.3390/polym17121635
Chicago/Turabian StyleJiang, Jikun, Shunda Wan, Cheng Wen, Li Tang, and Ning Xu. 2025. "Frontiers in Innovative Materials and Technologies for Oil–Water Separation" Polymers 17, no. 12: 1635. https://doi.org/10.3390/polym17121635
APA StyleJiang, J., Wan, S., Wen, C., Tang, L., & Xu, N. (2025). Frontiers in Innovative Materials and Technologies for Oil–Water Separation. Polymers, 17(12), 1635. https://doi.org/10.3390/polym17121635





