Investigation of the Effect of Exposure to Liquid Chemicals on the Strength Performance of 3D-Printed Parts from Different Filament Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Chemicals
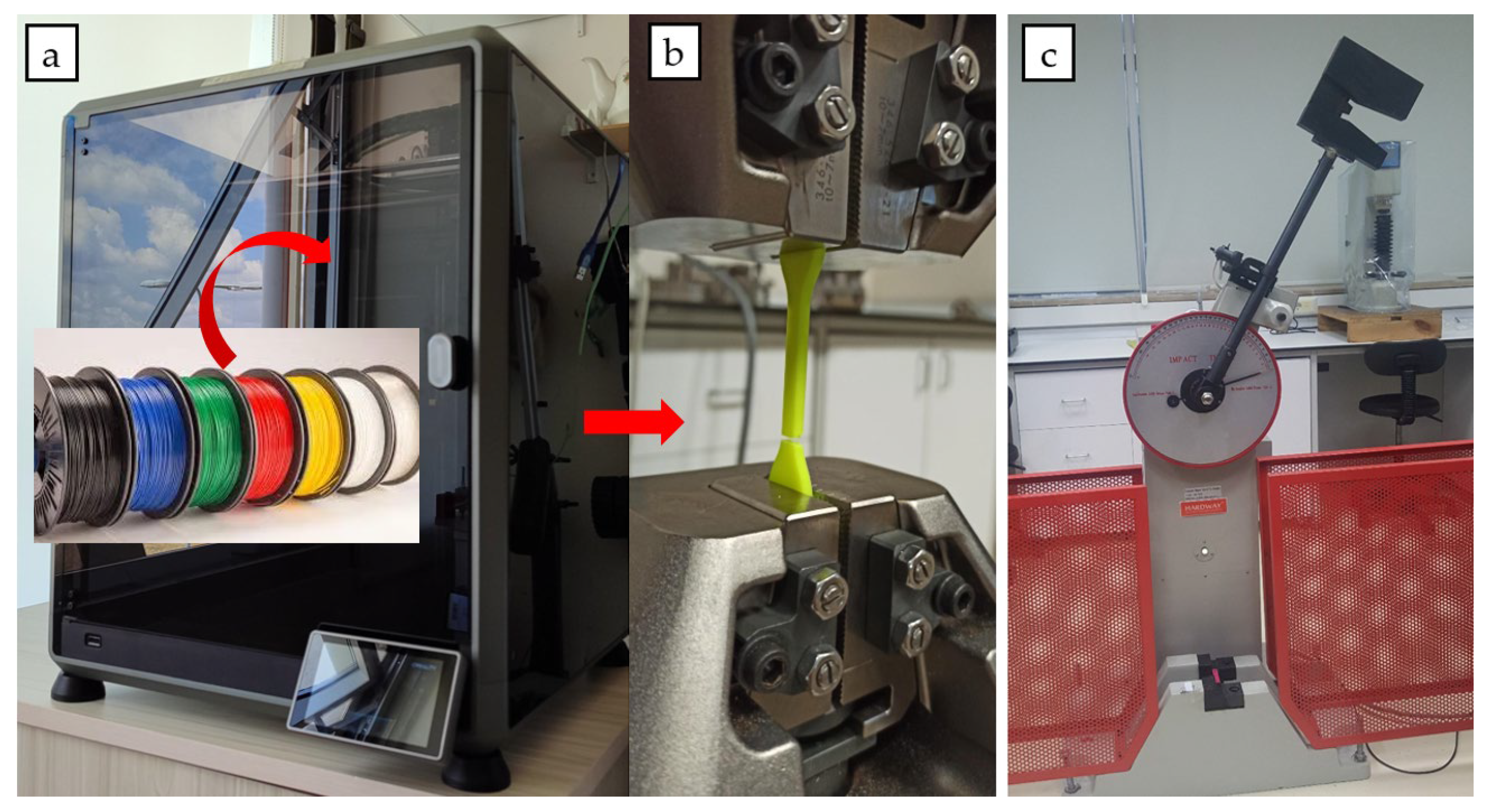
2.3. Specimen Preparation Experimental Procedure
3. Results
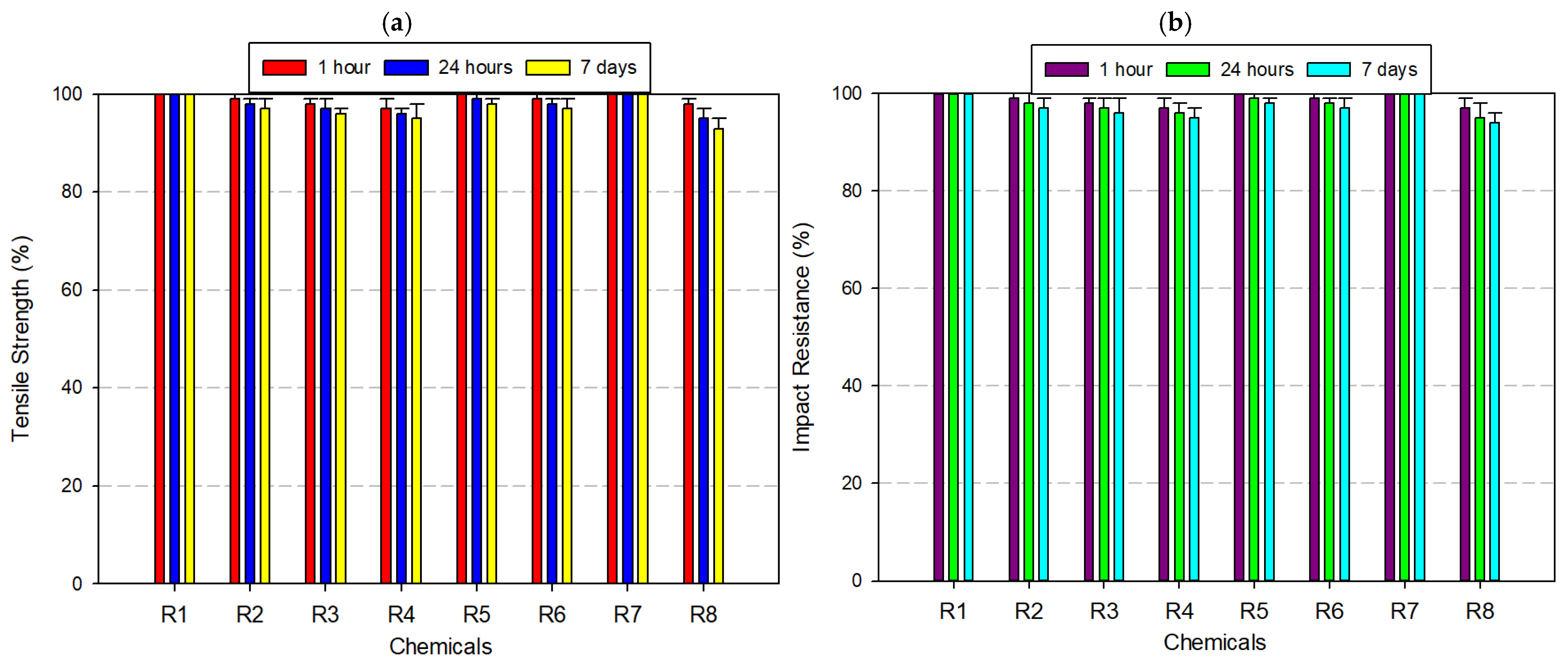
3.1. Tensile Strength and Izod Impact Test
3.2. Filaments Used in Chemical Resistance Analysis Tests Applied
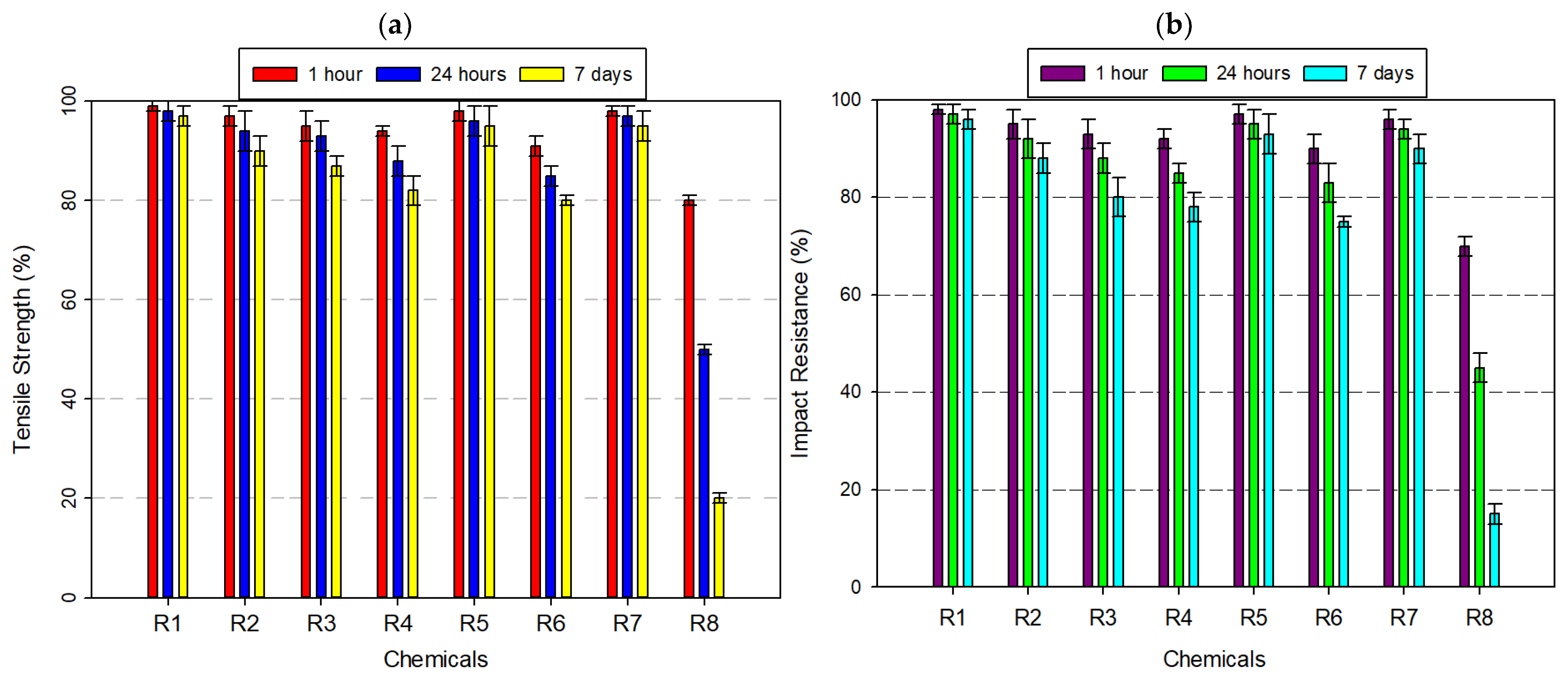
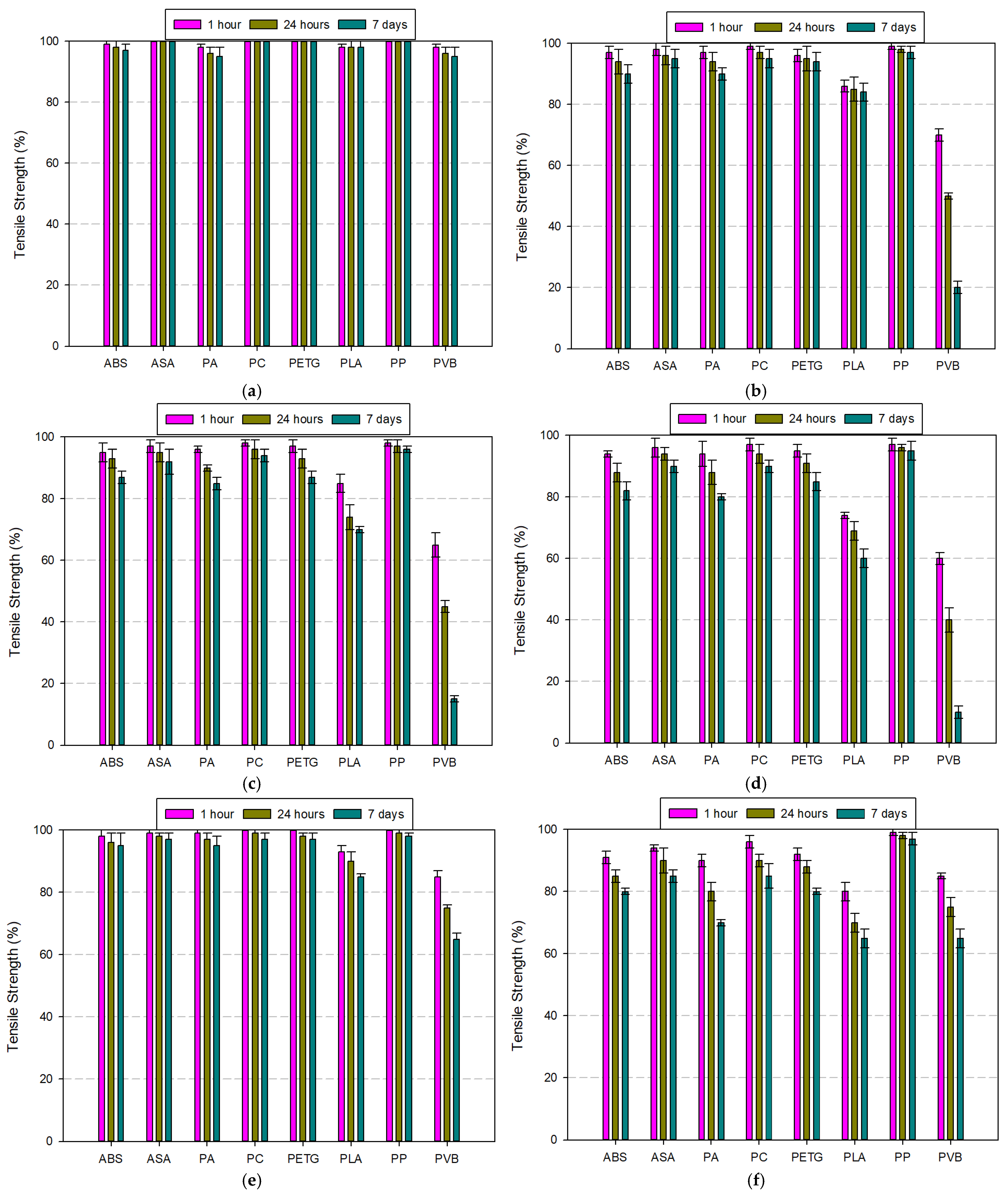
3.2.1. Tests Performed on Acrylonitrile Butadiene Styrene (ABS) Filament
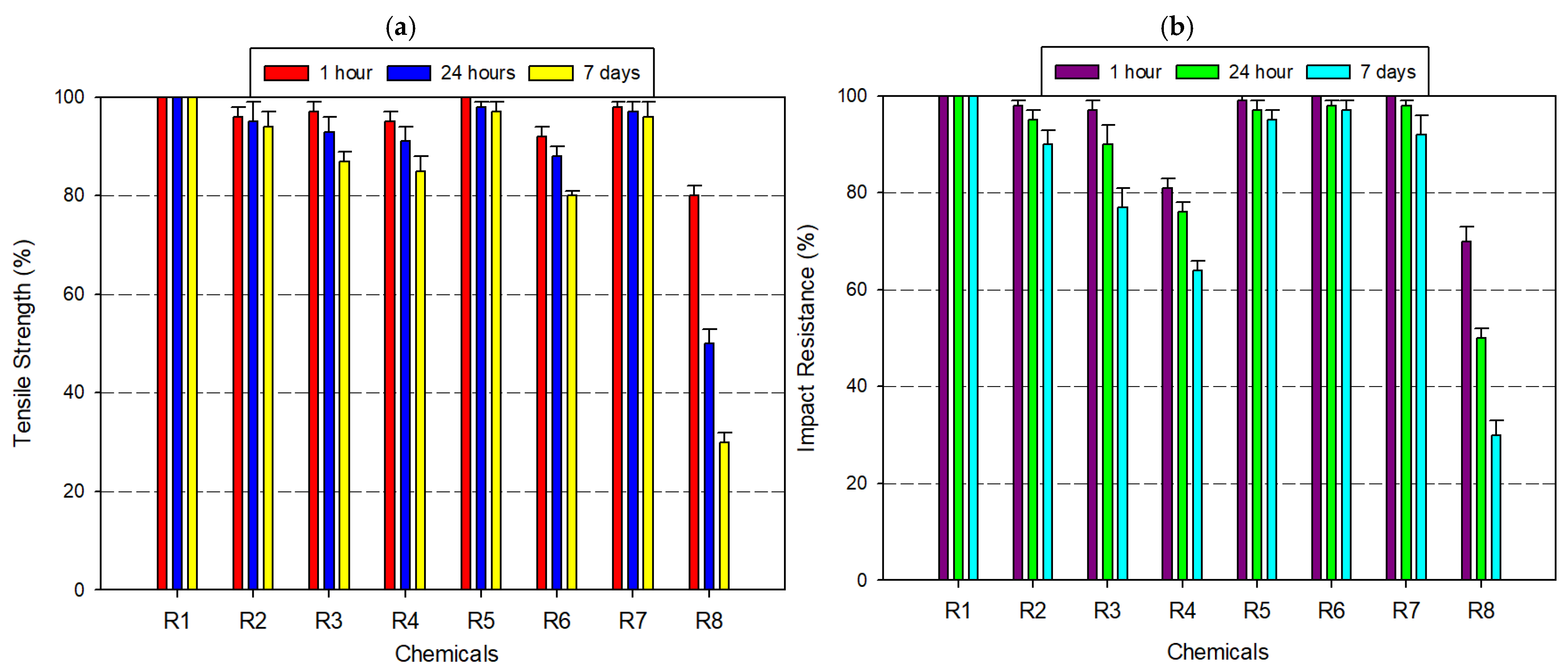
3.2.2. Tests Performed on Acrylonitrile Styrene Acrylate (ASA) Filament
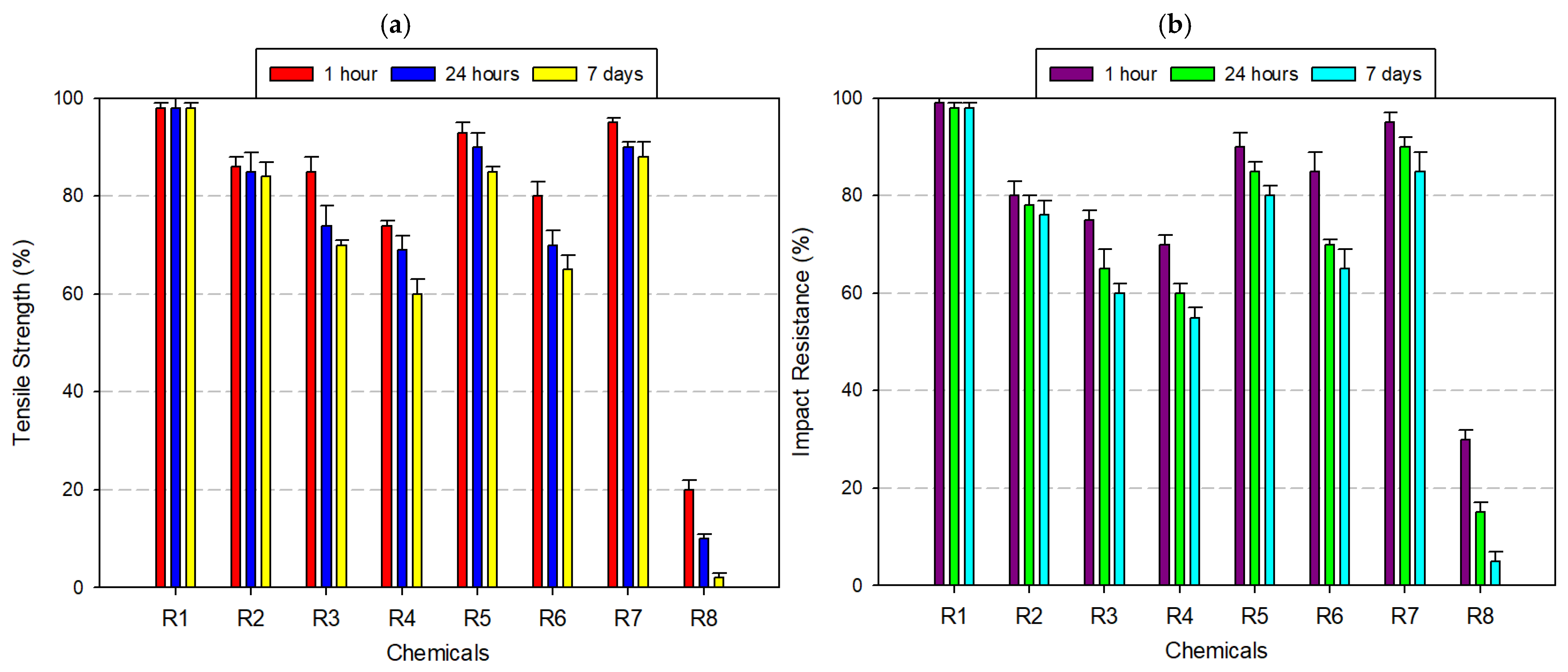
3.2.3. Tests Performed on Polyamide (PA) Filament
3.2.4. Tests Performed on Polycarbonate (PC) Filament
3.2.5. Tests Performed on Polyethylene Terephthalate Glycol (PETG) Filament
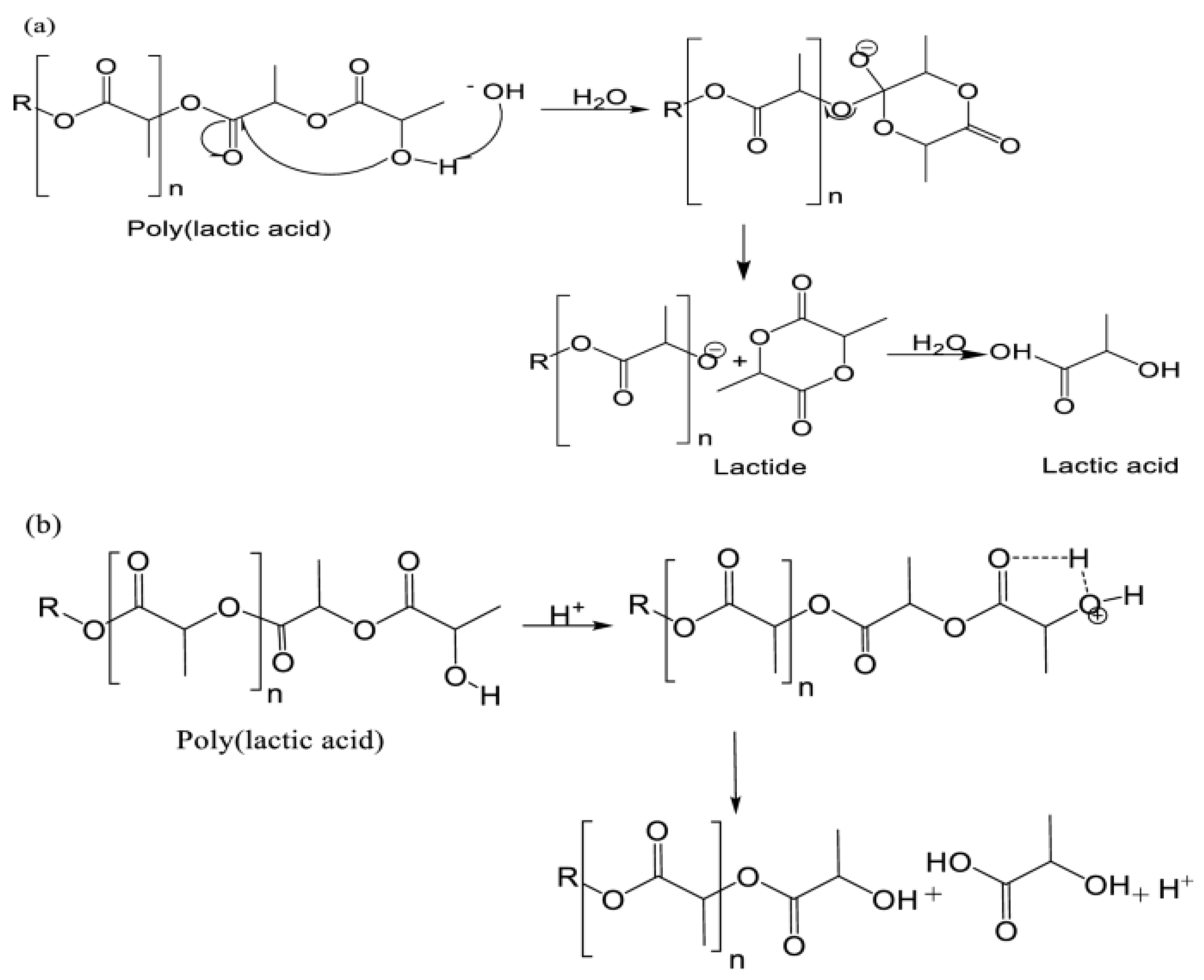
3.2.6. Tests Performed on Polylactic Acid (PLA) Filament
3.2.7. Tests Performed on Polypropylene (PP) Filament
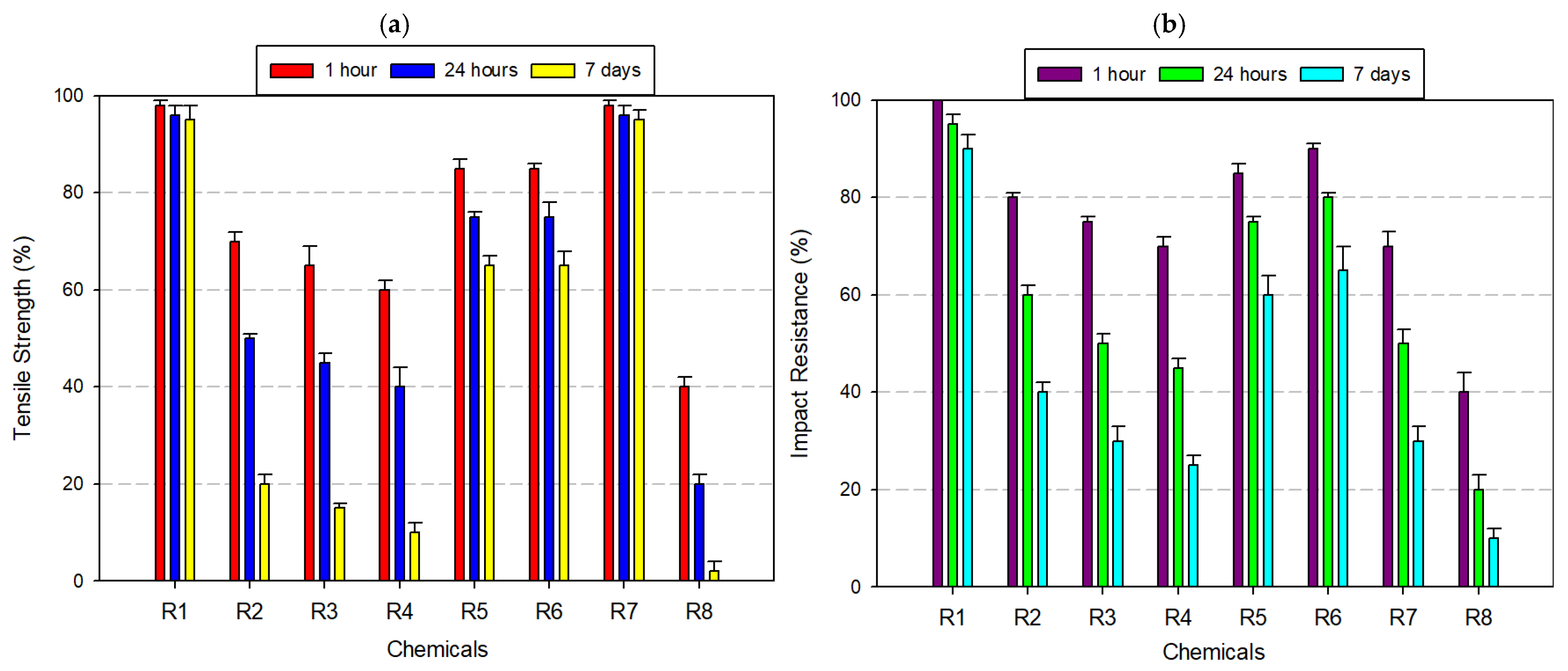
3.2.8. Tests Performed on Polyvinyl Butyral (PVB) Filament
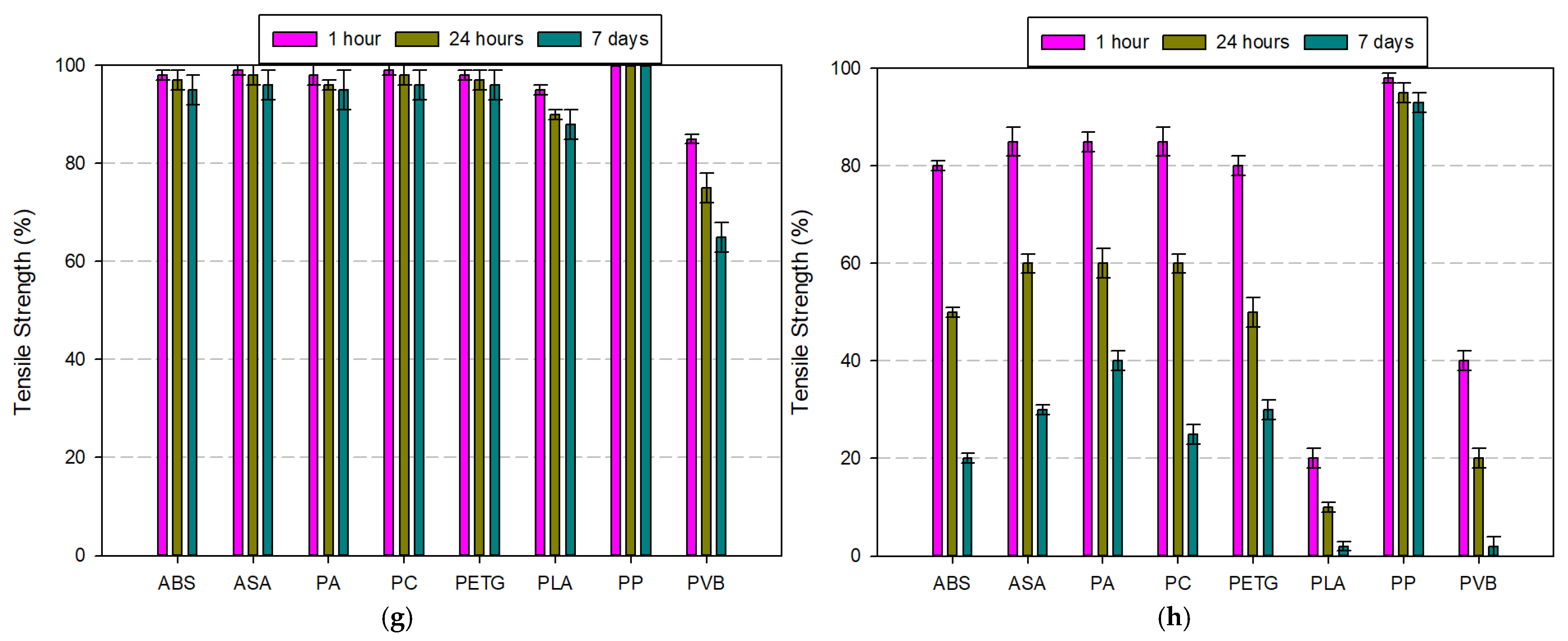
3.3. Comparison of Chemically Exposed Filaments
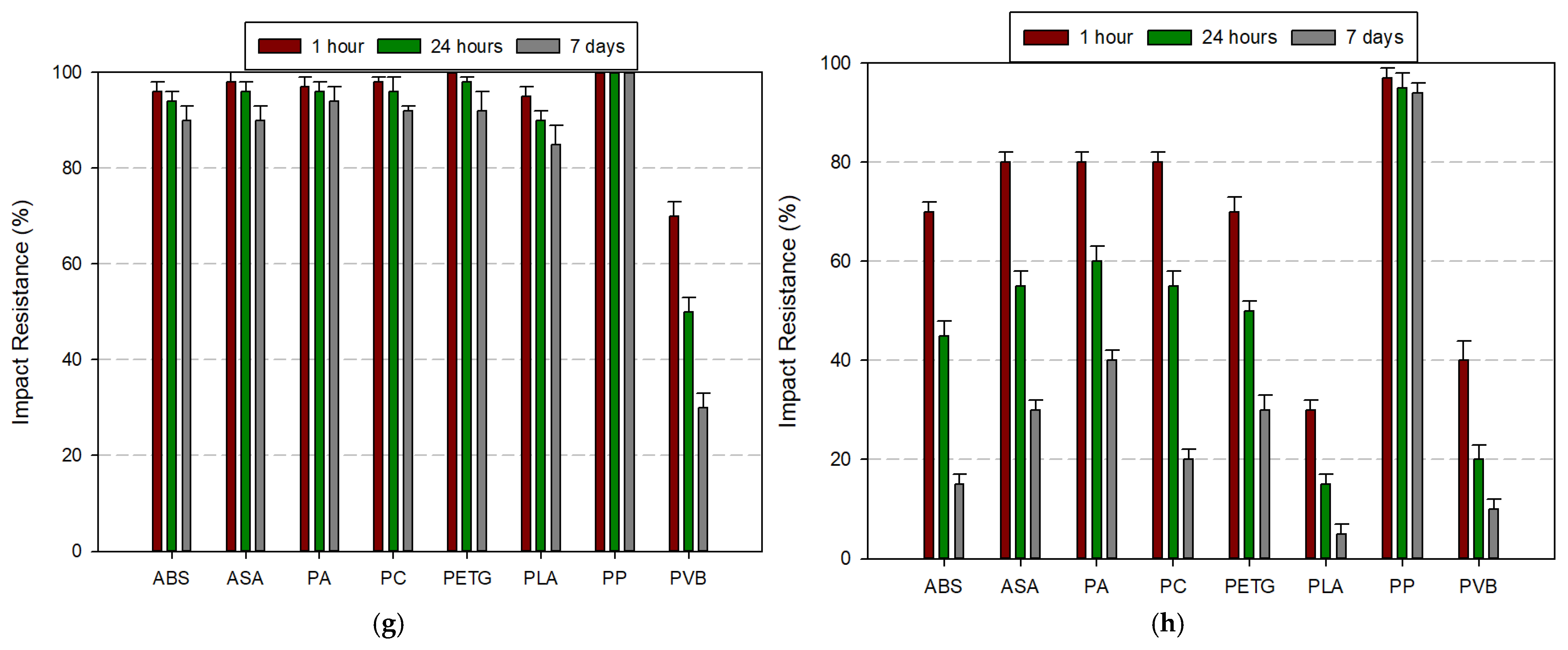
3.3.1. Tensile Strength Comparison
3.3.2. Impact Resistance Comparison
3.4. Correlation Analysis Between Tensile Strength and Impact Resistance
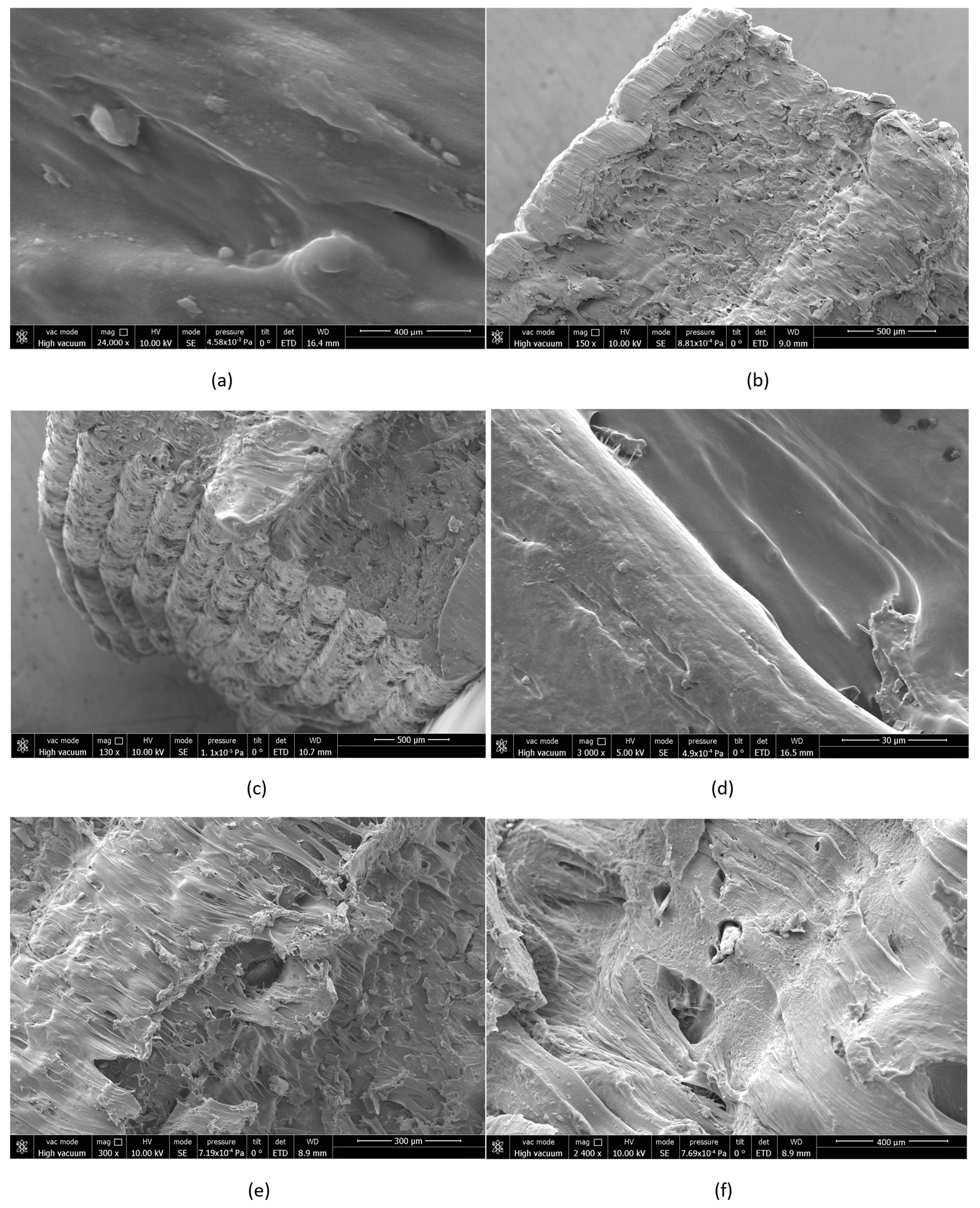
3.5. Scanning Electron Microscopy (SEM) Morphological Analysis
3.6. Summary Evaluation of Results According to Their Success Status
 ), yellow caution symbols (
), yellow caution symbols ( ), and red single (
), and red single ( ) or multiple caution symbols (
) or multiple caution symbols (
 and
and 

 ) effectively highlights the strength profile of each material. The number of red caution symbols is given in direct proportion to the losses in strength performance of the relevant filament type.
) effectively highlights the strength profile of each material. The number of red caution symbols is given in direct proportion to the losses in strength performance of the relevant filament type.
 or
or 

 ) symbols. This vulnerability is corroborated by Rezayat et al. (2024) and Csótár et al. (2025), who noted rapid loss of mechanical integrity in PLA under solvent-rich or alkaline environments [53,54].
) symbols. This vulnerability is corroborated by Rezayat et al. (2024) and Csótár et al. (2025), who noted rapid loss of mechanical integrity in PLA under solvent-rich or alkaline environments [53,54].4. Discussion
5. Conclusions
- PP is highly recommended for harsh chemical environments involving strong acids, oxidizers, or organic solvents, making it ideal for laboratory apparatus, fluid handling systems, and chemical-resistant mechanical enclosures;
- PETG and ASA, which maintained over 90% mechanical integrity in most conditions except acetone (99.5%), are suitable for consumer products, lightweight structural components, and UV-exposed outdoor applications;
- PLA and PVB, while cost-effective and esthetically advantageous, are best reserved for non-load-bearing and chemically benign contexts, such as educational prototypes, display parts, or biodegradable packaging;
- ABS, PC, and PA demonstrated moderate chemical resistance, and their use in solvent-prone or acidic environments should be approached with engineering caution and material-specific testing.
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Głowacki, M.; Mazurkiewicz, A.; Słomion, M.; Skórczewska, K. Resistance of 3D-printed components, test specimens and products to work under environmental conditions. Materials 2022, 15, 6162. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Jassim, M.; Ilcan, H.; Sahin, O.; Bayer, İ.R.; Sahmaran, M.; Koc, M. 3D printing of circular materials: Comparative environmental analysis of materials and construction techniques. Case Stud. Constr. Mater. 2023, 18, e02059. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Carrera, E.; Mena Córdova, N.; Fajardo, J.I.; Aliaga, P. Comparative analysis of fracture resistance between CAD/CAM materials for interim fixed prosthesis. Materials 2021, 14, 7791. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Alghamdi, R.; Al-Ghamdi, R.; Al-Jefri, A.; Akhtar, S.; Khan, S.Q.; al-Qarni, F.D. Wear and fracture resistance of 3D-printed denture teeth: An In vitro comparative study. J. Prosthodont. 2023, 32, 170–177. [Google Scholar] [CrossRef]
- Ronca, A.; Abbate, V.; Redaelli, D.F.; Storm, F.A.; Cesaro, G.; De Capitani, C.; Ambrosio, L. A Comparative Study for Material Selection in 3D Printing of Scoliosis Back Brace. Materials 2022, 15, 5724. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing–A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Habiba, R.; Amaro, A.; Trindade, D.; Moura, C.; Silva, R.; Antão, A.; Branco, R. Comparative Analysis of Impact Strength among Various Polymeric Materials for Orthotic Production. Polymers 2024, 16, 1843. [Google Scholar] [CrossRef]
- Kozior, T.; Mamun, A.; Trabelsi, M.; Sabantina, L. Comparative analysis of polymer composites produced by FFF and PJM 3D printing and electrospinning technologies for possible filter applications. Coatings 2022, 12, 48. [Google Scholar] [CrossRef]
- Valenti, C.; Federici, M.I.; Masciotti, F.; Marinucci, L.; Xhimitiku, I.; Cianetti, S.; Pagano, S. Mechanical properties of 3D printed prosthetic materials compared with milled and conventional processing: A systematic review and meta-analysis of in vitro studies. J. Prosthet. Dent. 2024, 132, 381–391. [Google Scholar] [CrossRef]
- Kartal, F. Abrasive Water Jet Machining of Carbon Fiber-Reinforced PLA Composites: Optimization of Machinability and Surface Integrity for High-Precision Applications. Polymers 2025, 17, 445. [Google Scholar] [CrossRef]
- Valls Esteve, A.; Lustig Gainza, P.; Adell Gomez, N.; Tejo Otero, A.; Englí Rueda, M.; Julian Alvarez, E.; Munuera, J. A state-of-the-art guide about the effects of sterilization processes on 3D-printed materials for surgical planning and medical applications: A comparative study. Int. J. Bioprinting 2023, 9, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tey, W.S.; Chen, J.; Zhu, W.; Liu, X.; Liu, T.; Zhou, K. Comparative study on 3D printing of polyamide 12 by selective laser sintering and multi jet fusion. J. Mater. Process. Technol. 2021, 288, 116882. [Google Scholar] [CrossRef]
- Borella, P.S.; Alvares, L.A.; Ribeiro, M.T.; Moura, G.F.; Soares, C.J.; Zancopé, K.; das Neves, F.D. Physical and mechanical properties of four 3D-printed resins at two different thick layers: An in vitro comparative study. Dent. Mater. 2023, 39, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Simoneti, D.M.; Pereira-Cenci, T.; Dos Santos, M.B.F. Comparison of material properties and biofilm formation in interim single crowns obtained by 3D printing and conventional methods. J. Prosthet. Dent. 2022, 127, 168–172. [Google Scholar] [CrossRef]
- Taşın, S.; Ismatullaev, A. Comparative evaluation of the effect of thermocycling on the mechanical properties of conventionally polymerized, CAD-CAM milled, and 3D-printed interim materials. J. Prosthet. Dent. 2022, 127, 173.e1–173.e8. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Mohseni, M.; Aslani, A.; Zahedi, R. Investigation of thermal performance and life-cycle assessment of a 3D printed building. Energy Build. 2022, 272, 112341. [Google Scholar] [CrossRef]
- Jain, S.; Sayed, M.E.; Shetty, M.; Alqahtani, S.M.; Al Wadei, M.H.D.; Gupta, S.G.; Sheayria, M.F. Physical and mechanical properties of 3D-printed provisional crowns and fixed dental prosthesis resins compared to CAD/CAM milled and conventional provisional resins: A systematic review and meta-analysis. Polymers 2022, 14, 2691. [Google Scholar] [CrossRef]
- Yan, R.; Meng, F.; Ke, G.; Jia, K. Comparative evaluation of the applicability of 3D printing mortar with different waste powders. Constr. Build. Mater. 2024, 443, 137825. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Vélez Chimbo, D.; Lupú, B.; Pacurucu, M.; Fárez, M.V.; Fajardo, J.I. Comparative Analysis of the Structural Weights of Fixed Prostheses of Zirconium Dioxide, Metal Ceramic, PMMA and 3DPP Printing Resin—Mechanical Implications. Dent. J. 2023, 11, 249. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Córdova, J.; Merchán, A.; Larriva, J.; Bravo, A.; Bernal, B.; Fajardo, J.I. Comparative Analysis of the Fracture Resistance of a Polymeric Material for 3D Printing and a Milled Polymethylmethacrylate Material as Interim Material for Fixed Partial Dentures: New Material Updated. Designs 2023, 7, 118. [Google Scholar] [CrossRef]
- Dimitrova, M.; Corsalini, M.; Kazakova, R.; Vlahova, A.; Chuchulska, B.; Barile, G.; Kazakov, S. Comparison between conventional PMMA and 3D printed resins for denture bases: A narrative review. J. Compos. Sci. 2022, 6, 87. [Google Scholar] [CrossRef]
- Otieno, D.B.; Bosire, G.O.; Onyari, J.M.; Mwabora, J.M. Comparative analysis of 3D-printed polylactic acid and acrylonitrile butadiene styrene: Experimental and Materials-Studio-based theoretical studies. J. Polym. Res. 2022, 29, 291. [Google Scholar] [CrossRef]
- Ogul, H.; Gultekin, B.; Bulut, F.; Us, H. A comparative study of 3D printing and sol-gel polymer production techniques: A case study on usage of ABS polymer for radiation shielding. Nucl. Eng. Technol. 2024, 56, 1943–1949. [Google Scholar] [CrossRef]
- Ali, M.H.; Batai, S.; Sarbassov, D. 3D printing: A critical review of current development and future prospects. Rapid Prototyp. J. 2019, 25, 1108–1126. [Google Scholar] [CrossRef]
- Pantea, M.; Ciocoiu, R.C.; Greabu, M.; Ripszky Totan, A.; Imre, M.; Țâncu, A.M.C.; Petre, A.E. Compressive and flexural strength of 3D-printed and conventional resins designated for interim fixed dental prostheses: An in vitro comparison. Materials 2022, 15, 3075. [Google Scholar] [CrossRef]
- Srinivas, D.; Dey, D.; Panda, B.; Sitharam, T.G. Printability, thermal and compressive strength properties of cementitious materials: A comparative study with silica fume and limestone. Materials 2022, 15, 8607. [Google Scholar] [CrossRef]
- Gad, M.M.; Alalawi, H.; Akhtar, S.; Al-Ghamdi, R.; Alghamdi, R.; Al-Jefri, A.; Al-Qarni, F.D. Strength and wear behavior of three-dimensional printed and prefabricated denture teeth: An in vitro comparative analysis. Eur. J. Dent. 2023, 17, 1248–1256. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Hozdić, E. Characterization and Comparative Analysis of Mechanical Parameters of FDM-and SLA-Printed ABS Materials. Appl. Sci. 2024, 14, 649. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pal, A.K.; Rodriguez, A.U.; Wu, F.; Misra, M.; Mielewski, D.F.; Mohanty, A.K. Recycled poly (lactic acid)–based 3D printed sustainable biocomposites: A comparative study with injection molding. Mater. Today Sustain. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Alarifi, I.M. Revolutionising fabrication advances and applications of 3D printing with composite materials: A review. Virtual Phys. Prototyp. 2024, 19, e2390504. [Google Scholar] [CrossRef]
- Kozior, T.; Hanon, M.M.; Zmarzły, P.; Gogolewski, D.; Rudnik, M.; Szot, W. Evaluation of the influence of technological parameters of selected 3D printing technologies on tribological properties. 3D Print. Addit. Manuf. 2024, 11, e1567–e1580. [Google Scholar] [CrossRef]
- Saidulu, D.; Srivastava, A.; Gupta, A.K. Enhancement of wastewater treatment performance using 3D printed structures: A major focus on material composition, performance, challenges, and sustainable assessment. J. Environ. Manag. 2022, 306, 114461. [Google Scholar] [CrossRef]
- León-Calero, M.; Reyburn Valés, S.C.; Marcos-Fernández, Á.; Rodríguez-Hernandez, J. 3D printing of thermoplastic elastomers: Role of the chemical composition and printing parameters in the production of parts with controlled energy absorption and damping capacity. Polymers 2021, 13, 3551. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Malysa, A.; Mikulewicz, M. Comparison of the compression and tensile modulus of two chosen resins used in dentistry for 3D printing. Materials 2022, 15, 8956. [Google Scholar] [CrossRef]
- Joo, H.; Cho, S. Comparative studies on polyurethane composites filled with polyaniline and graphene for DLP-type 3D printing. Polymers 2020, 12, 67. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Fu, J.; He, Y. A review of 3D printing technologies for soft polymer materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Shoaei, P.; Gallantree-Smith, H.; Pacheco, V.M.; Pamies, R.; Kjøniksen, A.L.; Pilehvar, S. Comparative analysis of 3D printing of Portland cement mortars with hydroxypropyl methylcellulose and microfibrillated cellulose as viscosity modifying agents. Mater. Des. 2024, 244, 113124. [Google Scholar] [CrossRef]
- Campos, M.R.D.; Oliveira, T.T.D.; Botelho, A.L.; Reis, A.C.D. Mechanical, Chemical, and Biological Properties of 3D-Printed Abutments: A Systematic Review. J. Adv. Oral Res. 2022, 13, 167–175. [Google Scholar] [CrossRef]
- Aydin, E.M.; Kara, B.; Bundur, Z.B.; Ozyurt, N.; Bebek, O.; Gulgun, M.A. A comparative evaluation of sepiolite and nano-montmorillonite on the rheology of cementitious materials for 3D printing. Constr. Build. Mater. 2022, 350, 128935. [Google Scholar] [CrossRef]
- Erokhin, K.S.; Gordeev, E.G.; Ananikov, V.P. Revealing interactions of layered polymeric materials at solid-liquid interface for building solvent compatibility charts for 3D printing applications. Sci. Rep. 2019, 9, 20177. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, B.; Cambriani, A.; Cespi, M.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. An overview of natural polymers as reinforcing agents for 3D printing. ChemEngineering 2021, 5, 78. [Google Scholar] [CrossRef]
- Benwood, C.; Anstey, A.; Andrzejewski, J.; Misra, M.; Mohanty, A.K. Improving the impact strength and heat resistance of 3D printed models: Structure, property, and processing correlationships during fused deposition modeling (FDM) of poly (lactic acid). ACS Omega 2018, 3, 4400–4411. [Google Scholar] [CrossRef]
- Kim, H.J.; Lim, S.W.; Lee, M.K.; Ju, S.W.; Park, S.H.; Ahn, J.S.; Hwang, K.G. Which three-dimensional printing technology can replace conventional manual method of manufacturing oral appliance? A preliminary comparative study of physical and mechanical properties. Appl. Sci. 2021, 12, 130. [Google Scholar] [CrossRef]
- Kaikai, X.; Yadong, G.; Qiang, Z. Comparison of traditional processing and additive manufacturing technologies in various performance aspects: A review. Arch. Civ. Mech. Eng. 2023, 23, 188. [Google Scholar] [CrossRef]
- Sikora, P.; Techman, M.; Federowicz, K.; El-Khayatt, A.M.; Saudi, H.A.; Abd Elrahman, M.; Chung, S.Y. Insight into the microstructural and durability characteristics of 3D printed concrete: Cast versus printed specimens. Case Stud. Constr. Mater. 2022, 17, e01320. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D256-10; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM: West Conshohocken, PA, USA, 2015.
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Romero, L.M.; Esquivel, S.E.; Montaño, M.C.; Medina-Muñoz, C.; Sánchez-Sanhueza, G.A.; Palacio, D.A.; Jaramillo, A.F.; Meléndrez, M.F. Polypropylene with clay–filled for fused filament fabrication: Comparative study of the mechanical performance of injected and 3D printed composite. Int. J. Adv. Manuf. Technol. 2024, 127, 1835–1848. [Google Scholar] [CrossRef]
- Rezayat, M.; Karamimoghadam, M.; Dezaki, M.L.; Zolfagharian, A.; Casalino, G.; Mateo, A.; Bodaghi, M. Enhancing Corrosion Resistance and Mechanical Strength of 3D-Printed Iron Polylactic Acid for Marine Applications via Laser Surface Texturing. Adv. Eng. Mater. 2024, 26, 2301123. [Google Scholar] [CrossRef]
- Csótár, H.; Szalai, S.; Kurhan, D.; Sysyn, M.; Fischer, S. Evaluating 3D-Printed Polylactic Acid (PLA)-Reinforced Materials: Mechanical Performance and Chemical Stability in Concrete Mediums. Appl. Sci. 2025, 15, 2165. [Google Scholar] [CrossRef]
- Kantaros, A.; Katsantoni, M.; Ganetsos, T.; Petrescu, N. The Evolution of Thermoplastic Raw Materials in High-Speed FFF/FDM 3D Printing Era: Challenges and Opportunities. Materials 2025, 18, 1220. [Google Scholar] [CrossRef] [PubMed]
- Jash, C.; Birla, P.N.; Naveenraj, R.; Shinde, M.D.; Waghulde, K.B.; Rane, S.B. Solution chemistry-assisted preparation of ZnO NPs–PLA nanocomposites and their filaments for fused filament fabrication 3D printing process. Prog. Addit. Manuf. 2025, 10, 2505–2518. [Google Scholar] [CrossRef]
- Rodriguez, E.J.; Marcos, B.; Huneault, M.A. Hydrolysis of polylactide in aqueous media. J. Appl. Polym. Sci. 2016, 133, 44152. [Google Scholar] [CrossRef]
- Codari, F.; Lazzari, S.; Soos, M.; Storti, G.; Morbidelli, M.; Moscatelli, D. Kinetics of the hydrolytic degradation of poly (lactic acid). Polym. Degrad. Stab. 2012, 97, 2460–2466. [Google Scholar] [CrossRef]
- Lazzari, S.; Codari, F.; Storti, G.; Morbidelli, M.; Moscatelli, D. Modeling the pH-dependent PLA oligomer degradation kinetics. Polym. Degrad. Stab. 2014, 110, 80–90. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-Van den Bosch, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly (lactic acid): Participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Van Nostrum, C.F.; Veldhuis, T.F.; Bos, G.W.; Hennink, W.E. Hydrolytic degradation of oligo (lactic acid): A kinetic and mechanistic study. Polymer 2004, 45, 6779–6787. [Google Scholar] [CrossRef]
- Cam, D.; Hyon, S.H.; Ikada, Y. Degradation of high molecular weight poly (L-lactide) in alkaline medium. Biomaterials 1995, 16, 833–843. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly (L-lactide). II. Hydrolysis in alkaline solution. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 59–66. [Google Scholar] [CrossRef]
- Razavi, M.; Wang, S.Q. Why is crystalline poly(lactic acid) brittle at room temperature? Macromolecules 2019, 52, 5429–5441. [Google Scholar] [CrossRef]
- Velghe, I.; Buffel, B.; Vandeginste, V.; Thielemans, W.; Desplentere, F. Review on the degradation of poly(lactic acid) during melt processing. Polymers 2023, 15, 2047. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-based sustainable food packaging materials: Challenges and applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation behavior of PBAT, PLA, and their blends in freshwater. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Kralin, A.; Kamjaikittikul, A.; Lertvilai, P.; Sirisaksoontorn, W.; Jamnongkan, T. Homogeneous biopolymer films based on PBAT for packaging applications. J. Mater. Sci. 2025, 60, 7410–7427. [Google Scholar] [CrossRef]







 : successful,
: successful,  : average,
: average,  : weak,
: weak, 
 : very weak,
: very weak, 

 : unsuccessful.
: unsuccessful.
 : successful,
: successful,  : average,
: average,  : weak,
: weak, 
 : very weak,
: very weak, 

 : unsuccessful.
: unsuccessful.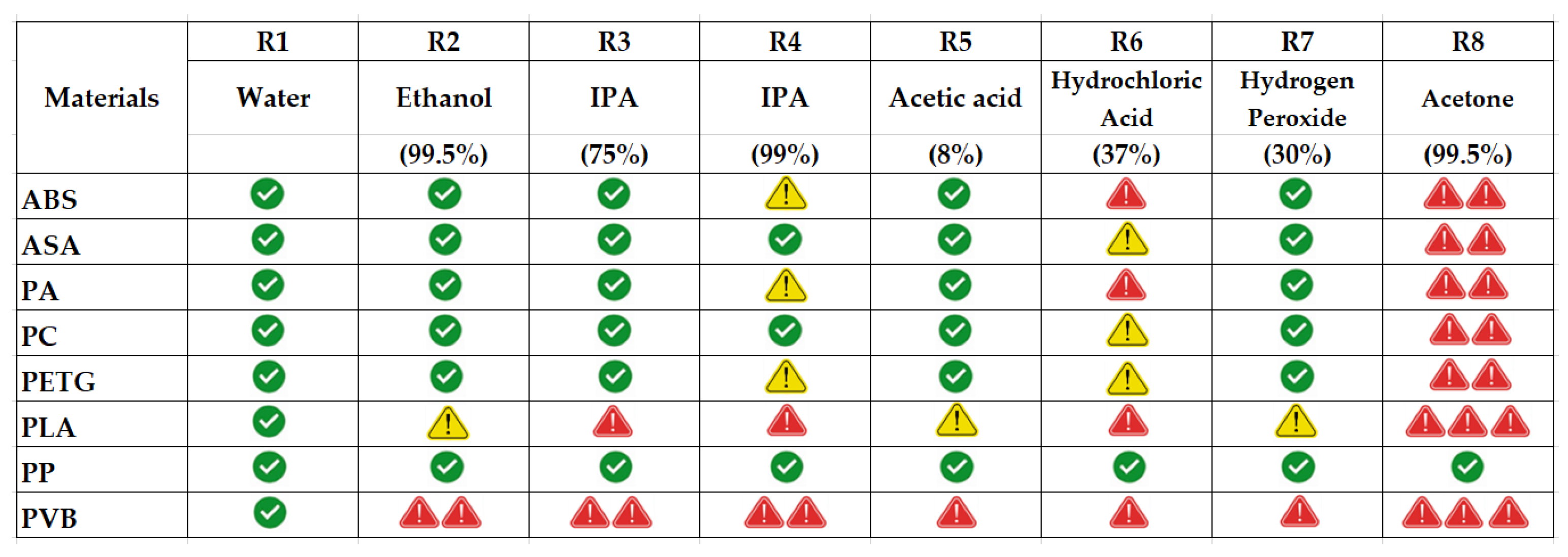
| Materials | Trade Names | Manufacturers | Countrys of Origin |
|---|---|---|---|
| ABS | ABS-M30 | Stratasys | Minnesota, USA |
| ASA | ASA-Pro | Polymaker | Houston, USA |
| PA | Nylon 230 | Taulman 3D | California, USA |
| PC | PC-Max | Polymaker | Houston, USA |
| PETG | PETG-X | Prusa | Prague, Czech Republic |
| PLA | PLA+ Premium | eSUN | Shenzhen, China |
| PP | PP-Natural | Ultimaker | Geldermalsen, Netherlands |
| PVB | BVOH Blend | Fillamentum | Hulin, Czech Republic |
| Material | Properties | Applications |
|---|---|---|
| ABS | High impact resistance, high heat resistance. | Automotive parts, electronic housings, toys. |
| ASA | UV and weather-resistant, good heat resistance. | Outdoor furniture, automotive parts, garden equipment. |
| PA | Flexible, high wear resistance, strong mechanical properties. | Gears, bearings, textile applications. |
| PC | High durability, impact-resistant, heat-resistant. | Safety glasses, electronic housings, machinery parts. |
| PETG | Strong, flexible, resistant to water and chemicals. | Durable prototypes, food packaging, chemical-resistant. |
| PLA | Biodegradable, derived from renewable resources. | Prototyping, educational prints, temporary projects. |
| PP | Chemical resistance, flexibility, low density. | Chemical containers, automotive parts, food packaging. |
| PVB | Transparency, smooth finish, good adhesion properties. | Laminated glass layers, decorative parts, adhesive layers. |
| Chemicals | Formulas | Codes | Properties | Applications |
|---|---|---|---|---|
| Water | H2O | R1 | Baseline, neutral substance | Testing material reactions, general-purpose solvent |
| Ethanol (99.5%) | C2H6O | R2 | Alcohol, solvent, disinfectant | Surface cleaning, hand sanitizers, alcoholic beverages |
| Isopropyl Alcohol (IPA) (75%) | C3H8O | R3 | Solvent, cleaning and sterilization agent | Cleaning, sterilizing surfaces, electronics |
| Isopropyl Alcohol (IPA) (99%) | C3H8O | R4 | Solvent, cleaning and sterilization agent | Cleaning, sterilizing surfaces, electronics |
| Acetic Acid (8%) | CH3COOH | R5 | Weak acid, antibacterial properties | Household cleaning, food preparation (vinegar) |
| Hydrochloric Acid (37%) | HCl | R6 | Strong acid, highly reactive | Industrial cleaning, chemical processing |
| Hydrogen Peroxide (30%) | H2O2 | R7 | Strong oxidizer, sterilizing agent | Medical sterilization, cleaning, bleaching |
| Acetone (99.5%) | C3H6O | R8 | Strong solvent, dissolves many plastics | Surface cleaning, paint thinning, nail polish remover |
| Parameter | Value/Description |
|---|---|
| Layer Height | 0.20 mm |
| Infill Density | 100% |
| Printing Speed | 50 mm/s |
| Build Orientation | Vertical (Z-axis) |
| Nozzle Temperature | PLA: 210–215 °C ABS/ASA: 260–270 °C PETG: 220–250 °C PA: 240–270 °C PP: 230–260 °C PVB: 210–240 °C PC: 260–310 °C |
| Bed Temperature | PLA: 60 °C ABS/ASA: 110 °C PETG: 50-80 °C PA: 50 °C PP: 65–85 °C PVB: 40–65 °C PC: 90–120 °C |
| Filament Storage | Stored in a desiccated cabinet with humidity maintained below 15% |
| Materials | Tensile Strength (MPa) Value ± SD | Izod Impact Test (J/m) Value ± SD |
|---|---|---|
| ABS | 35.03 ± 2.21 | 200.51 ± 5.37 |
| ASA | 43.15 ± 1.42 | 145.26 ± 3.07 |
| PA | 60.23 ± 1.17 | 84.12 ± 1.09 |
| PC | 62.09 ± 3.37 | 725.06 ± 8.46 |
| PETG | 40.31 ± 1.08 | 75.77 ± 1.34 |
| PLA | 54.28 ± 1.75 | 16.36 ± 1.25 |
| PP | 33.11 ± 2.05 | 150.51 ± 4.79 |
| PVB | 27.04 ± 1.34 | 30.06 ± 2.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaptan, A. Investigation of the Effect of Exposure to Liquid Chemicals on the Strength Performance of 3D-Printed Parts from Different Filament Types. Polymers 2025, 17, 1637. https://doi.org/10.3390/polym17121637
Kaptan A. Investigation of the Effect of Exposure to Liquid Chemicals on the Strength Performance of 3D-Printed Parts from Different Filament Types. Polymers. 2025; 17(12):1637. https://doi.org/10.3390/polym17121637
Chicago/Turabian StyleKaptan, Arslan. 2025. "Investigation of the Effect of Exposure to Liquid Chemicals on the Strength Performance of 3D-Printed Parts from Different Filament Types" Polymers 17, no. 12: 1637. https://doi.org/10.3390/polym17121637
APA StyleKaptan, A. (2025). Investigation of the Effect of Exposure to Liquid Chemicals on the Strength Performance of 3D-Printed Parts from Different Filament Types. Polymers, 17(12), 1637. https://doi.org/10.3390/polym17121637





